Abstract
Because an esophageal submucosa tumor (SMT) may be malignant despite its small size, a safe endoscopic resection method is needed in some small SMTs. Conventional endoscopic mucosal resection (EMR) may be simple, but incomplete pathologic resection margin status is common. We aimed to investigate the clinical outcomes of 2 kinds of EMR techniques (conventional EMR and EMR with band ligation device) and to evaluate the factors associated with incomplete pathologic resection.
We evaluated the medical records of 36 patients. All lesions were esophageal SMTs located in the submucosa or muscularis mucosa less than 10 mm in size by endoscopic ultrasound (EUS). The clinical outcomes based on the endoscopic procedures and factors associated with incomplete pathologic resection were evaluated.
The mean tumor size was 6.6 ± 4.1 mm. The overall en bloc and complete resection rates were 100% and 80.6%, respectively. No procedure-related complications, such as perforation and bleeding, were found. Univariate analysis showed that complete resection rates were higher in granular cell tumors than in leiomyomas (82.8% vs 17.2%, P = .029), tumors located in the submucosa layer than in the muscularis mucosa (96.6% vs 3.4%, P = .003), and in EMR with band ligation device than in conventional EMR (82.8% vs 17.2%, P < .001). Multivariate analysis showed that conventional EMR was the only significant factor associated with incomplete resection (OR, 35.594; 95% CI, 2.042–520.329; P = .014)
EMR with a band ligation device is an effective and safe treatment method for small esophageal SMT.
Keywords: biopsy, endoscopic resection, endoscopic ultrasound, submucosa tumor
1. Introduction
An esophageal submucosal tumor (SMT) is a tumor located beneath the mucosa. Although most small esophageal SMTs (less than 10 mm) are benign and asymptomatic, the tumor may be premalignant or malignant. Endoscopic forceps biopsy is a simple and reliable diagnostic tool for gastrointestinal epithelial tumors. However, endoscopic forceps biopsy is an unreliable diagnostic tool for the overlying mucosa of esophageal SMT because the lesion is located beneath the mucosa. Conventional endoscopy can provide useful information regarding SMT, including surface color, motility, and hardness. Because the malignant risk of small SMT with normal-appearing overlying mucosa is low, the regular follow-up endoscopic examination without further examination or resection may be acceptable.[1]
The differential diagnosis for esophageal SMT between potentially malignant and benign tumors is an important consideration when esophageal SMT is encountered. Endoscopic ultrasound (EUS) can provide more useful information regarding SMT compared with conventional endoscopy, including lesion size, layer of origin, and echogenicity.[2] Based on the data from conventional endoscopy and EUS findings, we can predict the malignant risk of a tumor to some extent. However, the diagnostic accuracy of EUS for SMT without tissue acquisition is reported to be 45.5% to 66.3%. Therefore, definite tissue diagnosis is important for esophageal SMT without normal-appearing mucosa.[3,4] Currently, several endoscopic methods to increase the diagnostic yield of gastrointestinal SMT have been introduced, such as bite on bite,[5] jumbo biopsy,[6] endoscopic mucosal resection (EMR),[7] endoscopic submucosal dissection,[8] endoscopic submucosal tunnel resection,[9] and EUS-guided fine needle aspiration (FNA) and biopsy.[10] However, no universally accepted tissue acquisition method with high diagnostic yield and low risk of complications has been described in the literature.
In the present study, we compared conventional EMR with EMR using a band-ligation device for small esophageal SMT (less than 10 mm). In addition, we analyzed the factors associated with incomplete pathologic resection margin status.
2. Patients and methods
2.1. Patients
We retrospectively reviewed the medical records of patients who underwent EMR of esophageal SMT at Pusan National University Yangsan Hospital in South Korea from January 2009 to January 2016. The present study included 36 patients who underwent EUS and EMR using the electrosurgical snare with or without the band-ligation device. All lesions were located in the muscularis mucosa or submucosa (2nd or 3rd layer by EUS) and were less than 10 mm in size. Written informed consent was obtained from all patients before performing the endoscopic procedures. The study was approved by the Ethics Committee of our Institutional Review Board (IRB No. L-2017–119).
