Abstract
Rationale:
Intracranial infection with Acinetobacter baumannii is a tough problem due to the presence of multiresistance and drugs poor penetration through the blood brain barrier (BBB). Tigecycline is effective to cure A baumannii, but it can only be used intravenously which is also difficult to pass BBB. So, it will be a breakthrough if intraventricular (IVT) tigecycline is used in the clinical therapy. However, this treatment has been reported quite rarely until now.
Patient concerns:
We described a case of a 50-year-old male worker whose clinical futures were high fever and cerebral rigidity after neurosurgery.
Diagnoses:
Intracranial infection with extensive drug resistant (XDR) A baumannii.
Interventions:
The patient was treated with IVT tigecycline.
Outcomes:
The symptoms of intracranial infection disappeared. The temperature of this patient decreased to normal and cerebral rigidity disappeared. The cerebrospinal fluid culture became negative, with normal levels of white blood cell, glucose and chlorine.
Lessons:
IVT tigecycline therapy maybe effective to intracranial infection with XDR A baumannii. However, more studies will further demonstrate the therapeutic values of IVT tigecycline to intracranial infection, and not only restricted to A baumannii infections.
Keywords: intracranial infection, intraventricular, tigecycline
1. Introduction
Acinetobacter baumannii is a kind of aerobic and gram-negative bacillus, and it has been increasingly involved as a cause of hospital-acquired infections and resulted in high morbidity and mortality rates.[1] During the recent years, A baumannii of multidrug resistance (MDR) and pandrug resistance has increased worldwidely because of the abuse of antibiotics and various mechanisms of resistance.[2] Postneurosurgical A baumannii infection is not rare in hospital which is usually MDR, even pandrug resistance to carbapenems, compound sulfamethoxazole and sulbactam.[3,4] Treatment of those infections is a tough problem due to the presence of multiresistance and drugs poor penetration through the blood brain barrier (BBB). Recent studies revealed that intraventricular (IVT) colistin therapy had been a reliable choice for mutiresistant Acinetobacter meningitis,[5] but the neurotoxicity limited its application, with a high incidence of 21.7%.[6] Although tigecycline showed a marvelous activity against multiresistant A baumannii in vitro, it has a poor penetration through BBB resulting in bad effect to the intracranial infection with A baumannii.[7] Besides, tigecycline can only be used as an intravenous drug at present. So, it will be a breakthrough if IVT tigecycline is used in clinical therapy. Until now, this treatment has been reported quite rarely. Here, we described a case infected with postneurosurgical extensive drug resistant (XDR) A baumannii and was successfully cured by IVT tigecycline therapy.
2. Case report
A male patient, 50 years old, accepted the operation of hematoma removal of the frontal and temporal lobes and decompression in local medical facility because of craniocerebral injury from a falling accident. And he was transferred to our hospital on September 12th, 2016 due to the high fever after surgery. The computed tomography imaging after admission was shown in Fig. 1. Then he was diagnosed as pneumonia and treated with meropenem. Later, the antibiotic was changed to tigecycline because the sputum culture showed he was infected with XDR A baumannii. The dose was 100 mg/day (50 mg twice a day for 10 days) and his condition improved gradually. However, a sudden onset of high fever came to this patient and his body temperature was 40 °C on October 13th, with symptoms of cerebral rigidity. Meanwhile, the laboratory tests for cerebrospinal fluid (CSF) showed a high level of white blood cell and a low level of both glucose and chlorine (Table 1). His CSF culture implied XDR A baumannii infection which was only sensitive to tigecycline (Table 2). So, intracranial infection was diagnosed, given his clinical manifestations and laboratory test results. In view of poor BBB permeability of tigecycline, off-label IVT injection was considered as an effective way to cure this patient. So, therapeutic schedule of IVT tigecycline was carried out after permission from the hospital ethics committee and his family members. We injected 3 mg tigecycline into the ventricular system every day from October 16th, closed the drainage tube for about 1 hour and open it again. Meanwhile, intravenous tigecycline (100 mg twice a day) and cefoperazone–sulbactam (3 g twice a day) were also added. The treatment was well tolerated by this patient. His fever improved 6 days later but the CSF culture was still positive. So, we added the dose of IVT tigecycline up to 4 mg twice a day and the CSF culture became negative 3 days later, with normal levels of white blood cell, glucose, and chlorine. At the same time, the temperature decreased to 37.2 °C and symptoms of cerebral rigidity disappeared. The CSF cultures were all negative for the next 3 days, which meant the intracranial infection was effectively cured. (The appearances of CSF before and after treatment of IVT tigecycline are shown in Fig. 2, and the laboratory tests for CSF in the period of treatment are shown in Table 1). After that, the IVT tigecycline therapy was stopped. Intravenous antibiotics were also discontinued 5 days later, and the patient was transferred to the rehabilitation unit to get the functional restoration.
