Abstract
Rationale:
Giant cell arteritis (GCA) combined with concomitant pulmonary embolism (PE) is extremely difficult to diagnose because of its low incidence and atypical clinical presentations.
Patient concerns:
A 62-year-old male developed fever of unknown origin.
Diagnoses:
Positron emission tomography/computed tomography (PET/CT) revealed increased glucose metabolism in the vascular walls of the ascending and descending aorta and pulmonary artery, leading to a diagnosis of GCA combined with PE.
Interventions:
The patient did not respond to regular antiviral and antibacterial treatment but was remised after steroid treatment.
Outcomes:
No specific autoantibodies were positive for this patient, and the patient did not respond to regular antiviral and antibacterial treatment. After diagnosed by PET/CT, the patient responded well to steroid treatment. Literature review found 16 cases of GCA diagnosed by PET/CT. Their median age was 68.5 (range, 21–87) years and 13 cases were female. PET/CT showed significantly increased metabolism in the ascending and descending aorta, abdominal aorta, and carotid artery. In 4 cases (including our own case), the mean maximum standardized uptake value was 4.2 ± 1.7 (range, 2.5–7.2). Six cases of GCA also had PE and 5 (6/7, 85.7%) cases were females, and the current case is the first male case of GCA combined with PE. Steroid therapy was initiated in all 5 cases. Complete remission was achieved in 4 cases and 2 patients died and the outcome of 1 patient was unknown.
Lessons:
Our case and the review highlight the value of PET/CT in diagnosing GCA combined with PE, suggesting that PET/CT is the preferred diagnostic tool for atypical patients presenting with fever or muscle pain.
Keywords: 18F-FDG, fever of unknown origin, giant cell arteritis, PET/CT, pulmonary embolism
1. Introduction
Giant cell arteritis (GCA) is a systemic vasculopathy mainly affecting the extracranial carotid branches and the aorta, and epidemiologically more common in people aged over 50 years.[1] A recent population-based study has revealed that patients with GCA are associated with a higher risk of venous thromboembolism including pulmonary embolism (PE).[2] The underlying mechanism could be that GCA occurring in large vessels such as the pulmonary arteries[3] leads to acute PE.
The incidence rate of PE in patients with GCA (7.7 per 1000 person-years) was approximately 4 times higher than that in healthy subjects (1.9 per 1000 person-years).[2] Pulmonary artery thrombosis caused by GCA is an extremely rare condition and the diagnosis is rather challenging. Positron emission tomography/computed tomography (PET/CT) scan has been more and more widely utilized in diagnosing large-vessel vasculitis. Compared to other image techniques such as magnetic resonance imaging (MRI) and arteriography, PET/CT is advantageous in detecting metabolic changes rather than anatomic changes. Studies have shown that metabolic changes in the arterial wall usually precede anatomic changes.[4] Furthermore, during the progress of vasculitis, recruitment, activation, migration, and infiltration of inflammatory cells usually precede the appearance of inflammatory edema; 18F-fluro-deoxyglueose (FDG) PET/CT is privileged in sensing changes in inflammatory cells; therefore, it is more sensitive at an earlier stage than an MRI scan.[5] Here, we report a case of PET/CT successfully diagnosing GCA of pulmonary artery with the presence of thrombi, and a review of relevant literature for the value of PET/CT in diagnosing GCA.
2. Case report
A 62-year-old male was admitted to our hospital on December 27, 2014 presenting with low-grade fever for 3 weeks and foot edema for 5 days. The patient developed a fever (37.3 °C) 22 days ago, with shiver, night perspiration, and headache on the right side of his head. He took some Chinese herbal medicines without consulting a doctor, and shiver, headache, and night perspiration disappeared; however, his body temperature continued to fluctuate between 37.6 and 37.8 °C. He received antiviral and antibacterial therapy with cefuroxime and amoxicillin at a community hospital, but no amelioration was seen. His fever worsened 5 days ago (38.9 °C), and he developed generalized body aches, bilateral foot edema, and cough with scant whitish sputum. Blood tests at our outpatient department showed—leukocytes: 12.8 × 109/L (normal range: 3.5–9.0 × 109), neutrophils: 78.3%, red blood cells (RBCs): 3.68 × 1012/L, hemoglobin: 124 g/L (normal range: 110–150 g/L), platelets: 227 × 109/L (normal range: 100–300 × 109/L). Urinalysis revealed proteinuria (0.5 g/L) and chest X-ray showed increased interstitial markings. The patient was intravenously injected with cephalosporins for 3 days, but his symptoms were aggravated, especially with increased body aches and swollen feet, lower extremity muscle pain, limited mobility. The patient showed in our emergency department and the blood tests revealed leukocytes 13.5 × 109/L; C-reactive protein (CRP) >200 mg/L (normal range: <8 mg/L), and d-dimer: 4.74 mg/L (normal range <0.5 mg/L). Urinalysis showed proteinuria was 0.7 g/L. Doppler ultrasound showed venous thrombi in the calf veins and arteriosclerosis of arteries of bilateral lower limbs. His hepatic and renal functions were normal, blood culture was negative, and abdomen ultrasound revealed no abnormality.
