Abstract
Background:
Texturing of breast implants is done to decrease the risk of associated complications. Each manufacturer utilizes unique and at times proprietary techniques to texture the surface of their implants. Little is known about the integrity of this surface structure texturing or the propensity for the surfaces to shed particulate matter. This study aimed to determine the extent of surface particulate shedding from 3 textured implants approved by the US Food and Drug Administration (FDA), which are manufactured by Allergan, Mentor, and Sientra.
Methods:
Control images of each of the 3 textured breast implants were obtained with scanning electron microscopy (SEM). A liquid adhesive, ethylene vinyl acetate (EVA) copolymer was then applied to the external shell of the implants, allowed to cool, and peeled from the surface. Images of the EVA copolymer were taken with SEM to qualitatively analyze displacement of surface particulate debris. Scanning electron microscopy imaging of the implants was repeated for qualitative comparisons with the control images.
Results:
The peeled copolymer of the 3 implants exhibited surface shedding. Comparison of the 3 breast implants showed the shedding to be greatest for the Allergan implant.
Conclusions:
This study highlights the dynamic surface material properties of the 3 FDA-approved breast implants. Shedding of particulate matter from the implant surfaces can be precipitated by moderate adhesion. Our qualitative examination of SEM findings showed more debris shed from the Allergan breast implants than from the Mentor or Sientra implants.
Keywords: breast implant, silicone shedding, surface debris, textured implant
Abstract
Historique:
La texturation des implants mammaires vise à réduire le risque de complications. Pour ce faire, chaque fabricant utilise des techniques uniques et exclusives. On ne sait pas grand-chose sur l’intégrité de la structure après texturation de la surface ni sur sa propension à excréter des particules. La présente étude vsait à déterminer l’étendue de l’excrétion des particules de surface de trois implants texturés approuvés par la Food and Drug Administration (FDA) des États-Unis, fabriqués par Allergan, Mentor et Sientra.
Méthodologie:
Les chercheurs ont obtenu des images témoin de chacun des trois implants mammaires texturés au moyen la microscopie électronique à balayage (MÉB). Ils ont ensuite appliqué un copolymère d’éthylène-acétate de vinyle (EAV) adhésif liquide sur la coquille extérieure des implants, l’ont laissé refroidir, puis l’ont décollé de la surface. Ils ont pris des images du copolymère EAV par MÉB pour procéder à l’analyse qualitative des débris des particules de surface qui s’étaient détachés. Ils ont repris des images des implants par MÉB pour procéder à des comparaisons qualitatives par rapport aux images témoins.
Résultats:
La pelure de copolymère des trois implants contenait des particules de surface. La comparaison entre les trois implants a révélé que l’implant Allergan excrétait plus de particules.
Conclusions:
La présente étude fait ressortir les propriétés dynamiques des matières de surface des trois implants mammaires approuvés par la FDA. L’excrétion de particules à la surface des implants peut être précipitée par une adhésion modérée. L’examen qualitatif des résultats de la MÉB a démontré que les implants mammaires Allergan excrétaient plus de débris que les implants Mentor ou Sientra.
Introduction
Since the introduction of breast implants in the 20th century, their construction has changed substantially—from implants made of polyurethane, polytetrafluoroethylene, and expanded polyvinyl alcohol formaldehyde to saline- and silicone-filled implants. In 1992, the paucity of published, scientific evidence regarding the safety of silicone-filled breast implants resulted in a moratorium on their use, mandated by the US Food and Drug Administration (FDA). The moratorium was lifted in 2006 after further high-quality, epidemiologic research was published, which showed that silicone implants were safe and effective.1 With lifting of the moratorium, silicone-filled implants were once again used in the field of reconstructive, restorative, and aesthetic plastic surgery. Since then, the design of breast implants has continued to evolve with the introduction of anatomically shaped implants, textured surface implants, and cohesive gel implants.2
Surface texturing of breast implants was introduced in an attempt to decrease some common complications of implant-based breast augmentation and reconstruction, such as capsular contracture and excessive movement of the implant within the breast pocket.3-8 The general structure of breast implants is similar: a highly cross-linked (cohesive) silicone gel housed in a silicone elastomer shell. However, the texturing processes used by individual manufacturers are unique. Allergan (Allergan, Inc, Irvine, California), Mentor (Mentor Corp, Santa Barbara, California), and Sientra (Sientra, Inc, Santa Barbara, California) are the 3 manufacturers with FDA-approved implants. Allergan uses a lost-salt technique to produce Biocell macro-texturing. The surface is created by dipping a chuck into uncured silicone, but before the surface dries, it is pressed into a bed of fine, granular salt and then cured in a laminar flow oven to create an irregular pattern of surface pores measuring 600 to 800 µm in diameter with depths of 150 to 200 µm.2,3,8 Mentor uses negative-contact polyurethane foam to stamp its Siltex breast implant surfaces. Specifically, a chuck is dipped into uncured silicone to form the shell after which the uncured silicone shell is pressed into polyurethane foam to imprint pores measuring 70 to 150 µm in diameter and 40 to 100 µm in height. Round implants have approximately 100 pores per inch, whereas the shaped implants have only 65 pores per inch.2,3,8 For proprietary reasons, Sientra does not disclose its texturing process. Although many studies have provided descriptions3 of the texturing process of the 3 manufacturers and the decrease in capsular contracture with textured implants, to our knowledge, none of the descriptions address how the texturing process may lead to residual surface debris that may be shed from the implant to the patient.
