Abstract
Graves disease is the most common cause of thyrotoxicosis. Although medical intervention with antithyroid drugs (ATDs) is commonly the first choice of treatment in Korea, the remission rate associated with this approach is not satisfactory. During ATD therapy, low or undetectable serum levels of thyroid-stimulating hormone (TSH) receptor antibodies (TRAbs) have been reported to affect the incidence of Graves disease remission. This study evaluated the correlation between serum 25-hydroxyvitamin D levels and TRAb levels, as well as the effect of 25-hydroxyvitamin D on the recurrence of Graves disease.
A total of 143 patients, who were diagnosed with Graves disease and treated with ATDs, were retrospectively included in our observational study. These patients were followed for more than 1 year after ATD discontinuation. The levels of serum 25-hydroxyvitamin D and TRAb (ie, thyroid-stimulating antibody [TSAb], as detected by bioassay, and TSH-binding inhibitory immunoglobulins [TBIIs]) were measured, and a thyroid function test was performed upon ATD discontinuation. Recurrence was evaluated every 3 months, and was defined as an occurrence of overt thyrotoxicosis during the follow-up period.
A total of 95 patients (66.4%) experienced recurrence with a median latency period of 182 days (ranging 28–1219 days). The serum 25-hydroxyvitamin D levels at the time of ATD discontinuation were not correlated with either TBII or TSAb. In the Cox proportional hazard regression analysis, higher free T4 levels (>1.4 ng/dL; hazard ratio [HR], 3.252; 95% confidence interval [CI], 1.022–10.347) and low levels of 25-hydroxyvitamin D (≤14.23 ng/mL) were associated with a higher probability of Graves disease recurrence (HR, 3.016; 95% CI, 1.163–7.819).
Lower serum 25-hydroxyvitamin D levels were associated with a higher incidence of Graves disease recurrence. Therefore, serum 25-hydroxyvitamin D might be an independent risk factor for predicting Graves disease recurrence after ATD discontinuation.
Keywords: Graves disease, prognosis, recurrence, thyrotropin-binding inhibitory immunoglobulin, vitamin D deficiency
1. Introduction
Vitamin D (25-hydroxyvitamin D) is known to play essential roles in the metabolism of calcium, phosphorous, and bone. Recently, it has been shown that vitamin D is related to autoimmune diseases in addition to its classic effects on bone metabolism. Vitamin D deficiency has been associated with several autoimmune diseases, such as multiple sclerosis,[1] Crohn disease,[2] rheumatoid arthritis,[3] systemic lupus erythematosus,[4] and type 1 diabetes mellitus.[5] In addition, clinical forms of autoimmune thyroiditis, such as Graves disease and Hashimoto thyroiditis, have also been reported to be associated with vitamin D deficiency.[6] Some studies suggest that the prevalence of vitamin D deficiency is higher among individuals with autoimmune thyroid disease than among healthy controls.[6,7] Regarding the association between serum vitamin D levels and Hashimoto thyroiditis, Bozkurt et al reported that serum vitamin D levels were significantly lower in patients with Hashimoto thyroiditis and that the severity of vitamin D deficiency was correlated with the thyroid volume, antibody levels, and duration of Hashimoto thyroiditis.[8] In another study of premenopausal Korean women, lower serum vitamin D3 levels were associated with the positivity of antiperoxidase antibody (TPO-Ab).[9]
Regarding Graves disease, Yasuda et al reported that vitamin D levels were significantly lower in patients with Graves disease and negatively correlated with thyroid volume.[10] Additionally, another study found that serum vitamin D levels were significantly lower in patients without remission of Graves disease than in patients with remission, or in control participants[11]; however, no significant association between serum vitamin D levels and thyroid-stimulating hormone (TSH) receptor antibody (TRAb) titers in the nonremission group was identified.[11] In contrast, Zhang et al reported that a lower vitamin D status was associated with increased TRAb titers in 70 Graves disease patients.[12] Therefore, the association between serum vitamin D levels and TRAb titers is currently inconclusive. Additionally, the levels of vitamin D that are sufficient to regulate the immune response of Graves disease patients remain unclear.
