Abstract
Background:
Esophageal cancer (EC) is a common cancer with high mortality because of its rapid progression and poor prognosis. Radiotherapy is one of the most effective treatments for EC. Three-dimensional conformal radiotherapy (3D-CRT) and intensity-modulated radiotherapy (IMRT) are 2 recently developed radiotherapy techniques. IMRT is believed to be more effective than 3D-CRT in target coverage, dose homogeneity, and reducing toxicity to normal organs. However, these advantages have not been demonstrated in the treatment of EC. This meta-analysis was performed to compare IMRT and 3D-CRT in the treatment of EC in terms of dose–volume histograms and outcomes including survival and toxicity.
Methods:
A literature search was performed in PubMed, Embase, and the Cochrane library databases from their inceptions to Dec 30, 2016. Two authors independently assessed the included studies and extracted data. The average percent irradiated volumes of adjacent noncancerous organs were calculated and compared between IMRT and 3D-CRT. The odds ratio of overall survival (OS), and radiation pneumonitis and radiation esophagitis was also evaluated.
Results:
Totally 7 studies were included. Of them, 5 studies (80 patients) were included in the dosimetric comparison, 3 studies (871 patients) were included in the OS analysis, and 2 studies (205 patients) were included in the irradiation toxicity analysis. For lung in patients receiving doses ≥20 Gy and heart in patients receiving dose = 50 Gy, the average irradiated volumes of IMRT were less than those from 3D-CRT. IMRT resulted in a higher OS than 3D-CRT. However, no significant difference was observed in the incidence of radiation pneumonitis and radiation esophagitis between 2 radiotherapy techniques.
Conclusion:
Our data suggest that IMRT-delivered high radiation dose produces significantly less average percent volumes of irradiated lung and heart than 3D-CRT. IMRT is superior to 3D-CRT in the OS of EC while shows no benefit on radiation toxicity.
Keywords: dose–volume histograms, esophageal cancer, intensity-modulated radiotherapy (IMRT), overall survival, radiation toxicity, three-dimensional conformal radiotherapy (3D-CRT)
1. Introduction
Esophageal cancer (EC) is the eighth most common cancer with an estimated 0.456 million new cases (3.2% of all cancers) and the sixth cause of cancer-related death with an estimated 0.4 million cancer deaths (4.9% of all cancer deaths) in 2012.[1] About 49% of all new cases and all cancer-related deaths occurred in China.[1] Moreover, EC at earlier stages does not present typical clinical symptoms; thus, it is always diagnosed at later stages and the 5-year survival rate of patients with ES is only 15% to 25%.[2–4]
Radiotherapy is one of the most effective treatments for cancer and plays an important role in the treatment of both resectable and unresectable ECs.[5,6] However, it is a great challenge to deliver radiation dose accurately with minimal toxicity.[6–8] In the past few decades, several advanced radiotherapy techniques, including three-dimensional conformal radiotherapy (3D-CRT), intensity-modulated radiotherapy (IMRT), image-guided radiotherapy, tomotherapy, intensity-modulated arc therapy, and volumetric modulated arc therapy, have been developed to increase the conformal degree of target areas as well as the radiation dose, and to decrease the toxicity to normal organs.[5,8,9]
3D-CRT is developed and proven in the late 1990s as a preferred treatment for cancer for its better target coverage and significantly decreased toxicity to normal organs compared to 2D-CRT. Later, the IMRT technique is proven to be more effective than 3D-CRT in target coverage, dose homogeneity, and reducing toxicity to normal organs.[10] The esophagus is an organ close to spinal cord, heart, and is surrounded by the lung. When radiotherapy is applied for treating EC, these organs of lung, heart, and spinal cord are the main 3 organs at risks (OARs).[9] Thus, the advantages of IMRT are important for these OARs. It has been reported that IMRT is superior to 3D-CRT in the treatment of nonsmall cell lung cancer and gynecologic malignancies in terms of treatment toxicity.[5,11] Several studies have compared IMRT and 3D-CRT in the treatment of EC. However, whether IMRT is superior to 3D-CRT in the treatment of EC remains controversial. Thus, we performed this meta-analysis to compare IMRT and 3D-CRT in the treatment of EC in terms of dose–volume histograms (DVHs) and outcomes including survival and toxicity.
