Abstract
Mouse embryonic stem cell (mESC) lines were derived by crossing heterozygous transgenic (tg) mice expressing green fluorescent protein (GFP) under the control of the rat tyrosine hydroxylase (TH) promoter, with homozygous alpha-synuclein (aSYN) mice expressing human mutant SNCAA53T under the control of the mouse Prion promoter (MoPrP), or wildtype (WT) mice. The expression of GFP and human aSYN was validated by immunocytochemistry in midbrain neuron cultures upon differentiation of mESC lines using stromal cell-derived inducing activity. These mESC lines can help to study the impact of human aSYN expression in neurons and oligodendrocytes, and also trace GFP-expressing midbrain neurons.
1. Resource details
We report the derivation of multiple mESC lines generated from the crossing between heterozygous transgenic mice expressing GFP under the control of the rat tyrosine hydroxylase (TH) promoter (Sawamoto et al., 2001), and homozygous aSYN mice expressing human mutant SNCAA53T under the control of the mouse prion promoter (MoPrP), also known as M83 (Giasson et al., 2002), or non-transgenic wild type (WT) mice expressing endogenous mouse Snca (Fig. 1, panel A). The mESC lines are referred to as TH∷GFP, TH∷GFP;PrP∷hSNCAA53T, PrP∷hSNCAA53T and WT. At the time of breeding, the mice were under C57BL/6 (B6) background. Blastocysts aged embryonic day 3.5 (E3.5; panel B) were isolated from pregnant female mice, and cultured separately on CF1 mouse irradiated feeders until the inner cell mass formed a primitive colony (panels C and D). Each colony was then passaged until the lines could be established (panel E). For each line derived, expression of the pluripotency marker Oct4 was confirmed by immunocytochemistry (panel F); the presence of GFP was confirmed by PCR (panel G). TH∷GFP neurons could be identified when TH∷GFP and TH∷GFP;PrP∷hSNCAA53T mESC lines were differentiated on a monolayer of PA6 stromal cells (panel H), using previously published protocols (Ganat et al., 2012; Kawasaki et al., 2000). Immunostaining for GFP in midbrain FoxA2-positive cultures revealed co-expression with β-III-tubulin (70 ± 11%) and with TH (59 ± 10%) on the day of analysis (D14 + 6 DIV). Generated TH∷GFP neurons could be purified by fluorescent-activated cell sorting (FACS) for GFP, and recovered in culture after sorting (data not shown). Transgenic expression of human aSYN was identified in TH∷GFP;PrP∷hSNCAA53T and PrP∷hSNCAA53T lines by immunocytochemistry (panel J and data not shown). Oligodendrocytes generated from human aSYN mESC lines also expressed human mutant SNCA (panel K).