In this initial study, we aimed to increase what is known about breast implant texturing to better understand the relationship between surface architecture and residual debris because we thought that this work might lead to additional research into the inflammatory response and capsule formation. To do so, we qualitatively evaluated the surface architecture of the 3 currently approved, textured breast implants for residual surface debris.
Methods
In collaboration with Arizona State University (ASU), we assessed the potential for displacement of surface particulate debris from 3 FDA-approved, textured, silicone-filled breast implants, which were obtained from the manufacturers: Allergan Natrelle Biocell, style 120, a round, high-profile implant (reference no. 120-500; volume, 500 mL; diameter, 13.5 cm; projection, 4.7 cm); Mentor MemoryGel (Siltex), style 4000, a round, high-profile implant (reference no. 354-4500; volume, 500 mL; diameter, 13.2 cm; projection, 5.4 cm); and the Sientra, a round, high-profile implant (reference no. 20621-505HP; volume, 505 mL; diameter, 13.4 cm; projection, 5.3 cm).
At the ASU laboratory facility, the implants were cut by hand into 1-cm square pieces by a single research specialist (K.M.) who wore gloves and cut each specimen in a sterile environment with a fresh blade. Images were then obtained by scanning electron microscopy (SEM) of the implant surfaces (Figure 1). In addition, we scanned the adhesive ethylene vinyl acetate (EVA) copolymer compound as a control, using the same SEM magnification (and microscope) after the adhesive had cured; the images did not show any inherent debris from the copolymer (Figure 2). Next, the EVA copolymer was heated to a maximum temperature of 120°C (range, 75°C-120°C) and was poured over each implant surface, allowed to cool, and then gently peeled from the implants. Because of the polymer’s silicone-like consistency, it can be applied at that temperature and then easily peeled from the surface. By manually applying and removing the polymer, we could draw any residual debris from the implant without distorting the underlying architecture. The same manual technique was used for all 3 implants, allowing any loose debris to be deposited into the EVA for analysis. The debris adhering to the EVA compound was analyzed by imaging the 1 cm2 imprint. The research specialist used the same SEM for each implant to visualize the residual debris. Finally, the implants were imaged again after the EVA compound was applied and after it was removed. The findings were qualitatively compared with those of the control implants for each implant studied.
Figure 1.
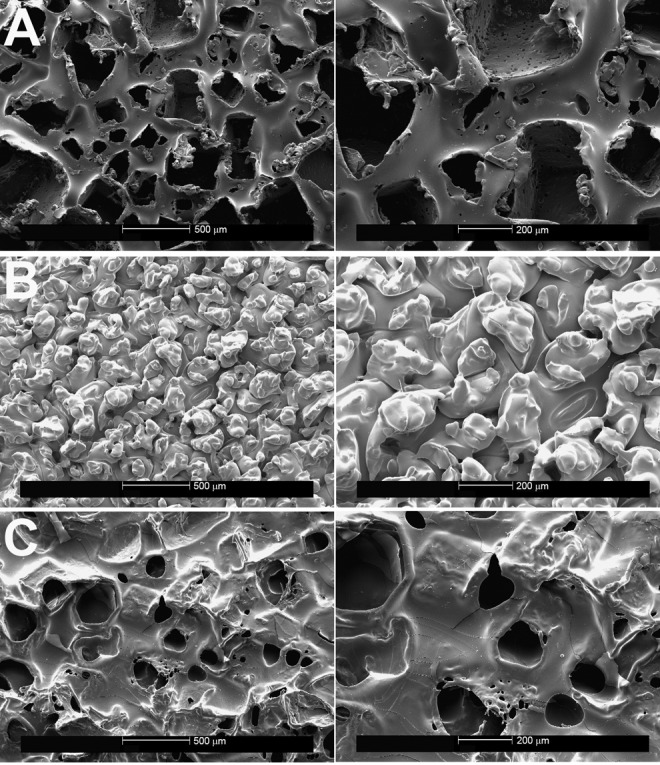
A, Control sections for the Allergan Natrelle Biocell implant, showing the lost-salt technique of surface texturing on scanning electron microscopy. Left panel (×50), right panel (×100). B, Control sections for the Mentor MemoryGel (Siltex) implant, demonstrating the negative imprint stamping technique on scanning electron microscopy. Left panel (×50), right panel (×100). C, Control sections for the Sientra implant, demonstrating the texturing resulting from the manufacturer’s proprietary technique on scanning electron microscopy. Left panel (×50), right panel (×100).
