Abstract
The aim of this study was to provide an overview of massive transfusion in Chinese hospitals, identify the important indications for massive transfusion and corrective therapies based on clinical evidence and supporting experimental studies, and propose guidelines for the management of massive transfusion. This multiregion, multicenter retrospective study involved a Massive Blood Transfusion Coordination Group composed of 50 clinical experts specializing in blood transfusion, cardiac surgery, anesthesiology, obstetrics, general surgery, and medical statistics from 20 tertiary general hospitals across 5 regions in China. Data were collected for all patients who received ≥10 U red blood cell transfusion within 24 hours in the participating hospitals from January 1 2009 to December 31 2010, including patient demographics, pre-, peri-, and post-operative clinical characteristics, laboratory test results before, during, and after transfusion, and patient mortality at post-transfusion and discharge. We also designed an in vitro hemodilution model to investigate the changes of blood coagulation indices during massive transfusion and the correction of coagulopathy through supplement blood components under different hemodilutions. The experimental data in combination with the clinical evidence were used to determine the optimal proportion and timing for blood component supplementation during massive transfusion. Based on the findings from the present study, together with an extensive review of domestic and international transfusion-related literature and consensus feedback from the 50 experts, we drafted the guidelines on massive blood transfusion that will help Chinese hospitals to develop standardized protocols for massive blood transfusion.
Keywords: coagulopathy correction, guidelines on massive blood transfusion, hemodilution, massive blood transfusion in China
1. Introduction
Blood transfusion plays an important life-saving role for patients with severe conditions during emergencies and those with potentially fatal conditions. Massive blood transfusion is often defined as a transfusion of more than 1 entire blood volume in 24 hours (h),[1–4] replacement of 50% of total blood volume within 3 hours,[3,4] or administration of ≥10 units (U) of red blood cell suspension (RBCs) to a patient within 24 hours.[5] As an important transfusion therapy in response to massive and uncontrolled hemorrhaging, massive transfusion is often associated with high rates of mortality and high risks of coagulopathy leading to higher incidence of multiorgan failure and mortality especially in trauma patients.[6–8] To reduce the incidence of dilutional complications resulting from large volumes of RBCs transfusion and lower the risk of mortality, many countries and regions have developed guidelines on massive blood transfusion.[3,4,9] Timely implementation of institutional massive transfusion protocols has significantly improved the resuscitation efficiency and patient outcomes.[10–13] However, currently in China, there are no guidelines on massive transfusion, nor consensus on the critical indications and the optimal timing and ratio of blood components delivery in the correction of coagulopathy. As a matter of fact, little is known about the current blood transfusion practice in response to massive hemorrhage in Chinese hospitals.
According to the Protocols of Clinical Transfusion Management issued by the Chinese Ministry of Health in 2012,[14] a request of 800–1600 mL transfusion within 24 hours needs approval of department directors; a request of >1600 mL transfusion must be submitted by department directors and approved by the Hospital Office of Medical Affairs, with the exception of emergency transfusion. Yet the protocols provide neither a definition of nor management guidelines for massive transfusion. With no clear definition of the indications for coagulopathy during massive transfusion and recommended corrective approaches, Chinese clinicians have to administer blood transfusions either based on their own judgment or by referring to foreign guidelines. Active bleeding occurs frequently during massive blood transfusions, which seriously affects the efficacy and clinical outcome of emergency resuscitation.
This study was aimed to accomplish the following goals: first, to understand the current practice of massive transfusion and patient outcomes in China by examining the demographic and clinical characteristics, pre and post-transfusion blood test results, volumes and ratios of transfused blood components, and outcomes of patients who received ≥10 U RBCs within 24 hours of hospitalization. Second, to identify important indications for massive transfusion used by Chinese clinicians and evaluate the variations in timing and ratio of blood components delivery in the correction of coagulopathy, the clinical features of coagulopathy, frequency of blood tests during massive transfusion, and their association with patient mortality. Additionally, to better understand the mechanism of coagulopathy and effective correction strategies, we designed an in vitro model of coagulopathy with a corrective approach to simulate in vivo coagulation during severe blood loss and collected data from laboratory experiments to verify our findings derived from clinical data. Third, based on the in vitro findings as well as evidence of clinical transfusion practice in Chinese hospitals and international massive transfusion guidelines, propose a definition of massive transfusion that fits the Chinese adult patient population, determine the objectives of massive blood transfusion protocol, and describe the important indications and strategies for massive transfusion management for Chinese clinicians.
2. Materials and methods
2.1. Clinical data collection
This retrospective study included 50 experts in clinical blood transfusion, cardiac surgery, trauma, anesthesiology, obstetrics, general surgery, and biostatistics from 20 tertiary general hospitals in 5 geographic regions in China (northwest, southwest, central south, north, and northeast). The number of beds varied greatly from hospital to hospital ranging from 2000 to 7000 beds and 20,000 to 65,000 annual surgeries in each hospital. From the blood bank electronic database at each hospital, all adult patients (≥18 years old) who received of ≥10 U of RBCs within 24 hours after admission during the period of January1 2009 to December 30 2010 were identified and included as the research subjects. Additional information on patient preoperative characteristics, intraoperative information, and postoperative outcomes was extracted from either computerized or paper medical charts, or surgery or anesthesiology records. We designed the data collection form “Questionnaire on Massive Blood Transfusion” that consisted of more than 60 items in 4 sections including patient demographic characteristics; type and volume of blood components transfused; perioperative complications and clinical condition within and after 24 hours of the transfusion; surgical and transfusion events; the laboratory test results before, during, and after blood transfusion such as routine blood test (RBT), blood coagulation tests (BCT), liver function test, kidney function test, and arterial blood gas analysis; adverse events after massive blood transfusion, and discharge status. All available blood test results and transfusion information at the following time points were collected: before RBCs transfusion and when RBCs transfusions reached the following volumes: 2U,4U,6U,8U,10U,12U,14U,16U,18U,20U,22U,24U,26U,28U,30U,40U. In general, 1U RBCs (140–172 mL) was derived from 200 mL whole blood; 1 U Fresh frozen plasma (FFP) was 100 mL; 1 bag of apheresis platelets (PLT) was 10 U (1 bag = 250–300 mL); 1 U PLT concentrate suspension (25–35 mL) was derived from 200 mL whole blood. Dedicated data collection staff from each participating hospital were selected and trained to complete the questionnaires according to the study protocol.
All collected questionnaire data were sent to the Blood Transfusion Department of Shaanxi Provincial People's Hospital. Data completeness, quality, and validity were assessed by a panel of experts in our research group based on the predetermined inclusion and exclusion criteria. Patients who underwent trauma, cardiac surgery, obstetric, or other general surgeries (including orthopedic, thoracic, general, urinary, hepatobiliary, and neurological surgery) were included in the study, whereas patients with coagulation disorders, hepatic failure due to medical causes, and coagulopathies were excluded. Details about the method, that is, the questionnaire, laboratory equipment and medical devices, and reagent utilization across all participating hospitals, as well as laboratory testing result validity, reliability, and overall data quality control have all been published.[15–21] This study was approved by the Institutional Review Board at all participating institutes.
