Abstract
Background
Atopic dermatitis (AD) and allergic contact dermatitis (ACD) are both forms of eczema and are common inflammatory skin diseases with a central role of Th22-derived IL-22 in their pathogenesis. Although prostaglandin E2 (PGE2) is known to promote inflammation, little is known about its role in processes related to AD and ACD development, including IL-22 up-regulation.
Objectives
To investigate whether PGE2 has a role in IL-22 induction and development of ACD, which has increased prevalence in patients with AD.
Methods
T-cell cultures and in vivo sensitization of mice with haptens were used to assess the role of PGE2 in production of IL-22. The involvement of PGE2 receptors and their downstream signals were also examined. The effects of PGE2 were evaluated using the oxazolone (OXA)-induced ACD mouse model. The relationship of PGE2 and IL-22 signaling pathways in skin inflammation were also investigated using genomic profiling in human lesional AD skin.
Results
PGE2 induces IL-22 from T cells through its receptors EP2 and EP4 and involves cyclic adenosine monophosphate (cAMP) signaling. Selective deletion of EP4 in T-cells prevents hapten-induced IL-22 production in vivo, and inhibition of PGE2 synthesis limits atopic-like skin inflammation in the OXA-induced ACD model. Moreover, both PGE2 and IL-22 pathway genes were coordinately up-regulated in human AD lesional skin, but were below significant detection levels after corticosteroid or ultraviolet band B (UVB) treatments.
Conclusions
Our results define a crucial role for PGE2 in promoting ACD by facilitating IL-22 production from T-cells.
Keywords: Allergic contact dermatitis, Atopic dermatitis, Prostaglandin E2, CD4+ T cells, Th22 cells, Th17 cells, Interleukin 22
Introduction
Atopic dermatitis (AD) and allergic contact dermatitis (ACD) are both forms of eczema and are common chronic inflammatory skin diseases. The prevalence of AD is up to 3-7% in adults and up to 25% among children1,2 and the prevalence of ACD is up to 7% among the general population3. ACD and AD potentially share common cellular mechanisms and there is an increased rate of ACD in patients with AD3. Histologic features of affected eczematous skin include epidermal hyperplasia and spongiosis, and infiltration of immune cells (T-cells, dendritic cells/DCs, eosinophils, etc) in the dermis1,3,4. Barrier dysfunction is also recognized as important for development of AD5–7. In response to antigen and hapten sensitization, keratinocytes are activated to produce pro-inflammatory cytokines. Concurrently skin resident innate immune cells like dendritic cells capture antigens and migrate to secondary lymphocytes, where they present antigens to T lymphocytes for their activation and differentiation into effector T cells, resulting in ACD initiation when the antigen or hapten is repeatedly applied to the skin3. Both type 1 and 2 helper T (Th1 and Th2) cells, characterized by production of IFN-γ or IL-4/IL-13, respectively, has been thought to contribute to ACD pathogenesis depending on the antigens8. For example, while the majority of haptens (e.g. 1-Fluoro-2,4-dinitrobenzene) induce a Th1-dominant response, some others like fluorescein isothiocyanate induce Th2-dominant responses9. While single hapten elicitation induces delayed-type hypersensititivity, as a mouse model of human contact dermatitis10, repeated hapten challenge results in a chronic late-phase and immediate-type hypersensitivity response, which are clinically relevant to the acute phase of AD in humans11–13. However, recent studies have shown that IL-17 and IL-22, produced by Th17 and other activated T cells including Th22 cells, are also critical in mediating the initiation and progression of ACD and AD14–20. In patients with ACD to nickel, there were upregulated levels of IL-22 in both serum and inflamed skin19,20. Furthermore, IL-22 levels in serum from patients with AD are higher than those from healthy individuals21,22, and IL-22+ T cells infiltrate into lesional AD skin23, where IL-22 induces epidermal hyperplasia and inhibits epidermal differentiation24,25. Importantly, a neutralizing anti-IL-22 antibody (NCT01941537) or targeting components of the IL-23/Th17 pathway, are currently being tested in clinical trials4,26. To refine future therapies, there is a need to better understand the mechanisms that drive IL-22 production in response to cutaneous antigen stimulation.
Prostanoids, including prostaglandin D2 (PGD2), PGE2, PGF2α, PGI2 and thromboxane A2, are bioactive lipid mediators that are generated from arachidonic acid by cyclooxygenases (COX) and then respective PG synthases. PGs have various roles in inflammatory skin diseases through regulating functions of immune cells, including Th1/Th17 T-cells, T regulatory cells, mast cells and DCs27. PGE2 is synthesized by microsomal prostaglandin E synthases (mPGES1 and mPGES2) and has essential roles in modulating various inflammatory responses by binding to PG receptors (EP1-4) on cell surfaces. Many cutaneous cells, including keratinocytes, mast cells, eosinophils, fibroblasts, DCs and lymphocytes, express PGs and PG receptors28. Increased PGE2 expression in biologically active amounts has been reported in both lesional and non-lesional skin from AD patients29. Blockade of PG production by a COX2 inhibitor was reported to enhance eosinophil infiltration and elevate IL-4 expression in lesions of an OVA-sensitized mouse model30. In contrast, PGE2 was also suggested to induce AD by favoring a Th2 immune milieu and directly enhancing B-cell production of IgE31,32. Furthermore, we and others have recently reported that PGE2 through EP2 and EP4 receptors augmented IL-17 and IL-22 productions, and blockade of PGE2 signaling during T-cell differentiation limited acute contact hypersensitivity33–35. These findings suggest that PGE2 may have both suppressive and provocative roles in the development of ACD and AD28. However, it is unclear how PGE2 regulates IL-22 production and chronic, atopic skin inflammation.
Here we report that PGE2 promotes IL-22 production from Th17 and Th22 T-cells through its receptors EP2 and EP4. This effect is mediated by cAMP-protein kinase A (PKA) signaling and induction of aryl hydrocarbon receptor (AHR), a transcription factor critical for both adaptive and innate IL-22 expression36. T-cell specific EP4 deficiency diminishes hapten-induced IL-22-expressing T cells in skin-draining lymph nodes (LNs). Accordingly, inhibition of PGE2 production limits skin inflammation in an animal model for ACD induced by repeated OXA challenges in mice. Furthermore, genes related to PGE2 signaling are over-expressed in human atopic lesional skin, and positively correlate with expression of IL-22 pathway genes. This relationship between expression of PGE2 and IL-22-related genes is no longer evident after successful corticosteroid or UVB treatments. These findings suggest that PGE2 facilitates ACD through promoting adaptive IL-22 signaling.
Methods
Mice
Wild-type C57BL/6 mice were purchased from Harlan UK. Lck-Cre mice were crossed to lox-flanked Ptger4 (EP4-floxed) mice37 to generate mice with selective EP4 deficiency in T cells (EP4cKO) as previously reported34,35. All mice were bred and maintained under specific pathogen-free conditions in accredited animal facilities in the University of Edinburgh and Kyoto University. Mice were aged >7 weeks old at the beginning of use and sex-matched. All experiments were conducted in accordance with the UK Scientific Procedures Act of 1986 and had local institutional ethical approval.
Reagents
Antibodies to mouse CD3 (clone 145-2C11), CD28 (clone 37.51), CD45 (clone 30-F11), CD4 (clone L3T4), CD8 (clone 53-6.7), IL-17A (clone eBio17B7) and IL-22 (clone IL22J0P) were from eBioscience or Biolegend. Mouse CD4 microbeads were from Miltenyi Biotec. Recombinant human TGF-β1 and recombinant mouse IL-6 and IL-23 were purchased from Biolegend. PGE2, 17-phenyl trinor PGE2 (EP1/3 agonist), Butaprost (EP2 agonist), CAY10598 or L-902,688 (EP4 agonist), PF-04418948 (EP2 antagonist), and L-161,982 (EP4 antagonist) were from Cayman. Db-cAMP, PKA Inhibitor 14-22 (PKI), CH-223191, oxalozone, indomethacin, phorbol myristate acetate (PMA), Ionomycin were from Sigma or Calbiochem.
