Abstract
The role of cytokines in diabetic retinopathy (DR) and effects of fenofibrate on cytokines were explored by observing changes in serum IL-1β, TNF-α, VEGF, and Lp-PLA2 in different stages of DR and the intervention effect of oral fenofibrate on cytokines.
In total, 190 patients with type 2 DR were enrolled and divided into 3 groups: diabetic without retinopathy (NDR) group (n = 30), nonproliferative diabetic retinopathy (NPDR) group (n = 80), and proliferative diabetic retinopathy (PDR) group (n = 80). According to whether or not to accept fenofibrate treatment, NPDR and PDR groups were further divided into the NPDR control (NPDR1) group (n = 40) and the NPDR treatment (NPDR2) group (n = 40), and the proliferative diabetic retinopathy control (PDR1, n = 40) group and the proliferative diabetic retinopathy treatment (PDR2) group (n = 40). At 12 weeks after fenofibrate treatment, serum IL-1β, TNF-α, VEGF, and Lp-PLA2 levels were detected.
In PDR and NPDR patients, levels of serum cytokines such as IL-1β (120.56 ± 27.32 pg/mL vs 112.34 ± 19.45 pg/mL vs 82.9 ± 13.8 pg/mL), TNF-α (125.86 ± 25.57 pg/mL vs 109.48 ± 20.15 pg/mL vs 80.7 ± 12.8 pg/mL), VEGF (166.65 ± 37.74 pg/mL vs 148.54 ± 36.27 pg/mL vs 88.97 ± 24.86 pg/mL), and Lp-PLA2 (172.34 ± 45.22 μg/L vs 154.66 ± 40.98 μg/L vs 125.88 ± 38.87 μg/L) were significantly higher than in diabetes patients without retinopathy. After fenofibrate treatment, serum IL-1β, TNF-α, VEGF, and Lp-PLA2 significantly decreased in NPDR and PDR patients.
Serum IL-1β, TNF-α, VEGF, and Lp-PLA2 play an important role in occurrence and development of diabetic retinopathy. Fenofibrate can reduce cytokine levels in DR patients and improve inflammatory response.
Keywords: diabetic retinopathy, fenofibrate, inflammatory cytokines, lipoprotein-associated phospholipase A2, vascular endothelial growth factor
1. Introduction
Diabetes has become a global problem. With the changes in people's life style and dietary structure, the incidence of this disease has increased year by year. The 7th diabetes map of the International Diabetes Federation revealed that the global number of diabetes patients in 2015 was up to 415 million and is expected to reach 642 million by 2040. The number of diabetes patients in China ranks first in the world. Epidemiological surveys in China revealed that the prevalence of diabetes in adults >20 years old is 11.6%.[1] Diabetic retinopathy (DR) is a common complication of diabetic microangiopathy, and hyperglycemia is an important factor in the occurrence and development of diabetic complications. Persistent hyperglycemia can induce many metabolic abnormalities, such as the activation of protein kinase C (PKC), increased activity of the polyol pathway, the accumulation of advanced glycation end products (AGEs), oxidative stress, and cytokine activation. These result in arteriosclerosis and microthrombosis, and eventually lead to DR.[2] Inflammatory cytokines play an important role in DR, but the detailed mechanism remains unclear.[3] The study conducted by Canning et al[4] revealed that lipoprotein-associated phospholipase A2 (Lp-PLA2) and its main enzyme-promoting production lysophosphatidylcholine (LPC) were closely related to damage induced to the blood-retinal barrier in DR, and that LPC could influence vascular permeability, and thereby impair the function of retinal vascular endothelial cells through VEGF receptor 2. There are no clinically effective drugs for preventing DR and the progression of nonproliferative diabetic retinopathy (NPDR) into diabetic proliferative retinopathy (PDR), except for the management of blood glucose, blood lipids, blood pressure, and other risk factors. Fenofibrate is the third generation of fibric acid derivative lipid-regulating drugs. In addition to the established lipid-lowering effect, it can inhibit the VEGF pathway, and thereby inhibit angiogenesis and maintain the integrity of the vascular endothelium.[5] Field trial and the Action to Control Cardiovascular Risk in Diabetes (ACCORD) Trial revealed that taking 200 mg of fenofibrate per day significantly decreased the possibility of macular edema and PDR in DR patients who need laser treatment, providing a new evidence for the application of fenofibrate for the prevention and treatment of DR.[6,7] In this study, we observed the changes of serum IL-1β, TNF-α, VEGF, and Lp-PLA2 in DR patients at different stages to further explore the role of cytokines in the pathogenesis of DR and explored the effect of fenofibrate in the treatment of DR by observing the intervention effect of oral fenofibrate on serum IL-1β, TNF-α, VEGF, and Lp-PLA2 in DR patients. Furthermore, the mechanism was also discussed.