2.2. Establishment of an in vitro model of coagulopathy and its correction protocol
Massive hemorrhage was simulated by an in vitro hemodilution model and a correction protocol for coagulopathy was developed. The study process is described in the references.[20]
2.3. Development of massive transfusion guidelines
Based on the clinical data derived from our multi-center study and the in vitro experimental results, as well as extensive review of domestic and international transfusion-related literature, laws, and regulations, and consultation with more than 50 experts, we developed the guidelines for massive blood transfusion for adult patients ≥18 years of age, especially those in general surgery, cardiac surgery, obstetric surgery, and trauma patients.[22]
2.4. Statistical analysis
All eligible clinical and experimental data were entered into the EPIDATA database (version 3.01; Epidata Association). The Double entry method was used to ensure data accuracy. Predictive Analytics Software Statistics for Windows version 18.0 (released 2009; SPSS Inc., Chicago, IL) was used for statistical analysis. The data on the demographic characteristics and clinical features were reported as the mean ± standard deviation (SD) or as counts and ranges. Categorical variables were analyzed by Chi-square statistics; continuous variables with normal distribution were analyzed by the Shapiro–Wilk test, analysis of variance or the Kruskal–Wallis test, as appropriate. Linear regression or logistic regression analysis was performed to describe the association between continuous or dichotomous outcomes and covariates. A 2-sided P < .05 was set as the statistically significant level.
3. Results
3.1. Summary of clinical data from 20 hospitals[15–21]
A total of 2000 surveys were distributed to 20 hospitals and 1753 (87.6%) completed questionnaires were collected, from which 1601 (91.3%) met all inclusion criteria. Among them, 553 (34.5%) received <10 U RBCs and 1048 (65.5%) received ≥10 U of RBCs within 24 hours of hospitalization. All data reported in the present study were based on the 1048 patients.
Patient demographics, clinical characteristics, pre- and post-transfusion blood test results, and mortality for the 1048 patients by clinical departments are displayed in Table 1. About half of these patients (51.6%) were from General Surgery, 25.5% Cardiac Surgery, 17.8% Trauma, and 5.1% Obstetrics. More male (n = 646, 61.6%) than female patients (n = 402, 38.4%) were included. Patients in obstetrics (mean age = 33 years) and trauma (mean age = 40 years) were younger than those in cardiac surgery (mean age = 46 years) and general surgery (mean age = 47 years). The average weight of all patients was about 60 kg, with considerable variations by gender and across departments. The average operation time and number of days in intensive care unit (ICU) care also varied across departments. The number of days in ICU in General surgery displays a very large variance (mean = 13 days, SD = ± 43 days), indicating the heterogeneity of clinical setting. No significant differences in the length of stay (LOS) were found between cardiac surgery, general surgery, and trauma, all being significantly longer than the LOS in obstetrics.
Table 1.
Demographic and clinical characteristics, pretransfusion blood indices, and summary of total transfused components for all patients.
There were significant differences in most pretransfusion blood and coagulation indices between the 4 departments (P's < .05). Mean preoperative hemoglobin (Hb) levels varied from 95 g/L in obstetrics, 108 g/L in trauma, 115 g/L in general surgery, to 132 g/L in cardiac surgery. The mean international normalized ratio (INR) was >1.5 in obstetrics but less than 1.5 in the other 3 departments. On average, patients in obstetrics and trauma received more RBCs transfusions (20 and 19 U per patient) than patients in cardiac surgery and general surgery (16 and 14 U per patient). Patients in cardiac surgery, obstetrics, trauma, and general surgery on average received 12, 11, 10, and 8 U of FFP, respectively.
The average mortality rate was 10.3%, including 49 cases in cardiac surgery (18.4%), 27 cases in trauma (14.4%), 29 cases in general surgery (5.4%), and 3 cases in obstetrics (5.7%).
3.2. Blood component supplement
All hospitals administered FFP to supplement RBCs transfusion at various time points. About 20% of patients, mostly in cardiac surgery, received FFP supplement when RBCs transfusion was initiated. After ≥6 U RBCs were transfused, about half of the patients (54.2%) were infused with FFP. When RBC transfusion reached ≥10 U, 72.4% of patients were given FFP supplement. In a separate analysis, we found that transfusion of FFP supplement also varied by departments. For example, plasma was often used in combination with RBCs at earlier stage of cardiac surgery operation and the RBCs:FFP ratio was maintained 1 to 1 throughout transfusion. However, in other departments, plasma was rarely used when RBCs infusion was less than 4 U. After ≥10 U RBCs were transfused, almost all patients from the 4 departments received plasma supplement at the RBCs:FFP ratio of 1.5:1. These data were summarized in previous publications by our group.[16,21]
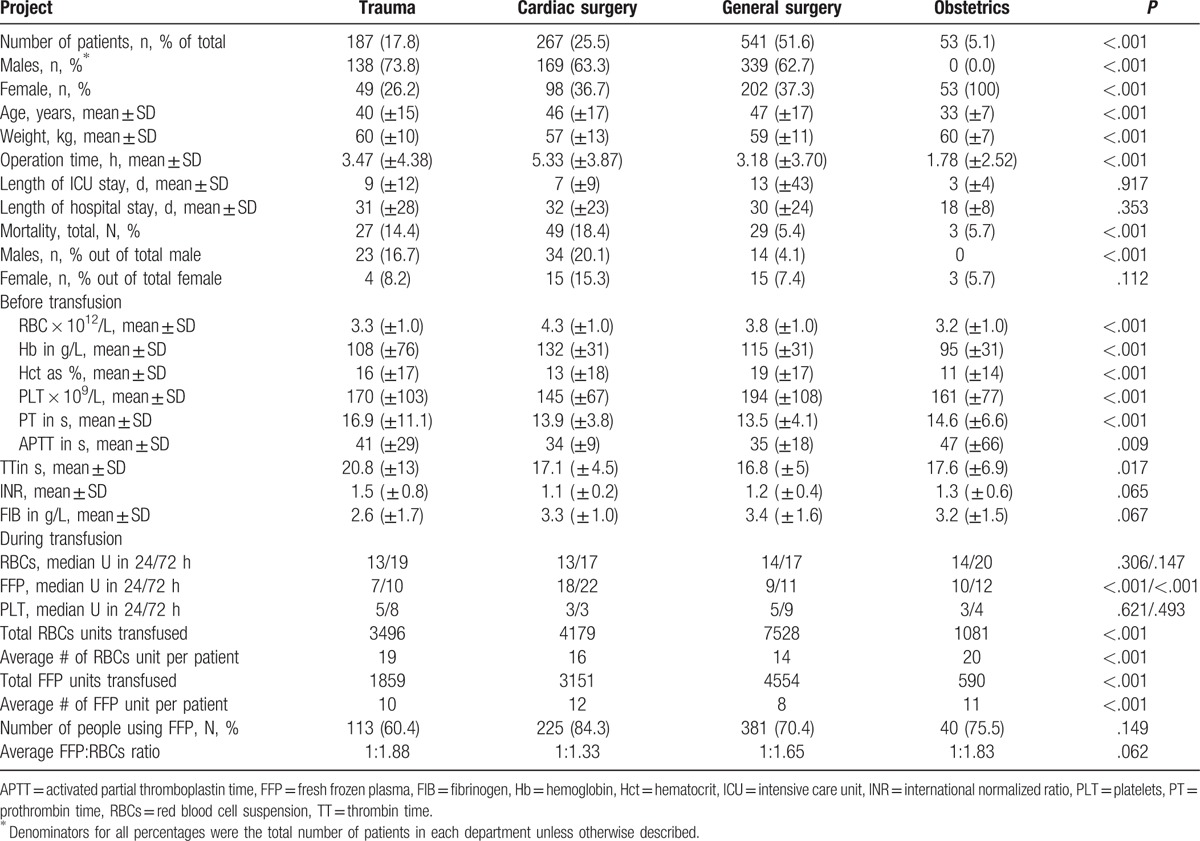
Due to their limited availability and accessibility in China, PLT and cryoprecipitate (CRYO) were transfused much less frequently and their dosages did not increase proportionally with the increase of RBC transfusion. Figure 1 presents the number of patients with FFP, PLT, and CRYO supplement at different stages of RBCs transfusion. Within 24 hours, when RBCs transfusion reached 10 U, 20 U, and 30 U, 72.4%, 78.3%, and 80.6% patients were provided with FFP supplement, respectively. However, only 4.4%, 6.7%, and 13.7% patients were given PLT and 16.4%, 24.2%, and 24.2% patients were given CRYO, respectively. The median number of RBCs, FFP, PLT, and CRYO units transfused within 24 hours was 25, 20, 6, and 5 respectively. Only 3 patients in obstetrics with low PLT counts had platelets infusion at the initiation of RBCs transfusion. An increase in PLT transfusion was found in a small number of trauma patients after they received 26 to 28 U RBCs, which might be random events. Similarly, an increase of CRYO infusion was observed only in cardiac surgery when transfused RBCs reached 30 U.[16]
Figure 1.