Admission examination showed that the patient's heart rate was 90 beats/min, blood pressure 130/80 mm Hg, and body temperature 38.6 °C. The patient presented increased pulsation of the right temporal artery and edema of the bilateral ankles and the dorsal feet. His laboratory results included leukocytes: 7.42 × 109/L, neutrophils: 80%, RBCs: 2.73 × 1012/L, hemoglobin: 87 g/L, platelets: 282 × 109/L, CRP: 19.5 mg/dL (normal range <0.8 mg/dL), erythrocyte sedimentation rate (ESR): 63 mm/h (normal<15 mm/h), procalcitonin: 0.61 ng/mL (normal range <0.5 ng/mL), and serum ferritin: 698.8 ng/mL. His antinuclear antibody (ANA) and antineutrophil cytoplasmic antibody (ANCA) were negative. His anticardiolipin antibody was 28 PL/mL (normal range <10 PL/mL), and his anti-double stranded deoxyribonucleic acid (dsDNA ) was 105 IU/mL (normal range <20 IU/mL), myeloperoxidase (MPO): 108 U/mL (normal range <20 U/mL), and proteinase 3 (PR3): 180 U/mL (normal range <20 U/mL). Anaerobic and aerobic blood culture was negative on 3 occasions, and urine and bone marrow culture was also unproductive. Bone marrow biopsy was unremarkable. T-Spot test for tuberculosis was negative. No tumor biomarkers were detected. His renal function was normal and urinalysis revealed no abnormality and his electromyography was also normal.
Lung CT scan of the patient showed bilateral mild emphysema, regional bulla, and scattered nodules (Fig. 1). Echocardiography showed slight enlargement of the right atrium, mild-to-moderate tricuspid insufficiency, mild bicuspid insufficiency, and pulmonary hypertension (estimated 54 mm Hg). The patient was given intensive antibiotic therapy, including moxifloxacin, piperacillin/sulbactam, imipenem, and vancomycin. Nonsteroid anti-inflammatory drugs (NSAIDs), anticoagulation agents, antiplatelet medications, and supportive therapy were also provided. His cough was lessened, but fever still remained and reached 39.2 °C. Repeated examination on January 4, 2015 showed leukocytes: 10.5 × 109/L, neutrophils: 82.1%, RBCs: 2.5 × 1012/L, hemoglobin: 81 g/L, platelets: 361 × 109/L, and CRP: 4.64 mg/dL.
Figure 1.
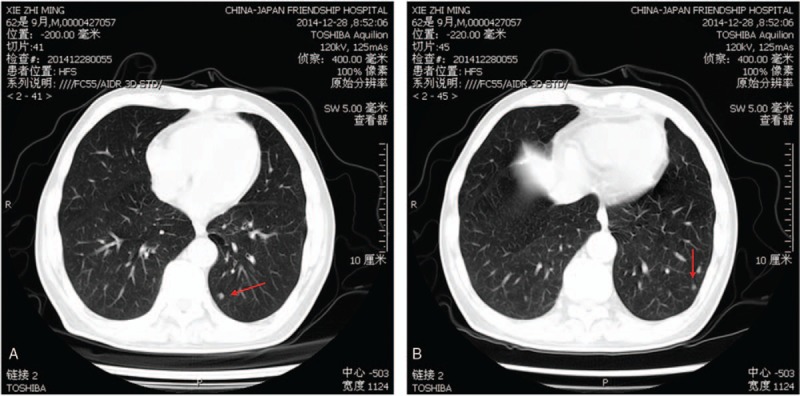
Computed tomography angiography shows cross-sections of the lungs. Mild emphysema, regional bulla, and scattered nodules can be seen from both lungs. Red arrows indicate nodules.
