Supplemental Digital Content is available in the text
Keywords: HDAC1, lung cancer, meta-analysis, survival
Abstract
Background:
Histone deacetylase 1 (HDAC1) is an important epigenetic factor, and is thought to be associated with the progression and prognosis of some types of cancer. HDAC1 has been reported to be overexpressed in lung cancer, but the correlation between HDAC1 overexpression and the clinical features or prognosis of lung cancer is controversial. In this study, we investigated the potential association between HDAC1 and lung cancer.
Materials and Methods:
Embase, Web of Science, PubMed, and other sources were searched for relevant studies. Pooled odds ratios (ORs) or hazard ratios (HRs) with 95% confidence interval (CI) were calculated to evaluate the association of HDAC1 with lung cancer risk.
Results:
Eight eligible studies were included in the final meta-analysis. We found that HDAC1 mRNA or protein expression was closely associated with the differentiation grade of lung cancer (OR = 2.36, 95% CI = 1.14–4.87, P = .02). In addition, the protein expression level of HDAC1 in squamous cell carcinoma was higher than that in adenocarcinoma (OR = 1.81, 95% CI = 1.13–2.90, P = .01). Finally, HDAC1 mRNA or protein expression was negatively correlated with the overall survival rate of patients with lung cancer (HR = 2.40, 95% CI = 1.48–3.88, P = .0004).
Conclusion:
In this meta-analysis, our results suggest that HDAC1 may serve as a good diagnostic and prognostic marker for lung cancer.
1. Introduction
Lung cancer presents an ever-increasing global public health threat, including several different subtypes, such as adenocarcinoma, squamous-cell carcinoma, large-cell carcinoma, and small-cell lung carcinoma. It was one of the most frequently diagnosed cancer and the leading cause of cancer death.[1] The overall survival (OS) rate for lung cancer remains low despite the advances in early diagnosis and clinical treatment over the last several decades.[2] Therefore, it is urgent to identify new biomarkers to screen out high-risk patients and predict lung cancer prognosis.
Histone deacetylase 1 (HDAC1) is an important epigenetic factor, which antagonizes the acetylation status of histone and nonhistone proteins. It has been well known that HDAC1 is tightly correlated with cancer development and progression. For example, HDAC1 silencing by siRNA resulted in cell cycle arrest, cell growth inhibition, and induction of apoptosis in breast and colon cancer cells,[3,4] whereas HDAC1 overexpression led to an increase in cell proliferation in prostate cancer cells,[5] indicating that HDAC1 stimulates cancer cell growth. In addition, HDAC1 was found to be recruited to the promoter of CDH1 to downregulate the expression of the cell adhesion molecule E-cadherin, thereby inhibiting cancer cell migration in pancreatic cancer.[6]
HDAC1 has been demonstrated to be overexpressed in many cancers, such as in breast, renal cell cancer, and classical Hodgkin's lymphoma, and HDAC1 expression is often associated with the clinical features and prognosis of cancer patients.[7–9] Therefore, HDAC1 may be a good diagnostic and prognostic marker for some types of cancer. HDAC1 is also overexpressed in lung cancer,[10,11] but the correlations between HDAC1 and clinical features and prognosis of patients with lung cancer are controversial.
In this study, we performed a systematic review and a meta-analysis to evaluate the correlation of HDAC1 expression with lung cancer. We found that HDAC1 expression was associated with the differentiation grade of lung cancer. In addition, the expression level of HDAC1 in squamous cell carcinoma was higher than that in adenocarcinoma. Finally, HDAC1 expression was negatively correlated with the OS rate of patients with lung cancer. Overall, this study is the first to systematically review the clinical value of HDAC1 in lung cancer.
2. Materials and methods
2.1. Search strategy and eligibility criteria
A systematic literature search was conducted for original articles analyzing the correlation between HDAC1 expression and the progression and prognosis of lung cancer in Embase, Web of Science, PubMed, and other sources. Studies were selected using the following keywords: “HDAC1” or “histone deacetylase 1” for HDAC1; “lung cancer”; or “lung neoplasms” for lung cancer. The search ended on December 27, 2016, and no lower date limit was used. No language restriction was applied, and the references of the relevant studies were also screened to check for potentially relevant articles.