Radioactive iodine (RAI) therapy, thyroidectomy, and antithyroid drugs (ATDs) have been shown to be effective and relatively safe for the initial treatment of Graves disease.[13] The results of a 2011 survey suggested that in the United States, 59.7% of clinical endocrinologists used RAI therapy for the primary treatment of Graves disease.[14] In contrast, ATD therapy has been the preferred primary treatment of Graves disease in Europe, Latin America, and Japan.[15] In Korea, the data suggest that 97.1% of clinical endocrinologists use ATD therapy as the primary treatment of choice for Graves disease.[16] If ATDs are chosen as the primary treatment option, medication should be continued for approximately 12 to 18 months and then considered discontinued if TSH and TRAb levels reach normality; however, another study indicated that remission of Graves disease was not achieved in 50% to 60% of patients treated with ATD therapy.[17] TRAb levels at the time of ATD discontinuation have been reported to be predictive of Graves disease relapse.[18]
Therefore, we aimed to examine the correlation between 25-hydroxyvitamin D levels at the time of ATD discontinuation and TRAb levels as well as the effect of 25-hydroxyvitamin D on Graves disease recurrence.
2. Methods
2.1. Study subjects
This study was approved by the Institutional Review Board of Chung-Ang University Hospital. We retrospectively identified 143 patients who were treated for Graves disease between March 2011 and April 2014 at Chung-Ang University Hospital, and who received at least 1 year of follow-up after ATD discontinuation. Of the included patients, 57 were newly diagnosed with Graves disease at our hospital, and 86 patients had been previously diagnosed with Graves disease and were referred to our hospital. The mean age was 39.4 years (range 16–71 years); there were 31 (21.5%) male subjects and 113 (78.5%) female subjects. All patients were treated with ATD, and we excluded patients treated with either RAI or thyroidectomy. We also excluded patients who were taking vitamin D supplements, pregnant, or diagnosed with cancer during the study period. Initial thyroid-stimulating antibody (TSAb) levels, as measured by a bioassay and TSH-binding inhibitory immunoglobulin (TBII) levels, were examined in 57 patients newly diagnosed with Graves disease.
ATD therapy was discontinued when total serum T3, free T4, and TSH levels were maintained within the normal range for more than 6 months with the following minimal maintenance doses of ATDs: methimazole ≤2.5 mg/d, carbimazole ≤5 mg/d, and propylthiouracil ≤50 mg/d. The levels of TBII, TSAb, and 25-hydroxyvitamin D at the time of discontinuation were measured for all study subjects. To adjust for seasonal differences in the 25-hydroxyvitamin D levels, we included in the models information regarding the season at the time of blood sampling. Following ATD discontinuation, the patients underwent routine thyroid function tests (TSH, free T4, and total T3 levels) and TBII measurements at 3-month intervals.
Recurrence was defined as concomitant suppression of TSH levels (less than 0.4 mU/L) and elevation of free T4 (>1.76 ng/dL) and/or T3 (>181 ng/dL) levels. Recurrence was determined by the date when thyroid function test was performed. Remission was defined as the maintenance of a normal thyroid status for at least 1 year after discontinuation of ATD therapy.
2.2. Laboratory measurements
Serum TSH (reference range, 0.55–4.78 mU/L; lower detection limit, 0.01 mU/L), free T4 (reference range, 0.89–1.76 ng/dL), and T3 (reference range, 60–181 ng/dL) were measured using a chemiluminescence immunoassay (Siemens Advia Centaur XP, Siemens Healthcare Diagnostics Inc, Tarrytown, NY). Sensitivity was 0.008 mU/L, 0.1 ng/dL, and 0.1 ng/mL, respectively. The inter-assay coefficients of variation (CV) were <5%, <5%, and <2%, and intra-assay CV were <5%, <4%, and <4%, respectively.
Levels of 25-hydroxyvitamin D (reference range of 4.2–150 ng/mL) were measured using a chemiluminescence immunoassay (Siemens Advia Centaur XP, Siemens Healthcare Diagnostics Inc, Tarrytown, NY). Sensitivity was 4.2 ng/mL. The inter- and intra-assay CV were ≤12% and ≤8%, respectively.
Antithyroglobulin and antimicrosomal antibody levels (reference range, 0–60 U/mL) were measured using a radioimmunoassay kit (B.R.A.H.M.S. GmbH, Hennigsdorf, Germany). Sensitivity was 5.5 U/mL. The inter-assay CV was <6% and <10% and intra-assay CV was <8% and <5%, respectively.
TBII levels (reference range, 0–1.75 IU/L) were evaluated using an automated electrochemiluminescence immunoassay kit (Elecsys Anti-TSHR, Roche Diagnostics, Mannheim, Germany). Sensitivity was 0.3 IU/L and inter- and intra-assay CV were <12% and <8%, respectively.