2. Materials and methods
This study was conducted in accordance with the declaration of Helsinki. This study was conducted with approval from the Ethics Committee of our hospital. Written informed consent was obtained from all participants.
2.1. Subjects
A total of 190 type-2 diabetes patients, who were admitted in the Endocrinology Department of our hospital from October 2014 to March 2016, were enrolled into this study. According to ophthalmoscopy and fundus fluorescein angiography results, 30 diabetes patients without retinopathy were assigned into the NDR group, and 80 NPDR patients (NPDR group) and 80 PDR patients (PDR group) were subjected to the experiment. According to whether or not to accept the fenofibrate treatment, the NPDR and PDR groups were further divided into the NPDR control (NPDR1) group (n = 40) and the NPDR treatment (NPDR2) group (n = 40), and the PDR control (PDR1) group (n = 40) and the PDR treatment (PDR2) group (n = 40). Exclusion criteria: (1) patients with liver and kidney insufficiency, malignant tumors, cardiovascular and cerebrovascular diseases, not strictly controlled hypertension (160/95 mm Hg, 1 mm Hg = 0.133 kPa), and acute and chronic infections; (2) patients with acute complications such as diabetic ketoacidosis and hyperosmolar coma in diabetes; (3) patients who recently used anticoagulant drugs, lipid-lowering drugs, and angiotensin-converting enzyme inhibitors (ACEI); (4) patients who were treated with retinal photocoagulation and drug therapy.
2.2. Research methods
2.2.1. Data acquisition
The patient's medical history, age, gender, duration of diabetes, and blood glucose control were collected, and the general situations of the selected subjects including height, weight, and blood pressure were measured.
2.2.2. Therapeutic methods
All subjects underwent visual acuity examination, ophthalmoscopy, and fundus fluorescein angiography to determine the fundus lesions and staging; and these subjects received conventional hypoglycemic, antihypertensive, and microcirculation therapies. Patients in the NPDR2 and PDR2 groups received 160 mmg/day of oral fenofibrate on the basis of the treatment in the control group, which lasted for 12 weeks.
2.2.3. Detected indexes
From all selected subjects, 5 mL of fasting venous blood was withdrawn for the detection of biochemical indexes, triglyceride (TG), total cholesterol (TC), high density lipoprotein cholesterol (HDL-C), and low density lipoprotein cholesterol (LDL-C) using an automatic biochemical detector. From each DR patient, approximately 8 mL of fasting venous blood was withdrawn before and after treatment. Then, the collected blood was centrifuged. Next, the serum was obtained, packed in different EP tubes, and stored at –80 °C for the determination of IL-1β, TNF-α, VEGF, and Lp-PLA2 levels by enzyme-linked immunosorbent assay (ELISA), ELISA kits of IL-1β(cat.no: DLB50),TNF-α(cat.no: DTA29),VEGF(cat.no: DVE06), and Lp-PLA2(cat.no: DPL70) were purchased from American R&D company. The measuring procedure was strictly conducted according to the manual instructions.