2. Materials and methods
This study was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. As it was based on previous publications, it did not require ethical approval or patient consent.
2.1. Search strategy
We performed a comprehensive literature review in PubMed, Embase, and Cochrane Library from inception to 30 December, 2016 using the following keywords
“intensity modulation radiation therapy,” “IMRT,” “three-dimensional conformal radiotherapy,” “3D-CRT,” “esophagus cancer,” “esophageal cancer,” and “esophageal neoplasm.” No language restrictions were imposed. In addition, manual searching was performed by screening references from retrieved original papers to identify any potentially eligible studies.
2.2. Study selection criteria and quality assessment
Only original studies were included. A study was selected if it provides information on DVHs of different OARs or outcomes (overall survival [OS] or toxicity) of those patients who had been treated with IMRT or 3D-CRT. Two reviewers evaluated the methodological quality of the included studies independently using the Newcastle-Ottawa Scale.[12] Studies with scores of 6 or higher were considered high quality and included.
2.3. Data extraction
Two reviewers extracted data from the eligible studies independently and used a standard form for data collection. The extracted information included the name of the first author, year of publication, country, number of patients, normal organs irradiated, prescribed radiotherapy dose, average percent irradiated volumes of OARs at different radiation doses in DVHs, OS, and incidence and the number of cases of radiation pneumonitis or esophagitis. The disagreement was resolved by consensus through a joint review of the manuscript.
2.4. Statistical analysis
RevMan (Version 5.3; Cochrane Collaboration, Oxford, UK) and STATA software (Version 12.0; Stata Corporation, College Station, TX) were used for the meta-analysis and statistics. The heterogeneity of all included studies was evaluated by calculating the I2 statistic. A fixed-effects model was applied when the I2 statistic < 50%, indicating that all included studies exhibited homogeneity. A random-effects model was applied when the I2 statistic > 50%. A P value < .05 was considered statistically significant. Potential publication bias was assessed by visual inspection of the funnel plot as well as the Egger regression asymmetry test. For the Egger test, P > .1 was considered as no publication bias.
3. Results
3.1. Study searching and characteristics of the included studies
A total of 60 relevant studies were identified during the initial search. After the initial screening, 14 potentially eligible studies were subjected to detailed assessment. Six studies for dosimetric comparison were excluded because 2 studies did not report the exact data of mean dose or the irradiated volumes of OARs, and 4 studies did not report the mean and standard deviation but the range of the irradiated volumes of OARs. One study for toxicity analysis was excluded because the incidence and number of cases of radiation pneumonitis or esophagitis were reported in a whole without grades. Ultimately, 7 studies were included.[7,8,13–17] Of them, 5 studies[7,8,13,14,16,17] were included in the dosimetric comparison meta-analysis, 3 studies [13,15,16] were included in the OS meta-analysis, and 2 studies [13,16] were included in the irradiation toxicity meta-analysis. The flow diagram of the article selection procedure is depicted in Fig. 1.
Figure 1.
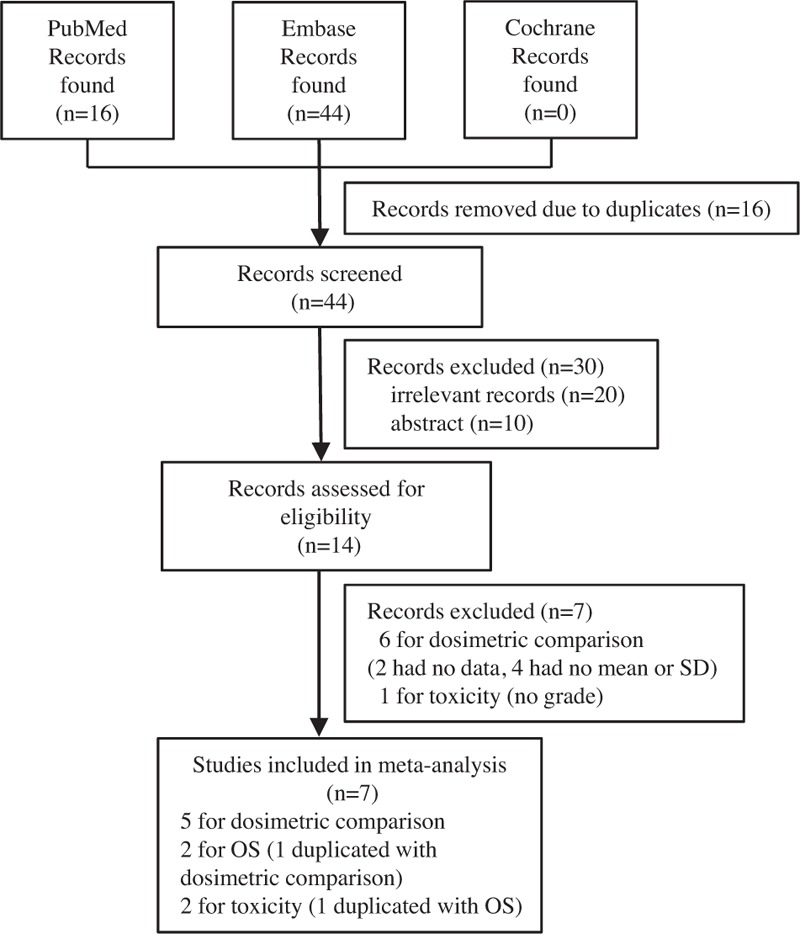
Flow chart of the identification of the meta-analysis. SD = standard deviation, OS = overall survival.