TSAb levels (reference range, 0%–140%) were measured by using a TSH receptor bioassay (Thyretain, Diagnostic Hybrids, Athens, OH). Sensitivity was 92% and inter- and intra-assay CV were <20% and <7%, respectively. Each assay was performed according to the manufacturer's instructions.
2.3. Thyroid volume estimation by ultrasonography
Thyroid ultrasonography was performed on 127 (89%) of the study subjects to evaluate, initially, the thyroid volume and number of nodules. The volume of each thyroid lobe was calculated using the following formula: Π/6(0.52)∗length∗width∗depth.[19]
2.4. Statistical analyses
Statistical analyses were performed using R (a free software program for statistical computing and graphics). Continuous variables were analyzed using 2-sample t tests to compare their distribution between the recurrence and remission groups and were expressed as the mean ± standard deviation (SD). Chi-square test was used to identify differences in the distribution of categorical variables. The correlations between serum 25-hydroxyvitamin D levels and the TBII and TSAb levels were examined using Spearman rank correlation coefficient. To identify risk factors for Graves disease recurrence, we performed univariate and multivariate analyses using the Cox proportional hazard model. Multivariate analysis included all potential risk factors for recurrence with a P value less than .2 in univariate analysis. Kaplan-Meier curves were calculated to measure cumulative rate of recurrence-free state. Using maximally selected rank statistics available in the maxstat package for R,[20] we calculated the cutoff values for free T4 (1.4 ng/dL) and 25-hydroxyvitamin D (14.23 ng/mL). To adjust for seasonal variations in 25-hydroxyvitamin D levels, we categorized patients into the following 4 seasonal groups based on the time of blood collection: group 1, June to August; group 2, September to November; group 3, December to February; and group 4, March to May.[21] A P value < .05 was considered statistically significant.
3. Results
3.1. Clinical characteristics of study subjects stratified by remission or recurrence of Graves disease
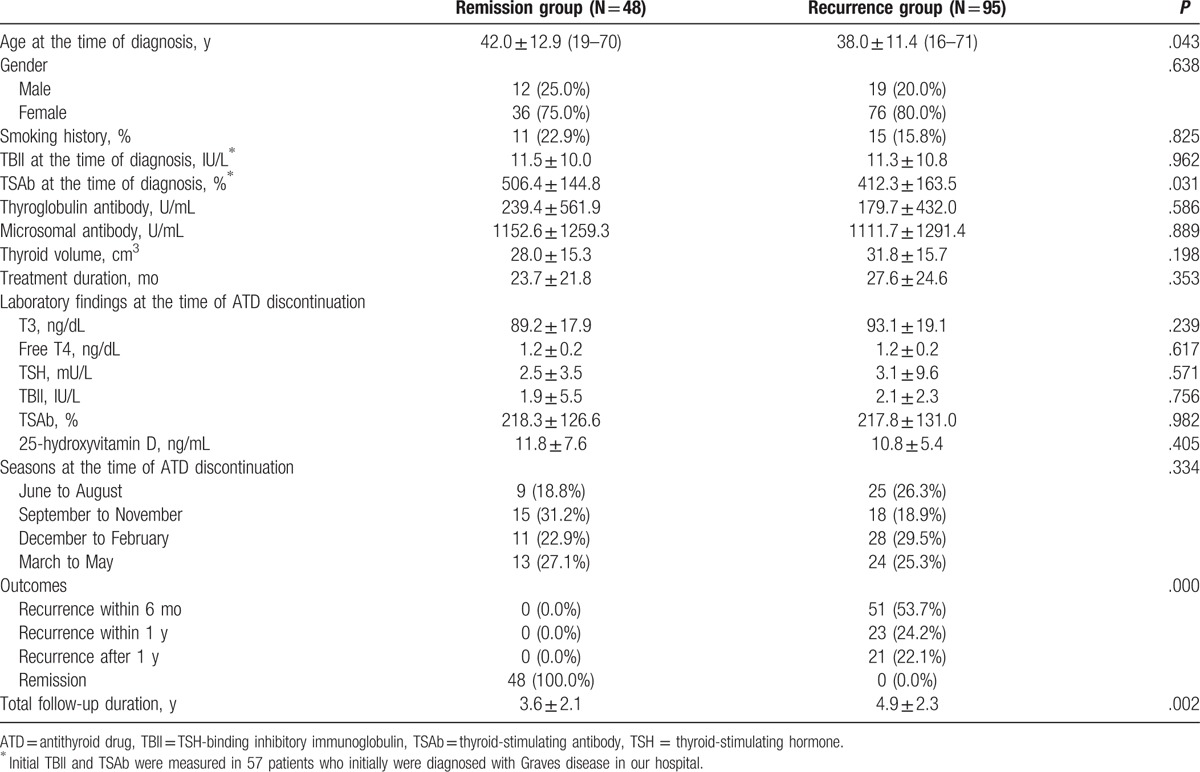
A total of 143 patients were followed, and recurrence occurred in 95 patients (66.4%) during the follow-up period. The median latency period prior to recurrence was 182 days (range 28–1219 days). Compared to members of the remission group (Table 1), members of the recurrence group were younger (38.0 ± 11.4 vs 42.0 ± 12.9 years, respectively; P = .043) and had lower initial TSAb levels (412.3 ± 163.5% vs 506.4 ± 144.8%, respectively; P = .031). The total follow-up period was longer in the recurrence group when compared to the remission group (4.9 ± 2.3 vs 3.6 ± 2.1 years, P = .002). However, the mean values of the thyroid function tests and the levels of TBII, TSAb, and 25-hydroxyvitamin D did not differ between the groups at the time of ATD discontinuation.
