Abstract
IMPORTANCE
Electronic cigarettes (e-cigarettes) have gained unprecedented popularity, but virtually nothing is known about their cardiovascular risks.
OBJECTIVE
To test the hypothesis that an imbalance of cardiac autonomic tone and increased systemic oxidative stress and inflammation are detectable in otherwise healthy humans who habitually use e-cigarettes.
DESIGN, SETTING, AND PARTICIPANTS
Cross-sectional case-control study of habitual e-cigarette users and nonuser control individuals from 2015 to 2016 at the University of California, Los Angeles. Otherwise healthy habitual e-cigarette users between the ages of 21 and 45 years meeting study criteria, including no current tobacco cigarette smoking and no known health problems or prescription medications, were eligible for enrollment. Healthy volunteers meeting these inclusion criteria who were not e-cigarette users were eligible to be enrolled as control individuals. A total of 42 participants meeting these criteria were enrolled in the study including 23 self-identified habitual e-cigarette users and 19 self-identified non–tobacco cigarette, non–e-cigarette user control participants.
MAIN OUTCOMES AND MEASURES
Heart rate variability components were analyzed for the high-frequency component (0.15–0.4 Hz), an indicator of vagal activity, the low-frequency component (0.04–0.15 Hz), a mixture of both vagal and sympathetic activity, and the ratio of the low frequency to high frequency, reflecting the cardiac sympathovagal balance. Three parameters of oxidative stress were measured in plasma: (1) low-density lipoprotein oxidizability, (2) high-density lipoprotein antioxidant/anti-inflammatory capacity, and (3) paraoxonase-1 activity.
RESULTS
Of the 42 participants, 35% were women, 35% were white, and the mean age was 27.6 years. The high-frequency component was significantly decreased in the e-cigarette users compared with nonuser control participants (mean [SEM], 46.5 [3.7] nu vs 57.8 [3.6] nu; P = .04). The low-frequency component (mean [SEM], 52.7 [4.0] nu vs 39.9 [3.8] nu; P = .03) and the low frequency to high frequency ratio (mean [SEM], 1.37 [0.19] vs 0.85 [0.18]; P = .05) were significantly increased in the e-cigarette users compared with nonuser control participants, consistent with sympathetic predominance. Low-density lipoprotein oxidizability, indicative of the susceptibility of apolipoprotein B–containing lipoproteins to oxidation, was significantly increased in e-cigarette users compared with nonuser control individuals (mean [SEM], 3801.0 [415.7] U vs 2413.3 [325.0] U; P = .01) consistent with increased oxidative stress, but differences in high-density antioxidant/anti-inflammatory capacity and paraoxonase-1 activity were not significant.
CONCLUSIONS AND RELEVANCE
In this study, habitual e-cigarette use was associated with a shift in cardiac autonomic balance toward sympathetic predominance and increased oxidative stress, both associated with increased cardiovascular risk.
Electronic cigarettes (e-cigarettes), first marketed in the United States in 2006, have gained unprecedented popularity, especially among young people.1,2 E-cigarettes are not actually cigarettes at all: there is no combustion and they contain no tobacco. Electronic cigarettes are handheld devices that, when puffed, deliver a heated, aerosolized mixture of nicotine, flavorings, and a humectant into the mouth and lungs of the user. Electronic cigarettes have created significant controversy in the medical community. They have been viewed as either a safer alternative to lethal tobacco cigarettes or as a gateway to expanding tobacco cigarette addiction.3–5 Unfortunately, scientific data supporting either side of the controversy are sparse.
More than 50 years ago, based on decades of observational data in habitual tobacco cigarette users, the Surgeon General of the United States warned the public about the lethality of tobacco cigarettes.6 Only years later were the mechanisms by which tobacco cigarettes led to adverse cardiovascular effects uncovered such as increased oxidative stress and inflammation, increased sympathetic activity, and enhanced platelet activity.7–9 Although tobacco cigarettes are widely recognized as the most common preventable cause of cardiovascular disease in the world, virtually nothing is known about the cardiovascular risks of e-cigarettes. Rather than wait decades for epidemiological data in habitual e-cigarette users to become available, we reasoned that investigations into several of the known mechanisms by which tobacco cigarettes increase cardiovascular risk would provide insights in the health risks of e-cigarettes.
In this study of habitual e-cigarette users, we focus on 2 critical mechanisms by which tobacco cigarettes are known to promote cardiovascular disease: (1) a shift in the cardiac sympathovagal balance toward sympathetic predominance as assessed by heart rate variability (HRV)9 and (2) increased systemic oxidative stress and inflammation.8 Abnormal HRV is present in tobacco cigarette smokers10,11 and has been shown in populations with and without known cardiac disease to identify those at increased risk for myocardial infarction and sudden cardiac death.12–14 Additionally, increased oxidative stress and inflammation are major mechanisms by which tobacco cigarettes initiate and propagate atherosclerosis. Each puff of tobacco cigarette smoke contains greater than 1015 free radicals.15 This promotes oxidative modification of low-density lipoprotein (LDL). Oxidized LDL is then taken up by macrophages forming foam cells, the instigators of atherosclerosis.8 The purpose of this study was to test the hypothesis that an imbalance of cardiac autonomic tone and increased systemic oxidative stress and inflammation are detectable in otherwise healthy humans who habitually use e-cigarettes.
