Abstract
To date, because of their rarity, the clinicopathological features and surgical outcomes of small bowel adenocarcinomas (SBAs) have been insufficiently explored. We evaluated the clinicopathological features and long-term outcomes of patients who underwent surgery for SBA.
This retrospective study (from 1999 to 2016) examined patients with SBA treated surgically at the China National Cancer Center/Cancer Hospital. Clinicopathological features, preoperative evaluation, surgical treatment, and outcome parameters were reviewed and analyzed.
Among the 241 patients studied, pancreaticoduodenectomies were performed in 51.0%, partial resection in 24.5%, palliative bypass surgery in 23.7%, and abdominal exploration in 0.8% of the patients. Majority of the patients were diagnosed at an advanced disease stage, and the duodenum was the most common tumor site. Postoperative complications occurred in 44.4% of the patients. Median overall and progression-free survival rates were 22.0 and 13.0 months, respectively. The 5-year overall and progression-free survival rates for patients with duodenal adenocarcinoma were 30.2% and 21.7%, respectively. Duodenal adenocarcinomas, lymph node metastases, distant metastases, poor differentiation, and lymphovascular invasion were associated with poor overall survival outcomes. The 3 factors associated with progression-free survival were the degree of differentiation, lymph node metastases, and distant metastases.
Surgery remains the mainstay of treatment for SBA. A poor prognosis could be owing to the site, metastasis, differentiation, and lymphovascular invasion; however, the prognosis may improve through early diagnosis and operation.
Keywords: overall survival, pathological feature, progression-free survival, small bowel adenocarcinoma, surgical treatment
1. Introduction
In our previous study,[1] we summarized the characteristics and outcomes of small bowel tumor as a whole, and here we summarize small bowel adenocarcinoma (SBA) in specific.
The incidence of small bowel malignancies is on the rise in recent years, but such malignancies remain relatively rare, accounting for only 1% to 3% of all gastrointestinal tumors.[2–4] Among small bowel malignancies, adenocarcinoma is the most common type,[5] followed by carcinoid tumors, lymphomas, and sarcomas.[6,7] Although the small bowel accounts for 70% to 80% of the total length and 90% of the surface of the gastrointestinal tract, SBA is 40 to 50 times less common than colorectal carcinoma.[8] SBA is most commonly located in the duodenum, with a decline in frequency toward the distal parts.[9]
Symptoms of SBA are often insidious and nonspecific, with nearly half the patients presenting with abdominal pain,[10] and current imaging examinations are nonspecific and lack evidence. These features result in troublesome diagnosis with a long latency period.[9] Despite increasing advances in imaging examinations in recent years, the early detection of SBA remains a big challenge reflected by the facts that majority of the patients with SBA are at the advanced stage when diagnosed.[5,11] This situation had a negative effect on the survival outcome.
For the treatment of SBA, surgery remains the mainstay strategy, wherein the surgical techniques differ with respect to the site and staging. However, even when treated with radical resection (R0) and adequate lymphadenectomy, the over 5-year survival rate remains poor (approximately 25%).[9] Previous studies revealed several independent prognostic factors indicating a poor outcome, including higher age; distal tumor sites (i.e., jejunum and ileum); increased tumor, node, and metastasis (TNM) stages; and lymph node metastasis.[7,10,12–14]
Owing to the rarity of these tumors, there is an ongoing lack of sufficient data to adequately characterize this patient population specially. A high-volume population report on this disease was presented by Halfdanarson et al in the Mayo Clinic,[10] which summarized 491 cases. In the present study, we made a comprehensive analysis of 241 consecutive patients with SBA and share our experience with SBA surgical treatments at a high-volume center in China. Although the present study included fewer cases, it summarized more data that were not mentioned in Halfdanarson et al's study,[10] including more detailed basic characteristic information (such as life style, basic diseases, and laboratory tests), more detailed tumor information (such as tumor size, degree of differentiation, lymphovascular invasion, perineural invasion, detail information on lymph metastasis, and genetic mutation), and more detailed surgical information (such as surgical time, resection margin, blood loss and transfusion, length of hospital stay, complication, metastasis, and recurrence at follow-up).
2. Methods
The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki (6th revision, 2008) and was revised and approved by the ethical committee of the China National Cancer Center.
A database of all patients with histologically verified adenocarcinomas of the small intestine who were diagnosed and operated on at the Department of Pancreatic and Gastric Surgery, China National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College between January 1999 and November 2016 was established. Histological or cytological confirmation was available for all patients. All tumors other than primary adenocarcinoma of the small intestine were excluded.
Trained investigators collected information from medical records, including sociodemographic characteristics, anthropometric measures, lifestyle habits, personal history of selected medical conditions, family history of selected cancers, symptoms, laboratory tests, imaging examinations, surgical and perioperative data, and pathological examinations. Vital status and progress information were ascertained by 2 methods: looking for medical records and making phone calls. Information on cause(s) of death was also collected. The TNM staging of tumors was adapted from the American Joint Committee on Cancer Cancer Staging Manual, 7th Edition (2010).[15]
2.1. Statistical analysis
Continuous data are presented as mean ± standard deviation/median and range. Continuous variables between different groups were compared using the t test and Mann–Whitney U test. Categorical data are expressed by frequencies and ratios. Discontinuous variables between different groups were compared using the χ2 test or Fisher exact test. Ranked data between different groups were compared using the Kruskal–Wallis test.
Overall survival (OS) and progression-free survival (PFS) rates were analyzed using the Kaplan–Meier product limit survival curve estimates and log-rank tests for comparison between groups. Survival curves include all SBA patients who underwent surgery at our center. OS was defined as the time from the date of surgery to the date of the end of follow-up or death. PFS was defined as the time from the date of surgery to the date of the end of follow-up, death, or progression. Survival times are expressed as median/mean ± standard error. Independent factors were identified through multivariate analysis using Cox proportional hazard analysis.
A 2-sided P value of <.05 was considered statistically significant. Statistical calculations were performed using IBM SPSS Statistics 20.0 (SPSS, Inc, Chicago, IL).