Table 1.
Clinical characteristics of study subjects.
3.2. Association between serum 25-hydroxyvitamin D levels and TBII and TSAb titers
The TBII and TSAb titers at the time of ATD discontinuation were not correlated with serum 25-hydroxyvitamin D levels (Fig. 1). In addition, when we compared the TBII and TRAb titers between the 3 groups of 25-hydroxyvitamin D levels (<10, 10–20, >20 ng/mL), no significant differences between the 25-hydroxyvitamin D levels and either the TBII or TSAb titers were identified (data not shown).
Figure 1.
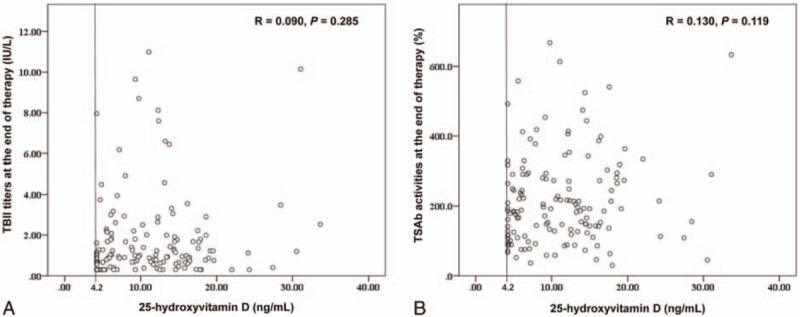
Correlation between serum 25-hydroxyvitamin D and TBII titers (A) or TSAb activities (B) at the end of therapy. TBII = TSH-binding inhibitory immunoglobulin, TSAb = thyroid-stimulating antibody.
3.3. Risk factors associated with Graves disease recurrence
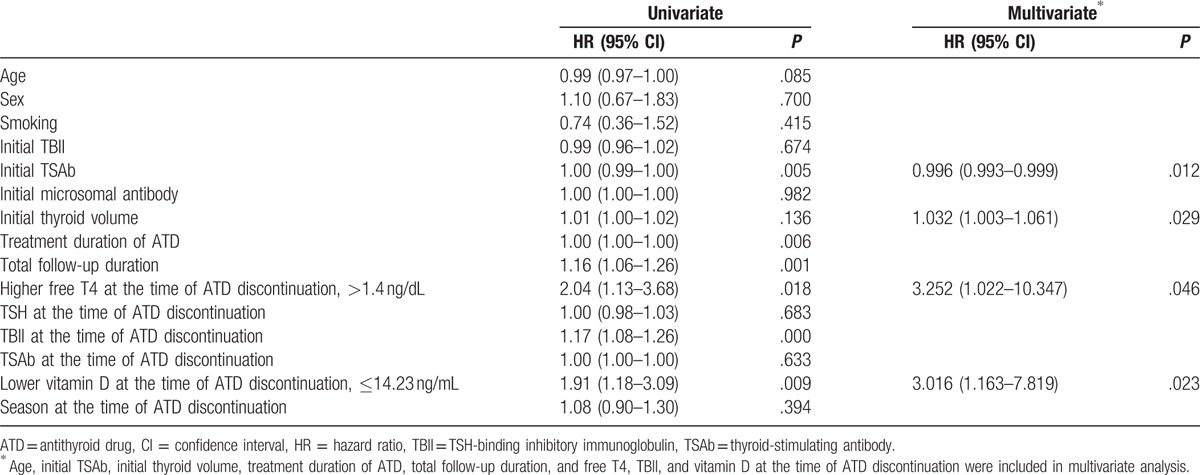
First, we constructed Cox proportional hazard regression models including all variables as continuous variables, except sex, smoking status, and season. In this model, high levels of free T4 at the time of ATD discontinuation were associated with Graves disease recurrence (hazard ratio [HR], 7.62; 95% confidence interval [CI], 1.11–52.60). In contrast, elevated 25-hydroxyvitamin D levels at the time of ATD discontinuation (HR, 0.933; 95% CI, 0.876–0.993) were