Methods
Study Population
Otherwise healthy habitual e-cigarette users between the ages of 21 and 45 years, who had used e-cigarettes most days for a minimum of 1 year, were eligible for the study if they met the following criteria: (1) no current tobacco cigarette smoking, (2) nonobese (body mass index ≤30 [calculated as weight in kilograms divided by height in meters squared]), (3) no known health problems, (4) not taking prescription medications except oral contraceptive pills, (5) alcoholic intake 2 or fewer drinks per day and no illicit drug use, and (6) not exposed to secondhand smoke or using licensed nicotine replacement therapies. Participants who were former tobacco cigarette smokers were eligible for the study if they had quit smoking more than 1 year prior to the study. Healthy volunteers meeting these inclusion criteria who were not e-cigarette users were eligible to be enrolled as control participants.
The experimental protocol was approved by the institutional Review board at the University of California, Los Angeles, and written informed consent was obtained from each participant.
A total of 42 participants meeting these criteria were enrolled in the study including 23 self-identified habitual e-cigarette users and 19 self-identified non–tobacco cigarette, non–e-cigarette user control participants. Two of the 23 e-cigarette users were eliminated when their plasma carboxy-hemoglobin levels were found to be elevated, consistent with recent tobacco cigarette use.16 One of the 19 control participants was eliminated when his plasma cotinine level was elevated, consistent with recent exposure to cigarettes.
Because the goal of the study was to investigate the effects of chronic, not acute, e-cigarette exposure, participants were asked not to use their e-cigarette on the day of the study. After abstaining from caffeine and e-cigarette use for at least 12 hours, volunteers were placed in a supine position in a quiet, temperature-controlled (21°C) room in the Human Physiology Laboratory located in the University of California, Los Angeles Clinical Translational Research Center. No cell phones or digital stimuli were permitted during the study, and during data acquisition, there was no unnecessary talking.
Heart Rate Variability
To avoid the potential influence of circadian rhythm or menstrual cycle phases on autonomic tone, participants were studied midday (between 10 AM–2 PM), and women were studied during the early follicular phase, confirmed by plasma estrogen and progesterone levels. All women had negative urine pregnancy test results on the day of the study.
Electrocardiogram electrodes were placed on the chest, and the participants then rested undisturbed for 10 minutes. The electrocardiogram was then recorded for 5 minutes during quiet rest and during 5 minutes of controlled breathing at a rate of 12 breaths per minute, a known stimulus for vagal tone.17,18 During controlled breathing, participants were cued visually by watching the second hand on a large clock to inhale every 5 seconds. Five-minute electrocardiogram recordings were analyzed using standard commercial software (LabChart7; Ad Instruments) in the frequency domain according to published guidelines.19 Three main spectral components were distinguished: high frequency (HF; 0.15–0.4 Hz), low frequency (LF; 0.04–0.15 Hz), and very LF (0.003–0.04 Hz). As recommended in the published guidelines, HRV is presented in normalized units to correct for differences in total power between the groups and in absolute units (microseconds squared).19 Time domain analysis was not applied to these recordings because a minimum of 20-minute recordings, and preferentially 24-hour recordings, are recommended for this methodology.19
Blood Tests
Venipuncture was performed by trained Clinical Translational Research Center nurses. Blood was drawn into preiced heparinized vacutainers and placed on ice. Blood was centrifuged to separate into plasma samples, which were frozen at −80°C in a cryopreservative solution20 for later analysis for the following antioxidant parameters: (1) LDL oxidizability, indicative of susceptibility of apolipoprotein B–containing lipoproteins to oxidation as previously reported,21 (2) high-density lipoprotein (HDL) antioxidant/anti-inflammatory capacity, expressed as an HDL antioxidant index, which assesses the ability of HDL to inhibit LDL oxidation monitored by conversion of a nonfluorescent dihydrodichlorofluorescein probe into the fluorescent dichlorofluorescein, performed as previously reported,22,23 and (3) paraoxonase-1 activity, a protective ester hydrolase enzyme associated with HDL in blood that prevents the formation of oxidized LDL,24 assayed by its ability to hydrolyze paraoxonsubstrate,23 described in detail in the eMethods in the Supplement.
Blood was also sent to the University of California, Los Angeles Clinical Laboratory for measurement of (1) nicotine (t1/2 1–2 hours) and the nicotine metabolite cotinine (t1/2 20 hours), (2) plasma carboxyhemoglobin (marker for tobacco cigarette but not e-cigarette use), and (3) inflammatory markers including C-reactive protein and fibrinogen.
Statistical Analysis
The Shapiro-Wilk statistic and normal quantile plots (not shown) were examined to determine whether continuous variables followed the normal distribution. If so, P values for comparing nonuser control individuals with e-cigarette users were computed using the t test, and the mean and its standard error are reported. Otherwise, P values were computed using the nonparametric Wilcoxon rank sum test, and the median and its standard error are reported. For binary data, such as sex, P values for nonuser control vs e-cigarette user comparisons were computed using Fisher exact test. For within-group paired comparisons (eg, controlled breathing and spontaneous breathing), the parametric P value was computed via the paired t test and the nonparametric P value was computed via the Wilcoxon signed rank test. Associations between 2 continuous variables were assessed using the nonparametric Spearman correlation. Missing data values were not imputed; only the observed data were used. Differences or associations were considered statistically significant when P was less than or equal to .05.
