Abstract
Rhinocerebral mucormycosis is an acute, rapidly fatal, fungal infection, classically involving the nasal mucosa and paranasal sinuses. It is an aggressive, opportunistic infection that frequently progresses to involve the orbit and cerebrum. Cerebral extension in immunocompromised patients is almost universally fatal. There are limited data on mucormycosis in pediatric immunocompromised patients in the literature, with only few reports on rhinocerebral involvement. The immunocompromised patients described in this report presented with suspected periorbital and nasal cellulitis, progressing rapidly to necrotic changes in nasal tissue and oral palatal mucosa. In these patients, the surgical resection of mucormycosis-infected tissue followed by flap reconstruction combined with medical treatment effectively treated the infection, allowed for the rapid resumption of chemotherapy and dramatically improved the quality of life for both the patient and their family.
Keywords: mucormycosis, craniofacial reconstruction, pediatric, hematological malignancy, immunocompromised, surgical resection, flap reconstruction
Abstract
La mucormycose rhinocérébrale est une infection fongique aiguë qui devient vite fatale et qui touche généralement les muqueuses nasales et les sinus paranasaux. C’est une infection agressive et opportuniste qui évolue souvent pour toucher l’orbite et le cerveau. Chez les patients immunodéprimés, l’atteinte cérébrale est presque toujours fatale. Les données sur la mucormycose chez les patients immunodéprimés d’âge pédiatrique sont limitées dans les publications, et seulement quelques articles traitent de l’atteinte rhinocérébrale. Les patients immunodéprimés décrits dans le présent rapport ont consulté à cause d’une présomption de cellulite périorbitale et nasale qui a évolué rapidement vers une nécrose des tissus nasaux et de la muqueuse oropalatine. Chez ces patients, la résection chirurgicale des tissus infectés par la mucormycose suivie d’une reconstruction par lambeau combinée à un traitement médical, a favorisé la résolution de l’infection, ce qui a permis une reprise rapide de la chimiothérapie et a considérablement amélioré la qualité de vie du patient et de sa famille.
Introduction
Rhinocerebral mucormycosis is an acute, rapidly fatal, fungal infection, classically involving the nasal mucosa and paranasal sinuses. It is an aggressive, opportunistic infection caused by organisms of Mucorales order and Zygomectes class.1-3 It frequently progresses to involve the orbit and cerebrum.4,5 Mucormycosis is commonly associated with immunocompromised patients, uncontrolled diabetes mellitus, chronic steroid use, lymphoma, leukemia, AIDS, and organ transplantation.1-8 Signs of infection are nonspecific but may include acute sinusitis, erythema over the sinuses, fever, periorbital edema, ptosis, proptosis, dark nasal, and/or palatine mucosal eschar.2,5
The pathological hallmark is invasion of blood vessel walls, thrombus formation, infarction of surrounding tissue, and black necrosis.1,4,9 Patients with locally invasive sinus mucormycosis have a mortality rate of 47% to 85%.6,8,10,11 Cerebral extension in immunocompromised patients is almost universally fatal with a rapid, fulminant progression and a reported median survival of 17 to 39 days.6,8,12
In patients with hematological malignancy, a combined surgical and medical antifungal chemotherapeutic treatment is described as the standard of care.1,4,6,8,9,13 With the advent of new antifungal regimens, the ability to modify chemotherapeutic agents and aggressive surgical treatment, we believe long-term survival can be accomplished in patients with pediatric leukemia. This report presents our experience with 2 pediatric cases diagnosed with aggressive rhinocerebral mucormycosis involving the cerebrum and their treatment.
Case 1
A 2-year-old male diagnosed with acute lymphoblastic leukemia (ALL) at 9 months of age. After 2 weeks of chemotherapy, he developed periorbital cellulitis, discolouration, and subsequent necrosis of the hard palate. Biopsies taken from the necrotic palate showed characteristic-branched hyphae, confirming the diagnosis of mucormycosis. Involvement extended from the hard palate and maxillary sinuses to the paranasal sinuses and cribriform plate. Two small abscesses were identified in the frontal lobes on computed tomography (CT). Two months after diagnosis, a ventriculoperitoneal shunt was inserted due to hydrocephalus and seizures. The patient was maintained on liposomal amphotericin at a low dose of 3.8 mg/kg for 1 year without surgical intervention. Surgical intervention was not initially considered because the patient had a poor prognosis. He was maintained on chemotherapy with daily 6 MP, weekly oral Methotrexate, and monthly Vincristine. With treatment, the patient went into remission; however, a year after the initial diagnosis he relapsed. With this relapse, he was reinduced with Vincristine, Prednisone, and Asparaginase. At this stage, there was almost complete collapse of the midface and nose (Figure 1). Intraoral examination revealed a completely necrotic hard palate and alveolus with only the lateral segments being vascular and viable (Figure 2). There was no significant facial skin loss.
Figure 1.
