Supplemental Digital Content is available in the text
Keywords: China, incidence, leukoaraiosis, onset, progression, risk factor
Abstract
Leukoaraiosis (LA) refers to white matter hyperintensities or white matter lesions (WMLs) on magnetic resonance imaging (MRI) scans of the brain; this disease is associated with an increased risk of stroke, dementia, and cognitive decline. The aims of the study are to assess the incidence of LA and its associated risk factors in a Chinese population.
A hospital-based cross-sectional study was conducted that included 4683 patients who were 40 years or older. Data collected included age, sex, hypertension, diabetes, smoking, drinking, homocysteine (HCY), and low-density lipoprotein cholesterol (LDL-C) levels in the blood in addition to brain MRI information. We examined the relationship of those putative risk factors with LA, LA occurrence, and LA progression through single-factor and multivariate analyses.
Of the total subjects, 58.3% (2731/4683 cases) suffered from LA. LA was more frequent amongst elderly females, particularly in those older than 60, compared to men. The incidence of LA increased with age. Age, sex, hypertension, diabetes, smoking, and HCY levels all were risk factors for LA. Amongst those risk factors, both smoking and high HCY levels were associated with the onset process of LA. Moreover, the multivariate logistic analysis revealed that both drinking and abnormal LDL-C levels were positive regulators in the progression process of LA.
This study revealed that the incidence of LA is high in hospitalized patients in China; moreover, age, sex, hypertension, diabetes mellitus, smoking, drinking, and abnormal HCY and LDL-C levels were found to be associated with overall LA risk, LA onset, or LA progression. These results provide insight into strategies for the prevention and treatment of LA.
1. Introduction
Leukoaraiosis (LA), also known as white matter lesions (WMLs), is a universal neuroimaging phenomenon that often occurs in elderly people.[1,2] It refers to white matter hyperintensities (WMHs) that are observed on magnetic resonance imaging (MRI) of the brain on T2-weighted and fluid-attenuated inversion recovery (FLAIR) sequences.[3,4] During the past 20 years, several epidemiological investigations on LA have been performed in Australia, Europe, and America.[5–10] The prevalence of LA is high in certain populations, and it increases with age.[5,10,11] Studies in healthy community-based populations have demonstrated that the prevalence of LA increased from 50.9% in healthy persons aged 44 to 48 years to 95% in healthy persons aged 60 to 90 years.[5,6] LA seems asymptomatic but it is not benign. Some reports also showed that LA could coexist with other geriatric disorders, such as dementia, Alzheimer disease, Parkinson disease, and stroke.[4,11] With the improvement in neuroimaging technology and the widespread use of MRI in the diagnosis of central nervous system disorders, that prevalence of LA is increasing rapidly in the worldwide. Moreover, LA has been confirmed to be associated with an increased risk of dementia, stroke, brain atrophy, abnormal gait, and urinary incontinence,[11–15] which could lead to disability, worse stroke outcome, poor quality of life, and high health care costs.[11,16] LA is even considered as an important MRI marker for cerebral small vessel disease progression.[17] However, the pathogenesis of LA remains unclear to date and it has long been debated. Moreover, LA is increasingly becoming a major public health challenge with the growth of the aging population. Therefore, exploring the comprehensive epidemiology of LA and screening for its clinical risk factors are urgently required for the understanding, prevention, and management of LA.
Except an investigation on risk factors for LA conducted in Zhengzhou which is a Northern city in China,[18] a comprehensively epidemiological study on LA in a large proportion of Chinese population is currently rare to date. Thus, we for the first time conducted a large-scale investigation on LA in Southern Chinese population. The aims of this study were as follows: to provide a whole-picture understanding of LA in hospitalized patients in China; to determine the effects of age, sex, hypertension, diabetes, and serum low-density lipoprotein-cholesterol (LDL-C) and homocysteine (HCY) levels, and epigenetic factors, such as smoking and drinking, on the risk and severity of LA; and to identify those risk factors as potential biomarkers for the diagnosis and management of LA.