Results
Baseline Characteristics
Although e-cigarette users were asked to abstain from using their e-cigarette on the day of the study, nicotine was present in plasma in 5 habitual e-cigarette users, consistent with recent use (range, 2.6–27.3 mg/L [to convert to micromoles per liter, multiply by 6.164]). These 5 e-cigarette users were excluded from further analysis; an analysis inclusive of these additional 5 e-cigarette users is available in eTables 1–5 in the Supplement. Plasma cotinine levels were elevated on the day of the study in 12 of the remaining 16 e-cigarette users, (range, 3.8–139 ng/mL, eFigure in the Supplement). Baseline characteristics of the 16 e-cigarette users and 18 nonusers are compared in Table 1. All parameters were within normal limits.
Table 1.
Baseline Characteristics
| Characteristic | Mean (SD) | P Value | |
|---|---|---|---|
| E-Cigarette User (n = 16) | Nonuser Control Participant (n = 18) | ||
| Age, y | 28.6 (1.4) | 26.6 (1.5) | .35 |
| Sex, No. | |||
| Male | 13 | 7 | .02 |
| Female | 3 | 11 | |
| BMI | 25.2 (0.8) | 23.0 (0.9) | .85 |
| Race/ethnicity, No. | |||
| African American | 1 | 2 | NA |
| Asian | 2 | 3 | NA |
| Hispanic | 2 | 2 | NA |
| White (non-Hispanic) | 11 | 11 | NA |
| Former smoker, No. | 10 | 2 | NA |
| Pack-years | 1.9 (0.5) | 0.6 (0.4) | NA |
| Interval since quitting, y | 2.3 (0.8) | 13 (7) | NA |
| E-cigarette use | |||
| Min/d | 241 (158) | NA | NA |
| Duration, y | 1.6 (0.5) | NA | NA |
| SBP, mm Hg | 115.8 (2.5) | 109.0 (2.6) | .07 |
| DBP, mm Hg | 73.5 (2.3) | 70.0 (2.0) | .27 |
| MAP, mm Hg | 87.6 (2.3) | 83.0 (2.0) | .15 |
| HR, bpm | 64.0 (2.0) | 63.0 (2.0) | .73 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); bpm, beats per minute; DBP, diastolic blood pressure; HR, heart rate; MAP, mean arterial pressure; NA, not applicable; SBP, systolic blood pressure.
Heart Rate Variability
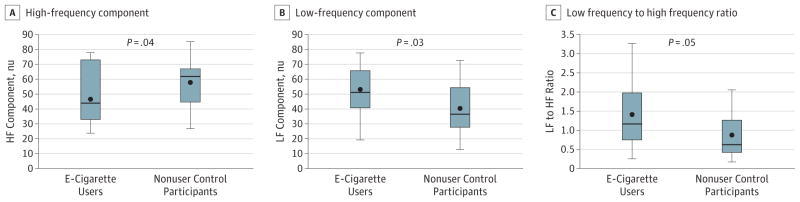
Heart rate variability components were analyzed for the HF component, an indicator of vagal activity, the LF component, a mixture of both vagal and sympathetic activity, and the ratio of the LF to HF, reflecting the cardiac sympathovagal balance (Figure 1; Table 2).19 The HF component was significantly decreased in the e-cigarette users compared with nonuser control participants (mean [SEM], 46.5 [3.7] nu vs 57.8 [3.6] nu; P = .04). The LF component (mean [SEM], 52.7 [4.0] nu vs 39.9 [3.8] nu; P = .03), and the LF to HF ratio (mean [SEM], 1.37 [0.19] vs 0.85 [0.18]; P = .05), were significantly increased in the e-cigarette users compared with nonuser control participants, consistent with sympathetic predominance even in the absence of recent e-cigarette use as verified by the absence of detectable nicotine in the plasma (Figure 1). Controlling for e-cigarette or nonuser control group, sex had no significant effect (data not shown) on HRV components.
Figure 1. Heart Rate Variability Components.
A, The high-frequency (HF) component, an indicator of vagal activity, was significantly decreased in the e-cigarette users compared with nonuser control individuals (mean [SEM], 46.5 [3.7] nu vs 57.8 [3.6] nu, P = .04). B and C, The low-frequency (LF) component (mean [SEM], 52.7 [4.0] nu vs 39.9 [3.8] nu, P = .03), and the LF to HF ratio (1.37 [0.19] vs 0.85 [0.18], P = .05), were significantly increased in the e-cigarette users compared with nonuser controls, consistent with sympathetic predominance. These findings were present even in the absence of recent e-cigarette use, as verified by the absence of detectable nicotine in the plasma.
Table 2.
Heart Rate Variability (Absolute Units)a
| HRV Parameter | Mean (SD), μs2 | P Value | |
|---|---|---|---|
| E-Cigarette User (n = 16) | Nonuser Control Participant (n = 18) | ||
| High frequency | 833.6 (295.7) | 1376.5 (574.2) | .33 |
| Low frequency | 455.5 (258.2) | 1316.0 (504.0) | .08 |
| Very low frequency | 896.0 (524.2) | 987.1 (432.5) | .59 |
| Total power | 1652.0 (720.5) | 4502.0 (1279.8) | .04 |
Abbreviation: HRV, heart rate variability.
Median values are displayed because these data followed a nonparametric distribution.
Correlation of HRV With E-Cigarette Burden
Plasma cotinine levels, an estimate of e-cigarette use, were significantly correlated with each of the HRV components: plasma cotinine levels were inversely related to HF component (rs, −0.34; P = .04) and directly related to the LF component (rs, 0.35; P = .03) and LF to HF ratio (rs, 0.36; P = .03).