2.3. Statistical processing
Data were analyzed using SPSS 17.0 software. All data were expressed as mean ± standard deviation 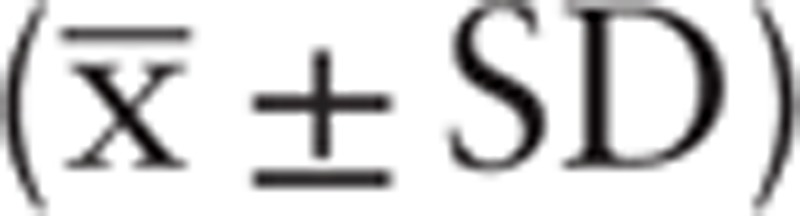 . Intergroup comparison was conducted using the t-test. Count data were evaluated using the X2-test. P < .05 was considered statistically significant.
. Intergroup comparison was conducted using the t-test. Count data were evaluated using the X2-test. P < .05 was considered statistically significant.
3. Results
3.1. Comparison of clinical data and serum cytokines among all groups
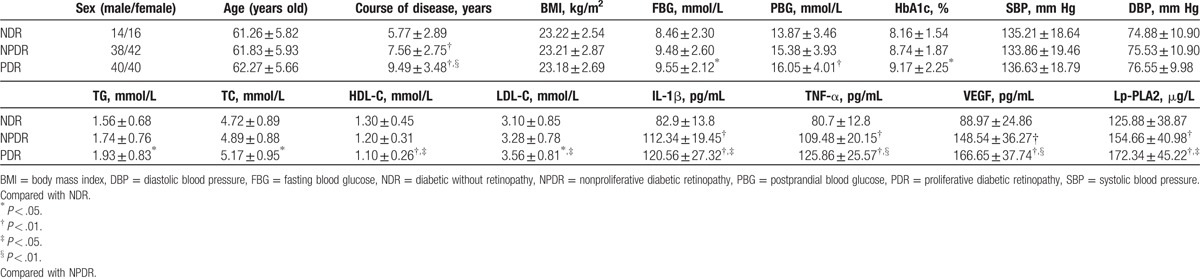
Differences in age, gender, BMI, and blood pressure among the NDR, NPDR, and PDR groups were not statistically significant, but the difference in rgw course of the disease was statistically significant. These results indicate that the degree of DR increased with the increase in the course of the disease. Differences in fasting blood glucose (FBG), postprandial blood glucose (PBG), HbA1c, TC, TG, HDL-C, and LDL-C levels between the PDR and NDR groups were statistically significant. Furthermore, these results indicate the influence of the levels of blood glucose and blood lipids on DR. Moreover, differences in serum VEGF, TNF-α, IL-1β, and Lp-PLA2 levels among the NDR, NPDR, and PDR groups were statistically significant (Table 1).
Table 1.
Comparison of clinical data and serum cytokines among all groups.
3.2. Changes in blood glucose, blood lipids, and cytokine levels in DR patients before and after treatment
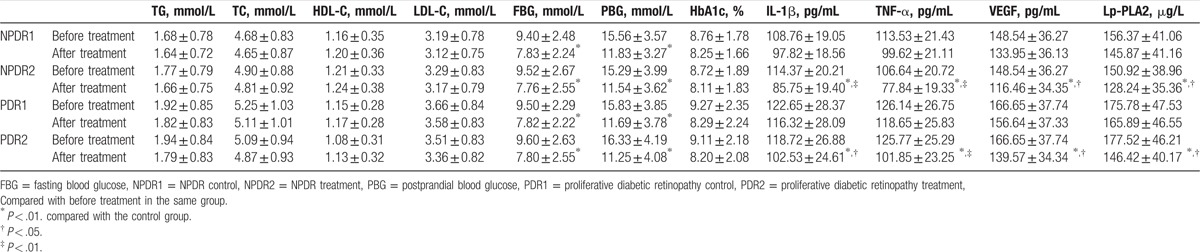
After hypoglycemic therapy, FBG and PBG in patients with PDR and NPDR improved. After 12 weeks of fenofibrate treatment, serum VEGF, TNF-α, IL-1β, and Lp-PLA2 levels significantly decreased in patients in both the NPDR and PDR groups. However, there was no significant change in the control group (Table 2).