Figure 2.
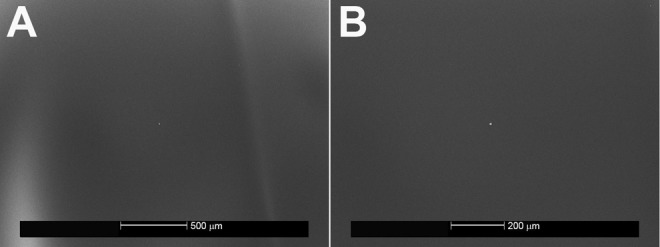
Control sections for the ethylene vinyl acetate (EVA) copolymer. A, ×50, B, ×100. No debris can be seen on the sections, demonstrating the smooth surface of the EVA compound.
Results
When the EVA copolymer was gently removed, each of the 3 implants showed shedding of surface debris on SEM compared with the mirror image surface of their controls. The Natrelle implant shed the most debris of the 3 implants (white flecks; Figure 3). Little surface debris was adherent to the EVA copolymer on the MemoryGel (Figure 4) and Sientra (Figure 5) implants at magnification ×200. In contrast, magnification ×50 showed shed debris on the surface of the Natrelle implant. The control EVA compound did not show debris on SEM magnification (Figure 2). As each SEM magnification increased, we could visualize even more surface debris on the Natrelle implant (Figure 3). Finally, the implants imaged after application and removal of the EVA copolymer did not show any change in surface topography when they were compared with the control implants (Figure 6).
Figure 3.
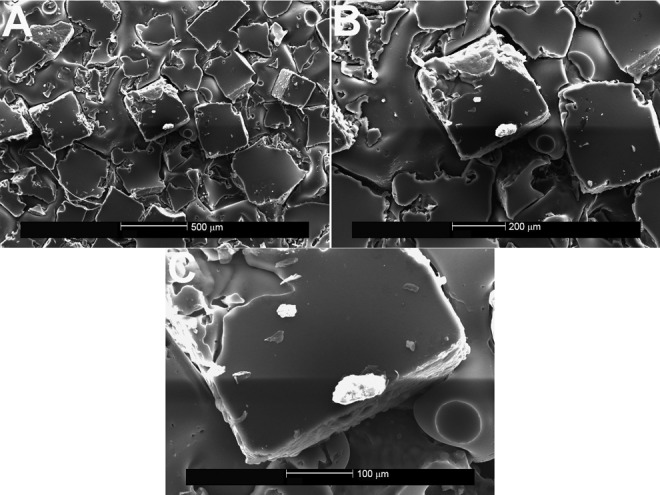
Ethylene vinyl acetate copolymer after spallation of the Allergan Natrelle Biocell implant, shown on scanning electron microscopy (A, ×50; B, ×100; C, ×250). The white flecks of material indicate shed particles of silicone from the surface topography.
Figure 4.
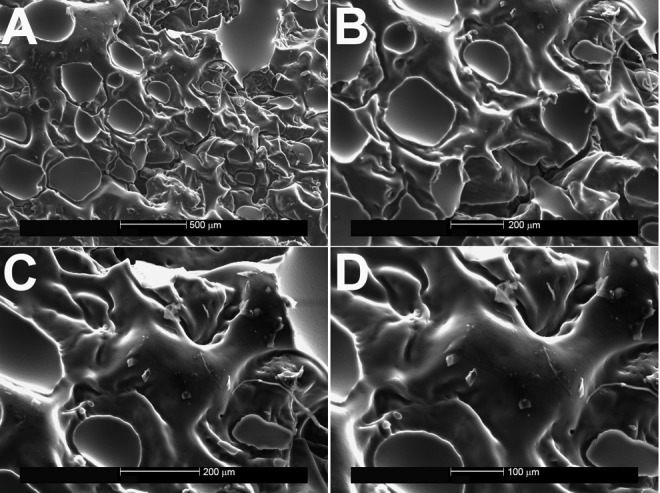
Ethylene vinyl acetate copolymer after spallation of the Mentor MemoryGel (Siltex) implant, shown on scanning electron microscopy (A, ×50; B, ×100; C, ×150; D, ×200).