Controlled Breathing (Vagal Maneuver)
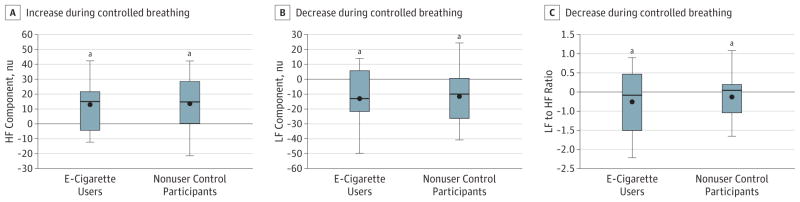
Within each group (e-cigarette users and nonuser control individuals), the HF component was significantly increased during controlled breathing compared with spontaneous breathing. Similarly, within each group, the LF and LF to HF ratio were decreased during controlled breathing compared with spontaneous breathing, consistent with a relative increase in cardiac vagal tone and decline in cardiac sympathetic influence (Figure 2). However, between e-cigarette users and nonuser groups, the magnitude of the increase in HF and decrease in LF and LF to HF ratio during controlled breathing were not different (Figure 2).
Figure 2. Heart Rate Variability During Controlled Breathing.
A, Within each group (e-cigarette users and nonuser control participants), the high-frequency (HF) component was significantly increased during controlled breathing compared with spontaneous breathing. Similarly, within each group, the low frequency (LF) (B), and LF to HF ratio (C) were decreased during controlled compared with spontaneous breathing, consistent with a relative increase in cardiac vagal and decline in cardiac sympathetic influence. However, between e-cigarette user and nonuser groups, the magnitude of the increase in HF and decrease in LF and LF to HF ratio during controlled breathing were not different.
aP = .05, within-group difference between controlled breathing and spontaneous breathing.
Oxidative Stress and Inflammation
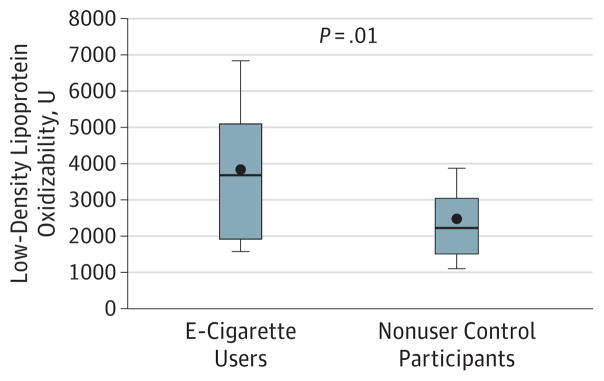
Low-density lipoprotein oxidizability, indicative of susceptibility of apolipoprotein B–containing lipoproteins to oxidation, was significantly increased in e-cigarette users (n = 12) compared with nonuser control participants (n = 18) (mean [SEM], 3801.0 [415.7] U vs 2413.3 [325.0] U, P = .01), consistent with increased oxidative stress (Figure 3). Paraoxonase-1 activity tended to be lower in the e-cigarette users (n = 12) compared with nonuser control individuals (n = 18) (mean [SEM], 649.9 [125.7] nmol p-nitrophenol/min/mL vs 892.8 [110.0] nmol p-nitrophenol/min/mL; P = .17), consistent with decreased protection against oxidative stress, although this difference did not meet statistical significance. High-density lipoprotein antioxidant index was not different between the groups (e-cigarette users [n = 12] vs nonusers [n = 18]: mean [SEM], 0.42 [0.05] U vs 0.38 [0.04] U; P = .55). Inflammatory markers, including fibrinogen (e-cigarette users [n = 15] vs nonusers [n = 17]: mean [SEM], 270.9 [12.6] mg/dL vs 251.9 [10.4] mg/dL; P = .24 [to convert to micromoles per liter, multiply by 0.0294) and C-reactive protein levels were not different between e-cigarette users and nonusers (abnormal in 3 e-cigarette users [n = 15] and 1 nonuser [n = 17]; P = .15).
Figure 3. Oxidative Stress.
Low-density lipoprotein oxidizability, indicative of susceptibility of apoB-containing lipoproteins to oxidation, was significantly increased in e-cigarette users (n = 12) compared with nonuser (n = 18) control participants (mean [SEM], 3801.0 [415.7] U vs 2413.3 [325.0] U, P = .01), consistent with increased oxidative stress.
Plasma cotinine levels were directly related to LDL oxidizability (rs, 0.35; P = .05) but not the other indices of oxidative stress measured.
Discussion
The major new findings in this study are that in otherwise healthy, habitual e-cigarette users compared with nonsmoking healthy control participants (1) HRV components are shifted toward sympathetic predominance and decreased vagal tone, the pattern found in patients with increased cardiovascular risk, including tobacco cigarette smokers,10,12–14 (2) systemic oxidative stress is increased, and (3) abnormalities of both HRV and oxidative stress are directly associated with e-cigarette burden. Importantly, these findings are not attributable to a transient pharmacological effect of nicotine because plasma nicotine levels were nondetectable at the time of the study. These findings are important for 2 reasons: first, because both increased cardiac sympathetic activity and increased oxidative stress are known mechanisms by which tobacco cigarettes increase cardiovascular risk,8,9 these findings have critical implications for the long-term cardiac risks associated with habitual e-cigarette use. Second, these findings mandate a reexamination of aerosolized nicotine and its metabolites. Nicotine, which is the major bioactive ingredient in e-cigarette aerosol, with its metabolites, may harbor unrecognized, sustained adverse physiologic effects that lead to an increased cardiovascular risk profile in habitual e-cigarette users.