Number of patients with RBCs transfusion and FFP, PLT, and CRYO supplement under different RBCs dosages. Cryo = cryoprecipitate, FFP = fresh frozen plasma, PLT = platelets, RBCs = red blood cell suspension.
3.3. RBCs dosage and mortality[17]
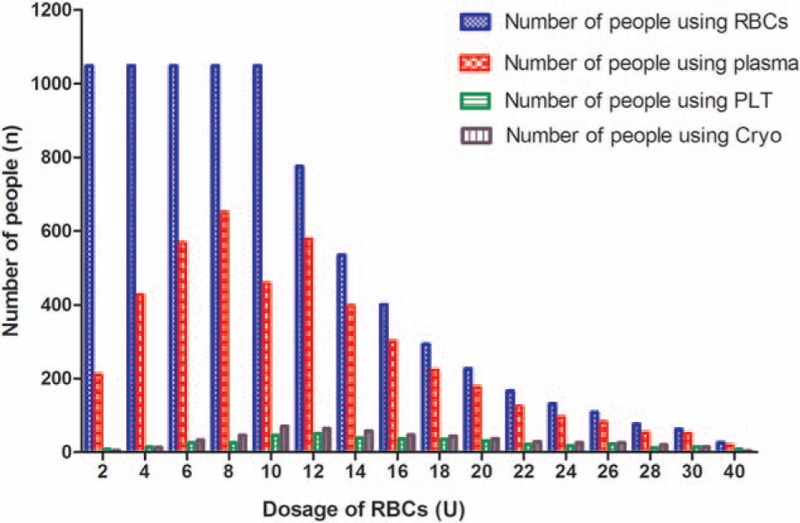
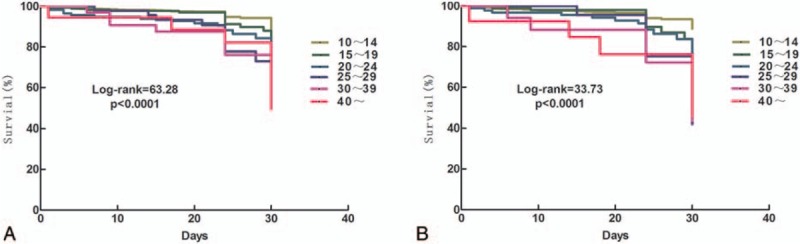
The association between the number of RBCs units transfused (RBCs dosage) and 30-day post-transfusion patient mortality was explored in the survival analysis. Figure 2A and B displays the Kaplan–Meier survival plots for post-transfusion 30-day mortality rates grouped by different RBC dosages within 24 and 72 hours. Without adjusting for covariates, the survival rates significantly decreased (therefore mortality increased) as the volume of transfused RBCs increased from 10 U to 40 U within 24 hours and 72 hours, (P's < .001). After adjusting for age, gender, weight, operation time, LOS, 24 hour plasma dosage, and number of days in ICU in logistic regression analysis, the volume of transfused RBCs used within 24 hours was an independent risk factor for patient mortality, increasing infusion of every 2 RBCs units was associated with about 2-folds likelihood of mortality (odds ratio, OR = 1.92; 95% confidence interval, CI: 1.56–2.33). Number of days in ICU was also significantly associated with mortality, the longer the stay in ICU, the higher the mortality rate (OR = 2.33, 95% CI: 1.14 – 4.76). Table 2 presents the results of the logistic regression analysis.
Figure 2.
Survival analysis on 30-day post-transfusion mortality rates by number of RBCs dosages transfused in 24 and 72 hours. (A) Kaplan–Meier survival plot for mortality rates by different RBCs dosage in 24 hours. (B) Kaplan–Meier survival plot for mortality rates by different RBCs dosage in 72 hours. RBCs = red blood cell suspension.
Table 2.
Logistic regression analysis on mortality during admission.
3.4. FFP: RBCs ratio and mortality[19]
As shown in Fig. 1, up to 72.4% of the patients with RBCs transfusion also received plasma supplement at an average FFP:RBCs ratio of 1:1.5 when RBCs transfusion reached ≥10 U. To explore the potential impact of FFP:RBCs ratio and patient outcome, we divided the patients into 3 groups based on the 24 hours and 72 hours FFP:RBCs ratios separately: 1:0.3 to 0.75 was the high ratio group (HR); 1:1 to 2 (1:0.75–2.3) was the medium ratio group (MR); 1:2.5 to 4 (1:2.3–4) was the low ratio group (LR). There was no significant difference in the distribution of the 3 groups across the 4 clinical departments (P = .295). Figure 3A and B displays the 30-day Kaplan–Meier survival plots for patients with different FFP:RBCs ratios during 24 hours and 72 hours transfusion, respectively. No significant differences in 30-day mortality rates were found among the 24 hour FFP:RBCs ratio groups (11.4%, 9.3%, and 11.8% for HR, MR, and LR, respectively, χ2 = 1.482, P = .197), although the medium ratio group maintained a slightly higher survival probability than the other 2 groups at 30-day post-transfusion. The advantage of medium 72 hour FFP:RBCs ratio over high and low ratio on 30-day mortality was apparent, with a significantly lower mortality rate in the MR group (7.3%) than in the HR (13.7%) and LR (10.4%) groups (χ2 = 9.874), P < .001.