Fig. 1.
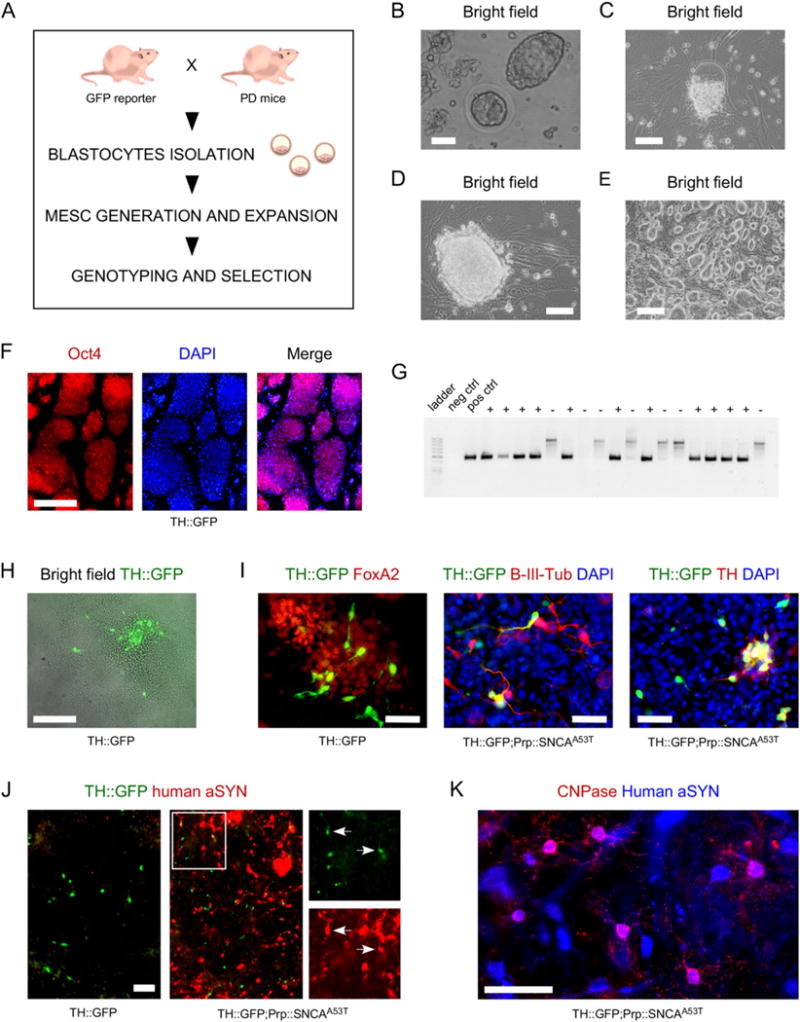
A- Schematic representation of the workflow to generate mESC lines. B- Bright field images of blastocysts aged E3.5, in culture medium, prior to seeding on MEFs. Scale bar = 50 μm. C- Inner cell mass migrating out to form a colony on MEFs. The zona pelucida can be identified above the newly formed colony. Scale bar = 50 μm. D- Seven day old newly formed colony. Scale bar = 50 μm. E- Culture of mESC line aged passage P4. Scale bar = 100 μm. F- Established mESC culture stained for pluripotency marker Oct4 (red); cell nuclei are counterstained with DAPI (blue). Scale bar = 100 μm. G- Example of gel allowing identification of clones positive for GFP revealed by the presence of a PCR product of 475 bp. “+” indicates positive clones; “−” indicates negative clones. H– Identification of TH∷GFP-expressing cells in a mESC colony differentiated on PA6 cells. Scale bar = 100 μm. I- TH∷GFP-positive cells co-express FoxA2 (87 ± 8%), β-III-tubulin (70 ± 11%) and TH (59 ± 10%). Mean ± SD. Scale bars = 50 μm. J-Differentiated TH∷GFP;Prp∷hSNCAA53T mESC lines, but not differentiated TH∷GFP mESC lines, do stain positive for human aSYN. Scale bar = 100 μm. K- Human aSYN expression identified in CNPase oligodendrocytes in Prp∷hSNCAA53T cultures. Scale bar = 100 μm.
Using similar approach, additional mESC lines could be derived using a Pitx3∷GFP reporter mouse (Zhao et al., 2004) and another aSYN mouse model of Parkinson’s disease, expressing human WT SNCA (Westerlund et al., 2008) (data now shown).
These mESC lines (Table 1) can be used to study human SNCA-induced pathology in mouse dopaminergic neurons and oligodendrocytes.
Table 1.
Table of mESC lines generated.
| Female | Male | Molecular analysis of the mESC lines |
|---|---|---|
| WT | TH∷GFP+/− | TH∷GFP+/− |
| WT | TH∷GFP+/− | WT |
| PrP∷hSNCAA53T+/+ | TH∷GFP+/− | TH∷GFP+/−; PrP∷hSNCAA53T+/− |
| PrP∷hSNCAA53T+/+ | TH∷GFP+/− | PrP∷hSNCAA53T+/− |
| WT | Pitx3∷GFP+/− | Pitx3∷GFP+/− |
| WT | Pitx3∷GFP+/− | WT |
| PrP∷hSNCAA53T+/+ | Pitx3∷GFP+/− | Pitx3∷GFP+/−;PrP∷hSNCAA53T+/− |
| PrP∷hSNCAA53T+/+ | Pitx3∷GFP+/− | PrP∷hSNCAA53T+/− |
| aSynP∷hSNCA+/+ | Pitx3∷GFP+/− | Pitx3∷GFP+/−; aSynP∷hSNCA+/− |
| aSynP∷hSNCA+/+ | Pitx3∷GFP+/− | aSynP∷hSNCA+/− |
2. Materials and methods
2.1. Derivation and maintenance of mouse embryonic stem cell lines
Animal handling and derivation of mESC was approved by the ethical committee for the use of laboratory animals at Lund University and the Swedish Work Environment Authority.