For further diagnosis, PET/CT scan was performed, which showed thickening of bilateral pleura and the pericardium, parapneumonic and pericardial effusions, and increased glucose metabolism in the vascular walls of the ascending and descending aorta and pulmonary artery (Fig. 2A and B). CT angiography showed mild PE in the anterior segment of the pulmonary artery in the superior lobe and pleural thickening of the left oblique fissure, and bilateral pleural effusions (Fig. 3). Therefore, the patient received an ultimate diagnosis of GCA combined with PE. NSAIDs and antibiotics were discontinued, and intravenous methylprednisolone (40 mg/d), albumin, and diuretics were given. After 2 days, his temperature returned to normal and muscle aches disappeared. Bilateral lower limb edema was lessened. Laboratory findings on January 9, 2015 showed leukocytes: 8.09 × 109/L, neutrophils: 68.8%, RBCs: 2.39 × 1012/L, hemoglobin: 78 g/L, and platelets: 387 × 109/L. Laboratory test on January 13, 2015 showed that CRP was 0.45 mg/dL. The patient was discharged and his ESR and CRP returned to normal at 6 months of follow-up.
Figure 2.
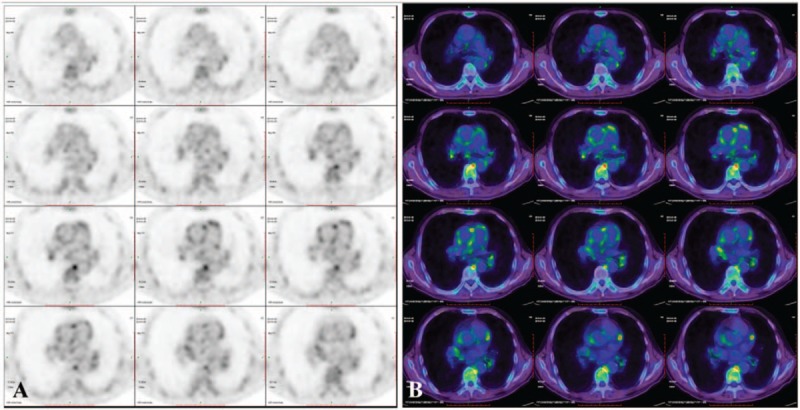
Fluro-deoxyglueose-positron emission tomography (A) and fused axial computed tomography (B) at baseline demonstrate diffuse, confluent radionuclide uptake in the walls of the ascending aorta, descending aorta, pulmonary aorta, the maximum standardized uptake value is 4.8.
Figure 3.
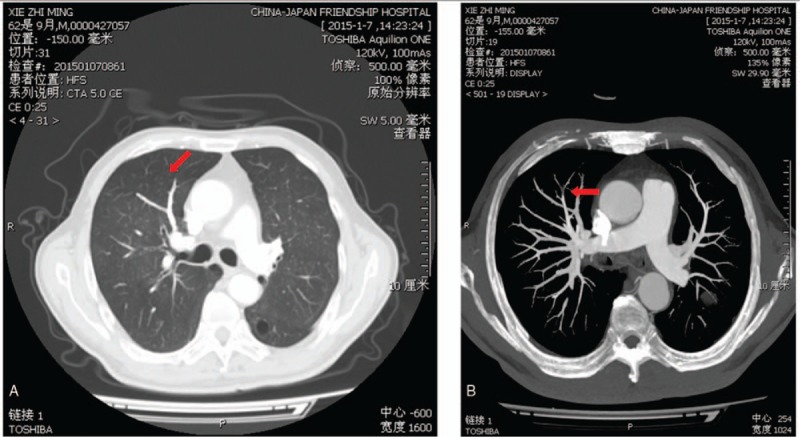
Computed tomography angiography shows cross-sections of the lungs. Red arrows indicate mild pulmonary embolism in the anterior segment of the pulmonary artery of the superior lobe.