2.2. Procedures
All endoscopic examination or endoscopic procedures were performed 4 endoscopists (CCW, KHW, PSB, and KSJ) who experienced more than 100 cases of endoscopic submucosal dissections. Diagnostic EUS using a mini-probe catheter (UM DP 20–25R; Olympus, Tokyo, Japan) was performed. In our institution, we recommend an annual follow-up endoscopic examination without resection for an esophageal SMT less than 10 mm with normal-appearing mucosa. We performed endoscopic forceps biopsy first for esophageal hard SMTs with abnormal patterns, such as color change, erosion, ulceration, or increasing size. We recommended endoscopic resection if endoscopic forceps biopsy results were inconclusive for SMT. If patients want to resect endoscopically for an esophageal SMT less than 10 mm with normal-appearing mucosa despite recommended regular checkups, we resected the tumor after explaining the possible complications (perforation and bleeding). In the present study, 2 kinds of endoscopic techniques were performed: conventional EMR or EMR using the band ligation device.
All procedures were performed using a single-channel endoscope (H260 or H290; Olympus Optical Co. Ltd., Tokyo, Japan) with an attached transparent cap with the patients under conscious sedation. We injected normal saline with an epinephrine and indigo carmine mixture into the submucosa around the tumor. Although the endoscopic procedures were selected based on the endoscopists’ decision, the main principle of the selected procedure type was that we usually performed conventional EMR if the tumor was elevated sufficiently after submucosa injection (polypoid elevation) to use the endoscopic electrosurgical snare (Fig. 1). However, if the elevated tumor after submucosal saline injection was difficult to use the electrosurgical snare (e.g., not polypoid elevation but broad base), we used EMR with a band ligation device (Fig. 2). For EMR with a band ligation device, we inserted an endoscope with a band ligation device attached tips of endoscope (Stiegmann-Goff ClearVue; ConMed, Boston, MA) into the esophagus after submucosal injection. After the tumor was aspirated into the ligator device, we underwent the elastic band ligation. Subsequently, endoscopic resection beneath the elastic band using a conventional endoscopic electrosurgical snare and electrosurgical generator (Endocut Q current, effect 3, cut duration 2, cut interval 5, VIO300D electrosurgical unit, ERBE, Tuebingen, Germany) was performed.
Figure 1.
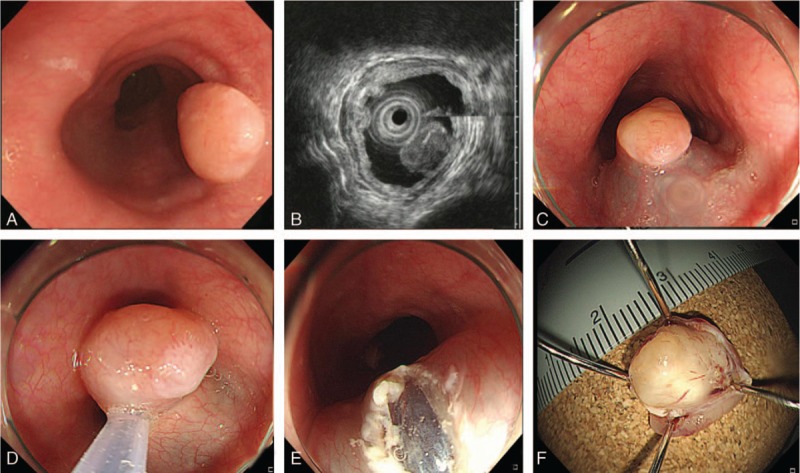
Conventional endoscopic mucosal resection with snare: (A) an esophageal submucosa tumor is found at the mid-esophagus. (B) A homogenous hypoechoic mass originating from the muscularis mucosa during endoscopic ultrasound. (C) Submucosa injection is done. (D) Tumor resection is performed. (E) Artificial ulcer after endoscopic resection is found. (F) Image of the resected specimen (10 mm). The diagnosis is granular cell tumor.