Table 2.
Changes in blood glucose, blood lipids, and cytokine levels in DR patients before and after treatment.
4. Discussion
DR is a common microvascular complication of diabetes. The main manifestation is microvascular disease in the eye ground, where peripheral cells in the eye ground are injured. This is the leading cause of blindness in adults. Long-term hyperglycemia can lead to pathological changes such as oxidative stress, changes in hemodynamic and cellular metabolism, cytokine activation, and so on. This would thereby result in lesions in all sizes of vessels and induce a variety of complications.[8] This study revealed that compared with patients in the NDR group, serum VEGF, TNF-α, IL-1β, and Lp-PLA2 levels significantly increased in patients in the NPDR and PDR groups. This indicate that serum IL-1β, TNF-α, VEGF, and Lp-PLA2 play certain roles in DR. Local retinal anemia and hypoxia in diabetes patients can increase the expression of VEGF. VEGF can promote the migration and proliferation of endothelial cells and angiogenesis, as well as increase the permeability of blood vessels, alter the activation state of certain genes in endothelial cells, upregulate the expression of plasma plasminogen activator, and induce the degeneration of the extracellular matrix and neovascularization, which play an important role in the development of DR.[9,10] VEGF interacts with other cytokines and promotes the progression of DR.[11] TNF-α can increase vascular permeability, stimulate the proliferation of endothelial cells, and thereby promote the formation of new blood vessels. TNF-α can also stimulate endothelial cells to synthesize and release interleukin-1, granulocyte macrophage colony-stimulating factor, intercellular adhesion molecule-1 and platelet activating factor, inhibit anticoagulant activity, induce abnormalities in retinal hemodynamics, and promote the occurrence of DR.[12] IL-1β is an important inflammatory response protein secreted by activated monocytes/macrophages. It can promote the expression of the adhesion molecule in microvascular endothelial cells, increase the adhesion and chemotaxis of neutrophils after ischemia, and impair the function of vascular endothelium.[13] Lp-PLA2 is a newly discovered inflammatory marker in recent years,[14,15] which is different from traditional inflammatory factors such as the C-reactive protein. Lp-PLA2 is mainly synthesized and secreted by mature monocytes and macrophages and regulated by inflammatory mediators. Lp-PLA2 can hydrolyze oxidized lecithin in low density lipoprotein, resulting in the vigorous production of oxidized nonesterified fatty acids and lyolecithin. This induces the occurrence of inflammation and causes vascular endothelial injury, promoting the occurrence of atherosclerosis. A previous study revealed that serum Lp-PLA2 was a predictor of atherosclerotic diseases, such as coronary heart disease, myocardial infarction, and cerebral infarction.[16] The study conducted by Staurenghi et al[17] revealed that Lp-PLA2 inhibitor darapladib can protect the damaged blood-retinal barrier in a high glucose environment and improve macular edema and retinopathy.