2.1. Review of the literature
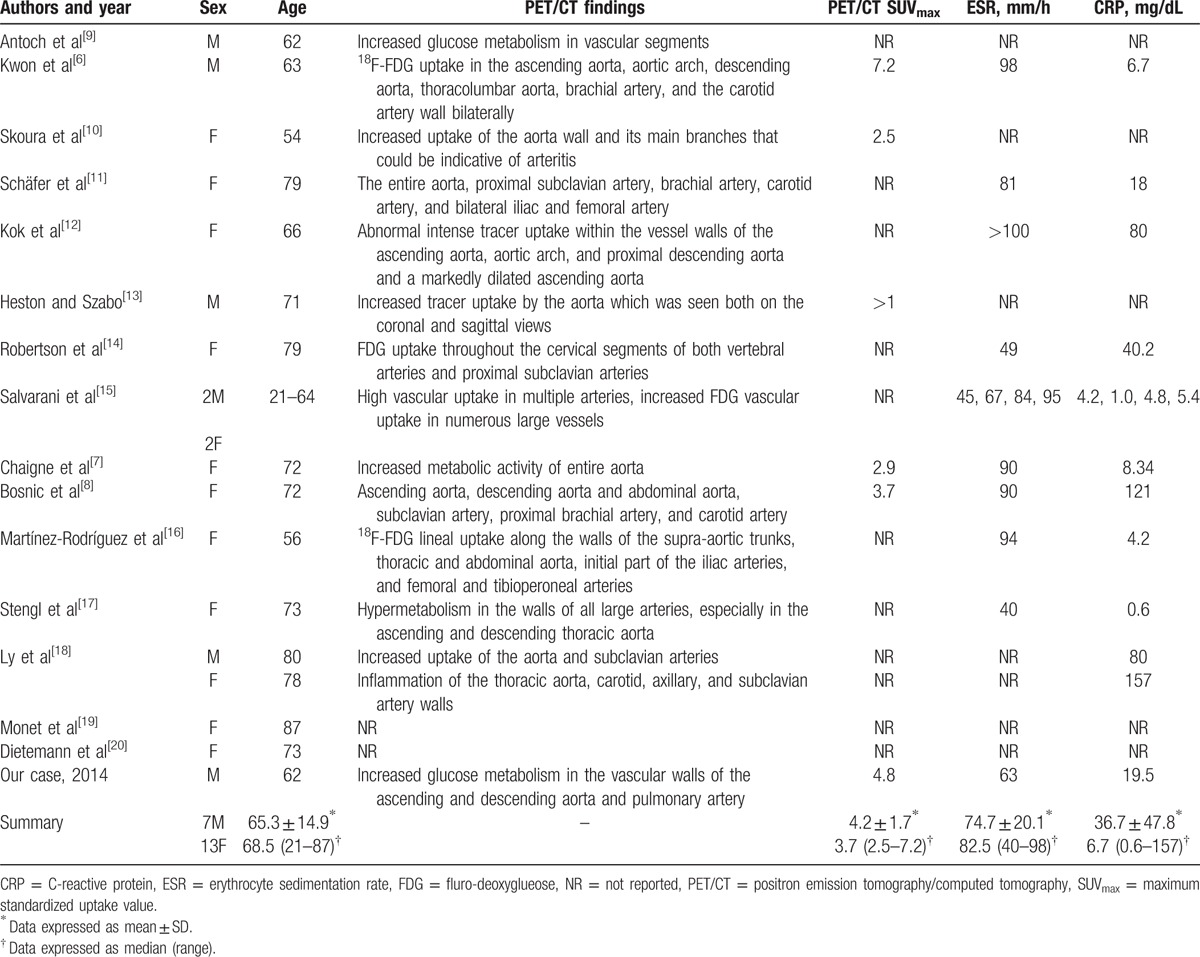
A PubMed search up to January 2016 using “PET/CT” and “giant cell arteritis” returned 40 papers, including 19 case reports. We reviewed all 20 cases. Their median age was 68.5 (range, 21–87) years and more than half of the patients (65%, 13/20) were female. The mean ESR was 74.7 ± 20.1 mm Hg, and the mean CRP was 36.7n and 74.7 dL. Totally, 16 cases of GCA were diagnosed using PET/CT. The PET/CT findings on GCA are summarized in Table 1. PET/CT in most cases show significantly increased metabolism in the ascending and descending aorta, abdominal aorta, and carotid artery, indicating large vessel vasculitis. In 4 cases (including our own case) where PET/CT standardized uptake values (SUV) is available, the mean SUVmax was 4.2 ± 1.7 (range, 2.5–7.2).[6–8]
Table 1.