identified as a protective factor for the recurrence of Graves disease. To examine the prognostic implications of the free T4 and 25-hydroxyvitamin D levels at the time of ATD discontinuation, we used the cutoff values calculated by using the maxstat package of R. In this analysis, the optimal cutoff points for 25-hydroxyvitamin D and free T4 were 14.23 ng/mL and 1.4 ng/dL, respectively (Fig. 2). Using these values, we performed additional Cox regression analyses. In the univariate analysis, higher TBII titers (>1.4 ng/dL; HR, 1.17; 95% CI, 1.08–1.26) and lower vitamin D levels (≤14.23 ng/mL; HR, 1.91; 95% CI, 1.18–3.09) at the time of ATD discontinuation were associated with the recurrence of Graves disease (Table 2). In multivariate analysis, when we used the stepwise backward elimination method, larger thyroid volumes (HR, 1.032; 95% CI, 1.003–1.061), higher free T4 levels (>1.4 ng/dL; HR, 3.252; 95% CI, 1.022–10.347), and lower vitamin D levels (≤14.23 ng/mL; HR, 3.016; 95% CI, 1.163–7.819) at the time of ATD discontinuation were identified as independent risk factors for the recurrence of Graves disease (Table 2).
Figure 2.
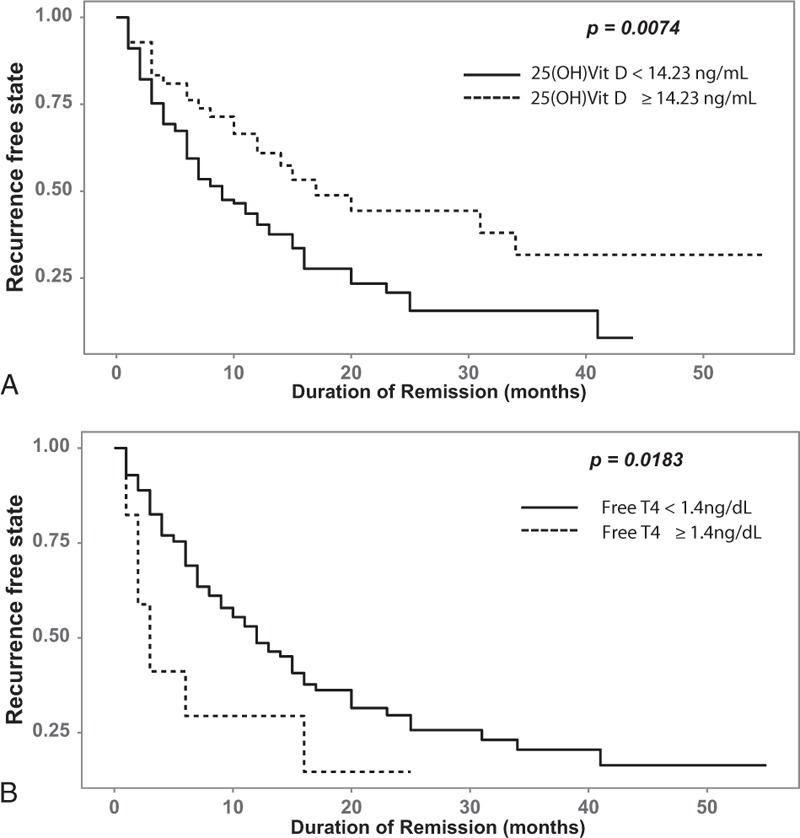
Kaplan-Meier curves and cutoff values of the recurrence-free state for 25-hydroxyvitamin D (A) and free T4 (B). 25(OH)Vit D = 25-hydroxyvitamin D.
Table 2.