The full text of each relevant study was carefully evaluated. The studies collected in this meta-analysis were required to meet the following criteria: HDAC1 expression was measured by real-time polymerase chain reaction (RT-PCR) or immunohistochemistry; the clinical features or prognosis of lung cancer were investigated; and the correlation of HDAC1 expression with clinical features and survival outcomes was analyzed. When several studies collected data from the same patient group, the most recent study was used; if the most recent study did not meet the inclusion criteria, the highest-quality study was included. Articles were excluded if they were case reports, letters, or reviews without original data; they focused on animal models or cancer cells; the expression of HDAC1 was determined by western blot; or the full text was unavailable. All evaluations were independently performed by 2 authors, Xiaoxu Song and Lin Pei, to ensure the accurate inclusion of studies. Disagreement between the 2 authors was adjudicated by Lin-Lin Cao. Eight articles were eligible for inclusion in this meta-analysis. This study is a meta-analysis, which extracted data from other original studies, so ethical approval was not necessary.
2.2. Data extraction
Two authors, Xiaoxu Song and Lin Pei, independently extracted data from the eligible studies. The following information was extracted from each included study: first author's name, publication year, country, sample size, age, HDAC1 detection method, the cut-off value, histology, stage, follow-up period, and outcome. If there were no original data for HDAC1 expression and only a histogram was provided, we used Engauge Digitizer 4.1 (http://digitizer.sourceforge.net) to extract the expression data. If the cut-off value of HDAC1 expression was not provided, the mean value of all samples was considered to be the cut-off value. In addition, if hazard ratios (HRs) for OS rates according to HDAC1 expression were not reported directly, the number of deaths and total samples in each article were extracted for HR calculation. If only the Kaplan–Meier curves were available, the survival data were extracted using Engauge Digitizer 4.1 and analyzed as described previously.[12]
2.3. Quality score assessment
Two reviewers (Xiaoxu Song and Lin Pei) independently assessed the quality of the included studies according to the Newcastle-Ottawa Scale (NOS) (http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp). The scale consists of 3 components related to sample selection, comparability, and ascertainment of exposure.
2.4. Statistical analysis
Analysis was conducted using Review Manager 5.3 (Cochrane Collaboration, Oxford, UK) by Lin-Lin Cao. The odds ratios (ORs) with 95% confidence intervals (CIs) were used to analyze the association between the expression level of HDAC1 and clinical features of lung cancer. HRs with 95% CIs were used to evaluate the correlation of HDAC1 expression with the OS of patients with lung cancer. The random-effect model was applied when I2 > 50%, and the fixed-effect model was used in the absence of between-study heterogeneity (I2 ≤ 50%). P values of <.05 were considered to be statistically significant. Publication bias was evaluated using the funnel plot.
3. Results
3.1. Study selection and characteristics of the included studies
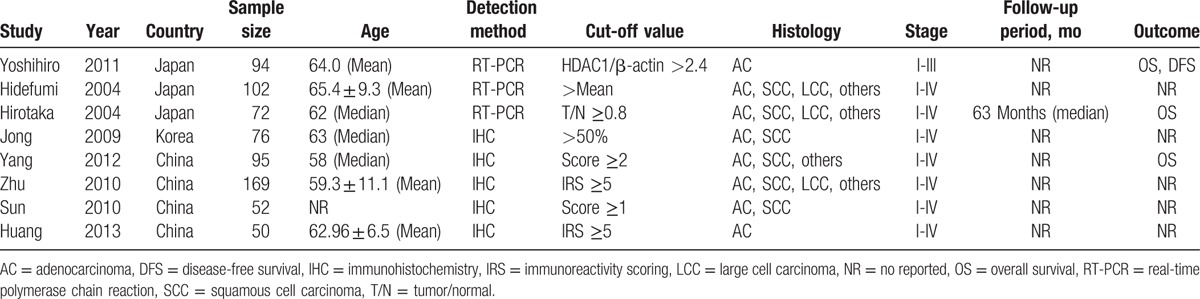
A total of 3932 articles were retrieved using the search strategy (Fig. 1). After the article titles and abstracts were checked, 3897 studies were excluded because of their irrelevance and duplication. Then, the remaining 35 articles were viewed in their entirety. Among the 35 articles, 27 were excluded according to the exclusion criteria. Finally, 8 studies[10,11,13–18] matched the criteria for this analysis. All characteristics of the 8 studies are listed in Table 1. Among the studies, 3 originated from Japan, 4 from China, and 1 from Korea. A total of 710 cases were enrolled. An immunohistochemistry assay was used in 5 studies, whereas an RT-PCR assay was used in 3 studies.