Figure 2.

Endoscopic mucosal resection with a band ligation device. (A) An esophageal submucosa tumor is found at the mid-esophagus. (B) A homogenous hypoechoic mass originating from the submucosa during endoscopic ultrasound. (C) Elastic band ligation is performed after submucosa injection. (D) Tumor resection with snare at beneath the elastic band is performed. (E) Artificial ulcer after endoscopic resection. (F) Image of the resected specimen (10 mm). The diagnosis is granular cell tumor.
Tumor size was determined using EUS. The procedure time was calculated from the photograph of the endoscopic procedure; before submucosal injection to after endoscopic hemostasis for artificial ulcer after endoscopic resection. We defined significant bleeding as a decrease of hemoglobin level more than 2 g/dL during or after the procedure. Perforation could be diagnosed during the endoscopic procedure or by the presence of subcutaneous emphysema.
After the specimens were sliced at 2-mm intervals, histopathologic type, invasion depth, and lateral and vertical resection margins were evaluated. Specimens were fixed in formalin, embedded in paraffin, and stained with hematoxylin and eosin for histologic examinations. A pathologic diagnosis was based on hematoxylin and eosin staining and, if possible, additional immunohistochemical staining using antibodies against CD34, CD117, S-100 protein, desmin, and smooth muscle actin. We defined en-bloc resection as resection of the tumor in 1 piece and complete resection as the absence of tumor cells at the resection margin. Incomplete resection was defined when the tumor cells were present in the resection margin or pathologists could not determine marginal status because of cautery artifact or crush injury.