Univariate and multivariate Cox proportional hazard models with backward elimination for predicting recurrence of Graves disease.
4. Discussion
In this study, we evaluated the correlation between 25-hydroxyvitamin D levels and TRAb titers as well as the effect of 25-hydroxyvitamin D on the recurrence of Graves disease. Serum 25-hydroxyvitamin D levels were not correlated with TRAb titers; however, when we used a cutoff value of 14.23 ng/mL, lower 25-hydroxyvitamin D levels at the time of ATD discontinuation were associated with higher rates of Graves disease recurrence.
Previous animal and small-scale human studies have evaluated the effect of vitamin D deficiency on the prognosis of Graves disease patients. Misharin et al investigated whether vitamin D deficiency could modulate Graves hyperthyroidism induced by thyrotropin receptor immunization in BALB/c mice and found that vitamin D-deficient BALB/c mice were more likely to develop persistent hyperthyroidism than mice with sufficient vitamin D levels.[22] In human studies, Graves disease patients receiving a 24-week course of combination treatment with methimazole and 1α-hydroxyvitamin D3 achieved normal thyroid function more rapidly than patients receiving methimazole alone.[23] In addition, Yasuda et al reported that compared with patients with Graves disease remission, serum 25-hydroxyvitamin D levels were significantly lower in patients without remission of Graves disease (18.2 ± 5.1 vs 14.5 ± 2.9 ng/mL, respectively; P < .005).[11] In addition, there was a review suggesting that vitamin D levels not only are related to the cause of Graves disease, but also may have a beneficial therapeutic effect on the remission of Graves disease.[24] Therefore, lower 25-hydroxyvitamin D levels might affect the recurrence of Graves disease.
Possible mechanisms underlying the association between lower 25-hydroxyvitamin D levels and the recurrence of Graves disease include the immunological modulatory effect of 25-hydroxyvitamin D, which is converted into calcitriol (an active form of vitamin D that binds vitamin D receptors [VDRs] by 1-alpha-hydroxylase [CYP27B1]).[25] Immune cells express both CYP27B1 and a degradation enzyme, 1,25-dihydroxyvitamin D3 24-hydroxylase (CYP24A1).[26] Additionally, nearly all immune cells, including T cells, B cells, and antigen-presenting cells (ie, macrophages and dendritic cells), express VDR.[27,28] Calcitriol inhibits the proliferation of Th1 cells as well as the expression of interleukin-2 (IL-2) and IFN-γ, whereas Th2 cell responses are promoted by calcitriol.[29] Additionally, calcitriol inhibits B cell proliferation, plasma cell differentiation, immunoglobulin secretion, and memory B cell generation.[30] In patients with Graves disease, B cells accumulate within the thyroid gland and secrete TRAb (IgG1 subclass)[31]; these subsequently increased levels of TRAb continuously stimulate thyroid cells and cause hyperthyroidism. Therefore, calcitriol may suppress the production of TRAb by suppressing B cells, thereby contributing to Graves disease remission.
Previous prospective studies reported that higher TRAb levels at the end of therapy, larger goiters, and smoking were identified as predictive factors for Graves disease relapse.[32,33] In our study, at the time of therapy discontinuation, large goiters, free T4 levels toward the upper normal limit (UNL), and lower vitamin D levels were significantly associated with the recurrence of Graves disease. These findings indicate that higher levels of free T4, even if the levels lie within the normal reference range, and vitamin D deficiency can be considered as independent risk factors for predicting Graves disease recurrence. High TBII titer at the time of ATD discontinuation was associated with the recurrence of Graves disease in univariate analysis. However, in our study, the risk of high TBII titer to the recurrence of Graves disease disappeared in multivariate analysis.
The current study has several limitations, the first of which is the retrospective design and the possibility of selection bias. All study subjects were retrospectively identified from one hospital (Chung-Ang University Hospital); and most lived in an urban area. Therefore, the patients included in our study may have been more likely to be vitamin D-deficient. In addition, since more severe patients were transferred to the university hospital, patients with a high risk for recurrence of Graves disease might have been included in our study group. Another limitation was that we could not consider the effects of polymorphisms of the VDR gene. A previous meta-analysis reported that in Asian populations, VDR gene polymorphisms such as ApaI, BsmI, and FokI were associated with Graves disease.[34] Lastly, serum 25-hydroxyvitamin D levels were measured 1 time during various seasons. To overcome this potential limitation, before performing the regression analysis, we divided the patients into 4 groups according to the season during which their blood was collected. Nevertheless, the results of our study have useful clinical implications in suggesting that, in addition to other known predictive factors such as large goiters and higher TRAb levels, vitamin D deficiency and free T4 levels toward the UNL might be considered as independent risk factors for predicting the outcome of Graves disease. These findings may affect clinical decision-making regarding the treatment of Graves disease. Based on our findings, vitamin D status should be evaluated regularly and, if vitamin D is insufficient, supplementation of vitamin D should be considered in patients with Graves disease.