Figure 1.
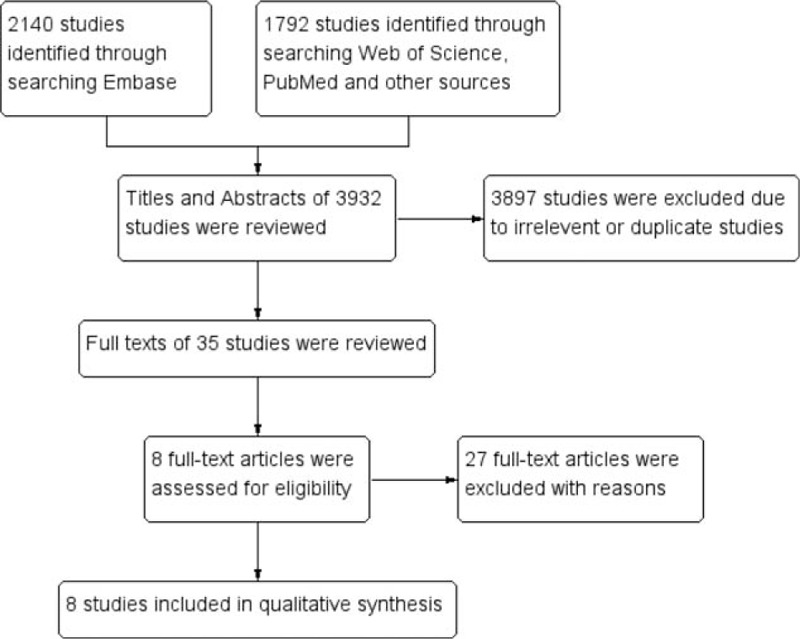
Methodological flow chart of study selection.
Table 1.
Main characteristics and results of the included studies.
3.2. Correlation of HDAC1 expression with the clinical features of lung cancer
Next, we analyzed the relationship between HDAC1 expression and the clinical features of lung cancer patients. As shown in Figure 2, there was no significant difference in the expression level of HDAC1 between tumors >3 cm and tumors ≤3 cm (OR = 0.78, 95% CI = 0.43–1.39, P = .40), indicating that HDAC1 expression was not associated with tumor size. In addition, no significant difference in HDAC1 expression was observed between stage III–IV lung cancer and stage I–II lung cancer (OR = 1.29, 95% CI = 0.64–2.61, P = .48) (Fig. 3). However, HDAC1 mRNA or protein expression level was higher in patients with low-differentiated lung cancer than that expressed in those with high-differentiated lung cancer (OR = 2.36, 95% CI = 1.14–4.87, P = .02) (Fig. 4). Finally, we analyzed the relationship between HDAC1 expression and the lymph node metastasis state of lung cancer. As shown in Figure 5, the groups positive and negative for lymph node metastasis did not show a significant difference in HDAC1 expression in lung cancer (OR = 1.51, 95% CI = 0.78–2.91, P = .22). Taken together, these results suggest that HDAC1 expression was tightly associated with the differentiation grade, but not with tumor size, tumor stage, and lymph node metastasis state of lung cancer.
Figure 2.