Literature regarding PET/CT aiding diagnosis of GCA.
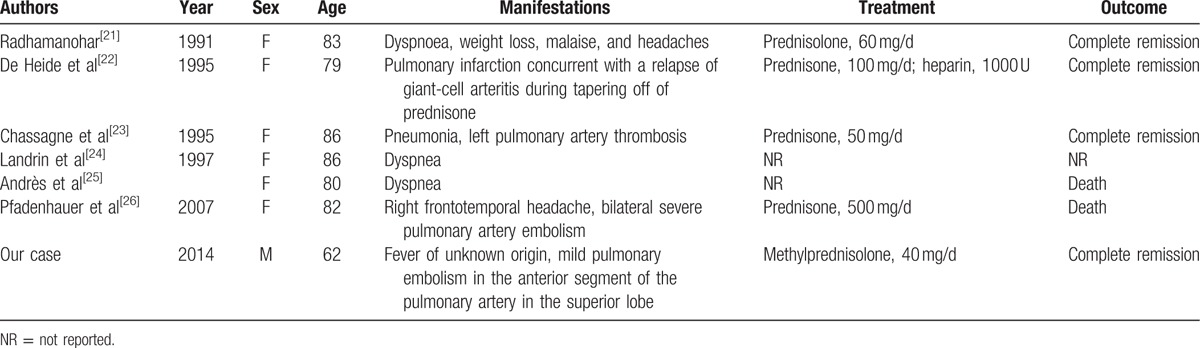
We searched for case reports published before January 2016 in PubMed on “giant cell arteritis and pulmonary embolism” for publications. Eleven case reports were identified, including 6 reports in English. We reviewed all 6 papers and their demographic and baseline characteristics are summarized in Table 2. Their age ranged from 62 (the current case) to 86 years with a median age of 82 years. Five (6/7, 85.7%) cases were females, and the current case is a male case of GCA combined with PE. In cases where treatment was described, steroid therapy was initiated in all 5 cases. Complete remission was achieved in 4 cases and 2 patients died and the outcome of 1 patient was unknown.
Table 2.
Reported cases of GCA combined with PE.
3. Discussion
GCA is the main cause of large-vessel vasculitis involved in the aorta and its major branches in elderly patients. The manifestations of GCA show wide variation from such typical symptoms as headache, scalp tenderness, visual disturbances, and jaw claudication to fever of unknown origin with the absence of cranial symptoms. Furthermore, GCA lacks specific serological markers, leading to misdiagnosis or missed-diagnosis.
Normally, GCA is a systemic disease, and pulmonary involvement is quite uncommon,[25] which happens only 2% in GCA patients.[2] Therefore, in some rare cases when GCA is combined with PE, the difficulty of diagnosis is largely increased. The current case presented fever of unknown origin as the initial manifestation, with muscle pain and nocturnal perspirations and elevations in plasma procalcitonin. Intensive antibiotic therapy neither brought down the body temperature, nor halted disease progression, indicating the presence of other causes instead of infection. Autoantibody test revealed that MPO and PR3 were positive, but ANCA was negative, and anti-dsDNA antibody was positive, but ANA was negative, which failed to explain the patient's illness. We also found that kidney was not involved and ANCA-associated small vessel vasculitis was ruled out.
GCA usually does not involve the lungs, and its combination with PE is even rarer. Our search of records in PubMed showed 6 reported cases of GCA combined with PE. All these cases were female and elderly (median age, 82.5 years). PE was diagnosed in these patients by CT angiograms or postmortem examination. Patients were typically managed with steroid therapy and complete recovery can be achieved in 4 out of 6 cases.
Recent literature has demonstrated a favorable diagnostic value of PET/CT in vasculitis and fever of unknown origin.[4] Therefore, we performed PET/CT scan to this patient and found increased glucose metabolism in the vascular walls of large vessels, multiple and uneven radiation concentrations, providing diagnostic clue for GCA. Along with CT angiography showing embolism in the anterior segment of the pulmonary artery, the diagnosis of GCA combined with PE was confirmed. Steroids were given to the patient and led to prompt recovery.