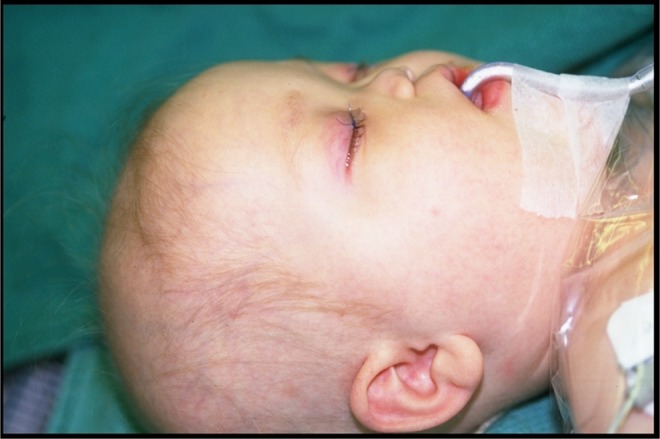
Case 1: Mucormycosis involving hard palate and maxillary sinues, paranasal sinuses, and cribiform plate resulting in almost complete collapse of midface and nose.
Figure 2.
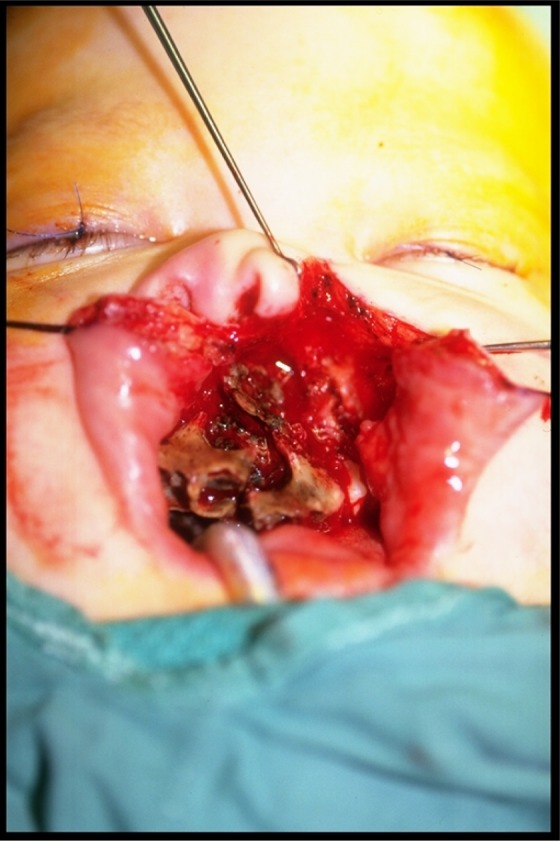
Case 1: Intraoral examination revealing necrotic hard palate and alveolus with only lateral segments vascular and viable.
With treatment, the patient went into remission at the age of 22 months and was scheduled for consolidation therapy. However, the patient was at risk for a flare of his colonized mucormycosis and therefore was transferred to our centre to surgically eradicate the infection before further chemotherapy. Through a lateral rhinotomy incision, the patient underwent a left medial maxillectomy, resection of the completely devitalized hard palate and nasal septum, and exenteration of the left ethmoid sinuses. Frozen section biopsies were taken to ensure no residual mucormycosis remained at the margins of the defect. The resultant defect spanned from the nasal root to the palate, the entire alveolus was gone and a remnant of normal posterior soft palate was left intact. Once the defect was clearly outlined and measured, it was decided to use the right fibula osteocutaneous flap for reconstruction. An osteotomy was made to recreate the curve in the anterior palate. The skin paddle was then inset into the soft palate and alveolus. The patient also required a dorsal nasal strut, which was reconstructed with a bone graft and plated to the frontal bone.
It was noticed the following day that the skin paddle was pale with poor bleeding on needle puncture and absent laser Doppler readings. The patient was taken back to the operating room (OR) immediately for an exploration of the anastomoses, which was patent. All tension on the skin paddle was released, and it was reinset without tension. Nine days after the original surgery, the patient was taken back to the OR for debridement and minimal revision of the flap post-partial dehiscence. The patient continued treatment with Liposmal amphotericin B at 50 mg and 50 cc D5 W given over 2 hours every 24 hours. Pathological specimens taken from the midface, mucosa next to medial wall of the left orbit, free flap and fragments of fibrous and bony tissue revealed chronic inflammation with no mucormycosis. The margins of the wound showed no signs of mucormycosis, and endotracheal and nasal aspirates were also clear of fungal infection.
The patient was discharged 2 weeks following the surgical revision (Figure 3) and at 6-month follow-up there were no signs of recurrent infection, and the flap was firmly in place and fully mucosalized. Further reconstruction was planned for when the patient reached school age; however, a recurrence of leukemia and fatal intracerebral bleed during reinduction chemotherapy caused the premature death of this patient.
Figure 3.