Figure 5.
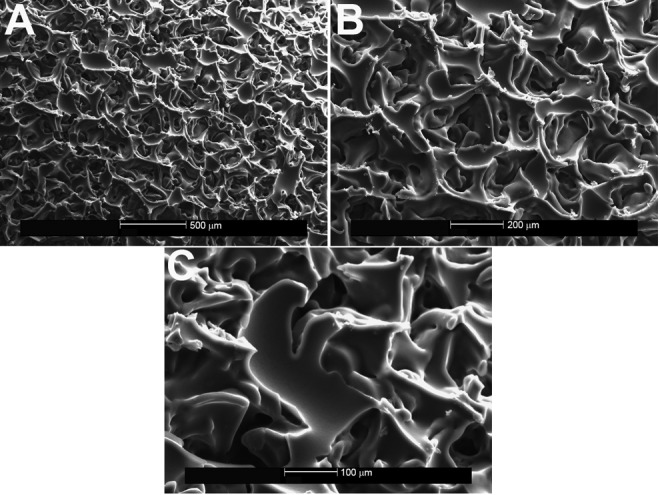
Ethylene vinyl acetate copolymer after spallation of the Sientra implant, shown on scanning electron microscopy (A, ×50; B, ×100; C, ×200). Minimal flecks of silicone were seen on the surface of the EVA compound after removal of the Sientra implant.
Figure 6.
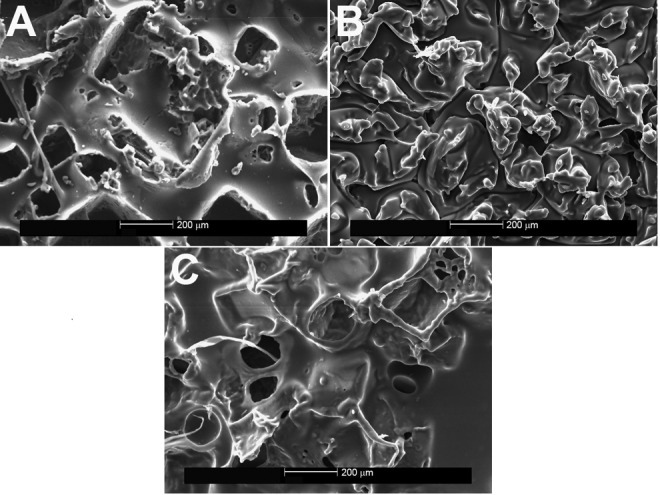
Implant surfaces after the ethylene vinyl acetate copolymer was applied and removed, shown on scanning electron microscopy (×100). A, Allergan Natrelle Biocell implant; B, Mentor MemoryGel (Siltex) implant; C, Sientra implant. Consistent topography can be seen, with no deformation of the texturing.
Discussion
Understanding the texturing process and how it affects the resultant shell of commonly used breast implants is important for plastic surgeons implanting these products. The texture of breast implants affects positioning, inflammatory mediators, capsule formation, and aesthetic result, thus making the process clinically important to success of the procedure. Previous studies clearly demonstrated an advantage of textured surface implants over smooth surface implants regarding capsular contracture.7 In their meta-analysis, Barnsley et al5 showed that textured surface implants were superior to smooth surface breast implants in decreasing the rate of capsular contracture. Furthermore, Barr et al8 studied commonly available silicone implants, including the Allergan Biocell and the Mentor Siltex, and offered a unique overview of their surface topography. However, they did not address the use of Sientra implants nor did they comment about the shedding of debris from the implant surfaces.
Although surface architecture is a very important feature, we aimed to address the possibility of shedding debris as a phenomenon of the texturing process. As shown by our results, each type of implant sheds differing amounts of residual debris from its surface, which may have clinical significance. In 1995, Thuesen et al9 reported no significant difference in capsule thickness or contracture rates between smooth and textured surface implants. However, they did find a significantly greater quantity of silicone particles in capsules of those patients who had textured expander prostheses. This was presumed to be due to the disrupted surface of the textured tissue expander. Although these were not permanent implants, the textured surface expanders had a different capsular composite than the smooth surface expanders, and findings from histologic samples showed silicone particles. This begs the question of whether the textured surface caused the shed debris: No silicone particles were found in the capsules from where the smooth expanders had been removed.9 Our results with textured surface implants support these earlier findings of Thuesen et al.9 We also saw shed debris on the surfaces of the textured implants, especially on the surface of the Allergan Natrelle, which shed more than the Mentor Siltex and Sientra implants. Therefore, we were able to show the difference in shedding among the 3 types of implants. However, at this early stage, our findings have limitations. Although the shed particles were identified by SEM, they were measured qualitatively, not quantitatively. This limitation needs to be addressed in future studies.