CT, MRI vascular reconstruction, and vascular ultrasonography are conventional vascular imaging modalities, which are more reliable to detect thickening of vessel wall and stenosis of lumens as the late signs of vasculitis but not sensitive for early signs. However, it is more important to establish more trustworthy methods to diagnose early vasculitis for early awareness and prompt therapy. PET/CT, which is mainly used for diagnosing and evaluating solid tumors and lymphoma, has been recently shown to detect metabolic changes in neutrophils, monocytes/macrophages, and activated lymphocytes by increased uptake of FDG in response to inflammation.[27] However, few authors provided data on SUVmax and only in 4 (20%, 4/20) GCA cases (including our own case) PET/CT SUV is available with a mean SUVmax of 4.2 ± 1.7.[6–8] Delineation by PET/CT would be clinically useful in identifying subtle cases of GCA and establishment of a cutoff SUVmax would facilitate diagnosis of GCA cases. Future studies involving an adequate cohort size would be required to provide a meaningful answer.
4. Conclusion
This is the first report on the application of PET/CT in early and accurate diagnosis of GCA combing with PE. This allows early administration of steroid therapy. Our case highlights the advantages of PET/CT in diagnosing GCA combing with PE, providing a diagnosing strategy for atypical patients and helping clinicians with quick diagnosis at an early stage of the disease to initiate suitable therapy promptly.
Footnotes
Abbreviations: GCA = giant cell arteritis, PE = pulmonary embolism, PET/CT = positron emission tomography/computed tomography, SUV = standardized uptake values.
Compliance with ethical standards.
Funding/support: The study was funded by the General Program of the National Natural Science Foundation of China (grant no. 81571603), the Major Research Plan of the National Natural Science of China (grant no. 91542121), the Youth Program of the National Natural Science Foundation of China (grant no. 81401363), the Capital Health Research and Development of Special Programs (grant no. 2014-4-4062), and China-Japan Friendship Hospital Youth Science and Technology Excellence project (2014-QNYC-B-04).
Research involving human participants: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent: Informed consent was obtained from all individual participants included in the study.
The authors have no conflicts of interest to disclose.
References
- [1].Tato F, Hoffmann U. Giant cell arteritis: a systemic vascular disease. Vasc Med 2008;13:127–40. [DOI] [PubMed] [Google Scholar]
- [2].Avina-Zubieta JA, Bhole VM, Amiri N, et al. The risk of deep venous thrombosis and pulmonary embolism in giant cell arteritis: a general population-based study. Ann Rheum Dis 2016;75:148–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Andrès E, Kaltenbach G, Marcellin L, et al. Acute pulmonary embolism related to pulmonary giant cell arteritis. Presse Med 2004;33:1328–9. [DOI] [PubMed] [Google Scholar]
- [4].Förster S, Tato F, Weiss M, et al. Patterns of extracranial involvement in newly diagnosed giant cell arteritis assessed by physical examination, colour coded duplex sonography and FDG-PET. Vasa 2011;40:219–27. [DOI] [PubMed] [Google Scholar]
- [5].Meller J, Strutz F, Siefker U, et al. Early diagnosis and follow-up of aortitis with [(18)F]FDG PET and MRI. Eur J Nucl Med Mol Imaging 2003;30:730–6. [DOI] [PubMed] [Google Scholar]
- [6].Kwon CM, Hong YH, Chun KA, et al. A case of silent giant cell arteritis involving the entire aorta, carotid artery and brachial artery screened by integrated PET/CT. Clin Rheumatol 2007;26:1959–62. [DOI] [PubMed] [Google Scholar]
- [7].Chaigne B, Jr, Magnant J, Favelle O, et al. 18 FDG PET/CT contribution in occult giant-cell arteritis. J Clin Rheumatol 2012;18:104–5. [DOI] [PubMed] [Google Scholar]