Figure 3.
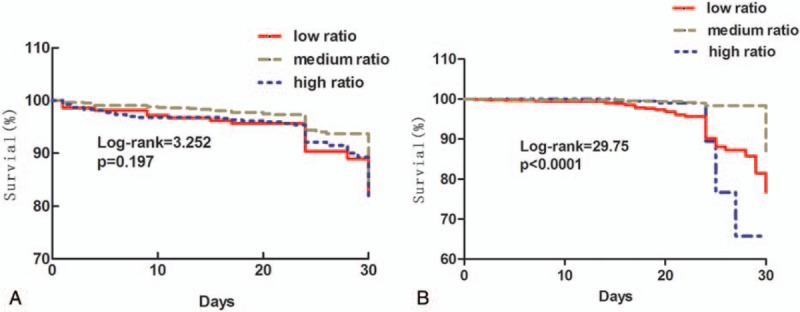
Survival analysis on 30-day post-transfusion mortality rates by transfused FFP:RBCs ratios in 24 and 72 hours. (A) Kaplan–Meier survival plot for 30-day mortality rates by different FFP:RBCs ratios in 24 hours. (B) Kaplan–Meier survival plot for 30-day mortality rates by different FFP:RBCs ratios in 72 hours. FFP = fresh frozen plasma, RBCs = red blood cell suspension.
3.5. Frequency of RBTs and BCTs during transfusion
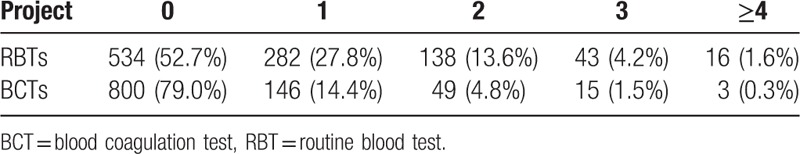
Overall, we obtained data from 1013 (97% of the 1048) patients regarding blood testing during transfusion. No information on blood tests was retrieved for 35 patients. Among the 1013 patients, 52.7% or 534 did not have any RBT during 24 hour transfusion. About 27.8%, 13.6%, 4.2%, and 1.6% of the 1013 patients had 1, 2, 3, and ≥4 RBTs within 24 hours during transfusion. For BCT, 79% or 800 patients did not have any test done, whereas 14.4%, 4.8%, 1.5%, and 0.3% had 1, 2, 3, and ≥4 tests within 24 hours of transfusion. Table 3 presents the frequencies of RBTs or BCTs performed within 24 hour during transfusion.
Table 3.
Frequency of routine blood tests (RBTs) and blood coagulation tests (BCTs) within 24 h transfusion.
3.6. Variations of PLT count during RBCs transfusion[18]
Among the 1048 patients, 646 received RBC transfusion without PLT supplement and had at least 1 PLT count measured at 1 of the 16 time points during the first 24 hours of transfusion. All PLT counts at the 16 predefined time points from these patients were selected and their association with the number of RBCs transfused was analyzed. One patient may contribute multiple PLT counts at various time points. When RBCs reached 18 U, the average PLT counts decreased to 72 × 109/L. Similarly, when patients (those underweight with the body mass index <18.5) were transfused with 0.3 units of RBCs per kilogram of body weight (i.e., 3 U/10 kg), the average PLT count decreased to below 75 × 109/L. Further laboratory testing found that the slight increase in the PLT count after RBC transfusion might be caused by the residual platelet-like fragments in RBCs that were mistaken as PLTs by the machine. Regardless, a linear statistical regression model found a negative correlation between the number of RBCs units transfused and subsequent PLT count (R2 = 0.641, P < .001). PLT count in these patients decreased with an increase in the number of RBCs units transfused. As shown in Fig. 4, the linear association was defined by the formula of Y = 127.3–2.441X.
Figure 4.
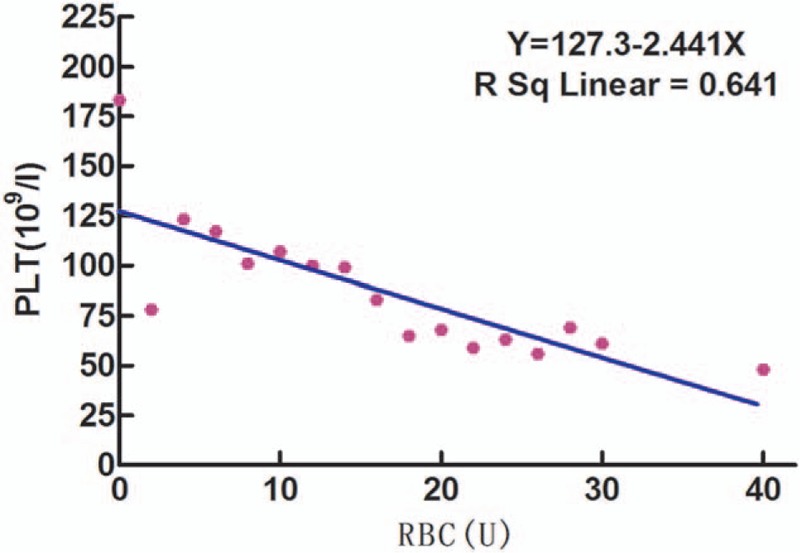
Linear regression trend between units of RBCs transfused and PLT count among patients with no PLT supplement during transfusion. PLT = platelets, RBCs = red blood cell suspension.