Oxazolone-induced ACD model
The OXA-induced mouse ACD model was induced as reported11. Briefly, mice were sensitized with 3% OXA in EtOH on shaved abdominal skin and after 5 days were repeatedly challenged with 0.6% OXA in EtOH on one ear once every two days for a total of 5 challenges. The opposing ear was challenged with vehicle (pure EtOH) to serve as a control. Mice were culled at 6 h after the last challenge and the ears and ear-draining LNs collected for further analysis. Ear samples were fixed with 10% neutral buffered formalin solution (Sigma), embedded in paraffin wax, and 5μm sections used for staining with hematoxylin and eosin (H&E). In some experiments, EP4cKO or control mice were sensitized with 0.5% dinitrofluorobenzene (DNFB), and 5 days later skin-draining LNs were collected for further analysis.
T-cell isolation and culture
Mouse CD4+ T-cells were isolated from spleens using autoMACS (Miltenyi). Cells were cultured in complete RPMI1640 medium containing 10% FBS and stimulated with plate-bound anti-CD3 (5 μg/ml) and anti-CD28 (5 μg/ml) antibodies plus various cytokines and other compounds as indicated in figures. For certain experiments, Th17 cells were differentiated from CD4+ T-cells by TGF-β1 and IL-6 for 3-4 d. IL-22 levels in supernatants were measured using mouse IL-22 enzyme-linked immunosorbent assay (ELISA) Ready-SET-Go! kits (eBioscience).
Surface and intracellular staining
For surface staining, cells were stained on ice for 30 min with anti-CD45, anti-CD3e, anti-CD4 and anti-CD8 Abs. For intracellular staining of IL-22, cells were stimulated with PMA and ionomycin for 4-5h in the presence of GolgiPlug (BD Bioscience). Cells were then harvested and fixed by BD Cytofix/Cytoperm Fixation Buffer (BD Bioscience) for 30min and then stained with anti-human/mouse IL-22 (clone IL22JOP, eBioscience) or anti-human IL-22 in BD Perm/Wash Buffer for 1h. Flow cytometry was performed on a BD LSRFortessa (BD Bioscience) and analyzed by FlowJo software (Tree Star).
Real-time PCR
RNA purification from T-cells was performed using the Rneasy Mini Kit (Qiagen). cDNA was obtained by reverse transcription using High-capacity cDNA Reverse Transcription Kits (ABI). Samples were analyzed by real-time PCR with SYBR Premix Ex Taq II (Tli RNase H Plus) kit (Takara) or GoTaq qPCR Master Mix (Promega) on the Applied Biosystem 7900HT Fast machine. The following primers were used. Gapdh forward, 5’-TGAACGGGAAGCTCACTGG-3’; Gapdh reverse, 5’-TCCACCACCCTGTTGCTGTA-3’. Il22 forward, 5'-CATGCAGGAGGTGGTACCTT-3'; Il22 reverse, 5'-CAGACGCAAGCATTTCTCAG-3'. Ahr forward, 5’-TGCACAAGGAGTGGACGA-3’; Ahr reverse, 5’-AGGAAGCTGGTCTGGGGTAT-3’. Expression was normalized to mouse glyceraldehyde-3-phosphate dehydrogenase (Gapdh) and presented as relative expression to control group by the 2–ΔΔCt method.
Gene expression of human skin biopsies from microarray datasets
Microarray gene expression data of human skin biopsies were retrieved from Gene Expression Omnibus datasets (GSE16161, GSE32924, GSE36842, GSE32473 and GSE27887)38–42. Patients information and skin sample have been described previously38–40,42. In brief, skin biopsy specimens were collected from patients with moderate-to-severe AD (Scoring of Atopic Dermatitis 20-97) and healthy volunteers under institutional review board–approved protocols (written consent obtained). Patients (age 16-81, mean age 40 from three cohorts) with an acute exacerbation of chronic AD and without any therapy for more than 4 weeks were included. Biopsy specimens were obtained from acute lesional skin which was actively involved, and erythematous lesions with atopic dermatitis and were frozen in liquid nitrogen for RNA extraction38–40,42. To standardize data across a wide range of experiments and to allow for the comparison of microarray data independent of the original hybridization intensities, gene expression levels were transformed to z-score values43. P values were calculated by nonparametric Wilcoxon-Mann-Whitney test, paired nonparametric tests with post-hoc Dunn’s multiple comparisons or paired 2-way analysis of variance (ANOVA) test with post-hoc Bonferroni's multiple comparisons test. Correlations between expression levels of two genes were calculated by nonparametric Spearman correlation test.
Statistical analyses
All data were expressed as mean ± SEM or scatter dot-plots in which each dot represents one mouse, one AD patient or healthy individual. Statistical significance between two groups was examined by the Student’s t-test or Mann-Whitney test, while the one-way and two-way ANOVA with post-hoc Bonferroni’s multiple comparisons test were used to evaluate multiple groups unless otherwise indicated in figure legends. Statistical analyses were performed using Prism 6 software (GraphPad) and a P<0.05 was considered as statistically significant.
Results
PGE2 promotes IL-22 production in vitro through its receptors EP2 and EP4
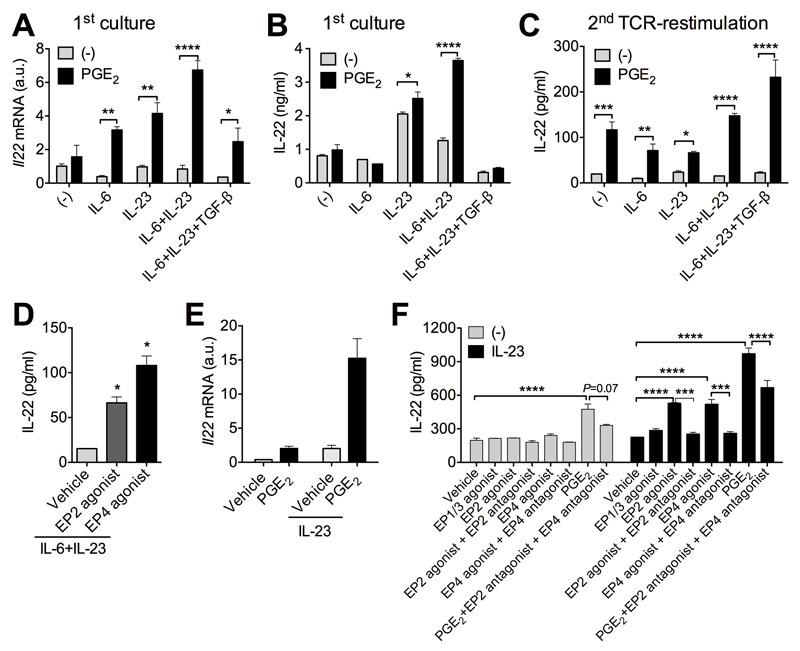
We have recently reported that PGE2 promotes IL-22 production from group 3 innate lymphoid cells (ILC3s)35. We thus evaluated whether PGE2 also promoted IL-22 production from T-cells. To address this question, we isolated CD4+ T-cells from mouse spleens, activated with anti-CD3 and anti-CD28 antibodies (Abs), and co-cultured with various cytokines. Addition of exogenous PGE2 enhanced Il22 gene expression with IL-6 or IL-23 alone, IL-6 + IL-23 (Th22 priming condition) or IL-6 + IL-23 + TGF-β (Th17 priming condition) (Fig 1A). IL-22 protein production in supernatants of primary cell cultures was also elevated by PGE2 in the presence of IL-23 or IL-23 + IL-6 (Fig 1B). It is important to note that these data are in agreement with previous findings44 that TGF-β inhibits IL-22 production even in the presence of PGE2 (Fig 1A,B). Moreover, when same numbers of PGE2-stimulated T-cells and control T-cells were washed and then re-stimulated with T-cell receptors (i.e. anti-CD3 and anti-CD28), PGE2-stimulated T-cells produced more IL-22 than control T-cells even in the presence of TGF-β (Fig 1C), suggesting that PGE2-treated cells have higher capability to produce IL-22 at the single cell level.
Figure 1. PGE2 promotes T cell production of IL-22 through its receptors EP2 and EP4.