- [8].Bosnic D, Barešić M, Padjen I, et al. Fever of unknown origin: large vessel vasculitis diagnosed by PET/CT. Rheumatol Int 2013;33:2417–21. [DOI] [PubMed] [Google Scholar]
- [9].Antoch G, Freudenberg LS, Debatin JF, et al. Images in vascular medicine. Diagnosis of giant cell arteritis with PET/CT. Vasc Med 2003;8:281–2. [DOI] [PubMed] [Google Scholar]
- [10].Skoura E, Giannopoulou C, Keramida G, et al. A case of fever of unknown origin: (18)F-FDG-PET/CT findings in Takayasu's arteritis. Hell J Nucl Med 2008;11:172–4. [PubMed] [Google Scholar]
- [11].Schäfer VS, Warrington KJ, Williamson EE, et al. Delayed diagnosis of biopsy-negative giant cell arteritis presenting as fever of unknown origin. J Gen Intern Med 2009;24:532–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kok J, Lin M, Patapanian H, et al. [18F]FDG PET/CT in the diagnosis of large vessel vasculitis. Intern Med J 2009;39:267–9. [DOI] [PubMed] [Google Scholar]
- [13].Heston TF, Szabo Z. Giant cell arteritis on 18F-FDG PET/CT. Clin Physiol Funct Imaging 2009;29:382–4. [DOI] [PubMed] [Google Scholar]
- [14].Robertson CE, Michet CJ, Jr, Hunt CH, et al. (18)F-FDG PET/CT may be useful when evaluating a patient for giant cell arteritis. Headache 2012;52:491–3. [DOI] [PubMed] [Google Scholar]
- [15].Salvarani C, Magnani L, Catanoso M, et al. Tocilizumab: a novel therapy for patients with large-vessel vasculitis. Rheumatology 2012;51:151–6. [DOI] [PubMed] [Google Scholar]
- [16].Martínez-Rodríguez I, Del Castillo-Matos R, Rubio-Vassallo A, et al. Diagnosis and assessment of the treatment response in a case of giant cell arteritis using (18)F-FDG PET/CT. Rev Esp Med Nucl Imagen Mol 2012;31:233–5. [DOI] [PubMed] [Google Scholar]
- [17].Stengl KL, Buchert R, Bauknecht H, et al. A hidden giant: Wallenberg syndrome and aortal wall thickening as an atypical presentation of a giant cell arteritis. BMJ Case Rep 2013;2013:bcr2012006994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ly KH, Stirnemann J, Liozon E, et al. Interleukin-1 blockade in refractory giant cell arteritis. Joint Bone Spine 2014;81:76–8. [DOI] [PubMed] [Google Scholar]
- [19].Monet A, Massonnat R, Merino B, et al. Crowned dens syndrome diagnosed on (1)(8)F-FDG PET/CT. Clin Nucl Med 2014;39:1041–2. [DOI] [PubMed] [Google Scholar]
- [20].Dietemann S, Noblet V, Imperiale A, et al. FDG PET findings of the brain in sudden blindness caused by bilateral central retinal artery occlusion revealing giant cell arteritis. Clin Nucl Med 2015;40:45–6. [DOI] [PubMed] [Google Scholar]
- [21].Radhamanohar M. Multiple pulmonary infarctions caused by giant cell arteritis. Postgrad Med J 1991;67:491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].De Heide L, Pieterman H, Hennemann G. Pulmonary infarction caused by giant-cell arteritis of the pulmonary artery. Neth J Med 1995;46:36–40. [DOI] [PubMed] [Google Scholar]
- [23].Chassagne P, Gligorov J, Dominique S. Pulmonary artery obstruction and giant cell arteritis. Ann Intern Med 1995;122:732. [DOI] [PubMed] [Google Scholar]
- [24].I. Landrin et al., Pulmonary artery thrombosis in giant cell arteritis. A new case and review of literature. (1996), vol. 148, 315–316. [PubMed] [Google Scholar]
- [25].Andrès E, Kaltenbach G, Marcellin L, et al. Acute pulmonary embolism related to pulmonary giant cell arteritis. Presse Med 2004;33:1328–9. [DOI] [PubMed] [Google Scholar]
- [26].Pfadenhauer K, Roesler A, Golling A. The involvement of the peripheral nervous system in biopsy proven active giant cell arteritis. J Neurol 2007;254:751–5. [DOI] [PubMed] [Google Scholar]
- [27].Umekita K, Takajo I, Miyauchi S, et al. [18F]fluorodeoxyglucose positron emission tomography is a useful tool to diagnose the early stage of Takayasu's arteritis and to evaluate the activity of the disease. Mod Rheumatol 2006;16:243–7. [DOI] [PubMed] [Google Scholar]