We recommended a periodic follow-up endoscopic examination (6–12 months after the first resection and annually after the first follow-up examination) to evaluate local recurrence, synchronous, or metachronous lesions.
2.3. Statistical analysis
Our analysis was based on individual patient outcomes. Univariate analysis was performed with a chi-square test or Fisher's exact test for categorical variables. Student's t-test was performed for continuous variables. Variables found to be statistically significant (P < .05) in the univariate analysis were included in a forward, stepwise, multiple logistic regression model, to identify factors associated with incomplete resection. A P < .05 was considered to be statistically significant. Statistical calculations were performed with PASW Statistics for Windows, Version 21.0 (SPSS Inc., Chicago, IL).
3. Results
3.1. Baseline characteristics of enrolled patients
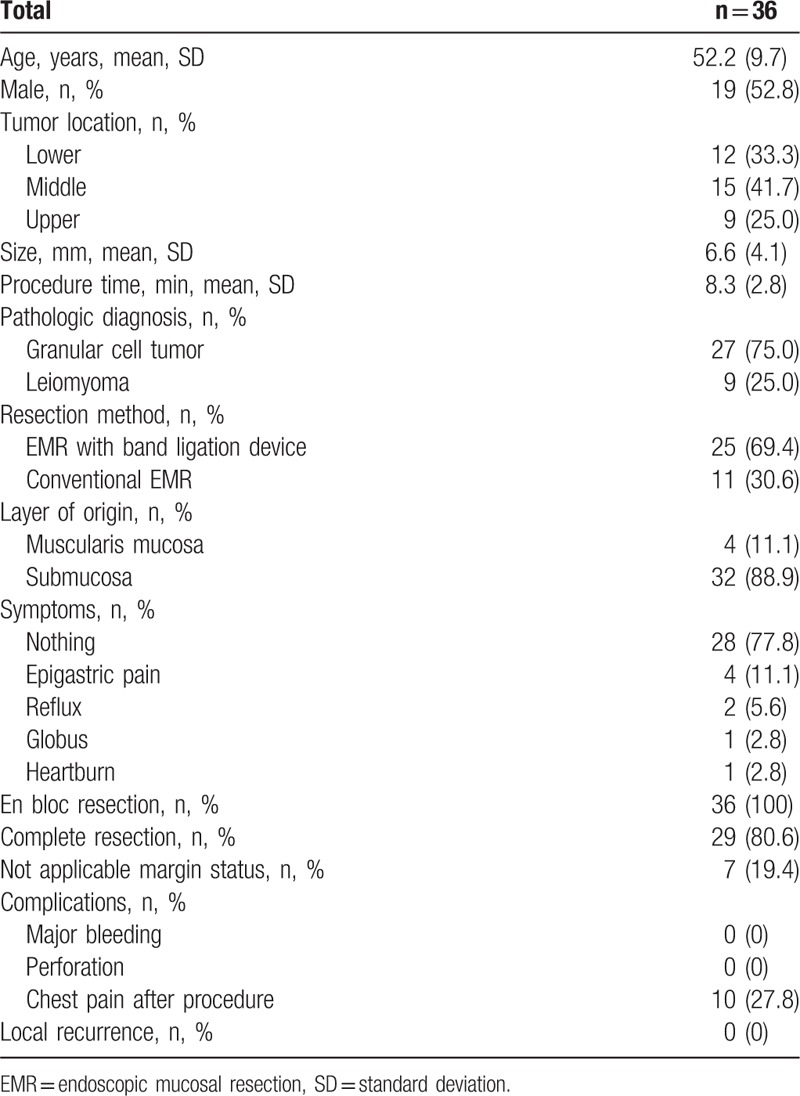
We evaluated 36 esophageal SMTs. The patients’ mean age was 52.2 ± 9.7 years (Table 1). The patient population predominantly consisted of men (19/36, 52.8%). The mean tumor size was 6.6 ± 4.1 mm. All lesions had hypoechoic echogenicity involving the muscularis mucosa or submucosa (2nd or 3rd layer by EUS). Most of the patients were asymptomatic (28/36, 77.8%). The overall en bloc and complete resection rates were 100% and 80.6%, respectively. Significant complications, such as major bleeding or esophageal perforation, were absent. Mild chest pain was relieved by analgesics in 27.8% of patients.
Table 1.
Baseline characteristics.