3. Results
A total of 241 patients (160 males and 81 females) were diagnosed with primary SBA at a median age of 58 years (range, 23–79 years) and underwent surgery between January 1999 and November 2016 at the Department of Pancreatic and Gastric Surgery, China National Cancer Center. Median follow-up was 14 months (range, 1–106 months). The duodenum was the most common site of tumor (n = 199; 82.6%; Table 1). All cases were solitary except 5 (all adenocarcinomas, 2 of which occurred at the second portion of the duodenum, D2, and 3 in the jejunum).
Table 1.
Basic characteristics and preoperative tests of patients of small intestinal adenocarcinomas.
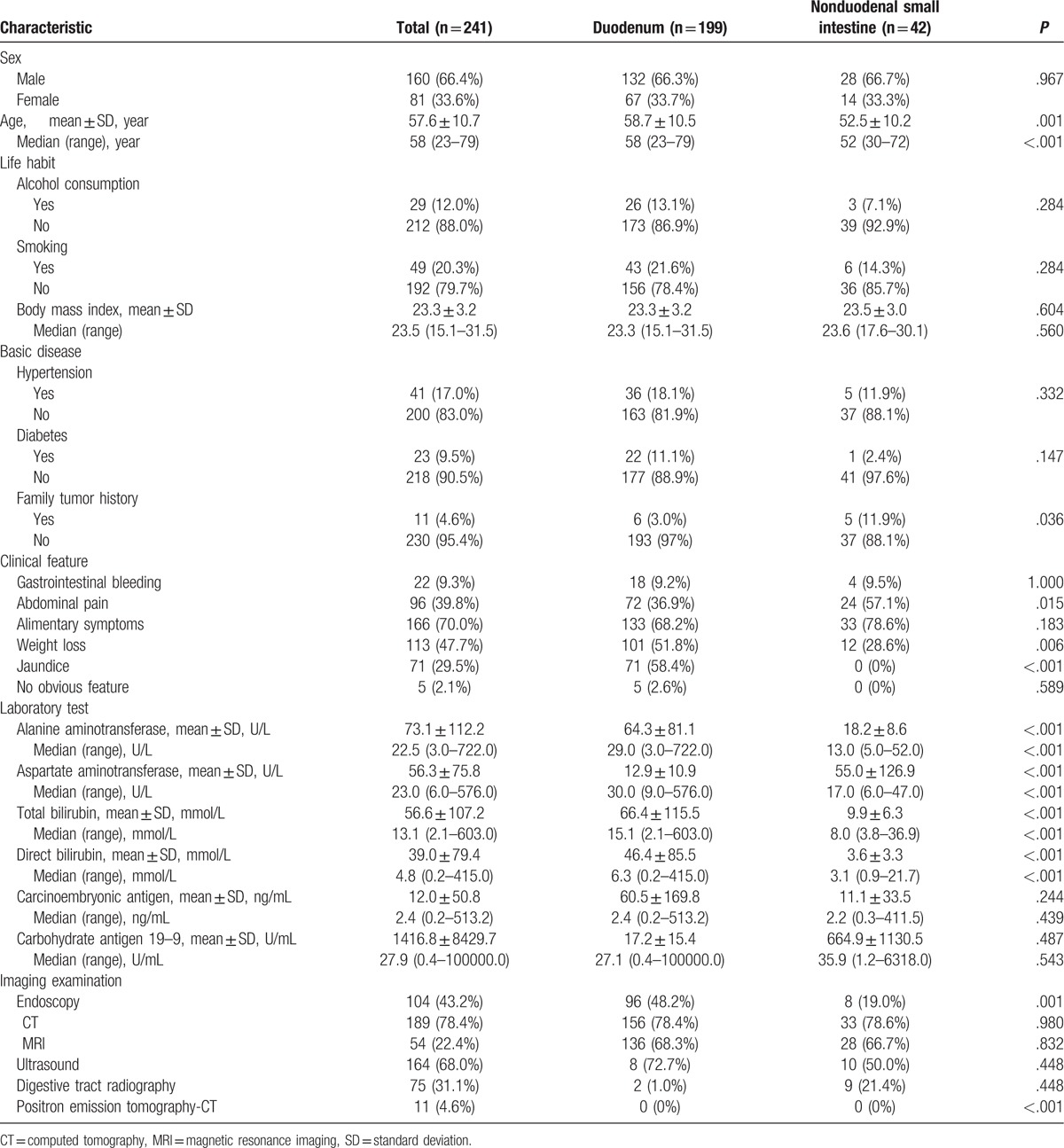
Patients with duodenal adenocarcinomas were older than those with nonduodenal small intestinal adenocarcinomas (P < .001). There was no significant difference between different sites in terms of sex, smoking, alcohol consumption, body mass index, hypertension, and diabetes. Family history of tumors differed between these 2 groups (P = .036), with the rate being slightly lower in the group with a history of duodenal carcinoma (Table 1). Four patients had celiac disease, 2 had Crohn disease, and only 1 had hereditary cancer syndromes. None of them had Meckel diverticulum or intestinal duplication. Alimentary symptoms were frequently noted at initial admission (n = 166; 70.0%), including nausea, vomiting, hiccups, and anorexia. Weight loss was documented in 113 cases (47.7%). Other common symptoms included abdominal pain (96, 39.8%), jaundice (71, 29.5%), and gastrointestinal bleeding (22, 9.3%). Five patients (2.1%) showed no symptoms. Jaundice, weight loss, and abdominal pain occurred more frequently in those with duodenal than in those with non-duodenal small intestinal tumors (P < .05; Table 1). Initial diagnosis was determined mainly by computed tomography (CT), ultrasound, and endoscopy (78.4%, 68.0%, and 43.2%, respectively; Table 1). In laboratory tests, transaminase and bilirubin levels were significantly higher in patients with duodenal adenocarcinomas than in those with tumors at other sites (P < .001). Pathological carbohydrate antigen 19–9 (CA 19–9) values were measured in 91 of the 190 patients (median, 27.9 U/mL), and an increased carcinoembryonic antigen (CEA) level was observed in only 39 of 194 patients (median, 2.4 ng/mL). There were no significant differences between the 2 groups in terms of tumor markers (Table 1).