Fenofibrate is an agonist of peroxisome proliferator activator receptor-α (PPAR-α). In addition to its effect in the lowering blood-fat, fenofibrate can improve vascular endothelial function, work against inflammation and oxidative stress, and inhibit angiogenesis.[18] Although DR is one of the common complications of diabetes, few are known on how to prevent DR and prohibit NPDR from transforming into PDR. Except for controlling blood glucose, blood lipids, blood pressure, and other risk factors, there is no clinically effective drug at present. In recent years, 2 large-scale clinical field tests and the ACCORD test confirmed the benefits of fenofibrate for DR.[19,20] However, the detailed mechanism remains unknown. The results of the field test revealed that the treatment of 200 mg/day of fenofibrate for DR patients can reduce the number of initial laser treatments, as well as delay the progression of DR and promote the absorption of macular edema, increasing the possibilities of macular edema and PDR in DR patients who need laser treatment by 30% and 31%, respectively. The study also pointed out that maintaining a low blood lipid level did not reduce the number of laser treatments. However, the oral use of fenofibrate can reduce the number of initial laser treatments for DR patients and delay the progression of DR. Both in the standard glucose-lowering group and intensive glucose-lowering group, the benefits of the concurrent use of fenofibrate could be observed.[21] This indicates that the beneficial effects of fenofibrate on DR are beyond the effect of inhibiting blood glucose and lipids. A study in Australia revealed that[22] among DR patients, the quality-adjusted life years (QALYs) increased by 0.09 in the fenofibrate treatment group compared with the control group, and the treatment costs of patients were reduced by 10,221 Australian dollars per QALY. The study conducted by Noonan et al[23] revealed that fenofibrate could affect the levels of blood lipids, inflammatory response, angiogenesis, and cell apoptosis by regulating the expression of various genes. An in vitro cell test revealed that fenofibrate could reduce the stress signaling pathway under a hyperglycemic state, improve the survival of retinal endothelial cells and epithelial cells, and reduce new corneal blood vessels. This study revealed that after 12 weeks of fenofibrate treatment, serum VEGF, TNF-α, IL-1β, and Lp-PLA2 levels significantly decreased in patients in the NPDR and PDR groups. This suggests that fenofibrate can reduce inflammatory cytokines in DR patients and may improve DR by improving inflammatory response. In addition to the verified lipid-lowering effect, fenofibrate may also be an effective drug for the prevention of DR. The clinical effect of fenofibrate treatment for DR and the detailed mechanism needs to be further explored.
Footnotes
Abbreviations: ACCORD = Action to Control Cardiovascular Risk in Diabetes, ACEI = angiotensin-converting enzyme inhibitors, AGEs = advanced glycation end products, DR = diabetic retinopathy, ELISA = enzyme-linked immunosorbent assay, HDL-C = high-density lipoprotein cholesterol, LDL-C = low-density lipoprotein cholesterol, LPC = lysophosphatidylcholine, Lp-PLA2 = lipoprotein-associated phospholipase A2, NDR = diabetic without retinopathy, NPDR = nonproliferative diabetic retinopathy, NPDR1 = NPDR control, NPDR2 = NPDR treatment, PDR = proliferative diabetic retinopathy, PDR1 = proliferative diabetic retinopathy control, PDR2 = proliferative diabetic retinopathy treatment, PKC = protein kinase C, TC = total cholesterol, TG = triglyceride.
Ethic statement: complied with ethical standards.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Xu Y, Wang L, He J, et al. Prevalence and control of diabetes in Chinese adults. JAMA 2013;310:948–59. [DOI] [PubMed] [Google Scholar]
- [2].Zeng J, Chen B. Epigenetic mechanisms in the pathogenesis of diabetic retinopathy. Ophthalmologica 2014;232:1–9. [DOI] [PubMed] [Google Scholar]
- [3].Hernández-Da Mota SE, Soto-Bahena JJ, Viveros-Sandoval ME, et al. Proinflammatory serum cytokines in diabetic retinopathy. Cir Cir 2015;83:100–6. [DOI] [PubMed] [Google Scholar]