3.2. Comparison between conventional EMR and EMR with band ligation device: univariate analysis
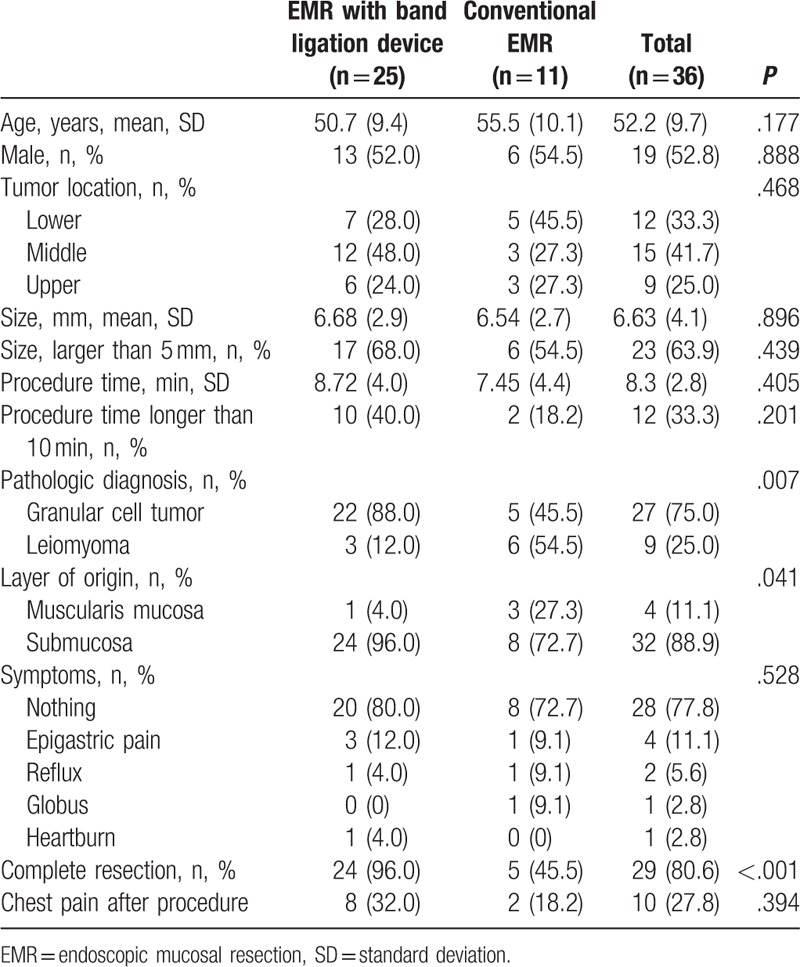
Mean age, sex predominance, tumor location, lesion size, and symptoms were not significantly different between treatment modalities (Table 2). EMR with band ligation device was more frequently performed for granular cell tumors (88.0% vs 45.5%, P = .007) and tumors originating from the submucosa layer (96.0% vs 72.7%, P = .041). The complete resection rate was higher in the EMR with a band ligation device group (96.0% vs 45.5%, P < .001). The reasons of incomplete resection in EMR were inapplicable margin status due to cautery artifact: conventional EMR (n = 6) and EMR with band-ligation device (n = 1). The regular follow-up examination was recommended for all patients with incomplete pathologic resection. No evidence of local recurrence during the follow-up period was found (range, 6–82 months).
Table 2.
Comparison between EMR with band ligation device and conventional EMR.
3.3. Factors associated with incomplete resection: univariate and multivariate analyses
Univariate analysis showed that the complete resection rate was associated with pathologic diagnosis, tumor origin layer, and endoscopic procedure types (Tables 3 and 4). Complete resection rates were higher in granular cell tumors than leiomyomas (82.8% vs 17.2%, respectively, P = .029), tumors originating from the submucosa layer than the muscularis mucosa (96.6% vs 3.4%, respectively, P = .003) and EMR with a band ligation device than the conventional EMR group (82.8% vs 17.2%, respectively, P < .001).
Table 3.
Comparison between complete and incomplete resection.
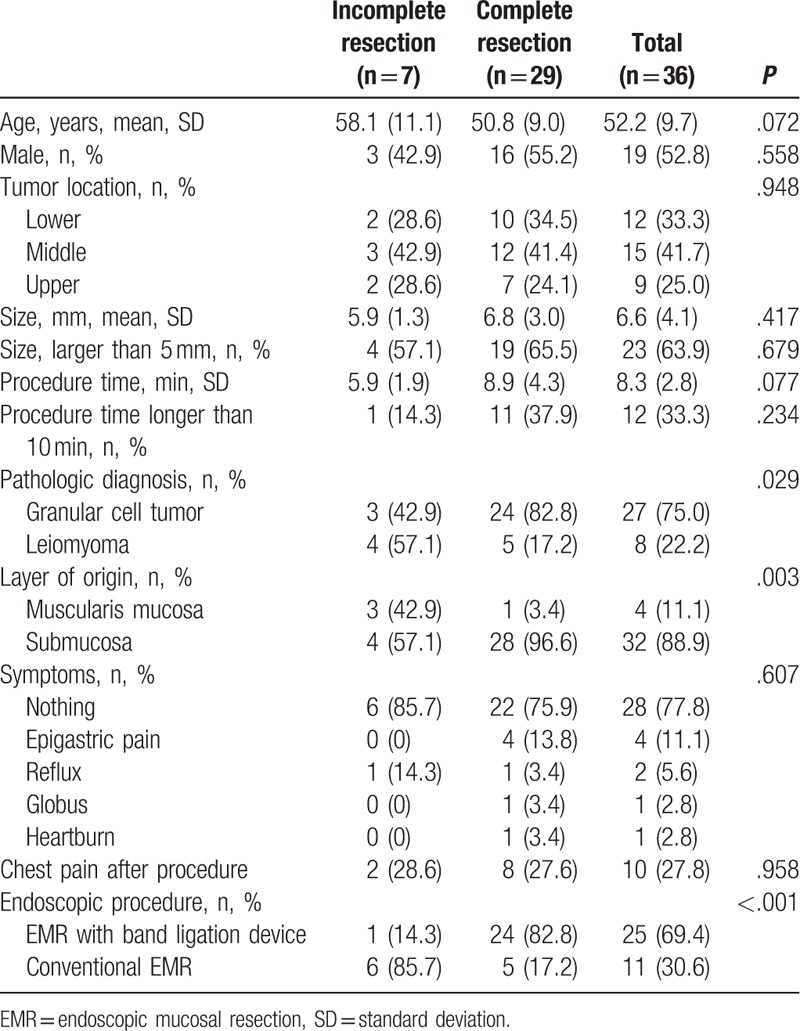
Table 4.