In 1993, Wickman et al4 reported differences between capsules around smooth and textured implants, which they analyzed using light and transmission electron microscopy. They found an obvious difference in collagen deposition, with a more robust reaction in capsules around textured implants. Danino et al3,10 compared capsular response and what they called a Velcro response with different implants by evaluating the implants with SEM. However, neither of these reports mentioned capsular silicone content or the possible shedding of silicone debris from the implant. Others have also compared capsular contracture with smooth and textured implants without noting debris shed from the implant surface as a source.6
Conclusion
This study highlights the dynamic surface material properties of 3 currently available, FDA-approved breast implants. Our findings show that shedding of particulate matter on implant surfaces can be precipitated by mild to moderate adhesion. Moreover, this study lays the groundwork for future investigations to explore the clinical significance of these findings for cosmetic breast augmentation and for breast reconstruction with respect to capsule formation and contracture, seroma formation, pro-inflammatory states, infection, and, possibly, breast implant–associated anaplastic large cell lymphoma.
Footnotes
Authors’ Note: Leland H. Webb, Victoria L. Aime, Annie Do, and Kenneth Mossman contributed to design, acquisition of data, data analysis, drafting, and revision of the manuscript. Raman C. Mahabir (principal investigator) contributed to design, acquisition of data, data analysis, drafting, and revision of the manuscript. Mayo Clinic does not endorse specific products or services included in this article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Heden P, Bone B, Murphy DK, Slicton A, Walker PS. Style 410 cohesive silicone breast implants: safety and effectiveness at 5 to 9 years after implantation. Plast Reconstr Surg. 2006;118(6):1281–1287. [DOI] [PubMed] [Google Scholar]
- 2. Maxwell GP, Gabriel A. The evolution of breast implants. Plast Reconstr Surg. 2014;134(1 suppl):12S–17S. [DOI] [PubMed] [Google Scholar]
- 3. Danino AM, Basmacioglu P, Saito S, et al. Comparison of the capsular response to the Biocell RTV and Mentor 1600 Siltex breast implant surface texturing: a scanning electron microscopic study. Plast Reconstr Surg. 2001;108(7):2047–2052. [DOI] [PubMed] [Google Scholar]
- 4. Wickman M, Johansson O, Olenius M, Forslind B. A comparison of the capsules around smooth and textured silicone prostheses used for breast reconstruction: a light and electron microscopic study. Scand J Plast Reconstr Surg Hand Surg. 1993;27(1):15–22. [DOI] [PubMed] [Google Scholar]
- 5. Barnsley GP, Sigurdson LJ, Barnsley SE. Textured surface breast implants in the prevention of capsular contracture among breast augmentation patients: a meta-analysis of randomized controlled trials. Plast Reconstr Surg. 2006;117(7):2182–2190. [DOI] [PubMed] [Google Scholar]
- 6. Fagrell D, Berggren A, Tarpila E. Capsular contracture around saline-filled fine textured and smooth mammary implants: a prospective 7.5-year follow-up. Plast Reconstr Surg. 2001;108(7):2108–2112. [DOI] [PubMed] [Google Scholar]
- 7. Hakelius L, Ohlsen L. Tendency to capsular contracture around smooth and textured gel-filled silicone mammary implants: a five-year follow-up. Plast Reconstr Surg. 1997;100(6):1566–1569. [DOI] [PubMed] [Google Scholar]
- 8. Barr S, Hill E, Bayat A. Current implant surface technology: an examination of their nanostructure and their influence on fibroblast alignment and biocompatibility. Eplasty. 2009;9:e22. [PMC free article] [PubMed] [Google Scholar]
- 9. Thuesen B, Siim E, Christensen L, Schroder M. Capsular contracture after breast reconstruction with the tissue expansion technique: a comparison of smooth and textured silicone breast prostheses. Scand J Plast Reconstr Surg Hand Surg. 1995;29(1):9–13. [DOI] [PubMed] [Google Scholar]
- 10. Danino A, Rocher F, Blanchet-Bardon C, Revol M, Servant JM. A scanning electron microscopy study of the surface of porous-textured breast implants and their capsules: description of the “Velcro” effect of porous-textured breast prostheses [in French]. Ann Chir Plast Esthet. 2001;46(1):23–30. [PubMed] [Google Scholar]