In the 1980s, clinical studies first recognized perturbations in HRV as a powerful independent predictor of increased mortality in patients following myocardial infarction.12 These perturbations in HRV reflect a relative increase in cardiac sympathetic nerve activity and a decrease in vagal tone.19 Since these early reports, abnormal HRV indicative of sympathetic predominance has been shown in numerous studies in diverse patient populations with and without known cardiac disease to identify patients who have increased cardiovascular mortality.14,25–28 In fact, this increased risk has been demonstrated to have a dose-response relationship, with the most severe HRV abnormalities conferring the greatest cardiovascular mortality.13,14 Adverse cardiovascular sequelae of increased sympathetic nerve activity include increased arrhythmia risk, heart failure, and fatal and nonfatal myocardial infarction.9
Habitual tobacco cigarette smokers have been found to have abnormal HRV, specifically, this same pattern of increased sympathetic cardiac activity accompanied by decreased cardiac vagal tone.18 This pattern of autonomic perturbation is found in habitual tobacco cigarette smokers who have abstained from tobacco cigarette smoking on the day of HRV measurement as well as in those who have smoked several tobacco cigarettes prior to the HRV measurement and in nonsmokers acutely and transiently exposed to secondhand smoke.11,29–31 Evidence supports the concept that nicotine exposure can alter HRV in tobacco cigarette smokers because acute oral nicotine ingestion in never-smokers also shifts the HRV balance toward sympathetic predominance.32 Acute nicotine exposure releases norepinephrine from postganglionic cardiac sympathetic nerve terminals, underlying this acute pharmacological effect.33 Surprisingly, in tobacco cigarette smokers who refrain from smoking 8 hours prior to HRV measurement, the LF to HF ratio has also been reported to be shifted compared with nonsmoking control individuals, consistent with persistently increased cardiac sympathetic activity even in the absence of acute nicotine exposure.11 Similarly, in our study of e-cigarette users, nicotine was not detectable in e-cigarette users at the time of the HRV recordings, consistent with a mechanism beyond the acute pharmacological effect of nicotine.
In this study, we also found evidence of increased oxidative stress in habitual e-cigarette users compared with nonusers. Low-density lipoprotein oxidizability is a measure of the susceptibility of LDL to oxidation, which increases in the presence of oxidative stress. The sensitivity of LDL to oxidation depends on its antioxidant contents, which determine its antioxidant potential. It has been shown that patients with diabetes and smokers have increased LDL oxidation.34 In addition, patients with diabetes have increased LDL oxidizability, as assessed by Cu2+–induced malondialdehyde formation in association with decreased LDL antioxidant potential, reflecting the presence of increased oxidative stress.35 Therefore, LDL oxidizability constitutes a useful measure of early oxidative stress. Each puff of smoke from a combusted tobacco cigarette releases enormous quantities of free radicals, and evidence is accumulating that e-cigarette aerosol also carries significant oxidative stress burden.33,36 Lerner et al36 have reported similar oxidants and reactive oxygen species reactivity in e-cigarette aerosols and tobacco cigarette smoke.36 This oxidative stress reportedly led to a cytotoxic response in oral epithelial cells invitro.37 However, other reports showed significant variability between e-cigarette liquids, with only 1 in 11 liquids tested inducing significant oxidative stress in cultured human endothelial cells.38 Nonetheless, it remains likely that the heated, aerosolized nicotine, the humectants (propylene glycol/glycerol), and/or flavorings, all known or potential airway irritants, could lead to the presence of reactive oxygen species in the human airway, in turn leading to systemic oxidative stress. Our e-cigarette users used a variety of flavored liquids and brands, all containing nicotine, suggestive of an oxidative effect that is ubiquitous from habitual e-cigarette use.
Limitations
Human studies rely on self-reporting for many of the behaviors that cannot be controlled when participants are away from the laboratory and thus are vulnerable to misstatements and misrecollections.39 To circumvent this problem, we required biochemical verification of e-cigarette use and absence of tobacco cigarette use.16,39 Nonetheless, we cannot be completely certain that 1 or more of our participants was not surreptitiously consuming tobacco products. We did not perform toxicology screening to eliminate marijuana and other drug exposures. Quantifying e-cigarette exposure is more difficult than tobacco cigarette exposure, which can be quantified by the number of tobacco cigarettes smoked per day. Although we did ask e-cigarette users how much time per day they used their e-cigarettes and how much liquid they used per day, answers were vague and varied on repeated questioning and were unreliable overall. Although measured only once, plasma cotinine levels seemed the most objective means to assess e-cigarette burden. There were more former smokers in the habitual e-cigarette user group compared with nonuser control individuals. This difference is unlikely to explain the difference in HRV or oxidative stress between the groups because several studies have confirmed that HRV components improve significantly, and cardiovascular risk similarly improves following tobacco cigarette cessation.40–44
Finally, the relative effect of tobacco cigarettes compared with e-cigarettes on autonomic balance and oxidative stress remains an important yet unanswered question. In contrast to our findings in e-cigarette users, Barutcu et al18 found that vagal modulation in response to controlled breathing was blunted in heavy tobacco cigarette smokers who had abstained from smoking the day of the study, compared with age-matched nonsmoker control participants. In our study, vagal responses to controlled breathing were not different between e-cigarette users and nonusers, perhaps indicative of a less severe abnormality of autonomic function associated with e-cigarettes compared with tobacco cigarettes.