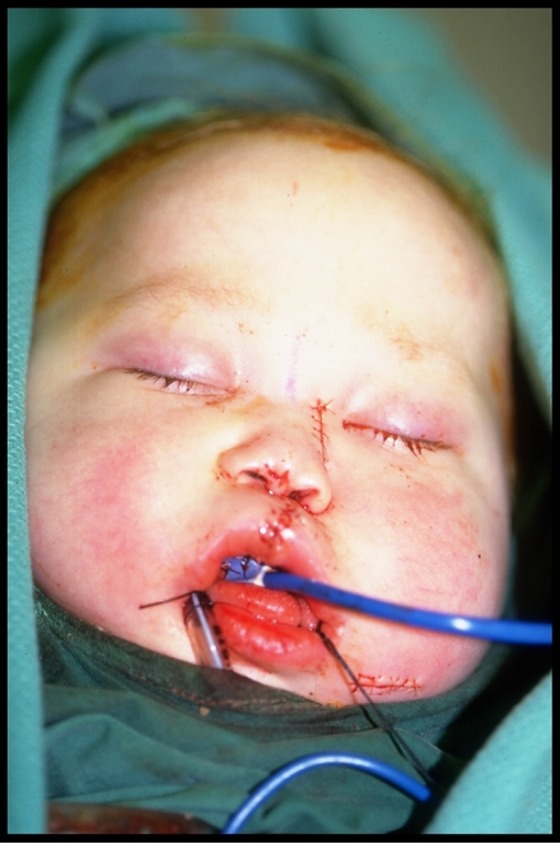
Case 1: Postoperative results after surgical resection of devitalized hard palate and nasal septum and exenteration of the left ethmoid sinuses. The defect was reconstructed with the right fibula osteocutaneous flap and a dorsal nasal strut.
Case 2
A 4-and-half-year-old boy was seen a week after starting a second induction of chemotherapy on for previously diagnosed acute myeloblastic leukemia (AML, M4 type). What had appeared to be a bacterial orbital cellulitis 4 days earlier, and was treated with antibiotics, had progressed to frank necrosis of the left and central aspect of the nose. He presented with orbital cellulitis and necrosis over the dorsum and left lateral nasal wall extending up to the forehead and across the midline. Biopsies that were taken showed mucormycosis (Figure 4).
Figure 4.
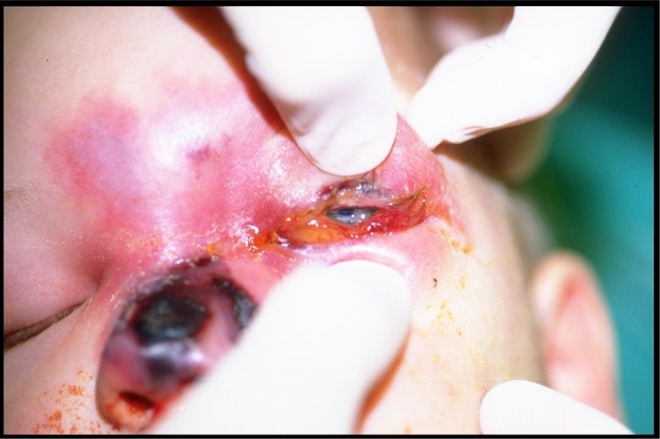
Case 2: Presenting with orbital cellulitis and frank necrosis over dorsum and the left lateral nasal wall extending to the forehead.
The patient was taken to the OR for urgent debridement, in conjunction with systemic antifungal therapy. Resections included full thickness skin over the dorsum of the nose, left alar cartilage, and alar rim. An over-the-forehead excision extended to the medial canthal region and dissected into the forehead in the region of the procerus. Elevation of skin flaps showed the perforating vessels to be thrombosed. The procerus, frontalis, and corrugator muscles were all necrotic and were, therefore, excised in the subcutaneous plane with extension on the less involved right side. Other areas of debridement included the origins of the levator muscles and some of the zygomaticus major and minor muscles. The ophthalmology service completed removal of the medial canthal tendon, lacrimal sac, and minimal medial orbital resection, whereas the otolaryngology service performed a left external ethmoidectomy, left inferior turbinectomy, and a left Caldwell-Luc resection (Figure 5). Pathology reports of the surgical specimen showed numerous broad, non-septate-branching hyphae within blood vessels infiltrating the vascular wall and also in the perivascular soft tissue consistent with diagnosis of zygomycosis. Three days after the initial procedure, there was further progression of the necrotic tissue and further debridement was required including resection of more zygomatic musculature and skin over the forehead and nasal region.
Figure 5.