The mice used to generate the mESC described in Table 1 were: heterozygous transgenic TH∷GFP mice (H. Okano laboratory at Keio University School of Medicine, Japan); heterozygous knock-in Pitx3∷GFP mice (M. Li Laboratory at Cardiff University, UK); homozygous M83 (Jackson Laboratory; http://www.jax.org/strain/004479); homozygous aSynP∷SNCA+/− which are transgenic mice expressing human WT SNCA driven by the 7 kb mouse SNCA promoter (known as F28 mice; Pekka Kallunki laboratory at H/S Lundbeck, Denmark); WT mice were accessible in house at Lund University. Mice were under B6 background, with the exception of the Pitx3∷GFP mice which originally were under mixed background, but at the time of the crossing with aSYN mice and WT mice had been back-crossed to B6 background.
Briefly, mice were euthanized 3.5 days after being plugged. The uterine horns were dissected out and blastocysts aged E3.5 were flushed out and squeezed out from the uterine horns in KES medium containing DMEM GlutaMax medium supplemented with 20% KnockOut Serum Replacement, 2 mM L-glutamine, 1% Nucleosides, 1% penicillin and streptomycin (All from ThermoFisher Scientific) and 1% non-essential amino acids (Millipore). Blastocysts were collected and seeded individually onto a layer of irradiated mouse embryonic fibroblasts (MEFs; MTI-GlobalStem), in KES medium, supplemented with leukemia inhibitor factor (LIF; 10,000 U/ml; Millipore) and MEK-inhibitor (40 μM; Selleck Chemicals). Cell culture medium was changed once blastocysts hatched and primitive mESC colonies were apparent, then every two days. Two to three weeks later, the whole cultures were individually passaged using 0.1% trypsin, then rinsed and transferred onto new irradiated MEFs in medium composed of 50% KES and 50% regular ES medium containing DMEM GlutaMax, 10% fetal bovine serum (FBS), 2 mM L-glutamine, 1% Nucleosides, 1% penicillin and streptomycin (All from ThermoFisher Scientific), 1% non-essential amino acids (Millipore), and 10,000 U/ml LIF (Millipore). Mouse ESC colonies were passaged several times until sufficient ES cells were available to make freeze-down stocks and prepare DNA for genotyping. Mouse ESC lines were treated in a prophylactic manner with Plasmocin (Invivogen), for 2 weeks.
2.2. Genotyping
The presence of the TH∷GFP and Pitx3∷GFP alleles was confirmed by PCR. Genomic DNA was extracted using conventional lysis buffer composed of 100 mM Tris (pH 8.0), 200 mM NaCl, 5 mM EDTA and 0.2% SDS in distilled autoclaved water supplemented with 1.5 mg/ml Proteinase K. PCR reaction was carried out using AccuPrime Supermix following manufacturer’s instructions (ThermoFisher Scientific) (Table 2).
Table 2.
Table of primer sequences used for validating the presence of reporter transgene.