The median size of the small intestinal adenocarcinomas was 4.0 cm (range, 1.0–20.0 cm), and nonduodenal small intestinal tumors were significantly larger than the duodenal ones (P = .001). Histopathologically, adenocarcinomas were classified into well- (n = 29; 13.0%), high–middle (n = 20; 9.0%), moderately (n = 88; 39.5%), middle–low (n = 35; 15.7%), and poorly (n = 51; 22.9%) differentiated. Tumor thrombus and perineural invasion occurred in 22.4% and 14.6%, respectively, among all small intestinal adenocarcinomas, and no significant difference was found between the 2 sites (P > .05; Table 2).
Table 2.
Pathological features of small intestinal adenocarcinomas.
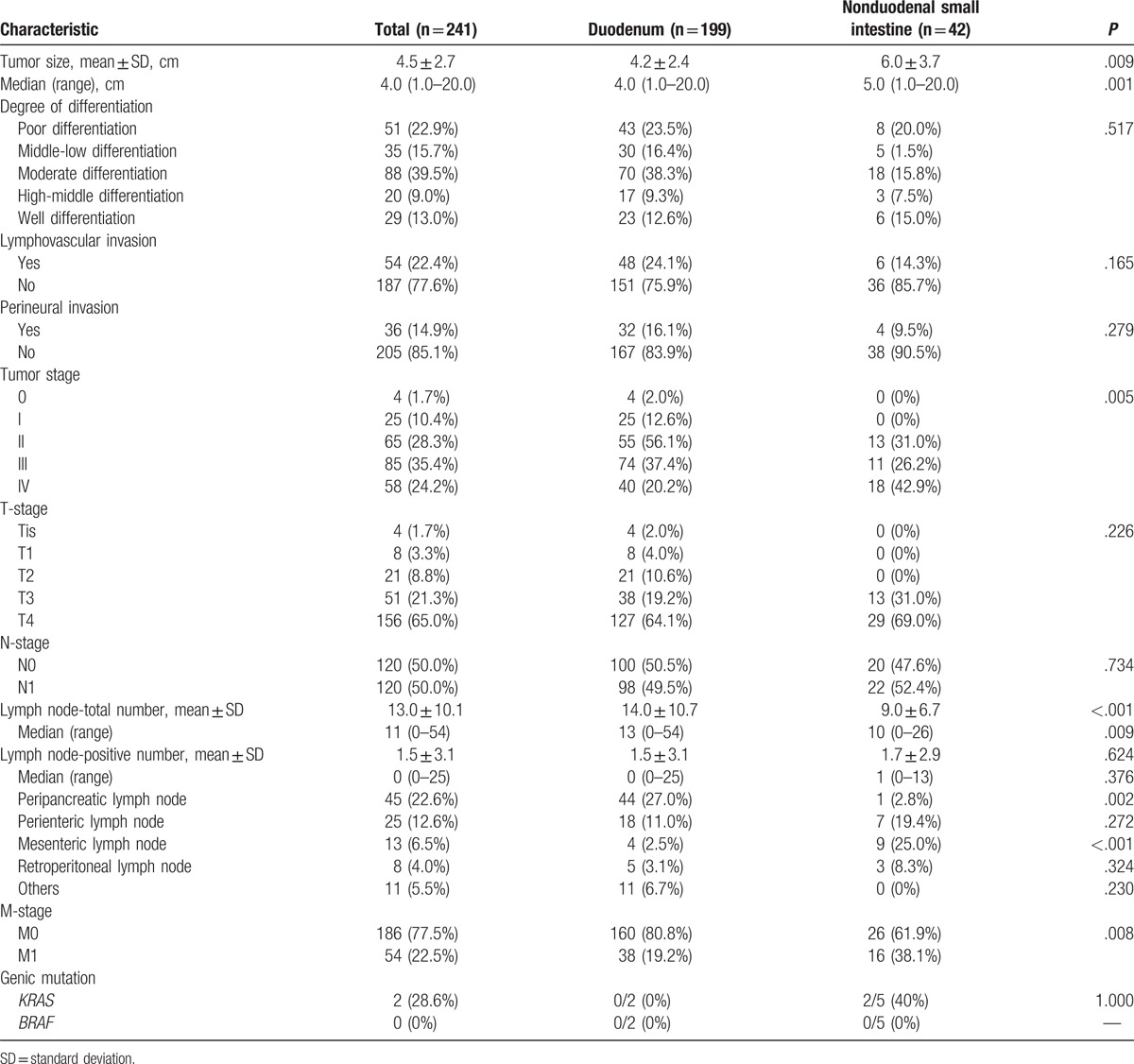
According to the Union for International Cancer Control TNM classification,[16] 86.3% of patients had stage pT3 (21.3%) or pT4 (65.0%) disease. According to the Clavien–Dindo classification, 4 patients (1.7%) had stage 0, 25 (10.4%) stage I, 65 (28.3%) stage II, and 85 (35.4%) stage III cancer, whereas the remaining 58 surgically treated individuals (24.2%) had stage IV cancer. The nonduodenal small intestinal adenocarcinomas were of later stage than the duodenal ones according to the TNM staging (P = .005; Table 2). Regardless of the surgical procedure, lymph node metastases were found in 120 patients (50.0%), whereas stage pN0 disease was observed in 120 (50.0%) (Table 1). The mean numbers of total and positive lymph nodes were 13.0 ± 10.1 and 1.5 ± 3.1, respectively. Positive lymph nodes were found in the peripancreatic (n = 45; 22.6%), perienteric (n = 25; 12.6%), mesenteric (n = 13; 6.5%), and retroperitoneal (n = 8; 4.0%) regions. Other regions with lymph node metastasis included the perigastric, para common hepatic artery, and hepatoduodenal ligament regions, and para left gastric artery lymph node metastases were found only in patients with duodenal adenocarcinomas. Distant metastasis was found in 54 patients (22.5%). Pathological M staging was late for nonduodenal small intestinal adenocarcinomas compared with that for duodenal adenocarcinomas (Table 2). Two patients with duodenal and 5 with nonduodenal adenocarcinomas had genetic testing for KRAS and BRAF, but only 2 patients with nonduodenal adenocarcinomas were positive for KRAS (Table 2).