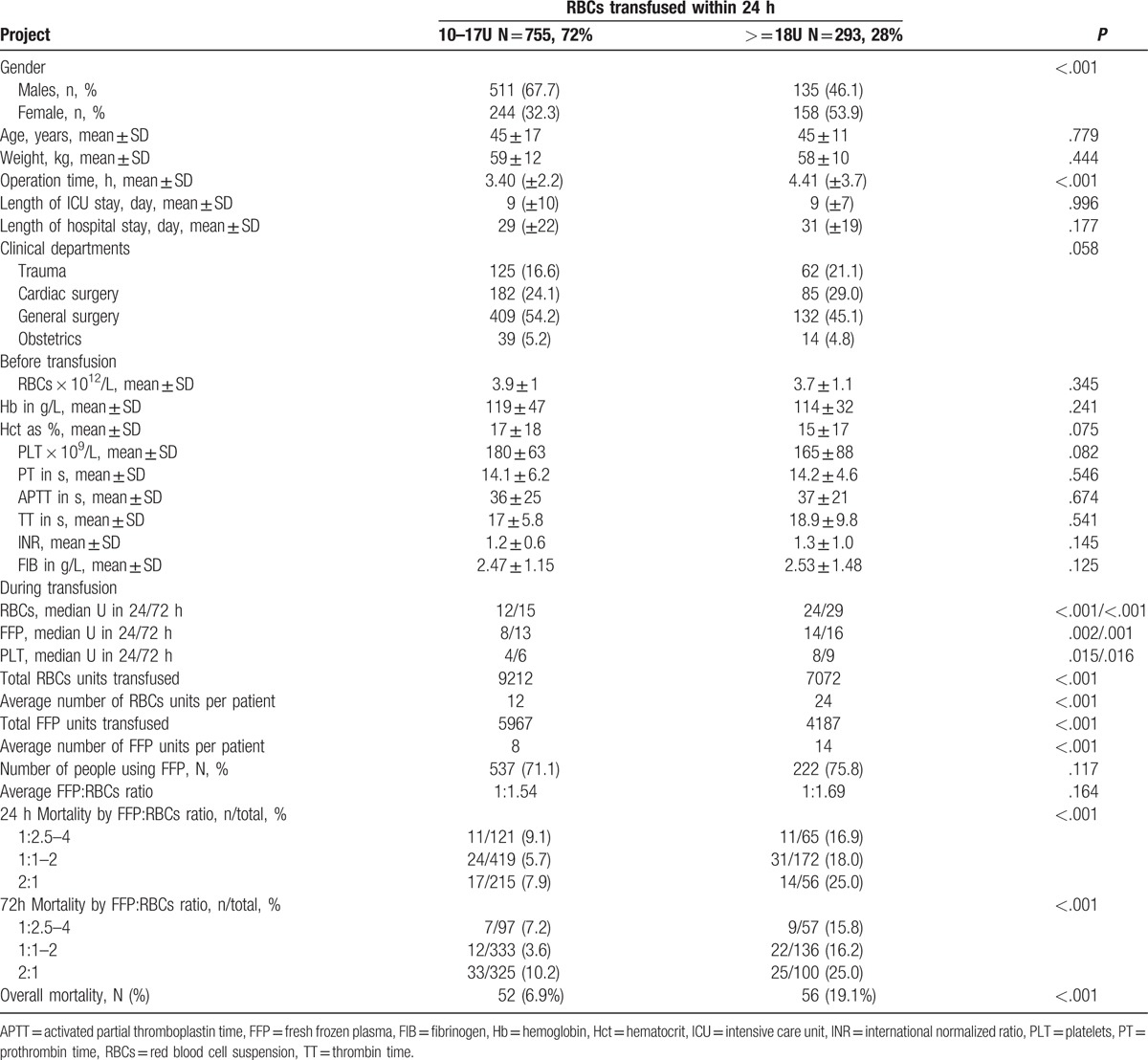
Based on these experimental findings and clinical evidence obtained from the 20 hospitals, we defined massive transfusion as a transfusion volume of RBCs ≥18U within 24 hours, or ≥0.3 U/kg within 24 hours.[18,22] According to this definition, among all the patients with ≥10 U RBCs within 24 hours in our study, 28% received massive transfusion. A follow-up analysis compared patient characteristics, clinical setting, pretransfusion blood and coagulation indices, and transfused components between the 2 groups (Table 4). There were no significant differences in age, weight, clinical departments, pretransfusion routine blood test and coagulation test results, ICU stay, and LOS between the 2 groups (P's >.05). However, patients with massive transfusion (≥18 U RBCs) received twice the amount of RBCs and FFP transfusion on average, had longer operation time (4.41 ± 3.7 hours vs 3.40 ± 2.2 hours) and higher overall mortality rate and at different FFP:RBCs ratios than those with <18 U RBCs (18.8% vs 7.02%), P's < .001. Females were more likely than males to receive massive transfusion (39.3% vs 20.9% respectively, P < .001).
Table 4.
Patient demographic and clinical characteristics, pretransfusion blood indices, and summary of transfused components by total number of RBCs units transfused within 24 hours.
4. Discussion
The present study was a joint research project among 20 participating hospitals in China. The goal of this study was to evaluate the current practice of massive transfusion in China and provide evidence-based data and guidelines for the development of domestic massive transfusion protocols. The ultimate objectives were to standardize the procedure of massive transfusion management, help clinicians to stop bleeding, and restore blood volume rapidly and efficiently in order to reduce the risks for adverse events associated with massive blood transfusion, and eventually improve the resuscitation success rate and patient outcomes. Efficient implementation of an effective massive transfusion protocol will also reduce potential waste of blood products, which has become a critical issue when the blood supply cannot meet the increasing demand of clinical transfusion in Chinese hospitals.[23,24]
Our findings indicated that: the first, during large volumes of RBCs transfusion, Chinese clinicians focus on the supplement of FFP at early stages of RBCs transfusion. This practice is in accordance with the finding that balanced use of plasma early in resuscitation was associated with improved survival rates especially among trauma patients.[25–28] However, insufficient consideration is given to the use of platelets and cryoprecipitate.[15,16] According to American Association of Blood Banks recommendation, early initiation of prophylactic PLT transfusion among adult patients with a platelet count of 10 × 109 cells/L or less may reduce the risk for spontaneous bleeding.[29] Cryoprecipitate and PLT transfusion were also reported to benefit the management of bleeding related to thrombolytic therapy.[30] However, due to the small number of patients who received PLT and CRYO transfusion, their small volumes of infusion, and the inconsistent RBCs:PLT:CRYO ratio and timing of infusion, we could not evaluate the effectiveness of these 2 components on patient outcomes in the present study. Future research is needed to investigate the immediate and long-term impact of PLT and CRYO supplement on Chinese patients with massive transfusion.
The second, total units of RBCs transfused within 24 hours were significantly associated with lower survival probability, even after adjusting for potential confounding factors such as operation time and ICU stay that are critical indicators of disease severity. Those who received massive transfusion (≥18 RBCs U in 24 hours) displayed significantly higher mortality rate (19.1%) than the other patients with 10–17 U in 24 hours (6.9%). Since no pre-transfusion differences were found between patients massive transfusion and those with <18 RBCs U in the first 24 hours, it is speculated that the higher patient mortality might be due to greater risks for respiratory and infectious complications associated with massive transfusion,[31] highlighting the importance of further investigation on the adverse events of massive transfusion and opportunities for improvement of massive transfusion management.
The third, the dilution and consumption of platelets and coagulation factors during massive transfusion often lead to coagulation complications. Therefore, early transfusion of FFP and PLT at appropriate ratios plays an important role in preventing coagulation disorders.[18,21,26–28] Our findings suggest that the amount of blood components replenished should increase proportionally to blood dilution levels at different time points, and that a medium FFP:RBCs ratio of 1:1.5 or the 1:1.3:0.9 RBCs:FFP:PLT ratio (based on in vitro experiments) is optimal to prevent common coagulation disorders and reduce fatality rate related to massive transfusion.[19] Our proposed ratios are the closest approximation to reconstituted whole blood and are in line with the 1:1:1 RBCs:FFP:PLT transfusion ratio recommended in the international literature.[9–13] However, our clinical data suggest that when the volume of FFP transfusion increased and was greater than that of RBCs within 24 hours or 72 hours, an increase in patient mortality followed. Further investigation is needed to understand the mechanisms of moderate FFP:RBCs ratios at early stages of transfusion.