| Primer name | Sequence |
|---|---|
| GFP_Forward | 5′ AAG TTC ATC TGC ACC ACC G 3′ |
| GFP_Reverse | 5′ TGC TCA GGT AGT GGT TGT CG 3′ |
| Pitx3_Forward (wild type) | 5′ TCC ATC GCC GCT TCT ATG GT 3′ |
| Pitx3_Forward (knock-in) | 5′ AGC CTC GAC TGT GCC TTC TA 3′ |
| Pitx3_Reverse | 5′ CCG GAG AGG CTG TGA ATT AC 3′ |
2.3. Generation of dopaminergic neurons
To obtain dopaminergic neuron cultures (which also contained CNPase-positive oligodendrocytes and GFAP-positive astrocytes), mESC lines were expanded on a monolayer of CF1 irradiated MEFs in ES medium, passaged and seeded as single cells on a layer of PA6 stromal cells at a concentration of 100 mESC per cm2 in SRM medium containing DMEM GlutaMax, 15% KnockOut Serum Replacement, 2 mM L-glutamine, 1% penicillin and streptomycin, 1% non-essential amino acids, 10uM β-mercaptoethanol (Ganat et al., 2012; Kawasaki et al., 2000). The medium was changed every other day, and from day 4 supplemented with 50 ng/ml SHH-C (R&D Systems) and 100 ng/ml FGF8 (ThermoFisher Scientific). At day 6, the cultures were grown using N2 medium composed of advanced DMEM:F12/Neurobasal medium (1:1), 15% KnockOut Serum Replacement, 2 mM L-glutamine, 1% penicillin and streptomycin, 10 μM β-mercaptoethanol, and supplemented with SHH-C and FGF8 (same concentrations as for day 4), plus 10 ng/ml FGF2 (ThermoFisher Scientific). Terminal differentiation was induced at day 10, by adding N2 media supplemented with 200 μM ascorbic acid (AA) (Sigma-Aldrich) and 20 ng/ml BDNF (R&D Systems). On day 14, cultures were dissociated using 1% trypsin, and cells were seeded on poly-L-ornithine/laminin-coated wells or prepared for FACS. Re-seeded cells were kept in culture for 6 days before subsequent fixing and immunocytochemistry.
2.4. Immunocytochemistry
Cultures were fixed with 4% paraformaldehyde for 30 min at 4 °C, rinsed three times with PBS and blocked for at least 1 h at room temperature with 10% donkey serum in PBS with 0.1% Triton-X (Sigma-Al-drich). Cells were incubated overnight at 4 °C with the following primary antibodies, in blocking solution: anti-aSYN (1:200, sc-7011-R, Santa-Cruz), anti-human aSYN (1:200, sc-12767, Santa-Cruz), anti-β-III-tubulin (1:500, T8660, Sigma-Aldrich), anti-CNPase (1:500, C5922, Sigma-Aldrich), anti-FoxA2 (1:250, sc-6554, Santa-Cruz), anti-GFP (1:500, AB16901, Millipore), anti-Oct4 (1:500, MAB4401, Millipore), anti-TH (1:500, AB152, Millipore; and 1:500, MAB318, Millipore), anti-β-III-tubulin (TUJ1, 1:500, PRB-435P, Covance). After three rinses with PBS, appropriate AlexaFluor-488, AlexaFluor-555 and AlexaFluor-647-labelled secondary antibodies (ThermoFisher Scientific) were used at 1:400 in PBS with 0.1% Triton-X and incubated for 1 h at room temperature in the dark. DAPI (ThermoFisher Scientific) was used for nuclei counterstaining (1:50,000). Images were acquired using an inverted epifluorescent microscope LRI - Olympus IX-73, cell counting was performed using ImageJ or metamorph.
2.5. FACS
Cells were detached using 0.1% trypsin on day 14 of the differentiation, re-suspended as single cells in 10% FBS in PBS and sorted based on the presence of GFP. Propidium iodide (ThermoFisher Scientific) at 10 μg/ml was used to discriminate dead cells from living cells. Cells were sorted using a FACS Aria-III machine. After sorting, cells were seeded on poly-L-ornithine/laminin-coated plates at 5000 cells per well in 5 μl droplets of N2 medium supplemented with BDNF and AA, topped up to 100 μl after 1 h, prior to imaging (data not shown).
Acknowledgments
We are thankful to Professors Anders Bjorklund, Pekka Kallunki, Meng Li and Hideyuki Okano for providing and facilitating access to the mice. This project was supported by the Crafoord foundation, the Bergvall foundation, the Kockska foundation, and the strategic area MultiPark to L.R.; and the Swedish and Chinese Medical Research Councils and the National Natural Science Foundation (81430025) to J.-Y.L.