Risk factor analysis associated with incomplete resection.
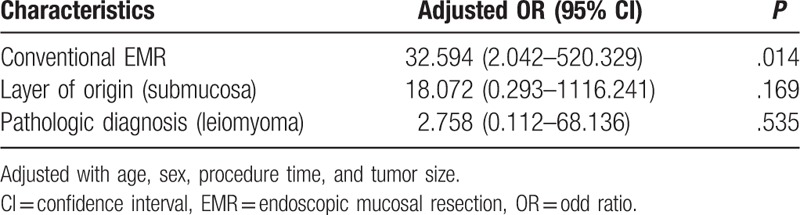
Multivariate analysis showed that the type of endoscopic procedure (conventional EMR) was the only significant factor associated with incomplete resection (OR, 35.594; 95% CI, 2.042–520.329, P = .014)
4. Discussion
In the present study, we evaluated effective complete resection associated with types of EMR techniques and the factors associated with incomplete resection. Although the en bloc resection rate was 100% regardless of endoscopic procedure types, the complete resection rate was higher in the EMR with a band ligation device (conventional EMR 45.5% vs EMR with a band ligation device 96.0%). In addition, the only significant factor associated with incomplete resection was a conventional EMR technique (OR, 35.594; 95% CI, 2.042–520.329; P = .014). Although the conventional EMR technique is a simple endoscopic resection method for esophageal SMT, conventional EMR was associated with difficulty in determining the pathologic margin status. Although we achieved 100% of en bloc resection rate using conventional EMR, the overlying mucosa of the SMT or tissue of the resection margin might be damaged extensively. In the present study, all the incomplete resection cases were associated with crush injury of the resection margin. Therefore, the pathologists reported inapplicable marginal status due to cautery injury. For patients with incomplete resection, we recommended regular follow-up examinations without additional resection because all lesions were benign tumors, such as leiomyoma and granular cell tumor. No evidence of local recurrence during the follow-up period was found (range, 6–82 months).