The fourth, the lack of laboratory monitoring of blood indices and coagulation factors during massive transfusion is consistent with a previous report in which among 35 surveyed hospitals in Sichuan (one of the most populated Chinese provinces) only 24.2% had systematic evaluations for the appropriateness and effect of blood component transfusion.[32] These findings underscore the great potential for improvement in clinical transfusion practice in China. Establishment of a standard massive transfusion protocol, in addition to the training and education of dedicated personnel for transfusion services and automated blood bank information systems as suggested by previous research,[32] is equally important to improve the efficacy of massive transfusion and optimize patient outcomes.
The fifth, based on our clinical data on patients without PLT supplement within 24 hours of transfusion and the in vitro experimental data on PLT variations, we defined massive blood transfusion as total transfusion volume of RBCs ≥18 U within 24 hours, or ≥0.3 U/kg within 24 hours.[18,22] The rationale for this definition is consistent with the recommended PLT threshold of 75 × 109/L for trauma patients.[33] Follow-up analysis comparing patient and clinical characteristics and pretransfusion blood testing results between patients with 10 to 17 U RBCs and those with ≥18 U RBCs within 24 hours found very few differences at baseline but significant differences in post-transfusion outcomes, providing further evidence to support this demarcation.
4.1. Massive transfusion guidelines and protocol[22]
The protocol consists of a Foreword and the following chapters: massive transfusion, preparation for and assessment of massive transfusion, laboratory testing, treatment of blood, procedure for massive transfusion, and so on.
Definition of massive transfusion. Massive transfusion is defined as a transfusion volume of ≧18 U RBC suspension within 24 hours for adult patients (1 U RBC suspension equals 200 mL whole blood) or a transfusion volume of ≧0.3 U/kg RBC suspension within 24 hours.
Goals of massive transfusion: (1) to maintain tissue perfusion and oxygen supply by restoring blood volume and treating anemia. (2) To prevent hemorrhage (while actively treating trauma or obstetric primary disorders). (3) To perform transfusion scientifically and rationally, and to minimize risks of transfusion and increase the resuscitation success rate. In addition, the risks of massive transfusion are clarified.[33–35]
The preparation for and assessment of massive transfusion chapter emphasizes: (1) effective communication and preparedness among hospital departments in case of emergency. (2) Transfusion preoperative preparation and assessment, and so on.
The laboratory testing chapter provides a detailed description of the following: (1) blood sample preparation and storage, (2) list of test items, and (3) frequency of RBT and BCT. Regulations on the frequency of these blood tests apply to the following situation: (a) continuous transfusion of ≥15 to 18 U RBCs in adults, or transfusion of ≥0.3 U/kg RBCs, requires PLT testing immediately. (b) Blood transfusion 1 to 1.5 times the patient's total blood volume requires complete blood count, conventional blood clotting, and blood gas testing every 1 to 2 hours to accurately reflect the blood clotting state and the clinical status of the patient. (c) During surgery, for every unit of blood transfused, when the total transfused volume reached the total blood volume, blood count and coagulation index should be checked, and special consideration should be given to tracking changes in PLT count and fibrinogen (Fib) levels. (d) The coagulation index should be checked after anticoagulant intervention and heparin neutralization during extracorporeal circulation surgery. (e) Thromboelastograph (TEG) is recommended for quick detection of coagulation status and PLT counts.[34]
The treatment of blood chapter regulates: (1) blood volume recovery and (2) blood component therapy.[15]
4.2. Suspended red blood cells (RBCs)
Function: RBCs are the principle means of oxygen delivery to body tissues, but they are not a means of volume expansion.[14] RBCs can facilitate hemostasis through PLT margination.[33] Experimental evidence shows that a relatively high hematocrit (Hct) is favorable for hemostasis in patients with massive blood loss, and risks increase when Hct is too low. Therefore, timely transfusion of RBCs is extremely important in case of massive blood loss.
Transfusion timing: transfusion of RBC suspension should be considered when blood loss reaches 30% to 40% of the patient's blood volume. If blood volume loss is above 40%: transfusion must be performed immediately. When Hb >100 g/L, massive transfusion is not recommended, but should be considered when Hb is <70 g/L. In cases where Hb is between 70 and 100 g/L, whether or not to perform transfusion is determined by the patient's condition, such as whether there is continuous bleeding, heart and lung function, and so on.[14,36]
Transfusion volume: during massive transfusion, for patients with good heart and lung function, Hb can be maintained at 80 to 100 g/L or Hct at 28% to 30%.
Laboratory tests: Hb and Hct should be checked every 1 to 2 hours. Nevertheless, RBC and Hb levels in cases of emergency sometimes cannot accurately reflect the degree of blood loss.[33] Often the blood loss is underestimated, especially in cases of occult bleeding and in young people, as with occult bleeding in obstetrics.
4.3. FFP
Function: to complement coagulation factors and expand blood volume, FFP is useful in patients with various clotting factor deficiencies, acute active bleeding and severe trauma, prevention of clotting factor dilution during massive blood loss, antiwarfarin therapy, and to correct known coagulation factor deficiency.[14,36]
Transfusion timing: to reduce patient mortality during massive transfusion, FFP should be transfused following 4 U of RBC suspension, and the FFP:RBC ratio should be 1:1 or 1:2 (1 U FFP equals 100 mL).[9–13] For patients with severe trauma, when RBC suspension transfusion was >3 to 5 U, FFP should be used as soon as possible.
Dose: our research results showed that transfusion of FFP at 15 to 30 mL/kg can reduce patient mortality. The amount of FFP should not exceed the amount of RBC suspension transfused within 24 to 72 hours of the FFP transfusion, namely FFP:RBCs = 1:1 or 1:2.[10] The recommended amount of FFP transfusion by the American Society of Anesthesiologists is (10–15) mL/kg.[9] A sufficient amount of FFP can correct Fib and multiple coagulation factor deficiencies. If Fib is <1.0 g/L, transfusion of cryoprecipitate should be considered.
Laboratory tests: it is critical to test coagulation every 1 to 2 hours.[37] The dilution of coagulation factors is one of the main reasons for coagulation disorders. Fib decreases at first, and then blood loss will be around 150% when Fib decreases to 1.0 g/L and blood loss will be around 200% when other coagulation factors’ activities decrease to 25%. Risks of coagulation disorders will increase when activated partial thromboplastin time (APTT) and prothrombin time (PT) increase to 1.5 times normal values.[9,38–42] However, our study of massive transfusion in China showed that conventional indices of coagulation, namely PT/ INR and APTT, did not clearly vary.[43] Compared with conventional clotting tests (PT/INR and APTT), TEG provides a better bedside assessment of in vivo clotting. Therefore, the initial use of TEG is recommended.[44]
4.4. PLT suspension
4.4.1. Function: hemostasis
Transfusion timing: (a) the current consensus for preventive Plt transfusion is a PLT count ≥50 × 109/L. In cases where the PLT count is <50 × 109/L, the estimated volume of RBCs for infusion or transfusion is up to twice the patient's blood volume. However, significant individual differences exist. Certain patients will have obvious bleeding when the PLT count is 75 × 109/L. Therefore, the threshold for preventive transfusion still should be determined based on comprehensive clinical judgment (for example, a PLT count >100 × 109/L is recommended for patients with central nervous system damage). (b) During therapeutic PLT transfusion, a count of 75 × 109/L is generally treated as a safety threshold[3] for patients who are unresponsive to or have ineffective hemostasis and electrocoagulation for active bleeding. When the count is less than 75 × 109/L, early transfusion of PLT should be performed if RBCs and plasma are transfused continuously. When the count is <50 × 109/L, PLT must be transfused. A PLT suspension should be transfused to maintain a level above 75 × 109/L with massive transfusion of RBCs >18U (if no laboratory data are obtained).