Figure 1.
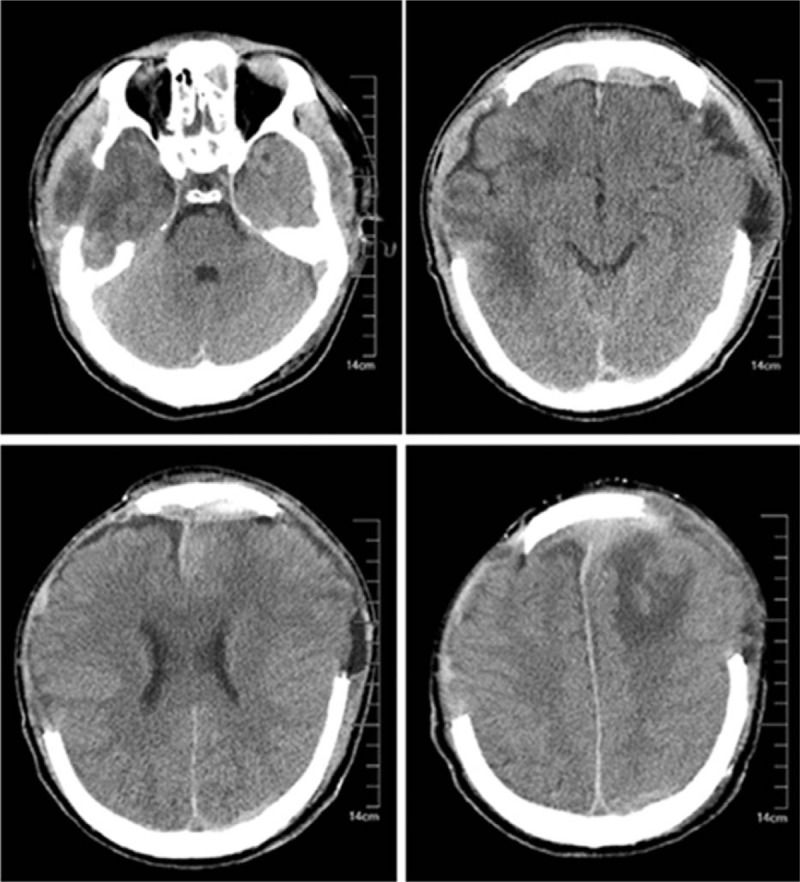
Computed tomography imaging after admission.
Table 1.
Laboratory tests for CSF in the period of treatment and follow-up.
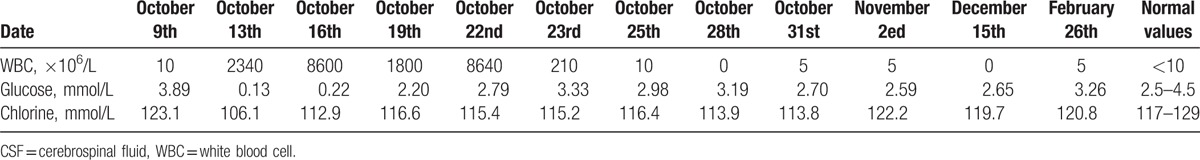
Table 2.
Antibiotics susceptibility tests for Acinetobacter baumannii in CSF.