A, Il22 mRNA expression in naïve CD4+ T cells stimulated with anti-CD3 and anti-CD28 (anti-CD3/CD28) antibodies (Abs) with indicated cytokines in the absence or presence of PGE2 (100 nM) for 3 days. B, IL-22 levels in supernatants of cultures in A. C. IL-22 levels in supernatants of naïve CD4+ T cells were cultured as in A and then the same numbers of T cells were re-stimulated with anti-CD3/CD28 Abs for another 3 days. D, IL-22 levels in supernatants of naïve CD4+ T cells were stimulated with anti-CD3/CD28 Abs with IL-6+IL-23 with or without an EP2 agonist (butaprost, 1 μM) or EP4 agonist (L-902,688, 1 μM) for 3 days and then the same numbers of T cells were re-stimulated with anti-CD3/CD28 Abs for another 3 days. E, IL-22 levels in supernatants from Th17 cells re-stimulated with anti-CD3/CD28 Abs without or with IL-23 and various small molecule compounds activating or inhibiting PGE2 receptors for 3 days. F, Il22 mRNA of re-stimulated Th17 cells with PGE2 and/or IL-23 for 3 days. EP1/3 agonist 17-phenyl trinor PGE2 was used at 1 μM while EP2 antagonist PF-04418948 and EP4 antagonist L-161,982 were used at 10 μM. All experiments were performed in duplicates or triplicates and repeated for 2-3 times independently. *P<0.05, **P<0.01, ***P<0.001 and ****P<0.0001.
To define which PGE2 receptor mediates IL-22 production, we cultured T-cells under Th22-priming conditions and selectively activated EP2 and EP4. Both EP2 and EP4 agonists individually mimicked augmentation of IL-22 production (Fig 1D). To further confirm the roles of PGE2-EP2/EP4 signaling in inducing IL-22 production of T-cells, we also cultured differentiated Th17 T-cells and found that PGE2 still promotes IL-23-driven IL-22 gene and protein expression (Fig 1E and 1F). Moreover, enhancement of IL-22 production by EP2 or EP4 agonists was prevented by co-treatment of antagonists against EP2 or EP4, respectively (Fig 1F); elevated IL-22 production by PGE2 was also diminished by co-administration of EP2 and EP4 antagonists (Fig 1F). In addition, PGE2 also increased IL-22 production in Th17 cells in the absence of other cytokines (Fig 1E and 1F), suggesting a potentially direct action of PGE2 to promote Il22 gene expression. These data indicate the involvement of EP2 and EP4 in PGE2 facilitation of IL-22 production from T cells in vitro.
Cyclic AMP promotes IL-22 production from T cells through the transcription factor aryl hydrocarbon receptor (AHR)
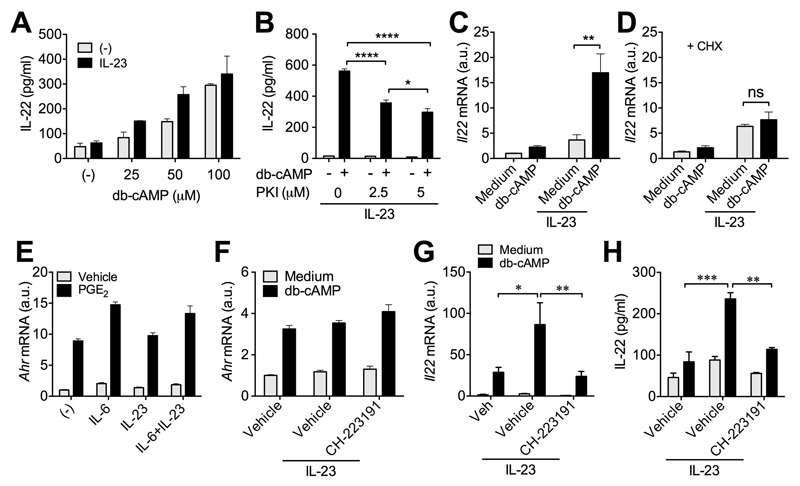
As both EP2 and EP4 receptors activate cAMP-PKA pathway in T cells33, we next examined whether cAMP mediates PGE2 facilitation of adaptive IL-22 production. Like PGE2, db-cAMP promoted IL-22 production in a concentration-dependent manner in either presence or absence of IL-23 (Fig 2A), confirming a direct action of cAMP on IL-22 induction. The increased IL-22 production by cAMP was prevented by PKI, a PKA inhibitor, in a concentration-dependent manner (Fig 2B). Cyclic AMP also up-regulated Il22 gene expression in IL-23-stimulated T-cells, which was prevented by cycloheximide, an inhibitor for eukaryote protein synthesis (Fig 2C and 2D), suggesting that the PGE2-cAMP pathway promoted adaptive IL-22 production through synthesis of new protein(s).
Figure 2. Cyclic AMP promotes IL-22 production from T cells through induction of aryl hydrocarbon receptor (Ahr).
A, IL-22 levels in supernatants from Th17 cells re-stimulated with anti-CD3/CD28 Abs without or with IL-23 and indicated concentrations of db-cAMP for 3 days. B, IL-22 levels in supernatants from Th17 cells re-stimulated with anti-CD3/CD28 Abs with IL-23, db-cAMP (100 μM) and indicated concentrations of PKI for 3 days. C,D, Il22 mRNA expression levels in Th17 cells re-stimulated with anti-CD3/CD28 Abs with IL-23 and db-cAMP in the absence (C) or presence (D) of cycloheximide (CHX, 1 μM) for 3 days. E, Ahr mRNA expression in naïve CD4+ T cells cultured with anti-CD3/CD28 Abs and indicated cytokines in the absence or presence of PGE2 for 3 days. F-H, Ahr (F) and Il22 (G) mRNA expression and IL-22 levels (H) in supernatants of Th17 cells cultured with anti-CD3/CD28 Abs, IL-23, db-cAMP and CH-223191 (10 μM) for 3 days. All experiments were performed in duplicates or triplicates and repeated for 2-3 times independently. *P<0.05, **P<0.01, ***P<0.001 and ****P<0.0001. ns, non significant.
The transcription factor AHR has been reported to regulate IL-22 production in both T-cells and ILC3s36. We thus investigated whether AHR contributes to PGE2-cAMP signaling-dependent increase in adaptive IL-22 production. We first checked that both PGE2 and cAMP up-regulated AHR gene expression in T-cells independently of cytokine stimuli (Fig 2E and 2F). Importantly, a small-molecule AHR inhibitor CH-223191 effectively suppressed cAMP-dependent IL-22 gene expression and protein production from T cells (Fig 2G and 2H) although it had no effect on AHR expression itself (Fig 2F). These results suggest that PGE2-cAMP signaling promotes IL-22 production from T-cells through induction of AHR.
PGE2-EP4 signaling promotes adaptive IL-22 production in vivo
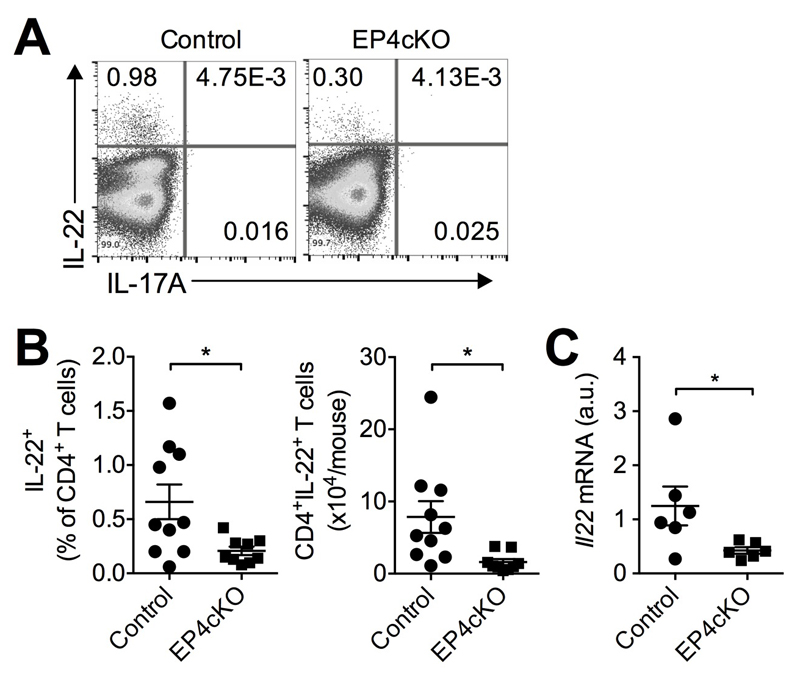
To investigate whether PGE2 promotes adaptive IL-22 production in vivo, we topically sensitized the abdominal skin of control mice and mice with specific EP4 deletion in T cells (EP4cKO mice34) with DNFB and measured IL-22-producing T-cells in skin-draining LNs 5 days after sensitization using flow cytometry. Both percentages and absolute numbers of IL-22-producing T-cells were markedly reduced in EP4cKO compared to control mice (Fig 3A and 3B). Furthermore, EP4 deficiency reduced Il22 gene expression in skin-draining LN CD4+ T cells (Fig 3C). These data indicate that PGE2-EP4 signaling promotes adaptive IL-22 production in vivo.