2. Materials and methods
2.1. Study population
This survey was a hospitalized population-based, cross-sectional, retrospective study that was conducted in the first affiliated hospital of Xiamen University, Xiamen, Fujian, China. The flow of subject recruitment for the study population is shown in the Figure 1. This study followed 9305 subjects who were hospitalized in the department of neurology in this hospital from 2008 to 2013. The inclusion criteria included subjects ≥40 years of age and subjects with LA or controls without LA. The following subjects were excluded: patients with lack of MRI data and sufficient medical record, subjects <40 years of age, subjects suffering from an intracerebral hemorrhage, a subarachnoid hemorrhage, an intracranial infection, toxic encephalopathy, Parkinson disease, ischemic heart disease, multiple sclerosis, hydrocephalus, and/or malignant tumor. After the exclusion of 2334 subjects who were younger than 40 years or whose brain FLAIR-MRI information was missing and 2288 subjects who were diagnosed with severe brain disorders and/or malignant tumor described in exclusion criteria, a total of 4683 subjects were included in the final analysis (Fig. 1). The remaining subjects were hospitalized in the department of neurology mainly due to stroke, headache, dizzy, and memory Loss. This study complied with the Declaration of Helsinki, and it was approved by the Institutional Review Board of Xiamen University.
Figure 1.
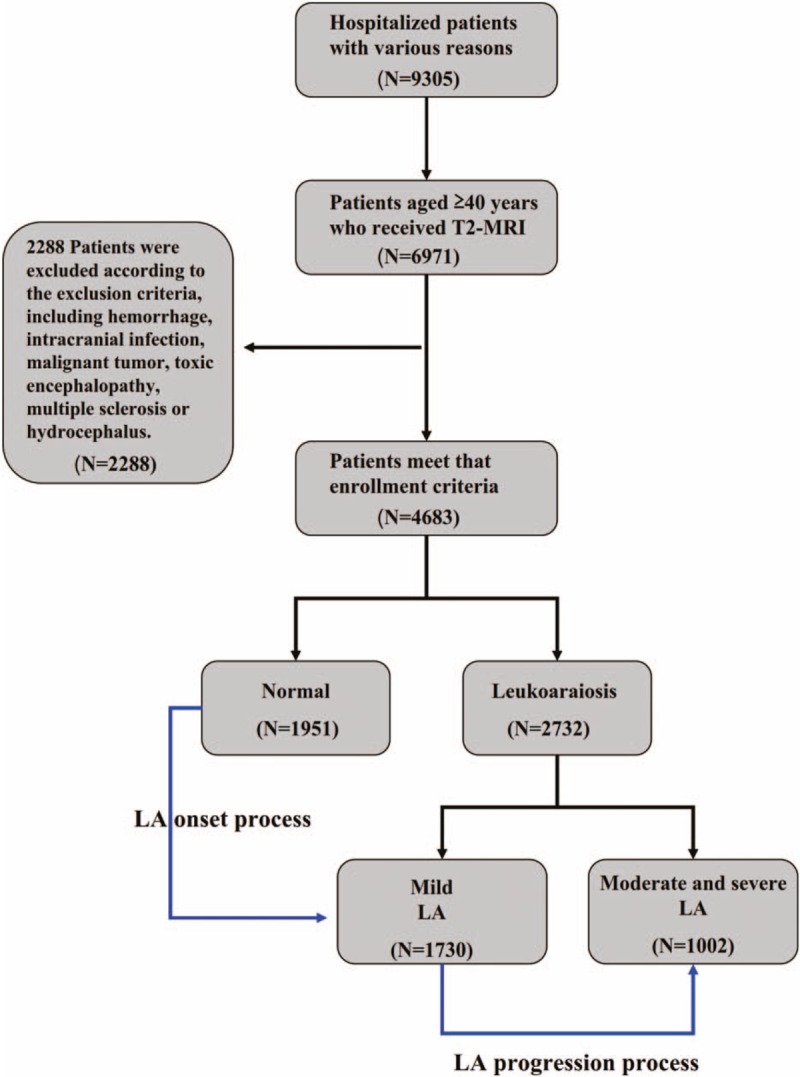
Flowchart of subject recruitment for the study population in the First Hospital affiliated with Xiamen University, including residents 40 years and older who showed normal neuroimaging or white matter hyperintensity on the T2-weighted FLAIR MRI scans of brain scans. LA = Leukoaraiosis, MRI = magnetic resonance imaging.