The main characteristics of all eligible studies are summarized in Tables 1–3. All studies were published from 2011 to 2014, with 4 from China, 2 from the USA, and 1 from Switzerland.
Table 1.
Main characteristics of the studies for dosimetric comparison.
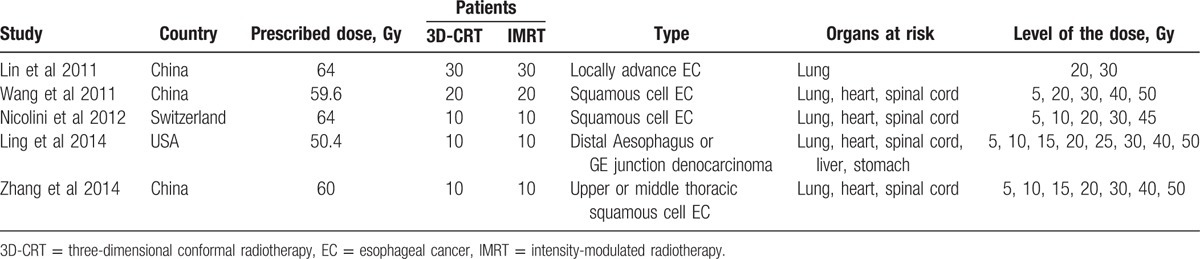
Table 3.
Main characteristics of the studies for irradiation toxicity.

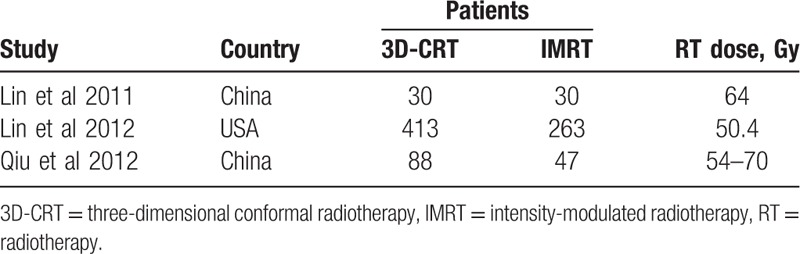
Table 2.
Main characteristics of the studies for overall survival.
3.2. Dosimetric comparison of 3D-CRT and IMRT for OARs
Lung, heart, and spinal cord are 3 mostly reported OARs in the radiotherapy treatment of EC. Mean or maximum dose of 3D-CRT and IMRT and irradiated volumes of these 3 organs at different irradiated levels were compared. For lung, the mean dose of 3D-CRT was significantly higher than that of IMRT, with mean difference dose of 2.18 (95% confidence interval [CI]: 0.83–3.53, P = .002) (Fig. 2). For lung in patients treated with <20 Gy, the irradiated volumes showed no difference between 2 radiotherapy techniques (mean volume difference: –3.40 [95%CI: –9.53 to 2.72] for V5, P = .28; 1.06 [95%CI: –4.62 to 6.73] for V10, P = .72). However, lung in patients treated with ≥20 Gy had significantly higher irradiated volumes for 3D-CRT than for IMRT (mean volume difference: 5.42 [95%CI: 3.54–7.30] for V20, P < .001; 3.85 [95%CI: 2.47–5.22] for V30, P < .001) (Fig. 2)
Figure 2.