In conclusion, lower serum 25-hydroxyvitamin D levels were associated with a higher incidence of Graves disease recurrence. Therefore, serum 25-hydroxyvitamin D levels might be an independent risk factor for predicting the outcome of patients with Graves disease after ATD discontinuation. To prove this hypothesis, future prospective studies are needed to determine the effect of vitamin D replacement on the recurrence of Graves disease.
Footnotes
Abbreviations: ATD = antithyroid drug, RAI = radioactive iodine, TBII = TSH-binding inhibitory immunoglobulin, TPO-Ab = antiperoxidase antibody, TRAb = thyroid-stimulating hormone (TSH) receptor antibody, TSAb = thyroid-stimulating antibody, TSH = thyroid-stimulating hormone, UNL = upper normal limit, VDR = vitamin D receptor.
The authors have no conflicts of interest to disclose.
References
- [1].Ascherio A, Munger KL, Simon KC. Vitamin D and multiple sclerosis. Lancet Neurol 2010;9:599–612. [DOI] [PubMed] [Google Scholar]
- [2].Cantorna MT. Vitamin D and its role in immunology: multiple sclerosis, and inflammatory bowel disease. Prog Biophys Mol Biol 2006;92:60–4. [DOI] [PubMed] [Google Scholar]
- [3].Merlino LA, Curtis J, Mikuls TR, et al. Vitamin D intake is inversely associated with rheumatoid arthritis: results from the Iowa Women's Health Study. Arthritis Rheum 2004;50:72–7. [DOI] [PubMed] [Google Scholar]
- [4].Kamen DL, Cooper GS, Bouali H, et al. Vitamin D deficiency in systemic lupus erythematosus. Autoimmun Rev 2006;5:114–7. [DOI] [PubMed] [Google Scholar]
- [5].Hypponen E, Laara E, Reunanen A, et al. Intake of vitamin D and risk of type 1 diabetes: a birth-cohort study. Lancet 2001;358:1500–3. [DOI] [PubMed] [Google Scholar]
- [6].Kivity S, Agmon-Levin N, Zisappl M, et al. Vitamin D and autoimmune thyroid diseases. Cell Mol Immunol 2011;8:243–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ma J, Wu D, Li C, et al. Lower serum 25-hydroxyvitamin D level is associated with 3 types of autoimmune thyroid diseases. Medicine 2015;94:e1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Bozkurt NC, Karbek B, Ucan B, et al. The association between severity of vitamin D deficiency and Hashimoto's thyroiditis. Endocr Pract 2013;19:479–84. [DOI] [PubMed] [Google Scholar]
- [9].Choi YM, Kim WG, Kim TY, et al. Low levels of serum vitamin D3 are associated with autoimmune thyroid disease in pre-menopausal women. Thyroid 2014;24:655–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Yasuda T, Okamoto Y, Hamada N, et al. Serum vitamin D levels are decreased and associated with thyroid volume in female patients with newly onset Graves’ disease. Endocrine 2012;42:739–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Yasuda T, Okamoto Y, Hamada N, et al. Serum vitamin D levels are decreased in patients without remission of Graves’ disease. Endocrine 2013;43:230–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Zhang H, Liang L, Xie Z. Low vitamin D status is associated with increased thyrotropin-receptor antibody titer in Graves’ disease. Endocr Pract 2015;21:258–63. [DOI] [PubMed] [Google Scholar]
- [13].Klein I, Becker DV, Levey GS. Treatment of hyperthyroid disease. Ann Intern Med 1994;121:281–8. [DOI] [PubMed] [Google Scholar]
- [14].Burch HB, Burman KD, Cooper DS. A 2011 survey of clinical practice patterns in the management of Graves’ disease. J Clin Endocrinol Metab 2012;97:4549–58. [DOI] [PubMed] [Google Scholar]
- [15].Wartofsky L, Glinoer D, Solomon B, et al. Differences and similarities in the diagnosis and treatment of Graves’ disease in Europe, Japan, and the United States. Thyroid 1991;1:129–35. [DOI] [PubMed] [Google Scholar]