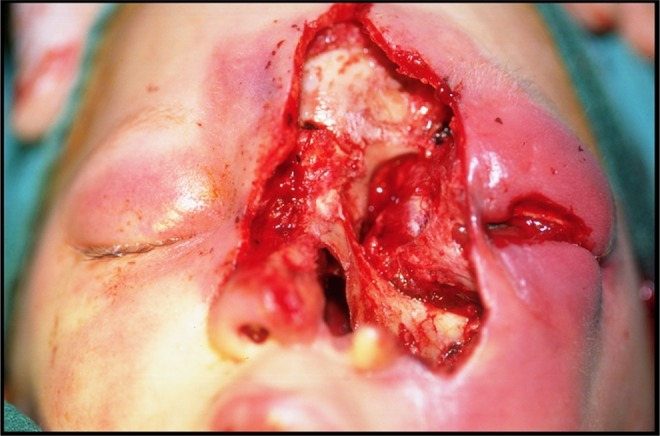
Case 2: Surgical debridement of dorsal aspect of the nose, left alar carilage and rim, procerus, frontalis and corrugator muscles, part of levator zygomaticus major and minor muscle, medial canthal tendon, lacrimal sac and part of medial orbit, left external ethmoidectomy and inferior turbinectomy and Caldweel-Luc resection.
Postoperative day (POD) 8, the patient underwent a third surgical debridement of the left periorbital structures. The patient returned to the OR POD 13 for his fourth extensive debridement (Figure 6) including enucleation of the left eye, debridement of the left cheek, forehead, and left maxillary sinus. The defect was temporarily reconstructed with rotation of a left cheek flap to cover exposed orbital apex and a split thickness skin graft to the nose (Figure 7).
Figure 6.
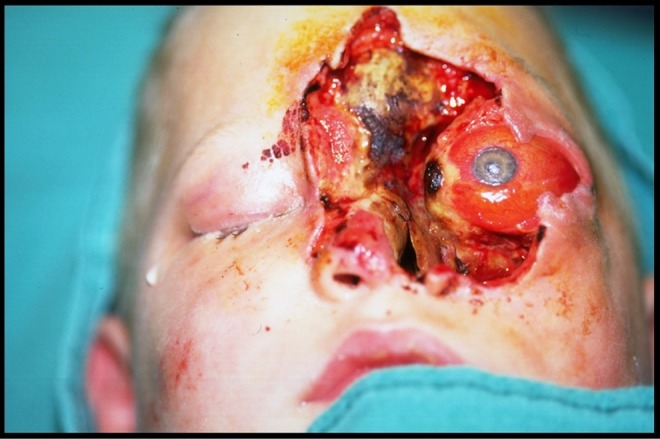
Case 2: Prior to the fourth extensive debridement including enucleation of the left eye and debridement of the left cheek, forehead, and maxillary sinsus.
Figure 7.
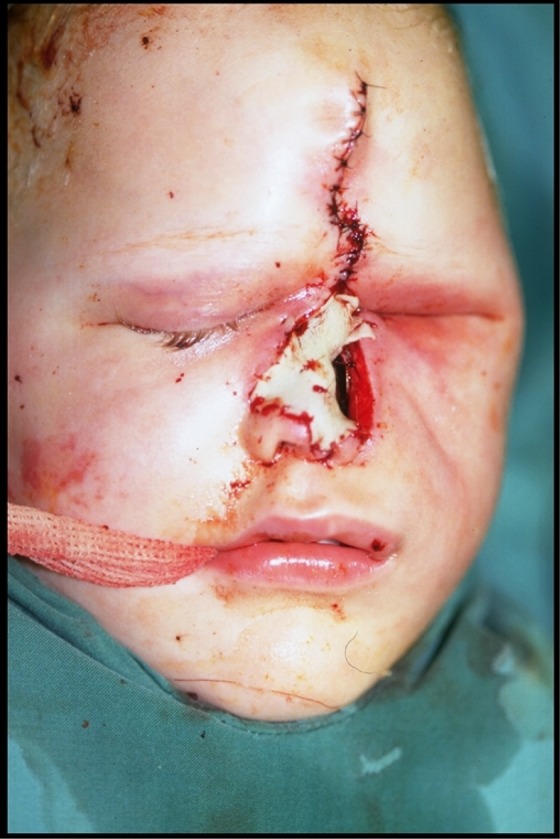
Case 2: Postoperative from the fourth extensive debridement with temporary reconstruction with rotation of the left cheek flap to cover exposed orbital apex and split thickness skin graft to the nose.
Nine days after this extensive debridement, the patient had a right-sided focal seizure, prompting a CT scan. It revealed bilateral frontal lobe abscesses extending to the ethmoid bone. Neurosurgery obtained an aspirate through a burrhole, which confirmed mucormycosis invasion. The patient was maintained on a course of intravenously amphotericin, fluconazole (for antifungal therapy), and phenobarbital and was discharged home 10 days later. Two-week follow-up revealed no clinical signs of infection and the flaps to be healing well. The multidisciplinary team decided to resume the first course of low-dose chemotherapy, this time with no decrease in neturophils (AML remission).
Eight months later, when it was confirmed he was in remission without any signs of active mucormycosis infection, the patient again returned to the OR for redebridement of the palate, left maxilla, and frontal bone (Figures 8 and 9) and closure of the palatal fistula, and enucleation defect. Reconstruction was achieved with a right osteomusculocutaneous iliac crest flap, which was osteotomized and hinged for the nasal dorsum. Two skin paddles were fashioned for the reconstruction and resurfacing of the enucleation defect and palate.