Figure 3. PGE2-EP4 signaling in T cells facilitates IL-22 production in vivo.
A, Representative flow cytometry dot-plot of IL-22 and IL-17 expression in skin-draining LN CD4+ T cells from T cell-specific EP4 deficient (EP4cKO, n=9) mice or control mice (n=10) 5 days post sensitization with 0.5% DNFB. B, Percentages and numbers of IL-22+ CD4+ T cells. Each dot represents one mouse. C, Il22 mRNA expression levels in CD4+ T cells isolated from skin-draining LNs of EP4cKO (n=6) or control (n=6) mice. Each dot represents one mouse. *P<0.05.
Endogenous PGE2-EP4 signaling in T cells promotes allergic contact dermatitis
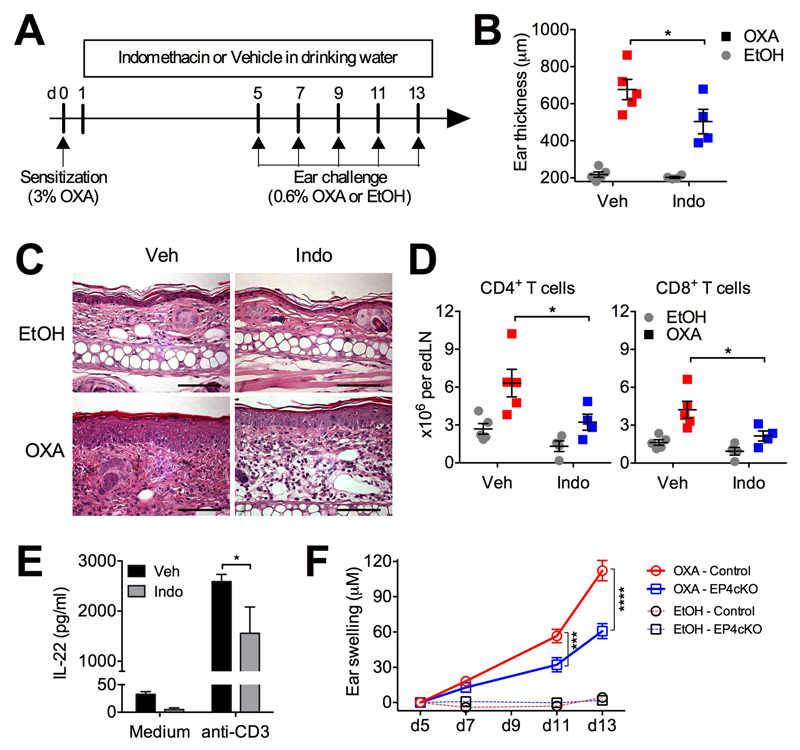
We next investigated whether PGE2 has a pathogenic role in development of allergic skin inflammation. To address this question, we used an animal model mimicking human ACD. We sensitized wild-type C57BL/6 mice with 3% oxalozone (OXA) on abdominal skin and after 5 days we repeatedly challenged mice with 0.6% OXA every two days for a total of 5 challenges. Indomethacin was administrated to mice in drinking water to inhibit endogenous PGE2 production. Once DCs in the skin capture the antigen OXA, they migrate to skin dLNs and present antigen to T cells for their activation. Because the migration of antigen-capturing DCs to skin dLNs peaks between 18-24h requires PGE2-EP4 signaling45, we only treated mice with indomethacin 24 h post the OXA sensitization (Fig 4A). Consistent with previous reports11, repeated challenge with OXA induced allergic skin inflammation (Fig 4B and 4C). Ears of OXA-challenged, indomethacin-treated mice showed decreased swelling compared to vehicle-treated mice (Fig 4B). Indomethacin had no effect on the thickness of vehicle (EtOH)-challenged ears. Compared to EtOH-challenged ears, histology of OXA-challenged ear skin showed features of eczema, namely parakeratosis, acanthosis and focal spongiosis with a dense and diffuse dermal infiltrate containing eosinophils (Fig 4C). However, in skin from the indomethacin-treated mice the epidermal changes are less pronounced, and the dermal infiltrates are markedly reduced (Fig 4C). Furthermore, whilst repeated OXA challenge recruited CD4+ and CD8+ T cells to ear-draining LNs (Fig 4D), indomethacin treatment reduced both this recruitment and the T-cell capacity to produce IL-22 (Fig 4E). To further investigated whether this pro-inflammatory effect of PGE2 on OXA-induced ACD development was mediated by EP4 and T cells, we performed this model on EP4cKO and control (EP4fl/fl) mice. OXA induced significantly less ear skin inflammation in EP4cKO mice compared to control mice (Fig. 4F), suggesting that PGE2-EP4 signaling in T cells promotes ACD development.
Figure 4. PGE2 exacerbates atopic skin inflammation in the repeated oxalozone (OXA) challenge model.
A-E, WT C57BL/6 mice were sensitized with 3% OXA on abdominal skin on (day 0) and then challenged with 0.6% OXA or EtOH on ears 5 days later. Challenge with OXA was repeated once every two days for a total of 5 challenges. Ears and ear-draining LNs were collected 6 h after the last OXA challenge (n=4-5). A, Schematic representation of the experimental protocol. B, Ear thickness. C, Ear histology. Scale bar, 50 μM. D, CD4+ and CD8+ T cells in ear-draining LNs. E, IL-22 production by ear-draining LN cells cultured with soluble anti-CD3 or medium only in vitro for 3 days. F, Swelling of ears from control and EP4cKO mice (n=9-13) sensitized and repeatedly challenged with OXA as in A but without indomethacin treatment. *P<0.05; ***P<0.001 and ****P<0.0001.
PGE2-dependent IL-22 production and signaling in human atopic lesional skin
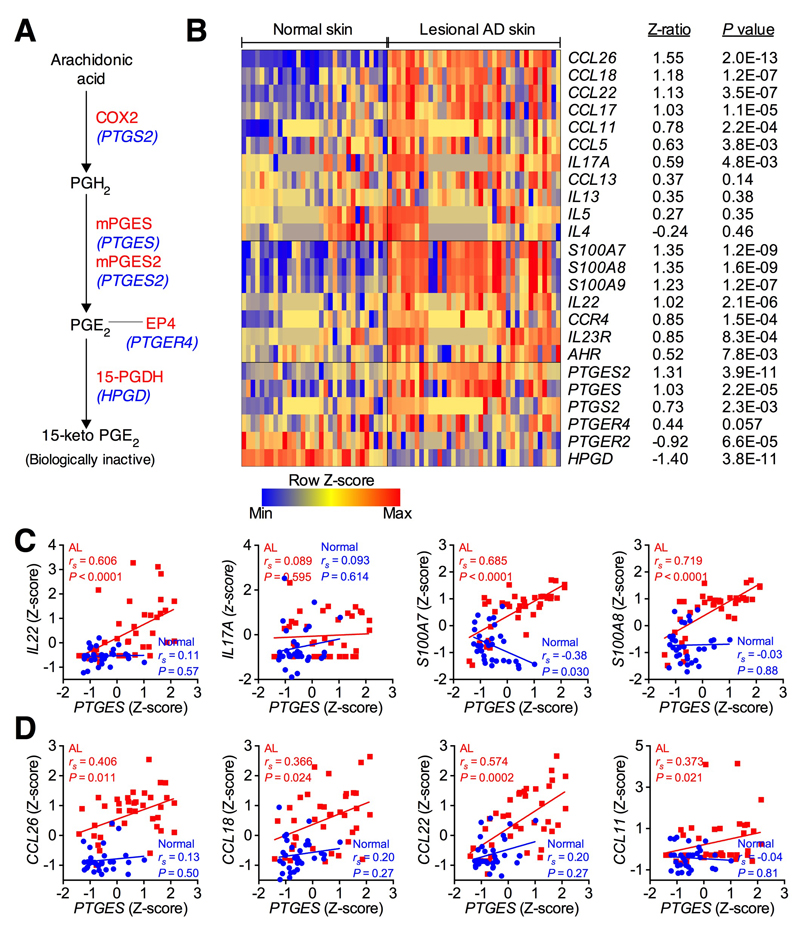
Because ACD and AD potentially share common cellular mechanisms4 and the repeated hapten challenge-induced animal model is relevant to the acute phase of AD in humans11, we next investigated whether PGE2-dependent adaptive IL-22 production and signaling can be found in human atopic skin. We analyzed mRNA expression of genes related to PGE2 metabolism (Fig 5A) and IL-22 signaling pathways in human lesional AD and normal skin biopsies. As reported previously39, mRNA expression levels for Th17/Th22 related genes (IL17A, IL22, IL23R, AHR), IL-22-induced products (e.g. S100A7, S100A8, S100A9), and skin-homing chemokines (CCR4) were over-expressed in lesional AD compared to normal skin (Fig 5B). Strikingly, mRNA expression levels for PGE2 synthases (PTGS2, PTGES, PTGES2) were also up-regulated in human atopic lesional skin, and expression levels for the PGE2 degradation enzyme HPGD (hydroxyprostaglandin dehydrogenase 15-(NAD)) was in contrast down-regulated in lesional AD skin (Fig 5B). These gene expression data are consistent with previous reports showing increased PGE2 levels in lesional AD skin21. There was a trend that expression of PTGER4 gene (encoding PGE2 receptor EP4) was up-regulated in lesional AD skin, but expression of PTGER2 (encoding PGE2 receptor EP2) was significantly down-regulated in lesional AD skin (Fig. 5B). Expression of Th2 cytokines (e.g. IL4, IL5, IL13) were not significantly up-regulated in lesional AD skin, however Th2 chemokines such as CCL26, CCL18, CCL22, CCL17, CCL11 and CCL5 were up-regulated in lesional AD skin compared to normal skin (Fig 5B). Interestingly, expression of IL-22 pathway genes showed strongly positive correlation with those of PTGES in biopsy samples from lesional AD but not normal skin (Fig 5C). There were no correlations between PTGES expression and Th2 cytokines (data not shown). However, weak correlations between PTGES gene expression and Th2 chemokines in lesional AD skin were observed (Fig 5D). These results suggest that PGE2 signaling is activated and positively correlates with the IL-22 signaling pathway and, probably, the Th2 pathway in human atopic skin.