- [16].Moon JH, Yi KH. The diagnosis and management of hyperthyroidism in Korea: consensus report of the Korean thyroid association. Endocrinol Metab 2013;28:275–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Abraham P, Avenell A, Park CM, et al. A systematic review of drug therapy for Graves’ hyperthyroidism. Eur J Endocrinol 2005;153:489–98. [DOI] [PubMed] [Google Scholar]
- [18].Ross DS, Burch HB, Cooper DS, et al. 2016 American Thyroid Association guidelines for diagnosis and management of hyperthyroidism and other causes of thyrotoxicosis. Thyroid 2016;26:1343–421. [DOI] [PubMed] [Google Scholar]
- [19].Knudsen N, Bols B, Bulow I, et al. Validation of ultrasonography of the thyroid gland for epidemiological purposes. Thyroid 1999;9:1069–74. [DOI] [PubMed] [Google Scholar]
- [20].Hothorn T, Lausen B. Maximally selected rank statistics in R. R News 2002;2:3–5. [Google Scholar]
- [21].Kim JR, Kim BH, Kim SM, et al. Low serum 25 hydroxyvitamin D is associated with poor clinicopathologic characteristics in female patients with papillary thyroid cancer. Thyroid 2014;24:1618–24. [DOI] [PubMed] [Google Scholar]
- [22].Misharin A, Hewison M, Chen CR, et al. Vitamin D deficiency modulates Graves’ hyperthyroidism induced in BALB/c mice by thyrotropin receptor immunization. Endocrinology 2009;150:1051–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kawakami-Tani T, Fukawa E, Tanaka H, et al. Effect of 1 alpha-hydroxyvitamin D3 on serum levels of thyroid hormones in hyperthyroid patients with untreated Graves’ disease. Metabolism 1997;46:1184–8. [DOI] [PubMed] [Google Scholar]
- [24].Rotondi M, Chiovato L. Vitamin D deficiency in patients with Graves’ disease: probably something more than a casual association. Endocrine 2013;43:3–5. [DOI] [PubMed] [Google Scholar]
- [25].Bikle D. Nonclassic actions of vitamin D. J Clin Endocrinol Metab 2009;94:26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Peelen E, Knippenberg S, Muris AH, et al. Effects of vitamin D on the peripheral adaptive immune system: a review. Autoimmun Rev 2011;10:733–43. [DOI] [PubMed] [Google Scholar]
- [27].Provvedini DM, Tsoukas CD, Deftos LJ, et al. 1,25-dihydroxyvitamin D3 receptors in human leukocytes. Science 1983;221:1181–3. [DOI] [PubMed] [Google Scholar]
- [28].Griffin MD, Lutz W, Phan VA, et al. Dendritic cell modulation by 1alpha,25-dihydroxyvitamin D3 and its analogs: a vitamin D receptor-dependent pathway that promotes a persistent state of immaturity in vitro and in vivo. Proc Natl Acad Sci USA 2001;98:6800–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Baeke F, Takiishi T, Korf H, et al. Vitamin D: modulator of the immune system. Curr Opin Pharmacol 2010;10:482–96. [DOI] [PubMed] [Google Scholar]
- [30].Chen S, Sims GP, Chen XX, et al. Modulatory effects of 1,25-dihydroxyvitamin D3 on human B cell differentiation. J Immunol 2007;179:1634–47. [DOI] [PubMed] [Google Scholar]
- [31].Weetman AP, Yateman ME, Ealey PA, et al. Thyroid-stimulating antibody activity between different immunoglobulin G subclasses. J Clin Invest 1990;86:723–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Schleusener H, Schwander J, Fischer C, et al. Prospective multicentre study on the prediction of relapse after antithyroid drug treatment in patients with Graves’ disease. Acta Endocrinol 1989;120:689–701. [DOI] [PubMed] [Google Scholar]
- [33].Nedrebo BG, Holm PI, Uhlving S, et al. Predictors of outcome and comparison of different drug regimens for the prevention of relapse in patients with Graves’ disease. Eur J Endocrinol 2002;147:583–9. [DOI] [PubMed] [Google Scholar]
- [34].Zhou H, Xu C, Gu M. Vitamin D receptor (VDR) gene polymorphisms and Graves’ disease: a meta-analysis. Clin Endocrinol 2009;70:938–45. [DOI] [PubMed] [Google Scholar]