- [4].Canning P, Kenny BA, Prise V, et al. Lipoprotein-associated phospholipase A2 (Lp-PLA2) as a therapeutic target to prevent retinal vasopermeability during diabetes. Proc Natl Acad Sci U S A 2016;113:7213–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Feng X, Gao X, Jia Y, et al. PPAR-α agonist fenofibrate decreased RANTES levels in type 2 diabetes patients with hypertriglyceridemia. Med Sci Monit 2016;22:743–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Sacks FM. After the fenofibrate intervention and event lowering in diabetes (FIELD) study: implications for fenofibrate. Am J Cardiol 2008;102:34L–40L. [DOI] [PubMed] [Google Scholar]
- [7].Wong TY, Simó R, Mitchell P. Fenofibrate—a potential systemic treatment for diabetic retinopathy? Am J Ophthalmol 2012;154:6–12. [DOI] [PubMed] [Google Scholar]
- [8].Zang J, Guan G. Study of pigment epithelium-derived factor in pathogenesis of diabetic retinopathy. Eye Sci 2015;30:81–8. [PubMed] [Google Scholar]
- [9].Behl T, Kotwani A. Exploring the various aspects of the pathological role of vascular endothelial growth factor (VEGF) in diabetic retinopathy. Pharmacol Res 2015;99:137–48. [DOI] [PubMed] [Google Scholar]
- [10].Wang J, Chen S, Jiang F, et al. Vitreous and plasma VEGF levels as predictive factors in the progression of proliferative diabetic retinopathy after vitrectomy. PLoS One 2014;9:e110531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Bromberg-White JL, Glazer L, Downer R, et al. Identification of VEGF-independent cytokines in proliferative diabetic retinopathy vitreous. Invest Ophthalmol Vis Sci 2013;54:6472–80. [DOI] [PubMed] [Google Scholar]
- [12].Sharma S, Purohit S, Sharma A, et al. Elevated serum levels of soluble TNF receptors and adhesion molecules are associated with diabetic retinopathy in patients with type-1 diabetes. Mediators Inflamm 2015;2015:279393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Mao C, Yan H. Roles of elevated intravitreal IL-1beta and IL-10 levels in proliferative diabetic retinopathy. Indian J Ophthalmol 2014;62:699–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Gong Y, Jin X, Wang QS, et al. The involvement of high mobility group 1 cytokine and phospholipases A2 in diabetic retinopathy. Lipids Health Dis 2014;13:156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Lupo G, Motta C, Giurdanella G, et al. Role of phospholipases a2 in diabetic retinopathy: in vitro and in vivo studies. Biochem Pharmacol 2013;86:1603–13. [DOI] [PubMed] [Google Scholar]
- [16].Maiolino G, Bisogni V, Rossitto G, et al. Lipoprotein-associated phospholipase A2 prognostic role in atherosclerotic complications. World J Cardiol 2015;7:609–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Staurenghi G, Ye L, Magee MH, et al. Darapladib, a lipoprotein-associated phospholipase A2 inhibitor, in diabetic macular edema: a 3-month placebo-controlled study. Ophthalmology 2015;122:990–6. [DOI] [PubMed] [Google Scholar]
- [18].Abcouwer SF. Direct effects of pparalpha agonists on retinal inflammation and angiogenesis may explain how fenofibrate lowers risk of severe proliferative diabetic retinopathy. Diabetes 2013;62:36–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Knickelbein JE, Abbott AB, Chew EY. Fenofibrate and diabetic retinopathy. Curr Diab Rep 2016;16:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Sharma N, Ooi JL, Ong J, et al. The use of fenofibrate in the management of patients with diabetic retinopathy: an evidence-based review. Aust Fam Physician 2015;44:367–70. [PubMed] [Google Scholar]
- [21].Keech AC, Mitchell P, Summanen PA, et al. Effect of fenofibrate on the need for laser treatment for diabetic retinopathy (FIELD study): a randomized controlled trial. Lancet 2007;370:1687–97. [DOI] [PubMed] [Google Scholar]
- [22].Valentine WJ, Pollock RF, Carr E, et al. Evaluating the cost-utility of fenofibrate treatment of diabetic retinopathy in Australia. Value in Health 2013;16:A442. [Google Scholar]
- [23].Noonan JE, Jenkins AJ, Ma JX, et al. An update on the molecular actions of fenofibrate and its clinical effects on diabetic retinopathy and other microvascular end points in patients with diabetes. Diabetes 2013;62:3968–75. [DOI] [PMC free article] [PubMed] [Google Scholar]