Figure 8.
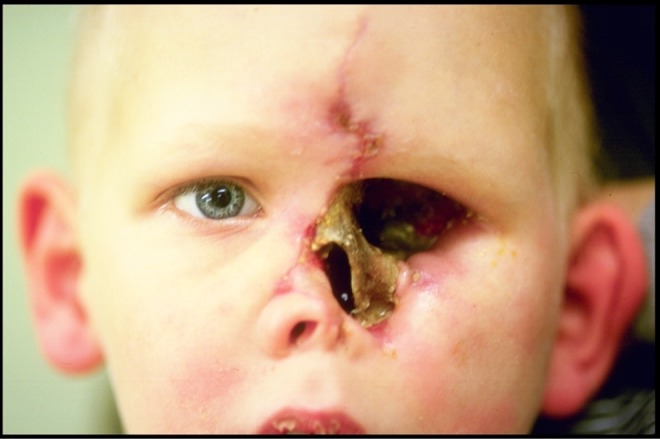
Case 2: Eight months postoperative from the fourth extensive debridement with confirmed remission.
Figure 9.
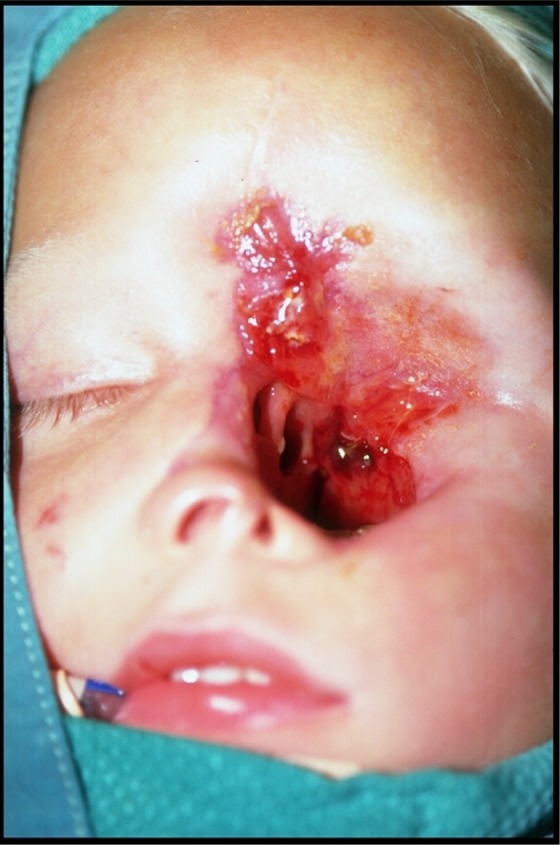
Case 2: Redebridement of palate, the left maxilla, and frontal bone with confirmed remission of mucormycosis, prior to closure of palatal fistula and enucleation defect with the right osteomusculocutaneous iliac crest flap.
Two weeks later, there were no signs of progressive necrosis. A small palatal fistula had developed at the site of his closure, giving the effect of hypernasal speech. The patient was discharged 2 weeks later and went on to attend school (Figure 10). Follow-up for the next year progressed well and the patient continued to thrive. Two years later, the patient had a right forehead flap for nasal reconstruction, the iliac skin paddle was partly excised and partly used for lining of the nose in the form of a turndown flap. The alveolus and zygoma reconstruction with the iliac crest was solid (Figure 11). The patient resumed a good quality of life with a functional but not aesthetically ideal reconstruction. Years later, the patient died of a complication of leukemia.
Figure 10.
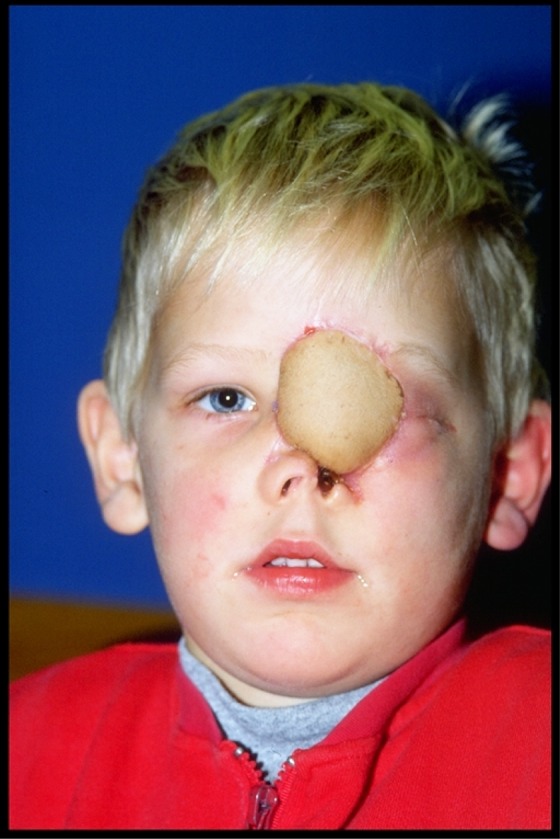
Case 2: Final result of reconstruction with the right osteomusculocutaneous iliac crest flap.