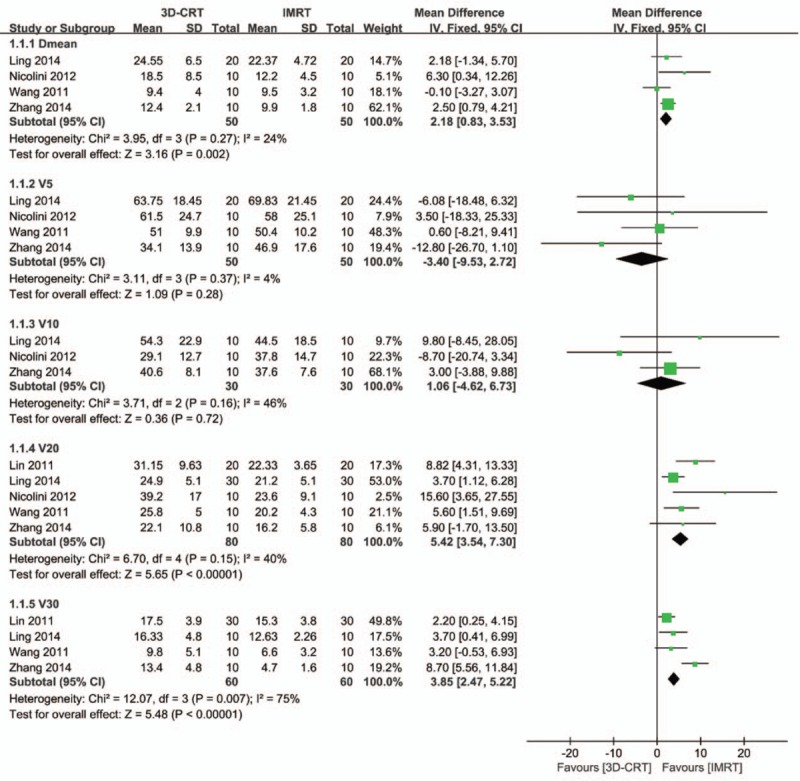
Forest plot showing the meta-analysis of the mean dose and the average percent volumes of the irradiated lung at different doses between 3D-CRT and IMRT. 3D-CRT = three-dimensional conformal radiotherapy, IMRT = intensity-modulated radiotherapy.
For the heart, the mean dose showed no difference between 2 radiotherapy techniques, with a mean difference dose of 0.17 (95%CI: –3.73 to 4.07, P = .93). For heart in patients treated with < 50 Gy, the irradiated volumes showed no difference between 2 radiotherapy techniques (mean volume difference: –1.06 [95%CI: –8.47 to 6.35] for V30, P = .78; 4.18 [95%CI: –1.77 to 10.13] for V40, P = .17). However, heart in patients treated with 50 Gy had significantly higher irradiated volumes for 3D-CRT than for IMRT (mean volume difference: 4.78 [95%CI: 0.88–8.68], P = .02) (Fig. 3).
Figure 3.

Forest plot showing the meta-analysis of the mean dose and the average percent volumes of irradiated heart at different doses between 3D-CRT and IMRT. 3D-CRT = three-dimensional conformal radiotherapy, IMRT = intensity-modulated radiotherapy.
The maximum dose in the spinal cord showed no difference between 2 radiotherapy techniques although the heterogeneity was very high (mean maximum dose difference: 1.82 [95%CI: –1.87 to 5.50], P = .33, I2 = 92%; Fig. 4).
Figure 4.
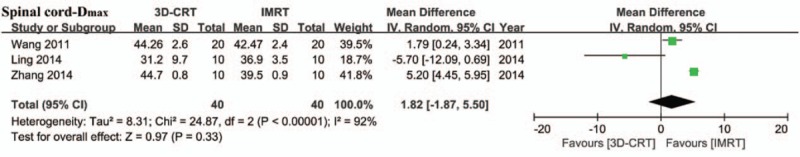
Forest plot showing the meta-analysis of the maximum dose for the spinal cord between 3D-CRT and IMRT. 3D-CRT = three-dimensional conformal radiotherapy, IMRT = intensity-modulated radiotherapy.