Dose: early high transfusion ratio of FFP and PLT suspension can improve patient survival,[26–28,44–46] as well as reduce the transfusion volume of the RBC suspension. A recommended transfusion ratio of RBCs:FFP:PLT suspension is 1:1:1 (1 U manually-collected PLT suspension is prepared from 200 mL whole blood; 1 bag of machine-collected PLT suspension is equivalent to a treatment dosage of 10 U PLT, and volume is between 200 and 250 mL).
Laboratory tests: the dilution of platelets is the main reason for coagulation disorders during massive transfusion. PLT count should be tested every 1 to 2 hours. In the meantime, the blood bank should provide a sufficient quantity of PLT to achieve an effective dose.
4.4.2. Cryoprecipitate and recombinant factor VIII (rFVIII)
Key function: to correct the lack of Fib and FVIII and treat severe bleeding.
Transfusion timing: for patients with diffuse intravascular coagulation and Fib < (80–100) mg/dL, occurrence of diffuse intravascular coagulation during massive transfusion, congenital abnormalities of fibrinogen, Hemophilia A, and von Willebrand disease.
Dose: 1 U cryoprecipitate contains fibrinogen 150 to 250 mg and FVIII 80 to 100 U and can be transfused according to the patient's laboratory test results.
4.4.3. Supplemental antifibrinolytic drugs
Tranexamic acid and aprotinin have been used as antifibrinolytic agents during massive blood transfusion.
Acknowledgments
We thank all the medical centers participating in this research: Shi-Jie Mu, Ai-Jun Xia and Xian-Qin Zhang from Xijing Hospital, the Fourth Military Medical University; Shu-ming Zhao from Southwest Hospital, the Third Military Medical University; Bi-Juan Li from Xiangya Hospital Center of South University; Dai-Yu Li from Affiliated Hospital of Luzhou Medical College; Wei Jiao from the People's Hospital of Guangxi Zhuang Autonomous Region; Li Tong from the First Affiliated Hospital of Kunming Medical University; Qing-Bao Meng from Shenzhen People's Hospital; Jie Li from the Fourth Clinical Medical College of Hebei Medical University; Shi-Ming Yang from Tangdu Hospital, the Fourth Military Medical University; Suo-Liang Yao from Xi’an Hong Hui Hospital; Cui-Ying Li from General Hospital of Chengdu Military Region; Mei-Ning Han from the Second Affiliated Hospital of Medical College of Xi’an Jiaotong University; Zhi-Xi Hu from Yan’an University Affiliated Hospital; Jin-Shan Jiao from the First Affiliated Hospital of Shanxi Medical University; Xian-Ping Lv from the First Affiliated Hospital of Zhengzhou University; Yan-Li Bai from Xi’an Central Hospital; Xiao-Xia Shi from Xianyang 215 Hospital; and Fang-Xiang Chen from Daping Hospital, the Third Military Medical University.
Footnotes
Abbreviations: APTT = activated partial thromboplastin time, BCT = blood coagulation test, CI = confidence interval, CRYO = cryoprecipitate, FFP = fresh frozen plasma, FIB = fibrinogen, Hb = hemoglobin, Hct = hematocrit, HR = high ratio, ICU = intensive care unit, INR = international normalized ratio, LR = low ratio, LOS = length of stay, MR = medium ratio, OR = odds ratio, PLT = platelets, PT = prothrombin time, RBCs = red blood cell suspension, RBT = routine blood test, SD = standard deviation, TEG = thromboelastograph, TT = thrombin time.
Funding: This study was supported by a grant from Johnson (China) Medical Equipment Co., Ltd.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Malone DL, Hess JR, Fingerhut A. Massive transfusion practices around the globe and a suggestion for a common massive transfusion protocol. J Trauma 2006;60:S91–6. [DOI] [PubMed] [Google Scholar]
- [2].Schuster KM, Davis KA, Lui FY, et al. The status of massive transfusion protocols in United States trauma centers: massive transfusion or massive confusion? Transfusion 2010;50:1545–51. [DOI] [PubMed] [Google Scholar]
- [3].Raymer JM, Flynn LM, Martin RF. Massive transfusion of blood in the surgical patient. Surg Clin North Am 2012;92:221–34. [DOI] [PubMed] [Google Scholar]
- [4].Massive transfusion protocol (MTP) for hemorrhagic shock. ASA committee on blood management. [Last accessed on 2014 Sep 27]. Available at: https://www.asahq.org/For-Members/About-ASA/ASACommittees/Committee-on-Blood-Management.aspx. Accessed 16.02.27. [Google Scholar]
- [5].Patil V, Shetmahajan M. Massive transfusion and massive transfusion protocol. Indian J Anaesth 2014;58:590–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Brohi K, Singh J, Heron M, et al. Acute traumatic coagulopathy. J Trauma 2003;54:1127–30. [DOI] [PubMed] [Google Scholar]
- [7].Maegele M, Lefering R, Yucel N, et al. Early coagulopathy in multiple injury: an analysis from the German Trauma Registry on 8724 patients. Injury 2007;38:298–304. [DOI] [PubMed] [Google Scholar]
- [8].Johansson PI, Stensballe J. Effect of haemostatic control resuscitation on mortality in massively bleeding patients: a before and after study. Vox Sang 2009;96:111–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].The American Society of Anesthesiologists. Practice guidelines for perioperative blood transfusion and adjuvant therapies. Anesthesiology 2015;122:241–75. [DOI] [PubMed] [Google Scholar]
- [10].Nunez TC, Dutton WD, May AK, et al. Emergency department blood transfusion predicts early massive transfusion and early blood component requirement. Transfusion 2010;50:1914–20. [DOI] [PubMed] [Google Scholar]
- [11].Cotton BA, Gunter OL, Isbell J, et al. Damage control hematology: the impact of a trauma exsanguination protocol on survival and blood product utilization. J Trauma 2008;64:1177–82. 1182-3. [DOI] [PubMed] [Google Scholar]
- [12].O’Keeffe T, Refaai M, Tchorz K, et al. A massive transfusion protocol to decrease blood component use and costs. Arch Surg 2008;143:686–90. 690-1. [DOI] [PubMed] [Google Scholar]
- [13].Riskin DJ, Tsai TC, Riskin L, et al. Massive transfusion protocols: the role of aggressive resuscitation versus product ratio in mortality reduction. J Am Coll Surg 2009;209:198–205. [DOI] [PubMed] [Google Scholar]
- [14].The Ministry of Health of People's Republic of China. Protocols of Clinical Transfusion Management in Medical Institutes. June 7, 2012. Available at: http://www.moh.gov.cn/mohzcfgs/s3576/201206/55072.shtml. Accessed 16.02.27. [Google Scholar]