Conclusions
In summary, in this cross-sectional study of non–tobacco cigarette smoking adults who habitually use e-cigarettes compared with nonuser control participants, evidence is presented demonstrating that e-cigarette use is not harmless. Habitual e-cigarette use is associated with a shift in cardiac autonomic balance toward sympathetic predominance and increased oxidative stress, both associated with increased cardiovascular risk. Further studies are required to determine whether these risks are similar to those associated with habitual tobacco cigarette use. However, the nonlinear relationship between number of tobacco cigarettes smoked per day and cardiovascular risk suggests that there may be a low threshold above which underlying physiologic processes are saturated45; habitual e-cigarette users may cross this threshold. On the basis of these studies, we can conclude that habitual e-cigarette use is associated with physiologic effects. Nonetheless, we cannot confirm causality on the basis of this single, small study; further research into the potential adverse cardiovascular health effects of e-cigarettes is warranted.
Supplementary Material
Key Points.
Question
Do habitual electronic cigarette users have increased cardiac sympathetic activity and oxidative stress, both risk factors for future adverse cardiac events?
Findings
In this cross-sectional case-control study of 42 otherwise healthy habitual electronic cigarette users and nonuser control individuals, heart rate variability was shifted toward increased sympathetic predominance, with the low frequency to high frequency ratio significantly increased. Furthermore, low-density lipoprotein oxidizability, which is a measure of oxidative stress, was significantly increased in habitual electronic cigarette users.
Meaning
Habitual electronic cigarette use is associated with physiologic effects. Further research into potential adverse health effects of electronic cigarettes is warranted.
Acknowledgments
Funding/Support: This study was supported by the Tobacco-Related Disease Research Program under the contract number TRDRP-XT320833 (Dr Middlekauff), American Heart Association, Western States Affiliate, Grant-in-Aid 15GRNT22930022 (Dr Middlekauff), the National Institute of Environmental Health Sciences, National Institutes of Health R56ES016959-06 (Dr Araujo), Training Grant in Molecular Toxicology (Ms Bhetraratana), Irma and Norman Switzer Dean’s Leadership in Health and Science Scholarship (Ms Moheimani) and the University of California, Los Angeles Clinical and Translational Science Institute grant UL1TR000124.
Footnotes
Correction: This article was corrected on March 22, 2016, to reflect changes to the author contributions section.
Conflict of Interest Disclosures: All authors have completed and submitted The ICMJE Form for Disclosure of Potential Conflicts of Interest and none were reported.
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Additional Contributions: We are grateful to G. Ramanathan, PhD, Department of Environmental Health Sciences, School of Public Health, University of California, Los Angeles, who contributed to the early stages of this work in blood collection and storage. No compensation was received.
Author Contributions: Dr Middlekauff had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Mss Moheimani and Bhetraratana contributed equally to the research and are considered cofirst authors.
Concept and design: Moheimani, Araujo, Middlekauff.
Acquisition, analysis, or interpretation of data: Moheimani, Bhetraratana, Yin, Peters, Gornbein, Araujo, Middlekauff.
Drafting of the manuscript: Moheimani, Middlekauff.
Critical revision of the manuscript for important intellectual content: Moheimani, Bhetraratana, Yin, Peters, Gornbein, Araujo, Middlekauff.
Statistical analysis: Moheimani, Gornbein.
Obtained funding: Araujo, Middlekauff.
Administrative, technical, or material support: Moheimani, Yin, Peters.
Supervision: Moheimani, Araujo, Middlekauff.
Role of the Funder/Sponsor: The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
References
- 1.Bhatnagar A, Whitsel LP, Ribisl KM, et al. American Heart Association Advocacy Coordinating Committee, Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology, and Council on Quality of Care and Outcomes Research. Electronic cigarettes: a policy statement from the American Heart Association. Circulation. 2014;130(16):1418–1436. doi: 10.1161/CIR.0000000000000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singh T, Marynak K, Arrazola RA, Cox S, Rolle IV, King BA. Vital signs: exposure to electronic cigarette advertising among middle school and high school students: United States, 2014. MMWR Morb Mortal Wkly Rep. 2016;64(52):1403–1408. doi: 10.15585/mmwr.mm6452a3. [DOI] [PubMed] [Google Scholar]
- 3.Avdalovic MV, Murin S. POINT: does the risk of electronic cigarettes exceed potential benefits? Yes. Chest. 2015;148(3):580–582. doi: 10.1378/chest.15-0538. [DOI] [PubMed] [Google Scholar]
- 4.Middlekauff HR. COUNTERPOINT: does the risk of electronic cigarettes exceed potential benefits? No. Chest. 2015;148(3):582–584. doi: 10.1378/chest.15-0540. [DOI] [PubMed] [Google Scholar]