Figure 11.
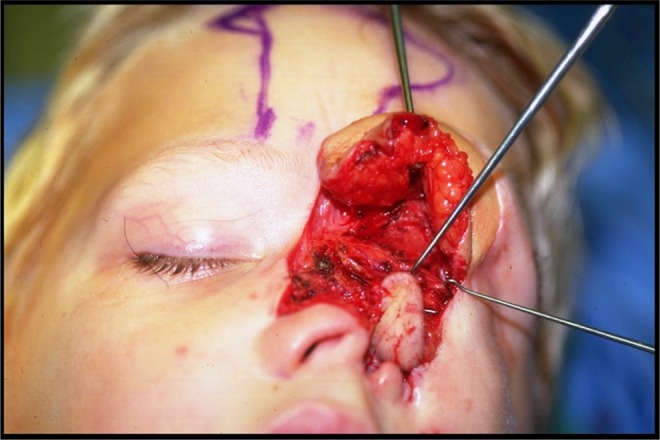
Case 2: The right forehead flap for nasal reconstruction with turndown flap of iliac skin paddle to line the nose.
Discussion
Mucormycosis is a rapidly progressive infection caused by ubiquitous fungi of the class Zygomycetes.1,3,7 The fungus is typically found in soil, dust, and bread moulds and is readily cultured from the human respiratory tract.4,7 They are normally non-invasive, non-pathogenic opportunists, often inhaled as spores of the organism, which convert into hyphae if invasion of the human tissue occurs.14,15
Predisposing factors are prolonged neutropenia, from chemotherapy and steroids, acidosis, dehydration, hyperglycemia, and diabetic ketoacidosis.1,2,6-8,15 Cytotoxic drugs are known to depress both humoral and cell-mediated immunity, induce neutropenia, and cause portals of entry by breakdown of epithelial barriers.2 Mucormycosis can occur in 6 clinical scenarios: rhinocerebral (most common), pulmonary, gastrointestinal, central nervous system (CNS), cutaneous, and miscellaneous regions (bone, kidney, and heart).3,7
Rhinocerebral mucormycosis classically involves the nasal mucosa with invasion of the paranasal sinuses and orbits. Earliest signs and symptoms can include lethargy, fever, headache, facial pain with nasal congestion, swelling of periorbital tissues, and dark nasal eschar or palatine mucosa.1,4,5,9 Orbital invasion is marked by the development of cellulitis, chemosis, proptosis, decreased vision, and orbital apex syndrome.1,16 Early diagnosis of mucormycosis is of paramount importance and wound cultures cannot be relied on. A high index of suspicion and low threshold for biopsy must be maintained in patients with rapidly developing soft tissue infection over the paranasal sinuses and periorbital region.1,3,6
Mucormycosis is detected by staining techniques, including haematoxylin and eosin, periodic acid-Schiff and methanamine silver nitrate stains.17 Characteristic histological findings are irregular, broad, non-septate, twisted, ribbon-like, fungal hyphae with the right angle branching.3,9,18 Fast identification of fungi in frozen sections is available with fluorescent microscopic stain using calcofluor white.19 Regardless of the tissue or organ involved, pathological hallmarks of mucormycosis include invasion of blood vessels with occlusion causing subsequent tissue reaction of thrombosis, ischemia, and necrosis.3,9 Embolization and acidosis promote fungal growth and decrease macrophage effectiveness, allowing rapid spread along injured blood vessels.2,20,21
There are limited data on mucormycosis in pediatric immunocompromised patients in the literature, with only few reports on rhinocerebral involvement (Table 1). In a study by Däbritz et al, there were a total of 5 of 12 patients reported with cerebral involvement of which only 1 survived. Another case series by Dehority et al demonstrated 1 of 6 patients having rhinocerebral involvement, which was the only fatality in the series.2 Three case reports have described successful treatment of rhino-cerebral mucormycosis, including a 12-year-old girl with ALL,1 a 7-year-old girl with ALL,18 and a boy with relapsed ALL solely treated with long-term amphotericin B in the absence of surgical debridement.22 In the latter case, survival was attributed to long-term antifungal use, neutrophil recovery, and slow ALL progression.22 In each of these cases the reconstruction was not documented. This is the first case report of reconstruction after cerebral mucormycosis.
Table 1.