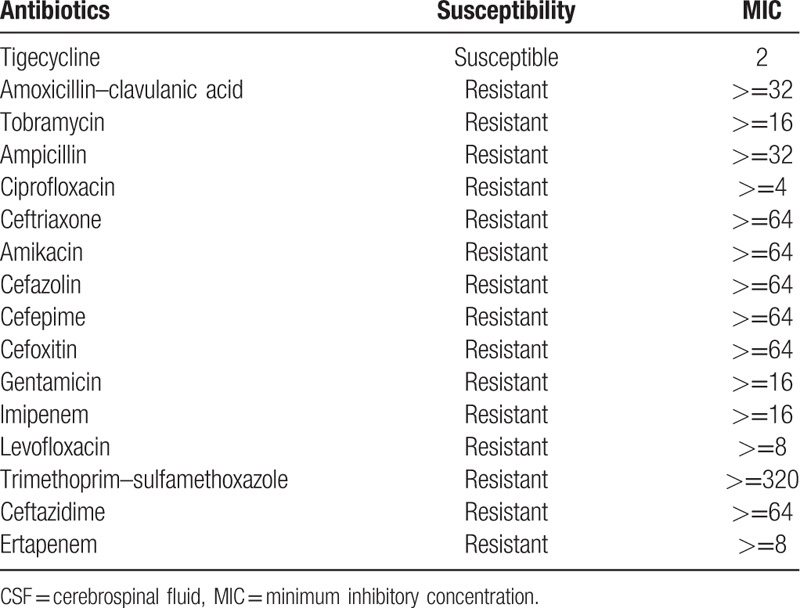
Figure 2.
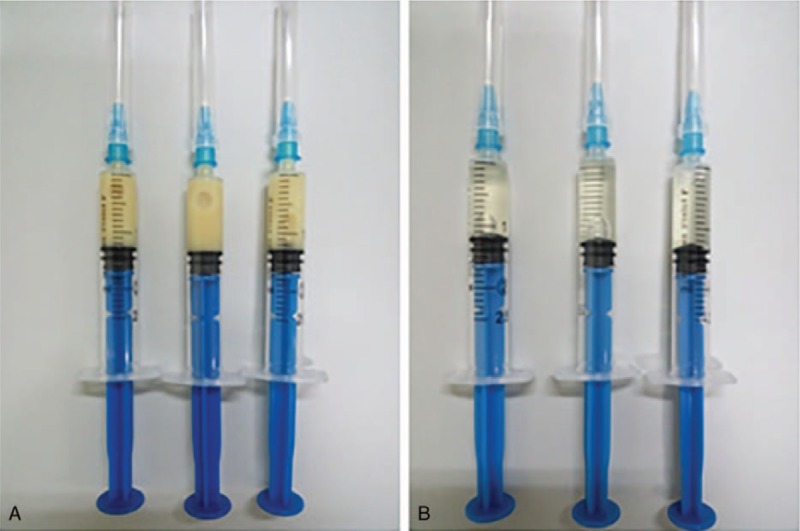
Appearance of CSF before and after treatment of IVT tigecycline. (A) Before treatment (October 13th, 2016). (B) After treatment (November 2nd, 2016). CSF = cerebrospinal fluid, IVT = intraventricular.
3. Follow-up
The patient was still in coma at 4 months of follow-up without symptoms of intracranial infection. Repeated CSF cultures were all negative and no radiological signs of neuroinflammation existed. The laboratory tests for CSF of follow-up (December 15th, 2016 and February 26th, 2017) are shown in Table 1.
4. Discussion
We reported a patient of intracranial infection with XDR A baumannii after the neurosurgery who was cured by the IVT tigecycline therapy. We found that IVT tigecycline was safe and effective for XDR A baumannii intracranial infection.
A baumannii intracranial infection with MDR or XDR has become a clinical challenge in recent years, especially the cases followed by neurological surgery with a mortality more than 15%.[4] Patients of intracranial infection have a poor outcome with mortality of up to 71%.[8] Many antibiotics have a considerable antibacterial activity to A baumannii in vitro, but they cannot reach the minimal inhibitory concentration in brain due to the low penetration to BBB. Hence, IVT antibiotics therapy for intracranial infections becomes a hot topic. Many studies revealed that IVT colistin could successfully treat patients with XDR A baumannii meningitis. However, the neurotoxicity of colistin restricted its further application.[5,6,9,10]
Tigecycline, a semisynthetic modified minocycline, is the first clinically available drug of glycylcyclines. Experimental studies have shown that it has an excellent antibacterial activity to a broad spectrum of MDR gram-positive and gram-negative bacteria in vitro.[11] Otherwise, tigecycline has a synergy effect with many other antimicrobials agents.[7] In the clinical work, intravenous tigecycline did not show an appealing effect for intracranial infection because of poor BBB penetration. We consider that IVT tigecycline maybe a potential choice for patients because no neurotoxic side effects were found in our case.