All patients underwent surgical treatment, and majority (n = 140; 58.6%) underwent surgery only. In total, 241 patients were treated with surgery, of whom the majority underwent pancreatoduodenectomy (n = 123; 51.0%), whereas 59 underwent small bowel segmental resection (24.5%). A palliative bypass surgery was performed in 57 patients (23.7%). Two patients underwent only exploratory laparotomy. Median operation time for patients with duodenal adenocarcinomas (240 minutes) was significantly longer compared with that for those with nonduodenal adenocarcinomas (135 minutes; Table 3), and 91 patients (38.1%) underwent adjuvant chemotherapy postoperatively. One patient in our study received bevacizumab combined with oxaliplatin and S-1 after a palliative operation, leading to a survival of 12 months. Only 2 of the current patients received neoadjuvant chemotherapy, one by intravenous injection and another by intervention (Table 3).
Table 3.
Treatments and perioperative features of small intestinal adenocarcinomas.
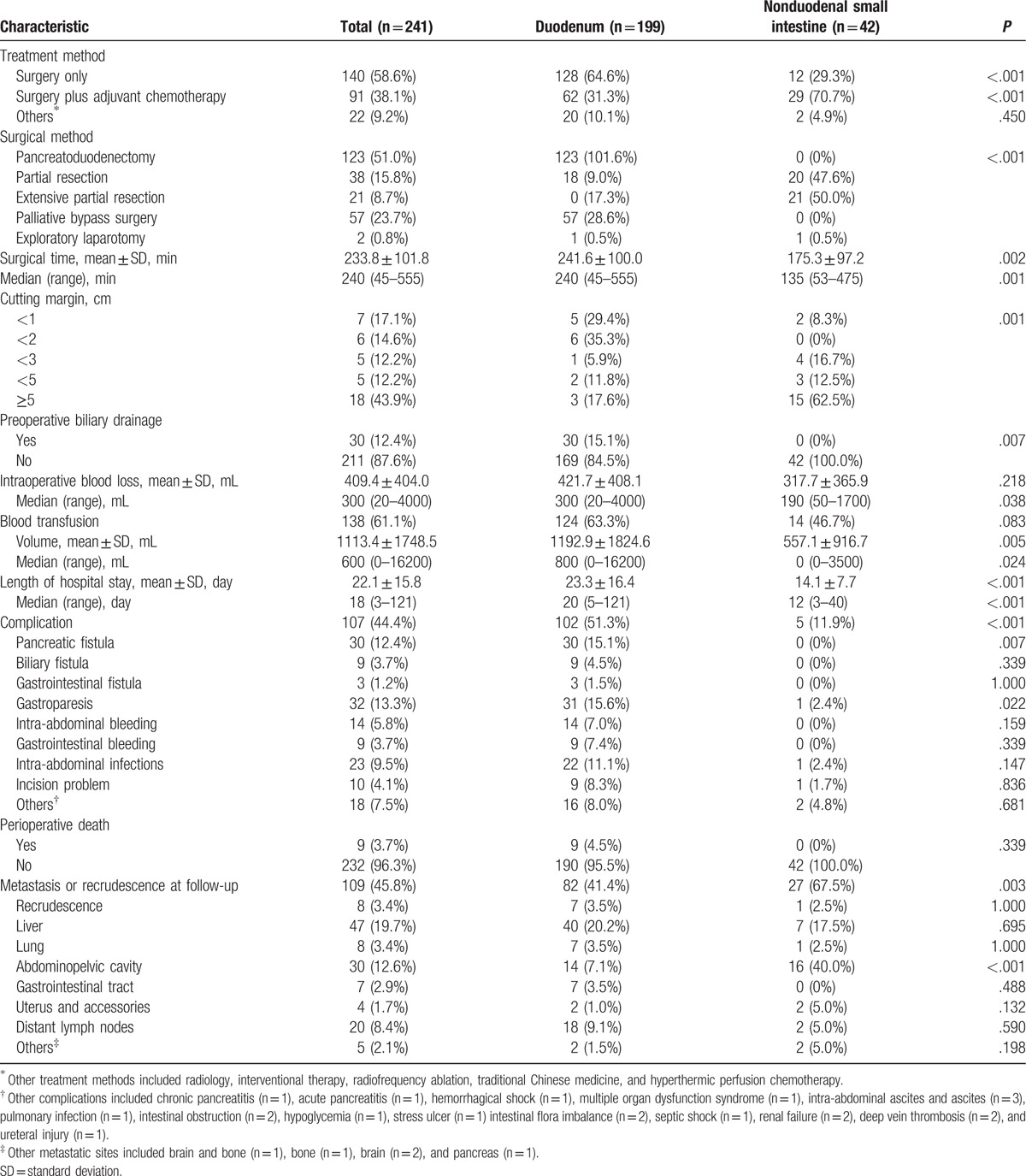
Preoperative biliary drainage was performed in 30 patients (12.4%), and 138 patients (61.1%) received blood transfusion during the perioperative period. The mean length of hospital stay was 18 days (range, 3–121 days), and patients with duodenal adenocarcinomas required more time to recover (P < .001; Table 3). Postoperative complications occurred in 107 patients (44.4%). The complications occurred more frequently in patients with duodenal adenocarcinomas. The pancreatic fistula rate for small intestinal adenocarcinomas was 12.4%. Other common complications included gastroparesis (13.3%), intra-abdominal infections (9.5%), and intra-abdominal bleeding (5.8%) (Table 3). Perioperative death occurred in 9 patients (all in the duodenal group), accounting for 3.7% of all patients: 4 owing to gastrointestinal bleeding; 3 owing to intra-abdominal bleeding (2 caused by a pancreatic fistula); 1 owing to small intestinal obstruction, stress ulcer, and multiple organ dysfunction syndrome; and 1 owing to renal failure (Table 3). During the follow-up period, metastasis or recurrence occurred in 109 patients (45.8%). The most common metastatic site was the liver (19.7%). Nonduodenal small intestinal tumors seemed to recur or metastasize more easily (Table 3).