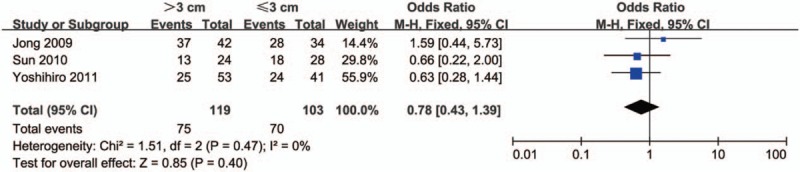
Forest plot of odds ratio (OR). Comparison of the expression level of histone deacetylase 1 (HDAC1) between tumors >3 cm and tumors ≤3 cm. CI = confidence interval.
Figure 3.
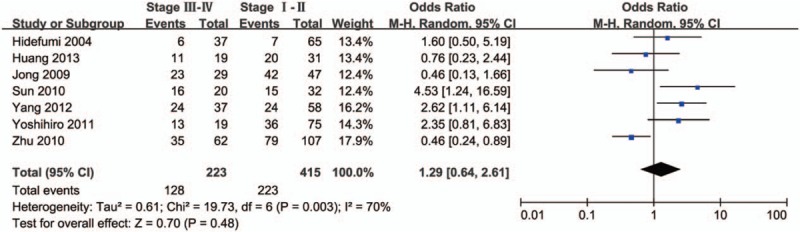
Forest plot of odds ratio (OR). Association between histone deacetylase 1 (HDAC1) expression and tumor stage in lung cancer. CI = confidence interval.
Figure 4.
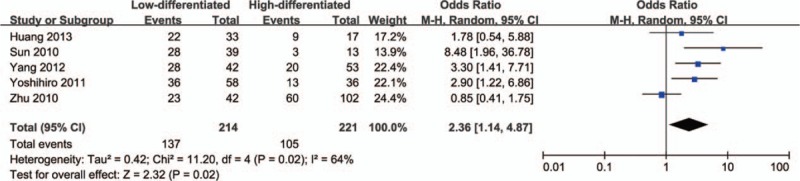
Forest plot of odds ratio (OR). Association between histone deacetylase 1 (HDAC1) expression and tumor differentiation grade in lung cancer. CI = confidence interval.
Figure 5.
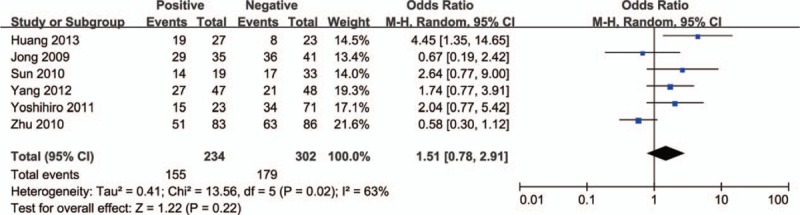
Forest plot of odds ratio (OR). Association between histone deacetylase 1 (HDAC1) expression and lymph node metastasis state in lung cancer. CI = confidence interval.
To determine whether there was a difference in HDAC1 expression in different pathological subtypes of lung cancer, we did a meta-analysis to see the difference between squamous cell carcinoma and adenocarcinoma. As shown in Figure 6, the pooled OR was 1.81 (95% CI = 1.13–2.90, P = .01), indicating that the protein expression level of HDAC1 in squamous cell carcinoma was higher than that in adenocarcinoma.
Figure 6.
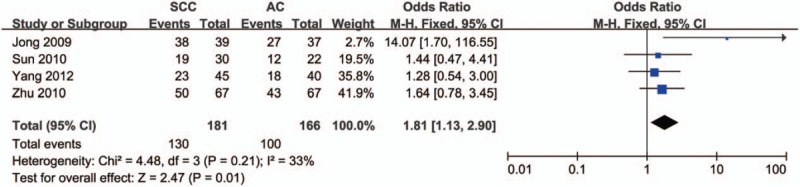
Forest plot of odds ratio (OR). Association between histone deacetylase 1 (HDAC1) expression and pathological subtypes of lung cancer. CI = confidence interval.