Figure 5. Over-expression of PGE2 signaling genes in human lesional AD skin which positively correlate with expression of IL-22 signaling genes.
A, Schematic depicting synthesis and metabolism of PGE2 mediated by COX2 (encoding by PTGS2), PGE synthases (encoding by PTGES or PTGES2) and 15-PGDH (encoding by HPGD), respectively. A PGE2 receptor EP4 (encoding by PTGER4) is also shown. B, Heat map of expression profiles of Th2 cytokine and chemokine genes and genes related to IL-22 and PGE2 pathways in human lesional AD skin (n=38) and normal skin (n=32). A color scale bar indicates the Z-score transformed values of microarray gene expression data38–40. Z-ratios represent the changes in gene expression levels between the normal and AD lesional groups43. P values were calculated by nonparametric Wilcoxon-Mann-Whitney tests. Probes with the largest Z-ratios were chosen when several probes represented single genes. C,D, Correlations of PTGES gene expression versus expression of IL-22 pathway genes (C) or Th2 chemokines (D) in atopic lesional and normal skins. P value was calculated by nonparametric Spearman correlation test.
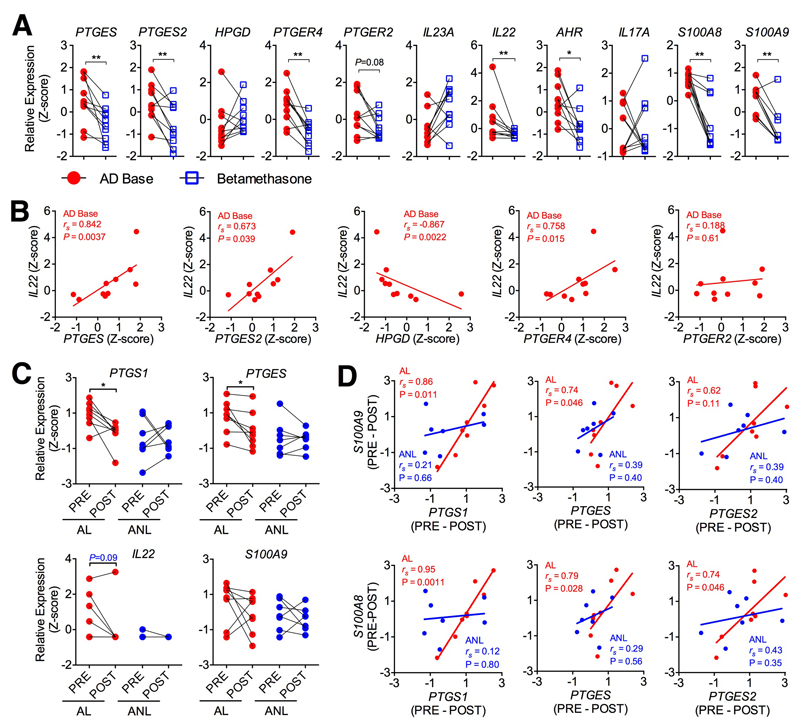
Finally, we investigated whether current therapies for atopic dermatitis modulate PGE2 signaling in human AD. We analyzed changes in expression levels of IL-22 and PGE2 signaling genes in lesional AD skin before and after treatments such as betamethasone (a corticosteroid) by re-analyzing a public microarray dataset41. Compared to baseline levels, treatments with betamethasone reduced IL22 gene expression in atopic lesional skin. Betamethasone also significantly reduced expression of IL-22-related genes such as AHR, S100A8, S100A9 (Fig 6A). Expression of PGE2 synthases (e.g. PTGES, PTGES2) and EP4 receptor (PTGER4) was significantly downregulated by betamethasone treatment, while EP2 receptor (PTGER2) expression was not changed (Fig 6A). This observation is consistent with findings that betamethasone treatment reduces PGE2 production46,47. Interestingly, expression of PTGES, PTGES2 and PTGER4, but not PTGER2, positively correlated with IL22 expression in atopic biopsies at baseline, while expression of HPGD, which mediates PGE2 degradation, negatively correlated with IL22 expression (Fig 6B). Furthermore, we have also reanalyzed gene expression of skin biopsies before and after treated with UVB from our previous dataset42. Expression of PGE2 synthases PTGS1 and PTGES in lesional AD skin were down-regulated after treatment with UVB (Fig 6C). Interestingly, although IL-22 gene expression was not changed by UVB treatment, most likely due to the small number of samples, changes in expression of IL-22 activated genes (e.g. S100A8 and S100A9) positively correlated with changes in expression of PGE2 synthases (i.e. PTGS1, PTGES and PTGES2) in atopic lesional, but not in non-lesional biopsies (Fig 6D and data not shown). Together, these data indicate that PGE2, most likely through the EP4 receptor, plays a role in IL-22 regulation in human skin.
Figure 6. Steroid and UVB therapies down-regulate PGE2 and IL-22 signaling pathway genes in human atopic lesional skin.
A, Expression profiles of genes related to PGE2 and IL-22 pathways in human lesional AD skin (n=10) at baseline (AD Base) or after treatment with betamethasone for 3 weeks. The z-score transformed values of microarray gene expression data41 were used. P value was calculated by paired nonparametric tests with post-hoc Dunn’s multiple comparisons test. B, Correlations of PTGES, PTGES2, HPGD, PTGER4 gene expression versus that of IL22 gene in human atopic lesional skins at baseline. P value was calculated by nonparametric Spearman correlation test. C, Expression of genes related to PGE2 and IL-22 signaling in human atopic lesional (AL, n=8) and non-lesional (ANL, n=7) skin before (PRE) or 12 weeks after (POST) treatment with UVB. The z-score transformed values of microarray gene expression data42 were used. P value was calculated by paired 2-way ANOVA test with post-hoc Bonferroni's multiple comparisons. D, Correlations of changes in expression of IL-22-induced genes (S100A8, S100A9) and PGE2 synthase genes (PTGS1, PTGES and PTGES2) before (PRE) and after (POST) UVB therapy in atopic AL or NL skins. P value was calculated by nonparametric Spearman correlation test.