Footnotes
Contribution
M.C., C.A., J.B. and C.V., maintenance, culture, differentiation and characterization of mESC lines. K.-S. K. and J.-Y.L., provision of study material and input on manuscript; L.R., derivation of mESC lines, manuscript writing together with M.C.
Resource table
|
| |
| Name of Stem Cell lines | TH∷GFP |
| TH∷GFP; PrP∷hSNCAA53T | |
| PrP∷hSNCAA53T | |
| WT | |
| Institution | University of Lund |
| Person who created resource | Laurent Roybon |
| Contact person and email | Laurent Roybon; Laurent.roybon@med.lu.se |
| Date archived/stock date | 21/11/2016 |
| Origin | Mouse blastocyst |
| Type of resource | Mouse embryonic stem cell lines |
| Sub-type | |
| Key factors | Tyrosine hydroxylase; SNCA |
| Authentication | Identity of cell line confirmed by PCR and immunocytochemistry (Fig. 1 and data not shown) |
| Link to related literature | http://www.ncbi.nlm.nih.gov/pubmed/11086981 |
| http://www.ncbi.nlm.nih.gov/pubmed/15016072 | |
| http://www.ncbi.nlm.nih.gov/pubmed/22751106 | |
| http://www.ncbi.nlm.nih.gov/pubmed/12062037 | |
| http://www.ncbi.nlm.nih.gov/pubmed/11353855 | |
| http://www.ncbi.nlm.nih.gov/pubmed/18790059 | |
| Information in public databases | |
| Ethics | The derivation of mESC was approved by the ethical committee for the use of laboratory animals at Lund |
| University and the Swedish Work Environment Authority. | |
|
| |
References
- Ganat YM, Calder EL, Kriks S, Nelander J, Tu EY, Jia F, Battista D, Harrison N, Parmar M, Tomishima MJ, et al. Identification of embryonic stem cell-derived midbrain dopaminergic neurons for engraftment. J Clin Invest. 2012;122:2928–2939. doi: 10.1172/JCI58767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giasson BI, Duda JE, Quinn SM, Zhang B, Trojanowski JQ, Lee VM. Neuronal alpha-synucleinopathy with severe movement disorder in mice expressing A53T human alpha-synuclein. Neuron. 2002;34:521–533. doi: 10.1016/s0896-6273(02)00682-7. [DOI] [PubMed] [Google Scholar]
- Kawasaki H, Mizuseki K, Nishikawa S, Kaneko S, Kuwana Y, Nakanishi S, Nishikawa SI, Sasai Y. Induction of midbrain dopaminergic neurons from ES cells by stromal cell-derived inducing activity. Neuron. 2000;28:31–40. doi: 10.1016/s0896-6273(00)00083-0. [DOI] [PubMed] [Google Scholar]
- Sawamoto K, Nakao N, Kobayashi K, Matsushita N, Takahashi H, Kakishita K, Yamamoto A, Yoshizaki T, Terashima T, Murakami F, et al. Visualization, direct isolation, and transplantation of midbrain dopaminergic neurons. Proc Natl Acad Sci U S A. 2001;98:6423–6428. doi: 10.1073/pnas.111152398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerlund M, Ran C, Borgkvist A, Sterky FH, Lindqvist E, Lundstromer K, Pernold K, Brene S, Kallunki P, Fisone G, et al. Lrrk2 and alpha-synuclein are co-regulated in rodent striatum. Mol Cell Neurosci. 2008;39:586–591. doi: 10.1016/j.mcn.2008.08.001. [DOI] [PubMed] [Google Scholar]
- Zhao S, Maxwell S, Jimenez-Beristain A, Vives J, Kuehner E, Zhao J, O’Brien C, de Felipe C, Semina E, Li M. Generation of embryonic stem cells and transgenic mice expressing green fluorescence protein in midbrain dopaminergic neurons. Eur J Neurosci. 2004;19:1133–1140. doi: 10.1111/j.1460-9568.2004.03206.x. [DOI] [PubMed] [Google Scholar]