3.3. OS
Three studies (871 patients) reported the 3-year OS after receiving 2 radiotherapies. Meta-analysis showed that the 3D-CRT group had a lower survival chance than the IMRT group (OR: 0.68 [95%CI: 0.52–0.90], P = .007). No significant heterogeneity was detected (I2 = 0%, P = .90) (Fig. 5).
Figure 5.
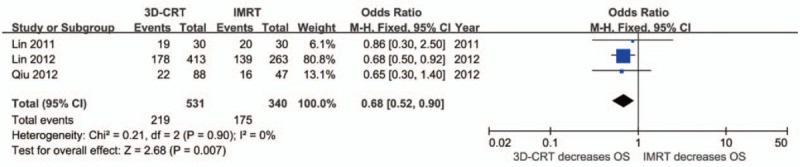
Forest plot showing the meta-analysis of the 3-year overall survival between 3D-CRT and IMRT. 3D-CRT = three-dimensional conformal radiotherapy, IMRT = intensity-modulated radiotherapy, OS = overall survival.
3.4. Toxicity
Two studies (205 patients) reported the incidence of radiation pneumonitis and radiation esophagitis with different grades. For the meta-analysis, we divided the population into 2 groups according to the toxicity grades: grade 0–1 and grade ≥2. Meta-analysis showed that there is no difference between 2 groups either in radiation pneumonitis or radiation esophagitis, no matter in different grades or as a whole (Fig. 6).
Figure 6.
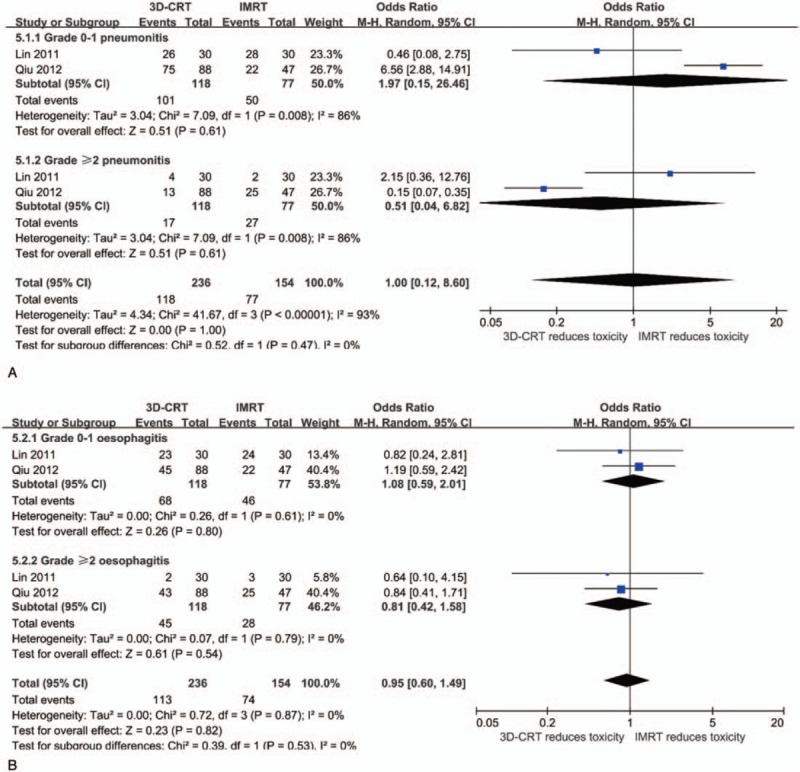
Forest plots showing the meta-analysis of the radiation pneumonitis (A) and radiation esophagitis (B) between 3D-CRT and IMRT. 3D-CRT = three-dimensional conformal radiotherapy, IMRT = intensity-modulated radiotherapy.
3.5. Publication bias
The publication bias for the current meta-analysis was difficult to estimate because the maximum number of the included study for meta-analysis was 5; thus, only meta-analyses that included more than 4 studies (mean radiotherapy dose for lung, V5, V20, and V30 of the lung) were subjected to publication bias assessment. Funnel plots seemed to be symmetrical on visual inspection for all analyses (Fig. 7). These were consistent with the results of Egger's regression tests which suggested no significant publication bias for the above 4 meta-analyses (P = .499, .928, .423, and .192, respectively).