- [15].Yang JC, Sun Y, Xu CX, et al. Application status of blood constituents during massive blood transfusion in some regions of China. Int J Clin Exp Med 2014;7:1775–80. [PMC free article] [PubMed] [Google Scholar]
- [16].Sun Y, Jin ZK, Xu CX, et al. Investigation of the current situation of massive blood transfusion in different surgical departments: a large multicenter study in China. Int J Clin Exp Med 2015;8:9257–65. [PMC free article] [PubMed] [Google Scholar]
- [17].Yang JC, Sun Y, Xu CX, et al. Correlation between red blood cell transfusion volume and mortality in patients with massive blood transfusion: a large multicenter retrospective study. Exp Ther Med 2015;9:137–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Yang JC, Sun Y, Xu CX, et al. Coagulation defects associated with massive blood transfusion: a large multicenter study. Mol Med Rep 2015;12:4179–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Yang JC, Xu CX, Sun Y, et al. Balanced ratio of plasma to packed red blood cells improves outcomes in massive transfusion: a large multicenter study. Exp Ther Med 2015;10:37–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Li L, Yang J, Sun Y, et al. Correction of blood coagulation dysfunction and anemia by supplementation of red blood cell suspension, fresh frozen plasma, and apheresis platelet: results of in vitro hemodilution experiments. J Crit Care 2015;30:220–1. [DOI] [PubMed] [Google Scholar]
- [21].Jin ZK, Sun Y, Dang QL, et al. The plasma and platelet are important in reducing the mortality in surgical massive blood transfusion: a large multicenter study in China. Int J Clin Exp Med 2015;8:1073–9. [PMC free article] [PubMed] [Google Scholar]
- [22].Yang JCXYSY. The guidelines for massive blood transfusion. Chin J Blood Transfus 2012;25:617–21. [Google Scholar]
- [23].China's “blood famine” drives patients to the black market. Available at: http://www.reuters.com/article/china-health-blood-idUSL4N0VN09020150214. Accessed 16.02.27. [Google Scholar]
- [24].Yin YH, Li CQ, Liu Z. Blood donation in China: sustaining efforts and challenges in achieving safety and availability. Transfusion 2015;55:2523–30. [DOI] [PubMed] [Google Scholar]
- [25].Sperry JL, Ochoa JB, Gunn SR, et al. An FFP:PRBC transfusion ratio ≥1:1.5 is associated with a lower risk of mortality after massive transfusion. J Trauma 2008;65:986–93. [DOI] [PubMed] [Google Scholar]
- [26].Gonzalez EA, Moore FA, Holcomb JB, et al. Fresh frozen plasma should be given earlier to patients requiring massive transfusion. J Trauma 2007;62:112–9. [DOI] [PubMed] [Google Scholar]
- [27].Zink KA, Sambasivan CN, Holcomb JB, et al. A high ratio of plasma and platelets to packed red blood cells in the first 6 hours of massive transfusion improves outcomes in a large multicenter study. Am J Surg 2009;197:565–70. [DOI] [PubMed] [Google Scholar]
- [28].Peralta R, Vijay A, El-Menyar A, et al. Trauma resuscitation requiring massive transfusion: a descriptive analysis of the role of ratio and time. World J Emerg Surg 2015;10:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kaufman RM, Djulbegovic B, Gernsheimer T, et al. Platelet transfusion: a clinical practice guideline from the AABB. Ann Intern Med 2015;162:205–13. [DOI] [PubMed] [Google Scholar]
- [30].Droubatchevskaia N, Wong MP, Chipperfield KM, et al. Guidelines for cryoprecipitate transfusion. BCMJ 2007;49:441–5. [Google Scholar]
- [31].Turan A, Yang D, Bonilla A, et al. Morbidity and mortality after massive transfusion in patients undergoing non-cardiac surgery. Can J Anaesth 2013;60:761–70. [DOI] [PubMed] [Google Scholar]
- [32].Liu Y, Lin J, Zhong L, et al. Blood transfusion practice: a survey in Sichuan, China. Transfus Apher Sci 2015;52:105–11. [DOI] [PubMed] [Google Scholar]
- [33].Stainsby D, MacLennan S, Hamilton PJ. Management of massive blood loss: a template guideline. Br J Anaesth 2000;85:487–91. [DOI] [PubMed] [Google Scholar]
- [34].Miller RD, Robbins TO, Tong MJ, et al. Coagulation defects associated with massive blood transfusions. Ann Surg 1971;174:794–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Kor DJ, Stubbs JR, Gajic O. Perioperative coagulation management-fresh frozen plasma. Best Pract Res Clin Anaesthesiol 2010;24:51–64. [DOI] [PubMed] [Google Scholar]
- [36].The Ministry of Health of the People's Republic of China. Specification for Clinical Transfusion Technology. 2000, 184th. [Google Scholar]
- [37].Hewitt PE, Machin SJ. ABC of transfusion. Massive blood transfusion. BMJ 1990;300:107–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Borgman MA, Spinella PC, Perkins JG, et al. The ratio of blood products transfused affects mortality in patients receiving massive transfusions at a combat support hospital. J Trauma 2007;63:805–13. [DOI] [PubMed] [Google Scholar]
- [39].Stansbury LG, Dutton RP, Stein DM, et al. Controversy in trauma resuscitation: do ratios of plasma to red blood cells matter? Transfus Med Rev 2009;23:255–65. [DOI] [PubMed] [Google Scholar]
- [40].Ronald DMiller. Massive blood transfusions: the impact of Vietnam military data on modern civilian transfusion medicine. Anesthesiology 2009;110:1412–6. [DOI] [PubMed] [Google Scholar]
- [41].Yuan S, Ferrell C, Chandler WL. Comparing the prothrombin time INR versus the APTT to evaluate the coagulopathy of acute trauma. Thromb Res 2007;120:29–37. [DOI] [PubMed] [Google Scholar]
- [42].Kozek-Langenecker S. Management of massive operative blood loss. Minerva Anestesiol 2007;73:401–15. [PubMed] [Google Scholar]
- [43].Rizoli SB, Boffard KD, Riou B, et al. Recombinant activated factor VII as an adjunctive therapy for bleeding control in severe trauma patients with coagulopathy: subgroup analysis from two randomized trials. Crit Care 2006;10:R178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Liumbruno G, Bennardello F, Lattanzio A, et al. Recommendations for the transfusion of plasma and platelets. Blood Transfus 2009;7:132–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Peiniger S, Nienaber U, Lefering R, et al. Balanced massive transfusion ratios in multiple injury patients with traumatic brain injury. Crit Care 2011;15:R68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Ho AM, Dion PW, Yeung JH, et al. Fresh-frozen plasma transfusion strategy in trauma with massive and ongoing bleeding. Common (sense) and sensibility. Resuscitation 2010;81:1079–81. [DOI] [PubMed] [Google Scholar]