3.3. Impact of HDAC1 expression on overall survival of lung cancer patients
Subsequently, we investigated the association between HDAC1 expression and the OS of lung cancer patients. The pooled HR with 95% CI is shown in Figure 7 (HR = 2.40, 95% CI = 1.48–3.88, P = .0004), indicating that patients with lung cancer with low HDAC1 mRNA or protein expression showed better OS than patients with high HDAC1 expression.
Figure 7.
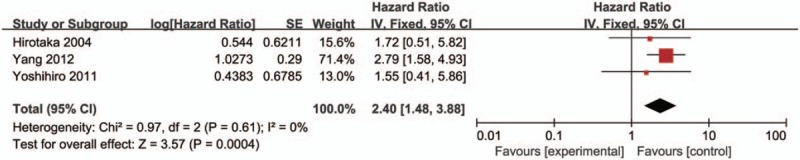
Forest plot of hazard ratio (HR). Association between histone deacetylase 1 (HDAC1) expression and the overall survival of lung cancer patients. CI = confidence interval.
3.4. Publication bias
Most of the funnel plots were almost symmetric, suggesting that there were no significant publication biases in these meta-analyses (Supplemental Figures 1–6).
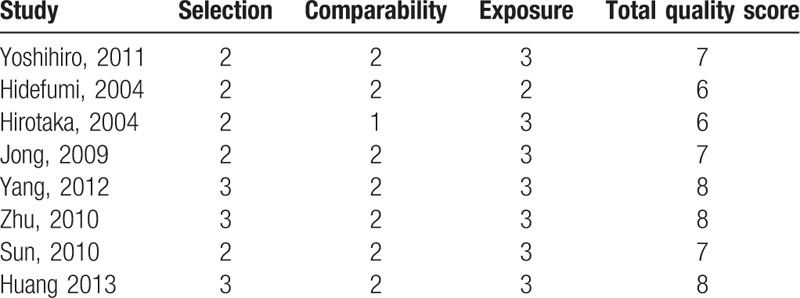
3.5. Quality assessment
The NOS was used to assess the methodological quality of the included studies. This scale allocated a total of 9 points for the quality of selection (4 points), comparability (2 points), and exposure (3 points). The NOS scores ranged from 0 to 9, and a score of ≥6 indicates a good quality. As shown in Table 2, all the studies had high quality.
Table 2.
Newcastle-Ottawa Scale for each included study.
4. Discussion
In our study, a combined analysis of 8 eligible clinical studies revealed the clinical value of HDAC1 expression in lung cancer. The meta-analysis results suggested that there was a tight correlation between HDAC1 expression and the differentiation grade of lung cancer. In addition, we performed a meta-analysis to determine the difference in HDAC1 expression between squamous cell carcinoma and adenocarcinoma, and found that the expression level of HDAC1 in squamous cell carcinoma was higher than that in adenocarcinoma. Finally, we found that patients with lung cancer with low HDAC1 expression showed better OS than those with high HDAC1 expression.
HDAC1 expression has been reported to be associated with the progression and prognosis of some types of cancer. For example, 2 studies suggested that HDAC1 is overexpressed in gastric cancer, and HDAC1 overexpression is closely correlated with the tumor stage, differentiation grade, or lymph node metastasis state of gastric cancer.[19,20] In addition, the expression level of HDAC1 in colorectal cancer tissues is also higher than that in noncancerous tissues, and the differences in HDAC1 expression in different tumor stages, differentiation grades, lymph node metastasis states, or distant metastasis states were observed as well.[4,21–24] These studies indicate that HDAC1 might be useful for clinical cancer diagnosis. However, the correlation between HDAC1 expression and the progression of lung cancer was controversial. In this analysis, the correlation between HDAC1 expression and differentiation grade of lung cancer, and the difference between squamous cell carcinoma and adenocarcinoma were observed, indicating that HDAC1 might be a good biomarker to distinguish different differentiation grades and different pathological subtypes of lung cancer. Therefore, it would be beneficial for the clinical diagnosis of lung cancer. Although there were no correlations between HDAC1 expression and tumor size, tumor stage, or lymph node metastasis, more studies concerning the relationship between HDAC1 and clinical features of lung cancer are needed.