Overall, among the 241 patients studied, 103 were alive at the end of follow-up (range, 1–106 months) and 136 had died. Median OS was 22.0 ± 3.2 months and median PFS was 13.0 ± 2.2 months. The 1-, 3-, 5-, and 10-year OS rates were 62.5%, 38.2%, 30.2%, and 16.9% and PFS rates were 51.5%, 30.3%, 21.7%, and 19.2%, respectively (Fig. 1). OS and PFS did not differ significantly among different sites (OS, P = .104; PFS, P = .402). The median OS was 20.0 ± 3.1 and 32.0 ± 11.7 months and the median PFS was 14.0 ± 2.2 and 11.0 ± 4.0 months for duodenal and non-duodenal small intestinal adenocarcinomas, respectively (Fig. 2A, 2B). OS and PFS differed significantly among the different TNM stages (P < .001). The mean OS was 87.2 ± 7.1, 56.9 ± 6.3, 26.3 ± 3.6, and 20.1 ± 3.7 months and the mean PFS was 78.7 ± 7.6, 50.8 ± 6.3, 18.5 ± 2.9, and 11.9 ± 2.4 months for stages 0 to I, II, III, and IV adenocarcinomas, respectively (Figs. 2 C and D). The OS rates were significantly related to the tumor size (P = .026), but the PFS rates were not (P = .071). The median OS was 26.0 ± 3.0 or 12.0 ± 2.6 months and the median PFS was 18.0 ± 3.0 or 9.0 ± 1.3 months for tumors with a size less than or no less than 5 cm, respectively (Figs. 2 E and F).
Figure 1.
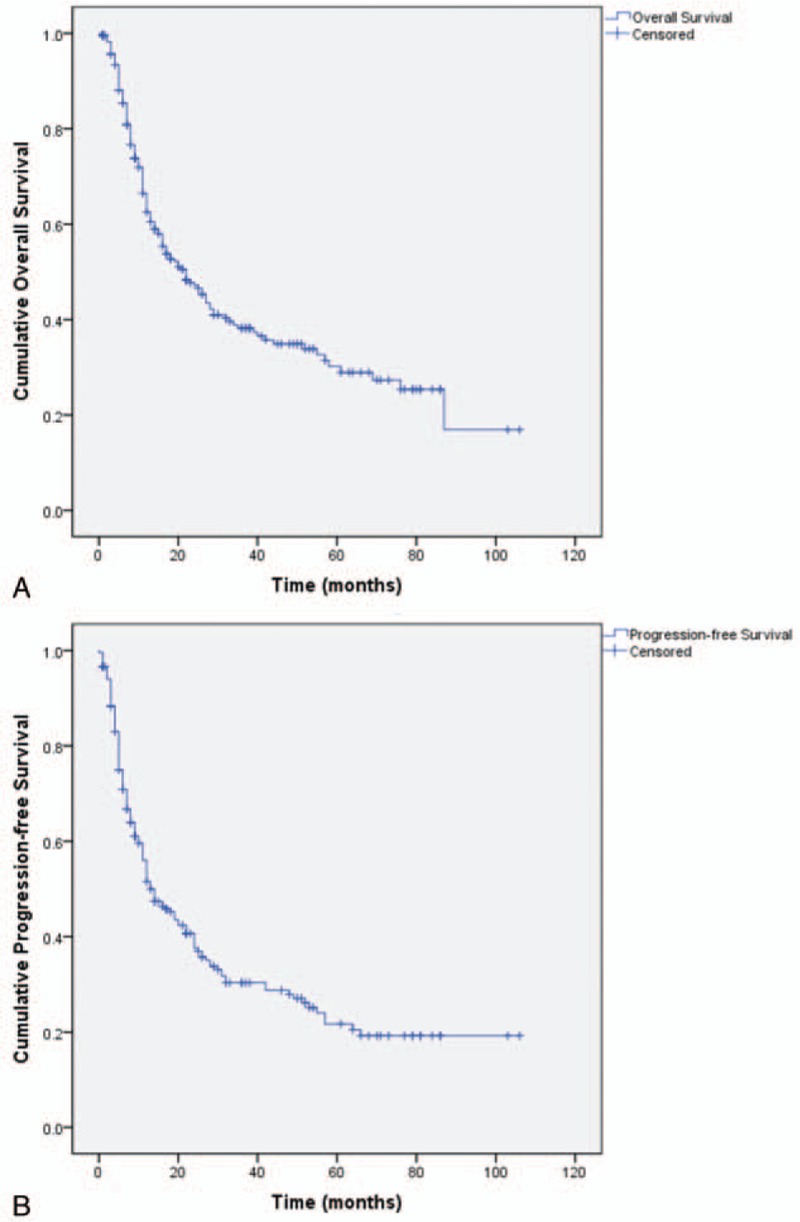
Kaplan–Meier survival graphs for overall survival (OS) or progression-free survival (PFS) of patients with small intestinal adenocarcinomas. (A) OS. (B) Overall PFS.
Figure 2.