In the present study, the selection of EMR techniques was different based on the pathologic results and layer of origin. The decision regarding which procedure should be performed depended on the endoscopists’ preference. In the present study, most of the leiomyomas were located in the muscularis mucosa, and the lesion was more sufficiently elevated after submucosal injection using the conventional electrosurgical snare. However, most of the granular cell tumors were located in the submucosa layer, and the height of the tumor elevation after submucosal injection was not sufficient to snare without a band ligation device. Perhaps, this difference between lesions affects the different procedure types based on the layer of the tumor origin and pathologic results. EMR with a band ligation device was introduced to overcome the drawbacks of snaring submucosa tumor using conventional EMR.[7,11] We can lift the SMT sufficiently to snare the SMT beneath the ligated elastic band by using EMR with a band ligation device. In this way, a sufficient marginal tissue could be obtained to determine the pathologic status of the resection margin.[7,11]
Various kinds of endoscopic tissue diagnostic methods for gastrointestinal submucosa tumors have been recently introduced. The simplest method may be to perform a repeat biopsy (biopsy on the same site) or to use a large bore forceps (jumbo biopsy forceps). Although the diagnostic yield of this technique was reported to be 58.9%, endoscopic hemostasis was needed in 34.9% of patients after endoscopic forceps biopsy.[5] Endoscopic submucosal dissection (ESD) has been used to improve the complete endoscopic resection rate for gastrointestinal superficial neoplasm. The advantages of ESD are higher en bloc and complete resection rate regardless of tumor size.[12] However, the ESD technique is more complex, has more complications, and needs longer procedure time than EMR.[13] The esophagus is a more difficult site to perform ESD compared with the stomach because the narrower esophageal lumen compared with the gastric lumen interferes with endoscopic manipulation. In the present study, the pathologic complete resection rate was 96% by EMR with a band ligation device. For esophageal SMTs less than 10 mm originating from the submucosa, EMR with a band ligation device is sufficient for institutions that cannot perform ESD.
In the present study, the pathologic results were granular cell tumor or leiomyoma. Further management strategies are not needed because leiomyoma is a benign tumor. The esophageal granular cell tumor originated Schwann cell are usually located in the submucosa.[14] Asymptomatic small esophageal granular cell tumor could be followed-up without resection. However, some authors recommend endoscopic resection because malignant transformation has been reported even in small-sized granular cell tumors, and the precise natural history of the lesion is unknown.[15–17] In our institutions, we generally recommend endoscopic resection for esophageal SMT with color change, increasing tumor size, or some different mucosal findings compared with the surrounding normal esophageal mucosa. Some patients wanted to resect the tumor because of anxiety regarding the discovery or future changes of the tumor. For these patients, endoscopic resection of the tumor can eliminate the anxiety.
This study has some limitations. First, selection biases were present because of the retrospective design of the study at an academic referral center. Therefore, the procedure types were not randomized. The type of endoscopic procedure was selected according to the endoscopist's preference during the procedure. Second, the small sample size of our study may be inadequate to generalize the study results. Third, the experience of the endoscopist could not be analyzed because of small sample size. A previous article of colon EMR reported that the experience of endoscopists was an important factor in the fellow in the fellow-treated group. However, the lesion size was important in the expert group.[18] Although the procedure types were no randomly distributed among endoscopists in the present study, all endoscopists experienced therapeutic endoscopic procedure more than 3 years (each endoscopist performs more than 100 cases endoscopic submucosal dissection, annually). And, the tumor sizes were less than 10 mm in diameter. Further prospective studies comparing another diagnostic modality may provide more accurate information.
In summary, the present study showed that both the conventional EMR and EMR with a band ligation device were a simple and safe resection modality for esophageal SMT less than 10 mm. However, EMR with band ligation device showed higher complete pathologic resection rate compared with conventional EMR. EMR with a band ligation device may be appropriate when resecting esophageal SMT less than 10 mm originating from the submucosa or muscularis mucosa rather and conventional EMR.