It has been demonstrated that HDAC1 expression was correlated with the OS of some types of cancer. For example, patients with gastric cancer with low HDAC1 expression showed better OS than those with high HDAC1 expression.[19,25] In addition, there was a tight correlation between HDAC1 expression and OS of patients with pancreatic cancer.[26] However, the correlation between HDAC1 expression and the prognosis of lung cancer was inconsistent in previous studies. In this analysis, we found that patients with lung cancer with low HDAC1 expression has better OS than those with high HDAC1 expression, indicating that HDAC1 might be a good prognostic marker for patients with lung cancer and could help screen out high-risk patients with lung cancer.
This study has several limitations. At first, the cut-off value to determine positive or negative expression of HDAC1 varied across the included studies. Secondly, the number of studies included for some analyses was insufficient, making the results less convincing. Finally, the cohorts included in this study were all from Asia, and regional bias existed. Therefore, the role of HDAC1 expression in lung cancer progression and prognosis warrants further study.
HDAC1 has been served as a target for cancer therapy, and some small molecule HDAC inhibitors have been used in clinical treatment of patients with several types of cancer, such as T-cell lymphoma and multiple myeloma.[27] Consistent with this, the present analysis revealed the important clinical value of HDAC1 expression in lung cancer. In conclusion, HDAC1 expression might be a good biomarker for the diagnosis and prognosis of patients with lung cancer.
5. Conclusions
This meta-analysis suggests that the expression level of HDAC1 might be a high risk factor for lung cancer, especially in Asian populations. Further research needs to be conducted in the population of other regions.
Acknowledgments
The authors thank all of the group members for helpful discussions.
Supplementary Material
Footnotes
Abbreviations: CI = confidence interval, HDAC1 = histone deacetylase 1, HR = hazard ratio, NOS = Newcastle-Ottawa Scale, OR = odds ratio, OS = overall survival, RT-PCR = real-time polymerase chain reaction.
L-LC, XS, and LP contributed equally to the article.
This work was supported by Beijing Natural Science Foundation grant 7164305.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7–30. [DOI] [PubMed] [Google Scholar]
- [2].Didkowska J, Wojciechowska U, Manczuk M, et al. Lung cancer epidemiology: contemporary and future challenges worldwide. Ann Transl Med 2016;4:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Senese S, Zaragoza K, Minardi S, et al. Role for histone deacetylase 1 in human tumor cell proliferation. Mol Cell Biol 2007;27:4784–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Weichert W, Roske A, Niesporek S, et al. Class I histone deacetylase expression has independent prognostic impact in human colorectal cancer: specific role of class I histone deacetylases in vitro and in vivo. Clin Cancer Res 2008;14:1669–77. [DOI] [PubMed] [Google Scholar]
- [5].Halkidou K, Gaughan L, Cook S, et al. Upregulation and nuclear recruitment of HDAC1 in hormone refractory prostate cancer. Prostate 2004;59:177–89. [DOI] [PubMed] [Google Scholar]
- [6].Aghdassi A, Sendler M, Guenther A, et al. Recruitment of histone deacetylases HDAC1 and HDAC2 by the transcriptional repressor ZEB1 downregulates E-cadherin expression in pancreatic cancer. Gut 2012;61:439–48. [DOI] [PubMed] [Google Scholar]
- [7].Zhang Z, Yamashita H, Toyama T, et al. Quantitation of HDAC1 mRNA expression in invasive carcinoma of the breast∗. Breast Cancer Res Treat 2005;94:11–6. [DOI] [PubMed] [Google Scholar]