Summary of Published Case Reports on Cerebral Mucormycosis Treatment and Survival in the Literature.
| Author | Patients | Treatment | Survival %, months |
|---|---|---|---|
| De Leonardis1 | 1 | Antifungal therapy +/−amphotericin B +/ − surgical debridement | 100% at 6 months |
| Däbritz et al6 | 5 | Surgical debridement | 20% at 20 months (609 days) |
| Dehority et al2 | 1 | Liposomal amphotericin + posaconazole + surgical debridement | 0% |
| Popa et al18 | 1 | A Amphotericin B + surgical debridement | 100% at 30 months |
| Wehl et al22 | 1 | Amphotericin B | 100% at 15 months |
As in our 2 patients, a combined approach of medical and surgical treatments is favoured, consisting of amphotericin B, reversal of immunosuppression, and aggressive surgical debridement. Other adjunctive forms of treatment may include local administration of antifungal agents and irrigation to control sino-orbital fungal infections.7,21 Hyperbaric oxygen has also been described as a modality of treatment.7 Oxygen is fungicidal in sufficient concentrations by creating superoxide radicals, and theoretically targets tissue hypoxia and acidosis that accompany vascular invasion by the fungus.23,24
The importance of controlling the underlying hematological malignancy with chemotherapy and maintaining some immunocompetance is imperative. The patients described in this report were immunocompromised and presented with suspected periorbital/nasal cellulitis, progressing rapidly to necrotic changes in nasal tissue and oral palatal mucosa. Patient 1 received immediate treatment with liposomal amphotericin B and delayed surgical intervention due to an initial unfavourable outlook. Patient 2 received amphotericin B and fluconazole, along with immediate surgical debridement. Intravenous liposomal amphotericin B at least 1 mg/kg/d (max 2.5-4.0 mg/kg/d) is recommended.2,7,25 Less toxic liposomal amphotericin (AriBisome) may be beneficial if side effects to amphotericin develop, or infection extends to the CNS. Due to lower toxic effects, higher total doses of liposomal amphotericin can be given 1.5 to 5 mg/kg/d.2,7,25,26
Along with amphotericin treatment, radical surgical debridement is required to eradicate the reservoir of the fungus.1,2,7,18,22 Multiple debridement may be warranted due to the rapidly progressive nature of the infection. Frozen sections are recommended during surgery to ensure resected margins are clear. Debridement should proceed to well-perfused bleeding tissues because of the vasoocclusive effects of mucormycosis. The cosmesis and possible loss of function should not take preference over a complete and adequate surgical excision. A question that still needs to be answered is should immediate or delayed reconstruction be done immediately after tumour excision with negative margins. In patient 1, it was felt that an adequate resection was achieved, but the patient’s urgent need to resume chemotherapy and remaining defect required immediate reconstruction. Patient 2 had multiple recurrences requiring serial surgical debridements until the tumour was totally eradicated.
In conclusion, a high index of suspicion should be maintained with any cellulites like periorbital process in immunocompromised children. A swift tissue biopsy will confirm the diagnosis of mucormycosis and a multidisciplinary approach should be initiated. This would include reversal of immunosuppression or any metabolic disorder, systemic and local antifungal medication, and prompt surgical excision of the infective tissues. Surgical excision should be done with frozen section control to ensure negative margins if immediate reconstruction is to be contemplated. It has been our experience that the surgical resection of mucormycosis-infected tissue has allowed for the rapid resumption of chemotherapy and dramatically improved the quality of life for both the patient and their family.
Footnotes
Authors’ Note: All authors have no commercial associations or other arrangement that may pose a conflict of interest in connection with this article. The patients discussed in this case report provided consent for photographs and discussion of their cases, respectively, in accordance with institutional policies.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. De Leonardis F, Perillo T, Giudice G, Favia G, Santoro N. Recurrent rhino-ocular-cerebral mucormycosis in a leukemic child: a case report and review of pediatric literature. Pediatr Rep. 2015;7(3):5938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dehority W, Willert J, Pong A. Zygomycetes infections in pediatric hematology oncology patients: a case series and review of the literature. J Pediatr Hematol Oncol. 2009;31(12):911–919. [DOI] [PubMed] [Google Scholar]