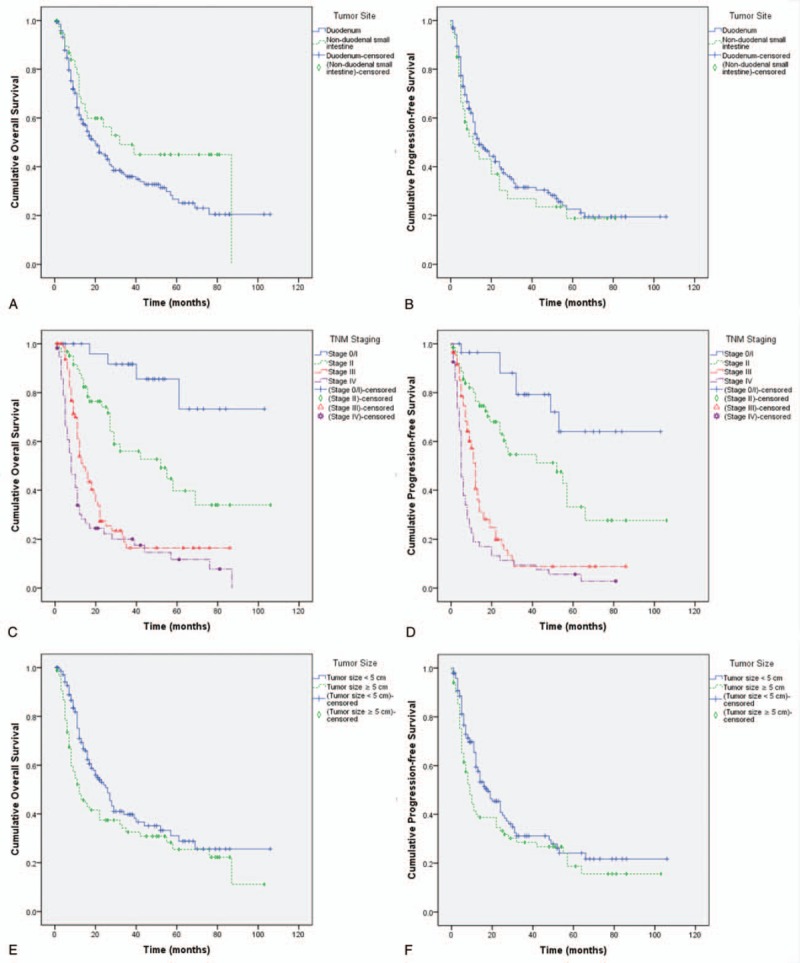
Kaplan–Meier survival graphs for overall survival (OS) or progression-free survival (PFS) of patients with small intestinal adenocarcinomas. (A) OS by tumor sites (Log Rank test, P = .104). (B) PFS by tumor sites (Log Rank test, P = .402). (C) OS by tumor staging (Log Rank test, P < .001). (D) PFS by tumor staging (Log Rank test, P < .001). (E) OS by tumor staging (Log Rank test, P = .026). (F) PFS by tumor staging (Log Rank test, P = .071).
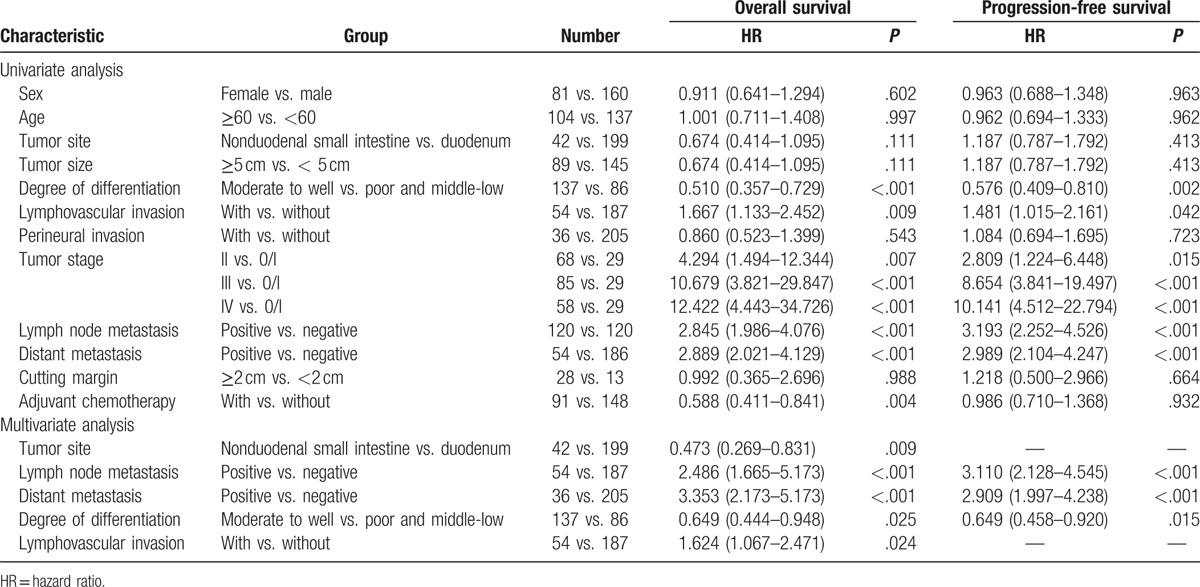
For adenocarcinomas, in the univariate analysis, many factors could affect OS, including the tumor size, degree of differentiation, lymphovascular invasion, tumor stage, lymph node and distant metastases, and adjuvant chemotherapy. Factors such as the degree of differentiation, lymphovascular invasion, tumor stage, and lymph node and distant metastases could also affect PFS. Using Cox regression models, 5 factors were associated with OS: the tumor site, degree of differentiation, lymphovascular invasion, and lymph node and distant metastases. The 3 factors associated with PFS were the degree of differentiation, tumor stage, and lymph node and distant metastases (Table 4).[1]
Table 4.
Univariate and multivariate analysis of overall survival and progression-free survival in patients with small intestinal adenocarcinomas[1].
4. Discussion
Although its incidence rates are on the rise, SBA is a rare tumor affecting approximately 1.45 per 105 males and 1.00 per 105 females each year, respectively.[17] In our study, 241 patients were included and SBA could be found everywhere in the small bowel. Approximately half of all SBAs arise in the duodenum, most commonly in the descending duodenum, 30% are located in the jejunum, and the remaining one-fifth occur within the ileum.[18] In our study, the duodenum, especially the second part, also was the most common site of tumor. To date, because of its rarity, the biology and carcinogenesis of SBA have been insufficiently explored and immunophenotyping and molecular characterization have not been finalized, leading to challenges in the determination of diagnostic methods and treatment.
Although the definitive etiology of SBA is unknown, several predisposing conditions and risk factors have been defined, including Crohn disease, hereditary cancer syndromes, Meckel diverticulum, intestinal duplication, and celiac disease.[4,19] Hereditary cancer syndromes included hereditary nonpolyposis colon cancer syndrome and familial colorectal polyposis, hereditary intestinal polyposis syndrome, and familial adenomatous polyposis. In the present study, the rate of these predisposing conditions and risk factors were very low.