- [8].Adams H, Fritzsche FR, Dirnhofer S, et al. Class I histone deacetylases 1, 2 and 3 are highly expressed in classical Hodgkin's lymphoma. Expert Opin Ther Targets 2010;14:577–84. [DOI] [PubMed] [Google Scholar]
- [9].Fritzsche FR, Weichert W, Roske A, et al. Class I histone deacetylases 1, 2 and 3 are highly expressed in renal cell cancer. BMC Cancer 2008;8:381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Sun Y, Chen L, Yan H, et al. The expression of HDAC1 and DNMT1 in non-small-cell lung cancer and its clinical significance. Heilongjiang Med Pharmacy 2010;33:77–8. [Google Scholar]
- [11].Park JH, Hong YS, Choi PJ, et al. The overexpression of histone deacetylase 1 and its relationship with p16INK4a gene hypermethylation in pulmonary squamous cell carcinoma and adenocarcinoma. Korean J Pathology 2009;43:107. [Google Scholar]
- [12].Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Han Y, Zhang Y, Yang LH, et al. X-radiation inhibits histone deacetylase 1 and 2, upregulates Axin expression and induces apoptosis in non-small cell lung cancer. Radiat Oncol 2012;7:183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Minamiya Y, Ono T, Saito H, et al. Expression of histone deacetylase 1 correlates with a poor prognosis in patients with adenocarcinoma of the lung. Lung Cancer 2011;74:300–4. [DOI] [PubMed] [Google Scholar]
- [15].Osada H, Tatematsu Y, Saito H, et al. Reduced expression of class II histone deacetylase genes is associated with poor prognosis in lung cancer patients. Int J Cancer 2004;112:26–32. [DOI] [PubMed] [Google Scholar]
- [16].Sasaki H, Moriyama S, Nakashima Y, et al. Histone deacetylase 1 mRNA expression in lung cancer. Lung Cancer 2004;46:171–8. [DOI] [PubMed] [Google Scholar]
- [17].Zhu X, Yang L, Wang H, et al. HDAC1 protein expression in non-small-cell lung cancer and its clinical significance. J Pract Med 2010;26:2720–2. [Google Scholar]
- [18].Jingsi Huang SZ. Analysis of Correlation Between Histone Acetylation and Expression of Fas and HDAC1 in Tissues of Lung Adenocarcinoma Carcinogenesis. Master's Thesis of Soochow University; 2013. [Google Scholar]
- [19].Yu S-Y, Hou X-L, Duan X-W, et al. Significance of expression of HDAC1 protein in gastric cancer. World Chin J Digestol 2015;23:5290–5. [Google Scholar]
- [20].Sudo T, Mimori K, Nishida N, et al. Histone deacetylase 1 expression in gastric cancer. Oncol Rep 2011;26:777–82. [DOI] [PubMed] [Google Scholar]
- [21].Huang BH, Laban M, Leung CH, et al. Inhibition of histone deacetylase 2 increases apoptosis and p21Cip1/WAF1 expression, independent of histone deacetylase 1. Cell Death Differ 2005;12:395–404. [DOI] [PubMed] [Google Scholar]
- [22].Thangaraju M, Carswell KN, Prasad PD, et al. Colon cancer cells maintain low levels of pyruvate to avoid cell death caused by inhibition of HDAC1/HDAC3. Biochem J 2009;417:379–89. [DOI] [PubMed] [Google Scholar]
- [23].Ozdag H, Teschendorff AE, Ahmed AA, et al. Differential expression of selected histone modifier genes in human solid cancers. BMC Genomics 2006;7:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Higashijima J, Kurita N, Miyatani T, et al. Expression of histone deacetylase 1 and metastasis-associated protein 1 as prognostic factors in colon cancer. Oncol Rep 2011;26:343–8. [DOI] [PubMed] [Google Scholar]
- [25].Weichert W, Roske A, Gekeler V, et al. Association of patterns of class I histone deacetylase expression with patient prognosis in gastric cancer: a retrospective analysis. Lancet Oncol 2008;9:139–48. [DOI] [PubMed] [Google Scholar]
- [26].Wang W, Gao J, Man XH, et al. Significance of DNA methyltransferase-1 and histone deacetylase-1 in pancreatic cancer. Oncol Rep 2009;21:1439–47. [DOI] [PubMed] [Google Scholar]
- [27].Qin HT, Li HQ, Liu F. Selective histone deacetylase small molecule inhibitors: recent progress and perspectives. Expert Opin Ther Pat 2017;27:621–36. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