- 3. Losee JE, Selber J, Vega S, Hall C, Scott G, Serletti JM. Primary cutaneous mucormycosis: guide to surgical management. Ann Plast Surg. 2002;49(4):385–390. [DOI] [PubMed] [Google Scholar]
- 4. Ferguson BJ. Mucormycosis of the nose and paranasal sinuses. Otolaryngol Clin North Am. 2000;33(2):349–365. [DOI] [PubMed] [Google Scholar]
- 5. Akoz T, Civelek B, Akan M. Rhinocerebral mucormycosis: report of 2 cases. Ann Plast Surg. 1999;43(3):309–312. [DOI] [PubMed] [Google Scholar]
- 6. Däbritz J, Attarbaschi A, Tintelnot K, et al. Mucormycosis in paediatric patients: demographics, risk factors and outcome of 12 contemporary cases. Mycoses. 2011;54(6): e785–e788. [DOI] [PubMed] [Google Scholar]
- 7. Zaoutis TE, Roilides E, Chiou C, et al. Zygomycosis in children: a systematic review and analysis of reported cases. Pediatr Infect Dis J. 2007;26(8):723–727. [DOI] [PubMed] [Google Scholar]
- 8. Roden MM, Zaoutis TE, Buchanan WL, et al. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis. 2005;41(5):634–653. [DOI] [PubMed] [Google Scholar]
- 9. Gokcil Z, Odabasi Z, Kutkcu Y, Umudum H, Vural O, Yardim M. Rhino-orbito-cerebral mucormycosis. J Neurol. 1998;245(10):689–690. [DOI] [PubMed] [Google Scholar]
- 10. Skiada A, Pagano L, Groll A, et al. European Confederation of Medical Mycology Working Group on Zygomycosis. Zygomycosis in Europe: analysis of 230 cases accrued by the registry of the European Confederation of Medical Mycology (ECMM) Working Group on zygomycosis between 2005 and 2007. Clin Microbiol Infect. 2011;17(12):1859–1867. [DOI] [PubMed] [Google Scholar]
- 11. Spellberg B, Walsh TJ, Kontoyiannis DP, Edwards J, Jr, Ibrahim AS. Recent advances in the management of mucormycosis: from bench to bedside. Clin Infect Dis. 2009;48(12):1743–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Peterson KL, Wang M, Canalis RF, Abemayor E. Mucormycosis: evolution of the disease and treatment options. Laryngoscope. 1997;107(7):855–862. [DOI] [PubMed] [Google Scholar]
- 13. Lewis RE, Kontoyiannis DP. Epidemiology and treatment of mucormycosis. Future Microbiol. 2013;8(9):1163–1175. [DOI] [PubMed] [Google Scholar]
- 14. Phulpin-Weibel A, Rivier A, Leblanc T, Bertrand Y, Chastagner P. Focus on invasive mucormycosis in paedi-atric haematology oncology patients: a series of 11 cases. Mycoses. 2013;56(3):236–240. [DOI] [PubMed] [Google Scholar]
- 15. Ribes JA, Vanover-Sams CL, Baker DJ. Zygomycetes in human disease. Clin Microbiol Rev. 2000;13(2):236–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gupta AK, Mann SB, Khosia VK, Sastry KV, Hundal JS. Non-randomized comparison of surgical modalities for paranasal sinus mycoses with intracranial extension. Mycoses. 1999;42(4):225–230. [DOI] [PubMed] [Google Scholar]
- 17. Song WK, Park H, Cinn YW, Rheem I, Pai H, Shin JH. Primary cutaneous mucormycosis in a trauma patient. J Dermatol. 1999;26(12):825–828. [DOI] [PubMed] [Google Scholar]
- 18. Popa G, Blag C, Sasca F. Rhinocerebral mucormycosis in a child with acute lym- phoblastic leukemia: a case report. J Pediatr Hematol Oncol. 2008;30(2):163–165. [DOI] [PubMed] [Google Scholar]
- 19. Monheit JE, Cowan DR, Moore DG. Rapid detection of fungi in tissues using calcofluor white and fluorescence microscopy. Arch Pathol Lab Med. 1984;108(8):616–618. [PubMed] [Google Scholar]
- 20. Stern LE, Kagan RJ. Rhinocerbral mucromycosis in patients with burns: Case report and review of the literature. J Burn Care Rehabil. 1999;20(4):303–306. [DOI] [PubMed] [Google Scholar]
- 21. Seiff SR, Choo PH, Carter SR. Role of local Amphotericin B for sino-orbito fungal infections. Ophthal Plast Reconstr Surg. 1999;15(1):28–31. [DOI] [PubMed] [Google Scholar]
- 22. Wehl G, Hoegler W, Kropshofer G, Meister B, Fink FM, Heitger A. Rhinocerebral mucormycosis in a boy with recurrent acute lymphoblastic leukemia: long-term survival with systemic antifungal treatment. J Pediatr Hematol Oncol. 2002;24(6):492–494. [DOI] [PubMed] [Google Scholar]
- 23. Kajs-Wyllie M. Hyperbaric oxygen therapy for rhinocerebral fungal infection. J Neurosci Nurs. 1995;27(3):174–181. [DOI] [PubMed] [Google Scholar]
- 24. Ferguson BJ, Mitchell TG, Moon R. Adjunctive hyperbaric oxygen for treatment of rhinocerebral mucormycosis. Rev Infect Dis. 1988;10(3):551–559. [DOI] [PubMed] [Google Scholar]
- 25. Strassar D, Kennedy RJ, Adam RD. Rhinocerebral mucormycosis. Therapy with amphotericin B lipid complex. Arch Intern Med. 1996;156(3):337–339. [DOI] [PubMed] [Google Scholar]
- 26. Handzel O, Landau Z, Halpern D. Liposomal amphotericin B treatment for rhinocerebral mucormycosis: how much is enough? Rhinology. 2003;41(3):184–186. [PubMed] [Google Scholar]