Footnotes
Abbreviations: EMR = endoscopic mucosal resection, ESD = endoscopic submucosal dissection, EUS = endoscopic ultrasound, FNA = fine needle aspiration, IRB = Institutional Review Board, SD = standard deviation, SMT = submucosa tumor.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Eckardt AJ, Wassef W. Diagnosis of subepithelial tumors in the GI tract. Endoscopy, EUS, and histology: bronze, silver, and gold standard? Gastrointest Endosc 2005;62:209–12. [DOI] [PubMed] [Google Scholar]
- [2].Hwang JH, Rulyak SD, Kimmey MB. American Gastroenterological Association I. American Gastroenterological Association Institute technical review on the management of gastric subepithelial masses. Gastroenterology 2006;130:2217–28. [DOI] [PubMed] [Google Scholar]
- [3].Karaca C, Turner BG, Cizginer S, et al. Accuracy of EUS in the evaluation of small gastric subepithelial lesions. Gastrointest Endosc 2010;71:722–7. [DOI] [PubMed] [Google Scholar]
- [4].Lim TW, Choi CW, Kang DH, et al. Endoscopic ultrasound without tissue acquisition has poor accuracy for diagnosing gastric subepithelial tumors. Medicine (Baltimore) 2016;95:e5246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Hunt GC, Smith PP, Faigel DO. Yield of tissue sampling for submucosal lesions evaluated by EUS. Gastrointest Endosc 2003;57:68–72. [DOI] [PubMed] [Google Scholar]
- [6].Buscaglia JM, Nagula S, Jayaraman V, et al. Diagnostic yield and safety of jumbo biopsy forceps in patients with subepithelial lesions of the upper and lower GI tract. Gastrointest Endosc 2012;75:1147–52. [DOI] [PubMed] [Google Scholar]
- [7].Hong JB, Choi CW, Kim HW, et al. Endoscopic resection using band ligation for esophageal SMT in less than 10 mm. World J Gastroenterol 2015;21:2982–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Li QL, Yao LQ, Zhou PH, et al. Submucosal tumors of the esophagogastric junction originating from the muscularis propria layer: a large study of endoscopic submucosal dissection (with video). Gastrointest Endosc 2012;75:1153–8. [DOI] [PubMed] [Google Scholar]
- [9].Chen T, Zhang C, Yao LQ, et al. Management of the complications of submucosal tunneling endoscopic resection for upper gastrointestinal submucosal tumors. Endoscopy 2016;48:149–55. [DOI] [PubMed] [Google Scholar]
- [10].Lee JH, Choi KD, Kim MY, et al. Clinical impact of EUS-guided Trucut biopsy results on decision making for patients with gastric subepithelial tumors >/ = 2 cm in diameter. Gastrointest Endosc 2011;74:1010–8. [DOI] [PubMed] [Google Scholar]
- [11].Ono A, Fujii T, Saito Y, et al. Endoscopic submucosal resection of rectal carcinoid tumors with a ligation device. Gastrointest Endosc 2003;57:583–7. [DOI] [PubMed] [Google Scholar]
- [12].Cao Y, Liao C, Tan A, et al. Meta-analysis of endoscopic submucosal dissection versus endoscopic mucosal resection for tumors of the gastrointestinal tract. Endoscopy 2009;41:751–7. [DOI] [PubMed] [Google Scholar]
- [13].Oka S, Tanaka S, Kaneko I, et al. Advantage of endoscopic submucosal dissection compared with EMR for early gastric cancer. Gastrointest Endosc 2006;64:877–83. [DOI] [PubMed] [Google Scholar]
- [14].Catalano F, Kind R, Rodella L, et al. Endoscopic treatment of esophageal granular cell tumors. Endoscopy 2002;34:582–4. [DOI] [PubMed] [Google Scholar]
- [15].Voskuil JH, van Dijk MM, Wagenaar SS, et al. Occurrence of esophageal granular cell tumors in The Netherlands between 1988 and 1994. Dig Dis Sci 2001;46:1610–4. [DOI] [PubMed] [Google Scholar]
- [16].Choong CK, Meyers BF. Benign esophageal tumors: introduction, incidence, classification, and clinical features. Semin Thorac Cardiovasc Surg 2003;15:3–8. [DOI] [PubMed] [Google Scholar]
- [17].Kahng DH, Kim GH, Park DY, et al. Endoscopic resection of granular cell tumors in the gastrointestinal tract: a single center experience. Surg Endosc 2013;27:3228–36. [DOI] [PubMed] [Google Scholar]
- [18].Choi JM, Lee C, Park JH, et al. Complete resection of colorectal adenomas: what are the important factors in fellow training? Dig Dis Sci 2015;60:1579–88. [DOI] [PubMed] [Google Scholar]