Figure 7.

Funnel plots for publication bias of selected meta-analyses. (A) Mean radiotherapy dose for lung, (B) V5 of the lung, (C) V20 of the lung, (D) V30 of the lung.
4. Discussion
Because IMRT can deliver irradiation dose more accurately to target organs while reducing the toxicity to normal organs, it seems to provide several advantages over conventional radiation technique for tumor treatment. However, its effect on EC treatment over 3D-CRT has not been fully investigated. In this meta-analysis, we compared DVHs, OS, and irradiation toxicity between IMRT and 3D-CRT.
For DVHs comparison, 5 studies have been included. The results showed that the average percent volumes of the irradiated lung at higher doses (20 and 30 Gy) were significantly lower in IMRT than in 3D-CRT, while showed no significant difference at lower doses (5 and 10 Gy), and the mean dose was significantly lower in IMRT than in 3D-CRT. Only the highest dose (50 Gy) resulted in a lower average percent volumes of irradiated heart in IMRT than in 3D-CRT, whereas two lower doses (30 and 40 Gy) showed no difference. Moreover, the mean dose showed no difference between 2 radiotherapy techniques. There was no difference in the spinal cord in terms of maximum dose between 2 radiotherapy techniques, whereas the heterogeneity was very high because the results from the included studies were contrary. However, 2 studies have been excluded because they reported the mean and range of the mean dose for lung. They showed that the mean dose was higher in IMRT than in 3D-CRT.[6,9] For heart and spinal cord, those excluded studies because of data presentation showed a similar trend as the included studies.[6,9,10] The dosimetric comparison results for 3D-CRT and IMRT were consistent with a previous report about the application of these 2 radiotherapy strategies on gynecologic malignancies.[11] It reported that IMRT-delivered high radiation dose produced significantly less average percent volumes of the irradiated rectum and small bowel than 3D-CRT, whereas low radiation dose showed no difference.[11]
For OS, 3 studies were included and the meta-analysis showed IMRT resulted in a better 3-year OS than 3D-CRT did. Moreover, for almost all reported outcomes (complete response, partial response, and 1-year, 2-year, 3-year, and 5-year OS), IMRT is superior to 3D-CRT.[13,15,16] However, when IMRT and 3D-CRT were applied for the treatment of nonsmall cell lung cancer, no difference in OS was observed between 2 techniques.[5]
For irradiation toxicity, pneumonitis and esophagitis were analysis, and only 2 studies were included. No matter for low grade (0–1) or high grade (≥2) irradiation toxicity, no difference was observed in both pneumonitis and esophagitis between 2 treatment techniques. One excluded study, which compared pulmonary and gastrointestinal complications when these 2 techniques were used to treat EC, reported that IMRT resulted in a lower incidence of complications than 3D-CRT did.[18] However, compared to 3D-CRT, IMRT significantly reduced the risk of radiation pneumonitis and increased the risk of radiation esophagitis when treating nonsmall cell lung cancer.[5]
There were several limitations for this meta-analysis. First, the included studies are limited especially for OS and irradiation toxicity analysis, and more studies are needed to draw a reliable conclusion. Second, the meta-analyses for OS and irradiation toxicity were based on retrospective studies with low evidence level, so the results may have been influenced by bias. Third, because of the limited studies included, the majority of the meta-analyses are hard to evaluate the publication bias by software.
In conclusion, our meta-analysis suggests that IMRT is superior to 3D-CRT in the OS of EC and significantly reduces the average percent irradiated volume of the lung resulting from >20 Gy doses and of the heart from 50 Gy, although it shows no advantages on reducing the incidence of radiation pneumonitis and esophagitis. Since the included studies for analysis are limited, further studies especially randomized trials are needed to confirm the advantages of IMRT over 3D-CRT in the treatment of EC.
Footnotes
Abbreviations: 3D-CRT = three-dimensional conformal radiotherapy, CI = confidence interval, DVHs = dose–volume histograms, EC = esophageal cancer, IMRT = intensity-modulated radiotherapy, OARs = organs at risks, OS = overall survival.
The authors have no funding and conflicts of interest to disclose.