In our case, the total course of IVT tigecycline treatment lasted for 2 weeks, and no obvious side effects were observed. The initial dose was 3 mg per day and then was added to 4 mg twice a day. We assumed that the course of treatment might be even shorter if we had started with a comparatively higher level of IVT tigecycline. In the following 4 months, no symptoms of intracranial infection happened to this patient. We revealed that IVT tigecycline might be a potential choice for intracranial infection, especially for MDR or XDR patients. However, only 1 patient was included in our case report, so we cannot predict the accurate rate of efficacy and its side effects. More studies will further demonstrate the therapeutic values of IVT tigecycline to intracranial infection, and not only restricted to A baumannii infection.
5. Conclusion
IVT tigecycline therapy maybe effective to intracranial infection with MDR or XDR A baumannii.
Footnotes
Abbreviations: BBB = blood brain barrier, CSF = cerebrospinal fluid, IVT = intraventricular, MDR = multidrug resistance, XDR = extensive drug resistant.
Y-QF and R-CZ are the cofirst authors.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Antunes LC, Visca P, Towner KJ. Acinetobacter baumannii: evolution of a global pathogen. Pathog Dis 2014;71:292–301. [DOI] [PubMed] [Google Scholar]
- [2].Neonakis IK, Spandidos DA, Petinaki E. Confronting multidrug-resistant Acinetobacter baumannii: a review. Int J Antimicrob Agents 2011;37:102–9. [DOI] [PubMed] [Google Scholar]
- [3].Cascio A, Conti A, Sinardi L, et al. Post-neurosurgical multidrug-resistant Acinetobacter baumannii meningitis successfully treated with intrathecal colistin. A new case and a systematic review of the literature. Int J Infect Dis 2010;14:e572–9. [DOI] [PubMed] [Google Scholar]
- [4].Karageorgopoulos DE, Falagas ME. Current control and treatment of multidrug-resistant Acinetobacter baumannii infections. Lancet Infect Dis 2008;8:751–62. [DOI] [PubMed] [Google Scholar]
- [5].Piparsania S, Rajput N, Bhatambare G. Intraventricular polymyxin B for the treatment of neonatal meningo-ventriculitis caused by multi-resistant Acinetobacter baumannii--case report and review of literature. Turk J Pediatr 2012;54:548–54. [PubMed] [Google Scholar]
- [6].Karaiskos I, Galani L, Baziaka F, et al. Intraventricular and intrathecal colistin as the last therapeutic resort for the treatment of multidrug-resistant and extensively drug-resistant Acinetobacter baumannii ventriculitis and meningitis: a literature review. Int J Antimicrob Agents 2013;41:499–508. [DOI] [PubMed] [Google Scholar]
- [7].Entenza JM, Moreillon P. Tigecycline in combination with other antimicrobials: a review of in vitro, animal and case report studies. Int J Antimicrob Agents 2009;34:1–8. [DOI] [PubMed] [Google Scholar]
- [8].Kim BN, Peleg AY, Lodise TP, et al. Management of meningitis due to antibiotic-resistant Acinetobacter species. Lancet Infect Dis 2009;9:245–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Khawcharoenporn T, Apisarnthanarak A, Mundy LM. Intrathecal colistin for drug-resistant Acinetobacter baumannii central nervous system infection: a case series and systematic review. Clin Microbiol Infect 2010;16:888–94. [DOI] [PubMed] [Google Scholar]
- [10].Ng J, Gosbell IB, Kelly JA, et al. Cure of multiresistant Acinetobacter baumannii central nervous system infections with intraventricular or intrathecal colistin: case series and literature review. J Antimicrob Chemother 2006;58:1078–81. [DOI] [PubMed] [Google Scholar]
- [11].Kaewpoowat Q, Ostrosky-Zeichner L. Tigecycline: a critical safety review. Expert Opin Drug Saf 2015;14:335–42. [DOI] [PubMed] [Google Scholar]