In particular, SBAs are diagnosed in patients in their fifth and sixth decades of life.[5] In our study, the median age at presentation was 58 years (range, 23–79 years). SBA is more prevalent in males than in females.[5,20] In the present study, we obtained similar results, and the males accounted for 66.4% of the total patients with SBAs.
There were no established specific imaging examination and diagnosis protocols, making the diagnosis challenging.[6] The insidious and nonspecific clinical manifestations and lack of specific tests are major factors contributing to the delayed diagnosis[7] reflected by the fact that T and N stages were advanced in most patients.[7,21] In terms of T staging, there were 90% stage T3 or T4 tumors at initial diagnosis.[7] In the present study, the stage was relatively late, as in the previous report. There were 143 stage II or IV adenocarcinomas (approximately 60%) and 156 patients (65.0%) stage T4 adenocarcinomas and lymph mode metastasis was observed in half of the patients. The delayed diagnoses in our study may have been caused by several factors, which have also been mentioned in previous studies.[6,7] First, these clinical manifestations are not specific. The frequent observations at initial admission were alimentary symptoms, weight loss, and abdominal pain. Second, the most frequently used imaging examinations were CT and ultrasound (78.4% and 68.0%, respectively). However, these could not provide specific diagnoses for small intestinal tumors. The current literature does not provide any recommendations on tumor marker determination in SBA patients. Also, our records are incomplete. Thus, more advanced screening methods, including capsule endoscopy and double-balloon endoscopy,[22,23] or protocols for early detection are urgently needed.
Owing to the rarity of SBA, evidence-based therapeutic recommendations and consensus are relatively limited. Until now, related studies were mostly small sample-sized and less conclusive. According to a previous report, approximately two-thirds of SBAs could be treated by potential resection when diagnosed.[11] Just as with malignancies in other parts of the gastrointestinal tract, surgical resection was the main treatment strategy and may be the only curative method for early stage disease.[2,9] All of our patients underwent surgical treatment. Of the 241 patients treated surgically, 51.0% underwent pancreatoduodenectomy and 24.5% underwent small bowel limited resection. Palliative bypass surgery was applied in 23.7% of the patients to reduce tumor-related intestinal or bile obstruction. In our study, the median hospital stay was 18 days, and it was relatively longer for abdominal surgery. This may be because of the high rate of complications.
The prognosis of SBA is poor. Previously, Overman et al[23] reported a distinctly poorer OS in patients with SBA than that in those with large bowel adenocarcinoma. Another study reported a 5-year rate of 25%.[9] In our study, the 5-year OS and PFS rates for patients with duodenal adenocarcinoma were 30.2% and 21.7%, respectively. Several factors could contribute to the poor prognosis, including nonspecific symptoms and lack of evidence-based diagnosis.
The Mayo Clinic conducted a study of 491 cases.[10] In this study, using univariate analysis, higher age, male sex, residual disease following resection, advanced TNM stage, and a lymph node ratio of ≥50% indicated a decreased OS, and using multivariate analysis, only age and TNM staging were the independent factors. Also, in the study performed by Cao et al,[12] the clinical tumor stage was significantly correlated with OS. Other reported independent prognostic factors included lymph node metastasis and distal tumor site.[7,10,12,13,24] In our study, 5 factors were related to OS (the tumor site, degree of differentiation, lymphovascular invasion, tumor staging, and lymph node and distant metastases) and 3 factors were related to PFS (the degree of differentiation, tumor stage, and lymph node and distant metastases).
Although SBA was treated by radical resection and adequate lymphadenectomy, the recurrence or metastasis rate remained high, leading to low OS and PFS rates. In many cases, chemotherapy after operation is necessary, especially in cases with a late TNM staging. A limited number of retrospective studies have reported the effect of adjuvant chemotherapy on survival,[21,25–28] most of which reported negative results.[21,25–27] However, recently, Ecker et al[28] conducted a large retrospective study that demonstrated that adjuvant chemotherapy could improve survival in patients with stage III SBA. In our study, using univariate analysis, adjuvant chemotherapy could improve the OS of patients who underwent surgery. However, it failed to be an independent factor in our study.
In a previous study, neoadjuvant radiochemotherapy showed an improved OS rate in patients undergoing R0 resection compared with that in those who underwent selective treatment.[25] Only 2 of the current patients received neoadjuvant chemotherapy, one by intravenous injection and another by intervention.
There are some drawbacks we cannot ignore. First, this is a retrospective study; thus, many confounding factors could affect the results. Second, the study period was too long for the treatment method and quality to be equivalent throughout. Third, the nonduodenal small intestine could be divided into jejunum and ileum, but many of the medical records could not provide detailed information. The advantages are that this study analyzed almost every aspect of the tumor and had a relatively large number of participants compared with some other studies.
5. Conclusions
SBA is a rare tumor. The clinical manifestations and examinations of SBA are nonspecific, making the diagnosis difficult. Surgery is a very important treatment for SBA. A poor overall survival outcome could be associated with the following factors: duodenal adenocarcinomas, lymph node metastases, distant metastases, poor differentiation, and lymphovascular invasion. The 3 factors associated with progression-free survival were the degree of differentiation, lymph node metastases, and distant metastases.
Acknowledgments
The authors thank Jia Jia for the help in data analysis.
Footnotes
Abbreviations: CA 19–9 = carbohydrate antigen 19–9, CEA = carcinoembryonic antigen, CT = computed tomography, OS = overall survival, PFS = progression-free survival, SBA = small bowel adenocarcinomas.
Funding: Our work was funded by CAMS Initiative Fund for Medical Sciences (CIFMS) (no. 2016-I2M-1-001).
Ethical approval: The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki (6th revision, 2008) and was revised and approved by the ethical committee of the China National Cancer Center.
The authors report no conflicts of interest.