Discussion
The cytokine IL-22, notably produced by Th22 and Th17 T-cells, is emerging as a key player in AD3,16,23, leading to a new therapy targeting IL-22 signaling in AD clinical trials (NCT01941537). Genome-wide association studies have identified that gene polymorphisms of the PGE2 receptor EP4 were associated with T cell-mediated human autoimmune and allergic inflammatory diseases48,49. However, the role of PGE2 in promoting IL-22 production and development of AD remain to be determined. To address this, ideally a model which showed the features of AD would be used. However, as no single animal model can fully capture all aspects of the human AD profile50 and there is acceptance that there are shared common cellular mechanisms between AD and ACD3, we have used a repeated hapten challenge ACD model to study the role of PGE2 signaling in IL-22+ T cells and development of chronic skin inflammation. We also analysed gene expression in human AD lesional skin before and after common treatments for AD to examine the PGE2-IL-22 aixs in the relevant human skin disease in both the chronic inflammatory phase and following therapeutic intervention.
We show that PGE2 markedly stimulates IL-22 production from T-cells, which complements our previous findings that PGE2 promotes innate IL-22 production from ILC3s35. It is worth noting that PGE2 promotes IL-22 production from freshly activated CD4+ T-cells under various conditions including Th17 and Th22-priming conditions. The effect of PGE2 was mimicked by activation of EP2 and EP4 receptors and was prevented by antagonists against these receptors, confirming the involvement of these two receptors in adaptive IL-22 production in vitro. Further studies showed that the cAMP-PKA signaling pathway, activated by engagement of EP2 and EP4, mediates PGE2 enhancement of IL-22 production through induction of newly expressed AHR. Our results thus uncover a new, targetable molecular mechanism for regulation of adaptive IL-22 by the PGE2-cAMP-AHR axis.
The role of PGE2 in promoting adaptive IL-22 production was also confirmed in vivo. T-cell specific EP4 deficiency led to large reduction of IL-22+ T-cells in skin-draining LNs in response to hapten sensitization and attenuated repeated OXA challenge-induced allergic skin inflammation in the mouse model of ACD, indicating a critical role of endogenous EP4 signaling in T-cells for IL-22 expression and function in vivo. This is similar to innate IL-22 expression which also requires PGE2-EP4 signaling35, suggesting a shared mechanism for regulating both the adaptive and innate IL-22 responses. Inhibition of endogenous PGE2 production by a COX inhibitor successfully prevented accumulation of T-cells in ear-draining LNs, reduced T-cell production of IL-22 and attenuated allergic skin inflammation induced by repeated OXA challenges. Given that PGE2 also promotes IL-17 production from both mouse and human Th17 T-cells33,51 and that IL-17 may participate in creating the ACD phenotype14–20, IL-17 may also be involved in the PGE2-facilitated ACD pathogenesis.
Facilitation of IL-22 production by PGE2 may contribute to several human inflammatory diseases such as AD and psoriasis52. Indeed, parallel up-regulations of IL-22 pathway genes, PGE2 synthases were observed in human atopic lesional skin, whereas the PGE2 degrading enzyme 15-PGDH (encoded by HPGD) was down-regulated in AD skin. This is consistent with previous observations showing increased PGE2 levels in lesional AD skin29. Moreover, our data indicate that IL-22 signaling positively correlated with PGE2 signaling in atopic lesional skin, and these correlations were absent in both normal skin or in lesional AD skin following successful steroid or UVB treatments. These findings potentially suggest a PGE2-dependent IL-22 production and signaling in human atopic skin.
Besides IL-22+ T-cells, Th2 cells (especially cutaneous lymphocyte antigen-positive population with skin-homing capacity) are expanded in severe AD53. Th2 cells suppress major terminal differentiation proteins (i.e. filaggrin and loricrin) and predominates in the acute phase of AD through production of cytokines (e.g. IL4 and IL13) and chemokines (e.g. CCL17, CCL18, CCL26, etc)40,54. The Th2 response is thus critical for AD pathogenesis. Inhibition of COX2 has been reported to enhance Th2 cytokine production and Th2 response to ovalbumin-induced epicutaneous sensitization30, suggesting a potential inhibitory role of PGs in Th2 cell-driven allergic inflammation. In human lesional AD skin, however, there was no correlations between the PGE2 pathway and Th2 cytokines and, in contrast, weak positive correlations between the PGE2 pathway and Th2 chemokines, suggesting a possible promoting role of PGE2 in Th2 response in AD. This may be because PGE2 promotes IL-17 and IL-22 production33–35,49 and subsequently IL-17 exacerbates Th2 type inflammation during the initiation of AD11,55.
We acknowledge this report has several limitations. For example, our analysis of gene expression profiles in human skin biopsies were retrieved from public GEO datasets and confirmation of these findings was not performed by methods with higher sensitivity such as real-time PCR using fresh biopsies. Moreover, cytokine expression in protein levels in different T-cell subsets were not measured by flow cytometry in skin of patients and animals. Furthermore, although in mice, the OXA-repeated challenge-induced chronic allergic skin inflammation represents a robust model, additional animal models are required in attempt to fully mimic most pathogenic and physiologic processes during the development of human AD. In addition, due to limitation of resources, the effects of PGE2 signaling in T cells, especially the involvement of both EP2 and EP4 in T cells, on initiation and progression of ACD could not be interrogated, e.g. using mice with T cell-specific deficiency of both EP2 and EP4 receptors. Future prospective studies are therefore required to further understand not only the role of PGE2 during perpetuating ACD and AD skin inflammation but also during their onset.
In conclusion, our data ascertain that PGE2 acts as a potent promoter of both adaptive and innate IL-22 production. We have previously found that PGE2 signaling promotes DC production of IL-23, a cytokine essential for IL-22 expression, as well as for proliferation and maturation of Th17 T-cells33. Taken together, our results highlight the PGE2 signaling pathway as an important stimulus of T-cell responses and ACD and, possibly, AD development. Thus targeting PGE2 synthesis or its receptors may represent a possible therapeutic strategy for the treatment of ACD and AD, and other inflammatory skin diseases such as psoriasis with an active role for IL-22, such as psoriasis.
Clinical Implications.
Allergic contact dermatitis is a common disabling disease characterized by elevated IL-22. The identification of a tightly regulated PGE2 driven pathway controlling IL-22 dysfunction offers a novel target for therapeutic intervention.
Capsule Summary.
Prostaglandin E2 promotes IL-22 production from T cells that mediates IL-22-driven development of allergic contact dermatitis.
Acknowledgements
We thank P. Ghazal (The University of Edinburgh) for critically reading and editing the manuscript and S. Johnston, W. Ramsay and F. Rossi for assistance with flow cytometry.
Funding:
This work was supported in part by the University of Edinburgh start-up funding and Wellcome Trust Institutional Strategic Support Fund (to C.Yao and H.J.M.), Asthma UK (to H.J.M.), Psoriasis Association UK (to R.B.W.), Medical Research Council (MRC) UK (A.G.R. C.T.R. and J.M.F. were supported by MR/K013386/1, and to S.M.A., R.B.W. and H.J.M.), NIH DK37097 and R56HL127218 and a VA Merit 1BX000616 (to R.M.B.), and Core Research for Evolutional Science and Technology (CREST) of Japan Science and Technology Agency (to S.N.). C.Yu and C.S. receive scholarship supports from the Chinese Scholarship Council and the University of Edinburgh, respectively. N.P. is supported by the Wellcome Trust Edinburgh Clinical Academic Training Programme.
Abbreviations used
- ACD
Allergic contact dermatitis
- AD
Atopic dermatitis
- AHR
Aryl hydrocarbon receptor
- ANOVA
Analysis of variance
- cAMP
Cyclic adenosine monophosphate
- CCR
Chemokine receptor
- COX
Cyclooxygenases
- Db-cAMP
Dibutyryl cAMP
- DC
Dendritic cell
- DNFB
Dinitrofluorobenzene
- IL-22
Interleukin 22
- ILC3
Group 3 innate lymphoid cells
- LN
Lymph node
- mPGES
Microsomal prostaglandin E synthases
- OXA
Oxazolone
- PGE2
Prostaglandin E2
- PKA
Protein kinase A
- Th
Helper T cells
- UVB
Ultraviolet band B
Footnotes
Disclosure of potential conflict of interest:
The authors declare that they have no relevant conflicts of interest.