- 5.Green SH, Bayer R, Fairchild AL. Evidence, policy, and e-cigarettes: will England reframe the debate? N Engl J Med. 2016;374(14):1301–1303. doi: 10.1056/NEJMp1601154. [DOI] [PubMed] [Google Scholar]
- 6.Schroeder SA, Koh HK. Tobacco control 50 years after the 1964 surgeon general’s report. JAMA. 2014;311(2):141–143. doi: 10.1001/jama.2013.285243. [DOI] [PubMed] [Google Scholar]
- 7.Ambrose JA, Barua RS. The pathophysiology of cigarette smoking and cardiovascular disease: an update. J Am Coll Cardiol. 2004;43(10):1731–1737. doi: 10.1016/j.jacc.2003.12.047. [DOI] [PubMed] [Google Scholar]
- 8.Csordas A, Bernhard D. The biology behind the atherothrombotic effects of cigarette smoke. Nat Rev Cardiol. 2013;10(4):219–230. doi: 10.1038/nrcardio.2013.8. [DOI] [PubMed] [Google Scholar]
- 9.Middlekauff HR, Park J, Moheimani RS. Adverse effects of cigarette and noncigarette smoke exposure on the autonomic nervous system: mechanisms and implications for cardiovascular risk. J Am Coll Cardiol. 2014;64(16):1740–1750. doi: 10.1016/j.jacc.2014.06.1201. [DOI] [PubMed] [Google Scholar]
- 10.Lucini D, Bertocchi F, Malliani A, Pagani M. A controlled study of the autonomic changes produced by habitual cigarette smoking in healthy subjects. Cardiovasc Res. 1996;31(4):633–639. [PubMed] [Google Scholar]
- 11.Hayano J, Yamada M, Sakakibara Y, et al. Shortand long-term effects of cigarette smoking on heart rate variability. Am J Cardiol. 1990;65(1):84–88. doi: 10.1016/0002-9149(90)90030-5. [DOI] [PubMed] [Google Scholar]
- 12.Kleiger RE, Miller JP, Bigger JT, Jr, Moss AJ. Decreased heart rate variability and its association with increased mortality after acute myocardial infarction. Am J Cardiol. 1987;59(4):256–262. doi: 10.1016/0002-9149(87)90795-8. [DOI] [PubMed] [Google Scholar]
- 13.Tsuji H, Larson MG, Venditti FJ, Jr, et al. Impact of reduced heart rate variability on risk for cardiac events. The Framingham Heart Study. Circulation. 1996;94(11):2850–2855. doi: 10.1161/01.cir.94.11.2850. [DOI] [PubMed] [Google Scholar]
- 14.Hillebrand S, Gast KB, de Mutsert R, et al. Heart rate variability and first cardiovascular event in populations without known cardiovascular disease: meta-analysis and dose-response meta-regression. Europace. 2013;15(5):742–749. doi: 10.1093/europace/eus341. [DOI] [PubMed] [Google Scholar]
- 15.Pryor WA, Stone K. Oxidants in cigarette smoke: radicals, hydrogen peroxide, peroxynitrate, and peroxynitrite. Ann N Y Acad Sci. 1993;686:12–27. doi: 10.1111/j.1749-6632.1993.tb39148.x. [DOI] [PubMed] [Google Scholar]
- 16.SRNT Subcommittee on Biochemical Verification. Biochemical verification of tobacco use and cessation. Nicotine Tob Res. 2002;4(2):149–159. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- 17.Driscoll D, Dicicco G. The effects of metronome breathing on the variability of autonomic activity measurements. J Manipulative Physiol Ther. 2000;23(9):610–614. doi: 10.1067/mmt.2000.110944. [DOI] [PubMed] [Google Scholar]
- 18.Barutcu I, Esen AM, Kaya D, et al. Cigarette smoking and heart rate variability: dynamic influence of parasympathetic and sympathetic maneuvers. Ann Noninvasive Electrocardiol. 2005;10(3):324–329. doi: 10.1111/j.1542-474X.2005.00636.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation. 1996;93(5):1043–1065. [PubMed] [Google Scholar]
- 20.Breton CV, Yin F, Wang X, Avol E, Gilliland FD, Araujo JA. HDL anti-oxidant function associates with LDL level in young adults. Atherosclerosis. 2014;232(1):165–170. doi: 10.1016/j.atherosclerosis.2013.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yin F, Lawal A, Ricks J, et al. Diesel exhaust induces systemic lipid peroxidation and development of dysfunctional pro-oxidant and pro-inflammatory high-density lipoprotein. Arterioscler Thromb Vasc Biol. 2013;33(6):1153–1161. doi: 10.1161/ATVBAHA.112.300552. [DOI] [PubMed] [Google Scholar]
- 22.Ramanathan G, Yin F, Speck M, et al. Effects of urban fine particulate matter and ozone on HDL functionality. Part Fibre Toxicol. 2016;13(1):26. doi: 10.1186/s12989-016-0139-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramanathan G, Araujo JA, Gornbein J, Yin F, Middlekauff HR. Cigarette smoking is associated with dose-dependent adverse effects on paraoxonase activity and fibrinogen in young women. Inhal Toxicol. 2014;26(14):861–865. doi: 10.3109/08958378.2014.965559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Watson AD, Berliner JA, Hama SY, et al. Protective effect of high density lipoprotein associated paraoxonase. Inhibition of the biological activity of minimally oxidized low density lipoprotein. J Clin Invest. 1995;96(6):2882–2891. doi: 10.1172/JCI118359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.La Rovere MT, Bigger JT, Jr, Marcus FI, Mortara A, Schwartz PJ ATRAMI (Autonomic Tone and Reflexes After Myocardial Infarction) Investigators. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. Lancet. 1998;351(9101):478–484. doi: 10.1016/s0140-6736(97)11144-8. [DOI] [PubMed] [Google Scholar]
- 26.Bigger JT, Jr, Fleiss JL, Steinman RC, Rolnitzky LM, Kleiger RE, Rottman JN. Frequency domain measures of heart period variability and mortality after myocardial infarction. Circulation. 1992;85(1):164–171. doi: 10.1161/01.cir.85.1.164. [DOI] [PubMed] [Google Scholar]