References
- [1].Zhang S, Zheng C, Chen Y, et al. Clinicopathologic features, surgical treatments, and outcomes of small bowel tumors: a retrospective study in China. Int J Surg 2017;43:145–54. [DOI] [PubMed] [Google Scholar]
- [2].Makino S, Takahashi H, Haraguchi N, et al. A single institutional analysis of systemic therapy for unresectable or recurrent small bowel adenocarcinoma. Anticancer Res 2017;37:1495–500. [DOI] [PubMed] [Google Scholar]
- [3].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5–29. [DOI] [PubMed] [Google Scholar]
- [4].Raghav K, Overman MJ. Small bowel adenocarcinomas—existing evidence and evolving paradigms. Nat Rev Clin Oncol 2013;10:534–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Poddar N, Raza S, Sharma B, et al. Small bowel adenocarcinoma presenting with refractory iron deficiency anemia - case report and review of literature. Case Rep Oncol 2011;4:458–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Lu Y, Frobom R, Lagergren J. Incidence patterns of small bowel cancer in a population-based study in Sweden: increase in duodenal adenocarcinoma. Cancer Epidemiol 2012;36:e158–63. [DOI] [PubMed] [Google Scholar]
- [7].Chang HK, Yu E, Kim J, et al. Adenocarcinoma of the small intestine: a multi-institutional study of 197 surgically resected cases. Hum Pathol 2010;41:1087–96. [DOI] [PubMed] [Google Scholar]
- [8].Overman MJ. Recent advances in the management of adenocarcinoma of the small intestine. Gastrointest Cancer Res 2009;3:90–6. [PMC free article] [PubMed] [Google Scholar]
- [9].Dabaja BS, Suki D, Pro B, et al. Adenocarcinoma of the small bowel: presentation, prognostic factors, and outcome of 217 patients. Cancer 2004;101:518–26. [DOI] [PubMed] [Google Scholar]
- [10].Halfdanarson TR, McWilliams RR, Donohue JH, et al. A single-institution experience with 491 cases of small bowel adenocarcinoma. Am J Surg 2010;199:797–803. [DOI] [PubMed] [Google Scholar]
- [11].Howe JR, Karnell LH, Menck HR, et al. The American College of Surgeons Commission on Cancer and the American Cancer Society. Adenocarcinoma of the small bowel: review of the National Cancer Data Base, 1985-1995. Cancer 1999;86:2693–706. [DOI] [PubMed] [Google Scholar]
- [12].Koo DH, Yun SC, Hong YS, et al. Adjuvant chemotherapy for small bowel adenocarcinoma after curative surgery. Oncology 2011;80:208–13. [DOI] [PubMed] [Google Scholar]
- [13].Cao J, Zuo Y, Lv F, et al. Primary small intestinal malignant tumors: survival analysis of 48 postoperative patients. J Clin Gastroenterol 2008;42:167–73. [DOI] [PubMed] [Google Scholar]
- [14].McLaughlin PD, Maher MM. Primary malignant diseases of the small intestine. AJR Am J Roentgenol 2013;201:W9–14. [DOI] [PubMed] [Google Scholar]
- [15].Stephen B. AJCC Cancer Staging Manual. New York, NY, USA: Springer; 2010. [Google Scholar]
- [16].Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 2010;17:1471–4. [DOI] [PubMed] [Google Scholar]
- [17].Pan SY, Morrison H. Epidemiology of cancer of the small intestine. World J Gastrointest Oncol 2011;3:33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Schwameis K, Schoppmann SF, Stift J, et al. Small bowel adenocarcinoma - terra incognita: A demand for cross-national pooling of data. Oncol Lett 2014;7:1613–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Palascak-Juif V, Bouvier AM, Cosnes J, et al. Small bowel adenocarcinoma in patients with Crohn's disease compared with small bowel adenocarcinoma de novo. Inflamm Bowel Dis 2005;11:828–32. [DOI] [PubMed] [Google Scholar]
- [20].Bilimoria KY, Bentrem DJ, Wayne JD, et al. Small bowel cancer in the United States: changes in epidemiology, treatment, and survival over the last 20 years. Ann Surg 2009;249:63–71. [DOI] [PubMed] [Google Scholar]
- [21].Poultsides GA, Huang LC, Cameron JL, et al. Duodenal adenocarcinoma: clinicopathologic analysis and implications for treatment. Ann Surg Oncol 2012;19:1928–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Pennazio M, Spada C, Eliakim R, et al. Small-bowel capsule endoscopy and device-assisted enteroscopy for diagnosis and treatment of small-bowel disorders: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy 2015;47:352–76. [DOI] [PubMed] [Google Scholar]
- [23].Lipka S, Rabbanifard R, Kumar A, et al. Single versus double balloon enteroscopy for small bowel diagnostics: a systematic review and meta-analysis. J Clin Gastroenterol 2015;49:177–84. [DOI] [PubMed] [Google Scholar]
- [24].Tsushima T, Taguri M, Honma Y, et al. Multicenter retrospective study of 132 patients with unresectable small bowel adenocarcinoma treated with chemotherapy. Oncologist 2012;17:1163–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kelsey CR, Nelson JW, Willett CG, et al. Duodenal adenocarcinoma: patterns of failure after resection and the role of chemoradiotherapy. Int J Radiat Oncol Biol Phys 2007;69:1436–41. [DOI] [PubMed] [Google Scholar]
- [26].Overman MJ, Kopetz S, Lin E, et al. Is there a role for adjuvant therapy in resected adenocarcinoma of the small intestine. Acta Oncol 2010;49:474–9. [DOI] [PubMed] [Google Scholar]
- [27].Aydin D, Sendur MA, Kefeli U, et al. Evaluation of prognostic factors and adjuvant chemotherapy in patients with small bowel adenocarcinoma who underwent curative resection. Clin Colorectal Cancer 2016;pii: S1533-0028(16)30149-9. doi: 10.1016/j.clcc.2016.08.002. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- [28].Ecker BL, McMillan MT, Datta J, et al. Efficacy of adjuvant chemotherapy for small bowel adenocarcinoma: a propensity score-matched analysis. Cancer 2016;122:693–701. [DOI] [PubMed] [Google Scholar]