References
- 1.Bieber T. Atopic Dermatitis. N Engl J Med. 2008;358:1483–94. doi: 10.1056/NEJMra074081. [DOI] [PubMed] [Google Scholar]
- 2.Malajian D, Guttman-Yassky E. New pathogenic and therapeutic paradigms in atopic dermatitis. Cytokine. 2015 Jun;73(2):311–8. doi: 10.1016/j.cyto.2014.11.023. [DOI] [PubMed] [Google Scholar]
- 3.Gittler JK, Krueger JG, Guttman-Yassky E. Atopic dermatitis results in intrinsic barrier and immune abnormalities: implications for contact dermatitis. J Allergy Clin Immunol. 2013;131:300–313. doi: 10.1016/j.jaci.2012.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Noda S, Krueger JG, Guttman-Yassky E. The translational revolution and use of biologics in patients with inflammatory skin diseases. J Allergy Clin Immunol. 2015;135:324–36. doi: 10.1016/j.jaci.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 5.Cork MJ, Danby SG, Vasilopoulos Y, Hadgraft J, Lane ME, Moustafa M. Epidermal barrier dysfunction in atopic dermatitis. J Invest Dermatol. 2009;129:1892–908. doi: 10.1038/jid.2009.133. [DOI] [PubMed] [Google Scholar]
- 6.Egawa G, Kabashima K. Multifactorial skin barrier deficiency and atopic dermatitis: Essential topics to prevent the atopic march. J Allergy Clin Immunol. 2016;138:350–8. doi: 10.1016/j.jaci.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 7.Kabashima K. New concept of the pathogenesis of atopic dermatitis: interplay among the barrier, allergy, and pruritus as a trinity. J Dermatol Sci. 2013;70:3–11. doi: 10.1016/j.jdermsci.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 8.Romagnani S. Immunologic influences on allergy and the TH1/TH2 balance. J Allergy Clin Immunol. 2004;113:395–400. doi: 10.1016/j.jaci.2003.11.025. [DOI] [PubMed] [Google Scholar]
- 9.Zhao Y, Balato A, Fishelevich R, Chapoval A, Mann DL, Gaspari AA. Th17/Tc17 infiltration and associated cytokine gene expression in elicitation phase of allergic contact dermatitis. Br J Dermatol. 2009;161:1301–1306. doi: 10.1111/j.1365-2133.2009.09400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Honda T, et al. Update of immune events in the murine contact hypersensitivity model: toward the understanding of allergic contact dermatitis. J Invest Dermatol. 2013;133:303–315. doi: 10.1038/jid.2012.284. [DOI] [PubMed] [Google Scholar]
- 11.Nakajima S, Kitoh A, Egawa G, Natsuaki Y, Nakamizo S, Moniaga CS, et al. IL-17A as an inducer for Th2 immune responses in murine atopic dermatitis models. J Invest Dermatol. 2014;134:2122–30. doi: 10.1038/jid.2014.51. [DOI] [PubMed] [Google Scholar]
- 12.Man MQ, et al. Characterization of a hapten-induced, murine model with multiple features of atopic dermatitis: structural, immunologic, and biochemical changes following single versus multiple oxazolone challenges. J Invest Dermatol. 2008;128:79–86. doi: 10.1038/sj.jid.5701011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jin H, He R, Oyoshi M, Geha RS. Animal models of atopic dermatitis. J Invest Dermatol. 2009;129:31–40. doi: 10.1038/jid.2008.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guttman-Yassky E, Lowes MA, Fuentes-Duculan J, Zaba LC, Cardinale I, Nograles KE, et al. Low expression of the IL-23/Th17 pathway in atopic dermatitis compared to psoriasis. J Immunol. 2008;181:7420–7. doi: 10.4049/jimmunol.181.10.7420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eyerich K, Pennino D, Scarponi C, Foerster S, Nasorri F, Behrendt H, et al. IL-17 in atopic eczema: linking allergen-specific adaptive and microbial-triggered innate immune response. J Allergy Clin Immunol. 2009;123:59–66. doi: 10.1016/j.jaci.2008.10.031. [DOI] [PubMed] [Google Scholar]
- 16.Souwer Y, Szegedi K, Kapsenberg ML, de Jong EC. IL-17 and IL-22 in atopic allergic disease. Curr Opin Immunol. 2010;22:821–6. doi: 10.1016/j.coi.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 17.Czarnowicki T, Krueger JG, Guttman-Yassky E. Skin barrier and immune dysregulation in atopic dermatitis: an evolving story with important clinical implications. J Allergy Clin Immunol Pract. 2014;2:371–379. doi: 10.1016/j.jaip.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 18.Simon D, Aeberhard C, Erdemoglu Y, Simon HU. Th17 cells and tissue remodeling in atopic and contact dermatitis. Allergy. 2014;69:125–131. doi: 10.1111/all.12351. [DOI] [PubMed] [Google Scholar]
- 19.Peiser M. Role of Th17 Cells in Skin Inflammation of Allergic Contact Dermatits. Clin Develop Immunol. 2013;2013:261037. doi: 10.1155/2013/261037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dyring-Andersen B, Skov L, Løvendorf MB, Bzorek M, Søndergaard K, Lauritsen JP, et al. CD4(+) T cells producing interleukin (IL)-17, IL-22 and interferon-γ are major effector T cells in nickel allergy. Contact Dermatitis. 2013;68:339–347. doi: 10.1111/cod.12043. [DOI] [PubMed] [Google Scholar]
- 21.Hayashida S, Uchi H, Takeuchi S, Esaki H, Moroi Y, Furue M, et al. Significant correlation of serum IL-22 levels with CCL17 levels in atopic dermatitis. J Dermatol Sci. 2011;61:78–9. doi: 10.1016/j.jdermsci.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 22.Ungar B, Garcet S, Gonzalez J, Dhingra N, da Rosa JC, Shemer A, et al. An integrated model of atopic dermatitis biomarkers highlights the systemic nature of the disease. J Invest Dermatol. 2016;4 doi: 10.1016/j.jid.2016.09.037. S0022-202X(16)32620-3. [DOI] [PubMed] [Google Scholar]
- 23.Nograles KE, Zaba LC, Shemer A, Fuentes-Duculan J, Cardinale I, Kikuchi T, et al. IL-22-producing “T22” T cells account for upregulated IL-22 in atopic dermatitis despite reduced IL-17-producing TH17 T cells. J Allergy Clin Immunol. 2009;123:1244–52. doi: 10.1016/j.jaci.2009.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nograles KE, Suárez-Fariñas M, Shemer A, Fuentes-Duculan J, Chiricozzi A, Cardinale I, et al. Atopic dermatitis keratinocytes exhibit normal T(H)17 cytokine responses. J Allergy Clin Immunol. 2010;125:744–746. doi: 10.1016/j.jaci.2009.12.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boniface K, Bernard FX, Garcia M, Gurney AL, Lecron JC, Morel F, et al. IL-22 inhibits epidermal differentiation and induces proinflammatory gene expression and migration of human keratinocytes. J Immunol. 2005;174:3695–702. doi: 10.4049/jimmunol.174.6.3695. [DOI] [PubMed] [Google Scholar]
- 26.Guttman-Yassky E, Ungar B, Noda S, Suprun M, Shroff A, Dutt R, et al. Extensive alopecia areata is reversed by IL-12/IL-23p40 cytokine antagonism. J Allergy Clin Immunol. 2016;137:301–304. doi: 10.1016/j.jaci.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 27.Hirata T, Narumiya S. Prostanoids as regulators of innate and adaptive immunity. Adv Immunol. 2012;116:143–74. doi: 10.1016/B978-0-12-394300-2.00005-3. [DOI] [PubMed] [Google Scholar]
- 28.Honda T, Kabashima K. Prostanoids in allergy. Allergology International. 2015;64:11–16. doi: 10.1016/j.alit.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 29.Fogh K, Herlin T, Kragballe K. Eicosanoids in skin of patients with atopic dermatitis: prostaglandin E2 and leukotriene B4 are present in biologically active concentrations. J Allergy Clin Immunol. 1989;83:450–5. doi: 10.1016/0091-6749(89)90132-2. [DOI] [PubMed] [Google Scholar]