- 27.Dekker JM, Crow RS, Folsom AR, et al. Low heart rate variability in a 2-minute rhythm strip predicts risk of coronary heart disease and mortality from several causes: the ARIC Study: Atherosclerosis Risk In Communities. Circulation. 2000;102(11):1239–1244. doi: 10.1161/01.cir.102.11.1239. [DOI] [PubMed] [Google Scholar]
- 28.Liao D, Carnethon M, Evans GW, Cascio WE, Heiss G. Lower heart rate variability is associated with the development of coronary heart disease in individuals with diabetes: the atherosclerosis risk in communities (ARIC) study. Diabetes. 2002;51(12):3524–3531. doi: 10.2337/diabetes.51.12.3524. [DOI] [PubMed] [Google Scholar]
- 29.Kobayashi F, Watanabe T, Akamatsu Y, et al. Acute effects of cigarette smoking on the heart rate variability of taxi drivers during work. Scand J Work Environ Health. 2005;31(5):360–366. doi: 10.5271/sjweh.919. [DOI] [PubMed] [Google Scholar]
- 30.Wilson MD, McGlothlin JD, Rosenthal FS, Black DR, Zimmerman NJ, Bridges CD. Ergonomics: the effect of occupational exposure to environmental tobacco smoke on the heart rate variability of bar and restaurant workers. J Occup Environ Hyg. 2010;7(7):D44–D49. doi: 10.1080/15459624.2010.483980. [DOI] [PubMed] [Google Scholar]
- 31.Dinas PC, Koutedakis Y, Flouris AD. Effects of active and passive tobacco cigarette smoking on heart rate variability. Int J Cardiol. 2013;163(2):109–115. doi: 10.1016/j.ijcard.2011.10.140. [DOI] [PubMed] [Google Scholar]
- 32.Sjoberg N, Saint DA. A single 4mg dose of nicotine decreases heart rate variability in healthy nonsmokers: implications for smoking cessation programs. Nicotine Tob Res. 2011;13(5):369–372. doi: 10.1093/ntr/ntr004. [DOI] [PubMed] [Google Scholar]
- 33.Haass M, Kübler W. Nicotine and sympathetic neurotransmission. Cardiovasc Drugs Ther. 1997;10(6):657–665. doi: 10.1007/BF00053022. [DOI] [PubMed] [Google Scholar]
- 34.Mol MJ, de Rijke YB, Demacker PN, Stalenhoef AF. Plasma levels of lipid and cholesterol oxidation products and cytokines in diabetes mellitus and cigarette smoking: effects of vitamin E treatment. Atherosclerosis. 1997;129(2):169–176. doi: 10.1016/s0021-9150(96)06022-4. [DOI] [PubMed] [Google Scholar]
- 35.Singh N, Singh N, Kumar Singh S, Kumar Singh A, Kafle D, Agrawal N. Reduced antioxidant potential of LDL is associated with increased susceptibility to LDL peroxidation in type ii diabetic patients. Int J Endocrinol Metab. 2012;10(4):582–586. doi: 10.5812/ijem.5029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lerner CA, Sundar IK, Watson RM, et al. Environmental health hazards of e-cigarettes and their components: oxidants and copper in e-cigarette aerosols. Environ Pollut. 2015;198:100–107. doi: 10.1016/j.envpol.2014.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ji EH, Sun B, Zhao T, et al. Characterization of electronic cigarette aerosol and its induction of oxidative stress response in oral keratinocytes. PLoS One. 2016;11(5):e0154447. doi: 10.1371/journal.pone.0154447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Putzhammer R, Doppler C, Jakschitz T, et al. Vapours of US and EU market leader electronic cigarette brands and liquids are cytotoxic for human vascular endothelial cells. PLoS One. 2016;11(6):e0157337. doi: 10.1371/journal.pone.0157337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Connor Gorber S, Schofield-Hurwitz S, Hardt J, Levasseur G, Tremblay M. The accuracy of self-reported smoking: a systematic review of the relationship between self-reported and cotinine-assessed smoking status. Nicotine Tob Res. 2009;11(1):12–24. doi: 10.1093/ntr/ntn010. [DOI] [PubMed] [Google Scholar]
- 40.Stein PK, Rottman JN, Kleiger RE. Effect of 21 mg transdermal nicotine patches and smoking cessation on heart rate variability. Am J Cardiol. 1996;77(9):701–705. doi: 10.1016/s0002-9149(97)89203-x. [DOI] [PubMed] [Google Scholar]
- 41.Harte CB, Meston CM. Effects of smoking cessation on heart rate variability among long-term male smokers. Int J Behav Med. 2014;21(2):302–309. doi: 10.1007/s12529-013-9295-0. [DOI] [PubMed] [Google Scholar]
- 42.Minami J, Ishimitsu T, Matsuoka H. Effects of smoking cessation on blood pressure and heart rate variability in habitual smokers. Hypertension. 1999;33(1 Pt 2):586–590. doi: 10.1161/01.hyp.33.1.586. [DOI] [PubMed] [Google Scholar]
- 43.Sandhu RK, Jimenez MC, Chiuve SE, et al. Smoking, smoking cessation, and risk of sudden cardiac death in women. Circ Arrhythm Electrophysiol. 2012;5(6):1091–1097. doi: 10.1161/CIRCEP.112.975219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thun MJ, Carter BD, Feskanich D, et al. 50-year trends in smoking-related mortality in the United States. N Engl J Med. 2013;368(4):351–364. doi: 10.1056/NEJMsa1211127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pope CA, III, Burnett RT, Krewski D, et al. Cardiovascular mortality and exposure to airborne fine particulate matter and cigarette smoke: shape of the exposure-response relationship. Circulation. 2009;120(11):941–948. doi: 10.1161/CIRCULATIONAHA.109.857888. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.