References
- [1].WHO Press, Stewart BW, Wild CP. World Cancer Report 2014. 2014. [Google Scholar]
- [2].Pennathur A, Gibson MK, Jobe BA, et al. Oesophageal carcinoma. Lancet 2013;381:400–12. [DOI] [PubMed] [Google Scholar]
- [3].Domper Arnal MJ, Ferrandez Arenas A, Lanas Arbeloa A. Esophageal cancer: risk factors, screening and endoscopic treatment in Western and Eastern countries. World J Gastroenterol 2015;21:7933–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Liang H, Fan JH, Qiao YL, et al. and prevention of esophageal squamous cell carcinoma in China. Cancer Biol Med 2017;14:33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Hu X, He W, Wen S, et al. Is IMRT superior or inferior to 3DCRT in radiotherapy for NSCLC? A meta-analysis. PLoS One 2016;11:e0151988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kole TP, Aghayere O, Kwah J, et al. Comparison of heart and coronary artery doses associated with intensity-modulated radiotherapy versus three-dimensional conformal radiotherapy for distal esophageal cancer. Int J Radiat Oncol Biol Phys 2012;83:1580–6. [DOI] [PubMed] [Google Scholar]
- [7].Wang D, Yang Y, Zhu J, et al. 3D-conformal RT, fixed-field IMRT and RapidArc, which one is better for esophageal carcinoma treated with elective nodal irradiation. Technol Cancer Res Treat 2011;10:487–94. [DOI] [PubMed] [Google Scholar]
- [8].Ling TC, Slater JM, Nookala P, et al. Analysis of intensity-modulated radiation therapy (IMRT), proton and 3D conformal radiotherapy (3D-CRT) for reducing perioperative cardiopulmonary complications in esophageal cancer patients. Cancers 2014;6:2356–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ghosh S, Kapoor R, Gupta R, et al. An evaluation of three dimensional conformal radiation therapy versus intensity modulated radiation therapy in radical chemoradiation of esophageal cancer: a dosimetric study. Clin Cancer Invest J 2012;1:65–70. [Google Scholar]
- [10].Chandra A, Guerrero TM, Liu HH, et al. Feasibility of using intensity-modulated radiotherapy to improve lung sparing in treatment planning for distal esophageal cancer. Radiother Oncol 2005;77:247–53. [DOI] [PubMed] [Google Scholar]
- [11].Yang B, Zhu L, Cheng H, et al. Dosimetric comparison of intensity modulated radiotherapy and three-dimensional conformal radiotherapy in patients with gynecologic malignancies: a systematic review and meta-analysis. Radiat Oncol 2012;7:197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Stang A. Critical evaluation of the Newcastle–Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603–5. [DOI] [PubMed] [Google Scholar]
- [13].Lin XD, Shi XY, Zhou TC, et al. [Intensity-modulated or 3-D conformal radiotherapy combined with chemotherapy with docetaxel and cisplatin for locally advanced esophageal carcinoma]. J Southern Med Univ 2011;31:1264–7. [PubMed] [Google Scholar]
- [14].Nicolini G, Ghosh-Laskar S, Shrivastava SK, et al. Volumetric modulation arc radiotherapy with flattening filter-free beams compared with static gantry IMRT and 3D conformal radiotherapy for advanced esophageal cancer: A feasibility study. Int J Radiat Oncol Biol Phys 2012;84:553–60. [DOI] [PubMed] [Google Scholar]
- [15].Lin SH, Wang L, Myles B, et al. Propensity score-based comparison of long-term outcomes with 3-dimensional conformal radiotherapy vs intensity-modulated radiotherapy for esophageal cancer. Int J Radiat Oncol Biol Phys 2012;84:1078–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Qiu R, Wang J, Wang YX, et al. Clinical contrast study of esophageal carcinoma treated with three-dimensional conformal radiotherapy and intensity modulated radiation therapy. Chin J Cancer Prevent Treat 2012;19:68–71. [Google Scholar]
- [17].Zhang WZ, Chen JZ, Li DR, et al. Simultaneous modulated accelerated radiation therapy for esophageal cancer: a feasibility study. World J Gastroenterol 2014;20:13973–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wang J, Wei C, Tucker SL, et al. Predictors of postoperative complications after trimodality therapy for esophageal cancer. Int J Radiat Oncol Biol Phys 2013;86:885–91. [DOI] [PMC free article] [PubMed] [Google Scholar]