- 30.Laouini D, Elkhal A, Yalcindag A, Kawamoto S, Oettgen H, Geha RS, et al. COX-2 inhibition enhances the TH2 immune response to epicutaneous sensitization. J Allergy Clin Immunol. 2005;116:390–6. doi: 10.1016/j.jaci.2005.03.042. [DOI] [PubMed] [Google Scholar]
- 31.Chan S, Henderson WR, Jr, Li SH, Hanifin JM. Prostaglandin E2 control of T cell cytokine production is functionally related to the reduced lymphocyte proliferation in atopic dermatitis. J Allergy Clin Immunol. 1996;97:85–94. doi: 10.1016/s0091-6749(96)70286-5. [DOI] [PubMed] [Google Scholar]
- 32.Fedyk ER, Phipps RP. Prostaglandin E2 receptors of the EP2 and EP4 subtypes regulate activation and differentiation of mouse B lymphocytes to IgE-secreting cells. Proc Natl Acad Sci U S A. 1996;93:10978–83. doi: 10.1073/pnas.93.20.10978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yao C, Sakata D, Esaki Y, Li Y, Matsuoka T, Kuroiwa K, et al. Prostaglandin E2-EP4 signaling promotes immune inflammation through Th1 cell differentiation and Th17 cell expansion. Nat Med. 2009;15:633–40. doi: 10.1038/nm.1968. [DOI] [PubMed] [Google Scholar]
- 34.Yao C, Hirata T, Soontrapa K, Ma X, Takemori H, Narumiya S. Prostaglandin E2 promotes Th1 differentiation via synergistic amplification of IL-12 signalling by cAMP and PI3-kinase. Nat Commun. 2013;4:1685. doi: 10.1038/ncomms2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Duffin R, O'Connor RA, Crittenden S, Forster T, Yu C, Zheng X, et al. Prostaglandin E2 constrains systemic inflammation through an innate lymphoid cell-IL-22 axis. Science. 2016;351:1333–8. doi: 10.1126/science.aad9903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cella M, Colonna M. Aryl hydrocarbon receptor: Linking environment to immunity. Semin Immunol. 2015;27:310–4. doi: 10.1016/j.smim.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schneider A, Guan Y, Zhang Y, Magnuson MA, Pettepher C, Loftin CD, et al. Generation of a conditional allele of the mouse prostaglandin EP4 receptor. Genesis. 2004;40:7–14. doi: 10.1002/gene.20048. [DOI] [PubMed] [Google Scholar]
- 38.Guttman-Yassky E, Suárez-Fariñas M, Chiricozzi A, Nograles KE, Shemer A, Fuentes-Duculan J, et al. Broad defects in epidermal cornification in atopic dermatitis identified through genomic analysis. J Allergy Clin Immunol. 2009;124:1235–44. doi: 10.1016/j.jaci.2009.09.031. [DOI] [PubMed] [Google Scholar]
- 39.Suárez-Fariñas M, Tintle SJ, Shemer A, Chiricozzi A, Nograles K, Cardinale I, et al. Nonlesional atopic dermatitis skin is characterized by broad terminal differentiation defects and variable immune abnormalities. J Allergy Clin Immunol. 2011;127:954–64. doi: 10.1016/j.jaci.2010.12.1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gittler JK, Shemer A, Suárez-Fariñas M, Fuentes-Duculan J, Gulewicz KJ, Wang CQ, et al. Progressive activation of T(H)2/T(H)22 cytokines and selective epidermal proteins characterizes acute and chronic atopic dermatitis. J Allergy Clin Immunol. 2012;130:1344–54. doi: 10.1016/j.jaci.2012.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jensen JM, Scherer A, Wanke C, Bräutigam M, Bongiovanni S, Letzkus M, et al. Gene expression is differently affected by pimecrolimus and betamethasone in lesional skin of atopic dermatitis. Allergy. 2012;67:413–23. doi: 10.1111/j.1398-9995.2011.02747.x. [DOI] [PubMed] [Google Scholar]
- 42.Tintle S, Shemer A, Suárez-Fariñas M, Fujita H, Gilleaudeau P, Sullivan-Whalen M, et al. Reversal of atopic dermatitis with narrow-band UVB phototherapy and biomarkers for therapeutic response. J Allergy Clin Immunol. 2011;128:583–93. doi: 10.1016/j.jaci.2011.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cheadle C, Vawter MP, Freed WJ, Becker KG. Analysis of Microarray Data Using Z Score Transformation. J Mol Diagn. 2003;5:73–81. doi: 10.1016/S1525-1578(10)60455-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rutz S, et al. Transcription factor c-Maf mediates the TGF-β-dependent suppression of IL-22 production in TH17 cells. Nat Immunol. 2011;12:1238–1245. doi: 10.1038/ni.2134. [DOI] [PubMed] [Google Scholar]
- 45.Kabashima K, Sakata D, Nagamachi M, Miyachi Y, Inaba K, Narumiya S. Prostaglandin E2-EP4 signaling initiates skin immune responses by promoting migration and maturation of Langerhans cells. Nat Med. 2003;9:744–9. doi: 10.1038/nm872. [DOI] [PubMed] [Google Scholar]
- 46.Inada T, Kushida A, Sakamoto S, Taguchi H, Shingu K. Intrathecal betamethasone pain relief in cancer patients with vertebral metastasis: a pilot study. Acta Anaesthesiol Scand. 2007;51:490–494. doi: 10.1111/j.1399-6576.2007.01272.x. [DOI] [PubMed] [Google Scholar]
- 47.Zulfakar MH, Abdelouahab N, Heard CM. Enhanced topical delivery and ex vivo anti-inflammatory activity from a betamethasone dipropionate formulation containing fish oil. Inflamm Res. 2010;59:23–30. doi: 10.1007/s00011-009-0065-z. [DOI] [PubMed] [Google Scholar]
- 48.Kurz T, Hoffjan S, Hayes MG, Schneider D, Nicolae R, Heinzmann A, et al. Fine mapping and positional candidate studies on chromosome 5p13 identify multiple asthma susceptibility loci. J Allergy Clin Immunol. 2006;118:396–402. doi: 10.1016/j.jaci.2006.04.036. [DOI] [PubMed] [Google Scholar]
- 49.Hinds DA, McMahon G, Kiefer AK, Do CB, Eriksson N, Evans DM, et al. A genome-wide association meta-analysis of self-reported allergy identifies shared and allergy-specific susceptibility loci. Nat Genet. 2013;45:907–11. doi: 10.1038/ng.2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ewald DA, Noda S, Oliva M, Litman T, Nakajima S, Li X, et al. Major differences between human atopic dermatitis and murine models, as determined by using global transcriptomic profiling. J Allergy Clin Immunol. 2017;139:562–571. doi: 10.1016/j.jaci.2016.08.029. [DOI] [PubMed] [Google Scholar]
- 51.Boniface K, Bak-Jensen KS, Li Y, Blumenschein WM, McGeachy MJ, McClanahan TK, et al. Prostaglandin E2 regulates Th17 cell differentiation and function through cyclic AMP and EP2/EP4 receptor signaling. J Exp Med. 2009;206:535–48. doi: 10.1084/jem.20082293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Noda S, Suárez-Fariñas M, Ungar B, Kim SJ, de Guzman Strong C, Xu H, et al. The Asian atopic dermatitis phenotype combines features of atopic dermatitis and psoriasis with increased TH17 polarization. J Allergy Clin Immunol. 2015;136:1254–1264. doi: 10.1016/j.jaci.2015.08.015. [DOI] [PubMed] [Google Scholar]
- 53.Czarnowicki T, Gonzalez J, Shemer A, Malajian D, Xu H, Zheng X, et al. Severe atopic dermatitis is characterized by selective expansion of circulating TH2/TC2 and TH22/TC22, but not TH17/TC17, cells within the skin-homing T-cell population. J Allergy Clin Immunol. 2015;136:104–115. doi: 10.1016/j.jaci.2015.01.020. [DOI] [PubMed] [Google Scholar]
- 54.Sehra S, Yao Y, Howell MD, et al. IL-4 regulates skin homeostasis and the predisposition toward allergic skin inflammation. J Immunol. 2010;184:3186–90. doi: 10.4049/jimmunol.0901860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dhingra N, Guttman-Yassky E. A possible role for IL-17A in establishing Th2 inflammation in murine models of atopic dermatitis. J Invest Dermatol. 2014;134:2071–2074. doi: 10.1038/jid.2014.141. [DOI] [PMC free article] [PubMed] [Google Scholar]