2.2. Data collection and evaluation
We collected the clinical data, including sex, age, the diagnosis and treatment of hypertension and diabetes, smoking and drinking status, and the results of the FLAIR-MRI of the brain, and several biochemical variables, such as serum LDL-C and HCY levels. The enrolled patients were older than 40 years, and they were divided into 5 groups according to age: 40 to 49, 50 to 59, 60 to 69, 70 to 79, and ≥80 years old. Hypertension was considered a systolic blood pressure ≥140 mm Hg and/or a diastolic blood pressure ≥90 mm Hg or requiring treatment with oral antihypertension drugs, according to the 1999 World Health Organization (WHO) Guidelines for the prevention and treatment of hypertension.[19] Diabetes was determined as a fasting glucose level ≥7.0 mmol/L or 2-hour postglucose load glucose levels ≥ 11.1 mmol/L, according to the diagnostic criteria of WHO for diabetes.[20] Cigarette smoking was defined as having smoked at least 10 cigarettes daily for at least 2 years. Drinking was defined as having a history of chronic alcoholism for 10 years. The LDL-C level was divided into 3 groups: <2.07 mmol/L, ≥2.07 mmol/L and ≤3.36 mmol/L and >3.36 mmol/L. The normal LDL-C level was considered to vary from 2.07 to 3.36 mmol/L. The level of HCY was divided into ≤15 μmol/L (normal HCY levels) and >15 μmol/L (the cutoff for hyperhomocysteinemia). Fasting plasma glucose, total cholesterol, and LDL-C concentrations were determined in enzymatic assays using Hitachi 7170 automatic biochemical analyzed (Hitachi, Tokyo, Japan). HCY level was assayed by high-performance chromatography with fluorometric detection, and was measured using the commercial kits supplied by Beijing Strong Biotechnologies Inc (BSBE, Beijing, China). FLAIR-MRI of the brain was performed using 3.0T Siemens Sonata (Siemens Medical Solutions, Erlangen, Germany) scanners using image acquisition. The MRI protocol included T1, T2-weighted images, and axial FLAIR. The scanning protocol used FLAIR sequence: echo time[TE], 94 ms; repetition time[TR], 9000 ms; inversion time[TI], 2500 ms; field of view[FOV], 22 × mm; matrix, 256 × 256−512 × 512; slice thickness, 5 mm; slice spacing, 1 mm.
2.3. Definition and classification of LA
LA was defined as cerebral WMLs detected by the FLAIR MRI technology using 3.0 T Siemens Sonata (Siemens Medical Solutions) scanners. According to the Fazekas scale, LA is often divided into 2 broad categories: periventricular WMLs and deep/subcortical WMLs.[13] Given that LA shows heterogeneous forms of WMLs that could reflect different progression states and clinical consequences, we moreover divided LA into 3 groups, “mild LA,” showing punctate foci and/or “caps” or pencil-thin lining; “moderate LA,” showing beginning confluence of foci and/or smooth “halo,” and “severe LA,” showing large confluent abnormalities, according to the severity of the WMLs (Fig. 2). Normal was defined as the absence of cerebral WMH signals on the several sections of the FLAIR MRI scans (Fig. 2). Both an experienced neurologist (QL) and a trained neuroradiologist (J-HY) who was blinded to all clinical information independently assessed the neuroimaging and then reached a consensus during the analysis of the MRI data. When the disagreement occurred, another neurology specialist (Q-LM, C-XL, or S-JT) made the final diagnose.
Figure 2.
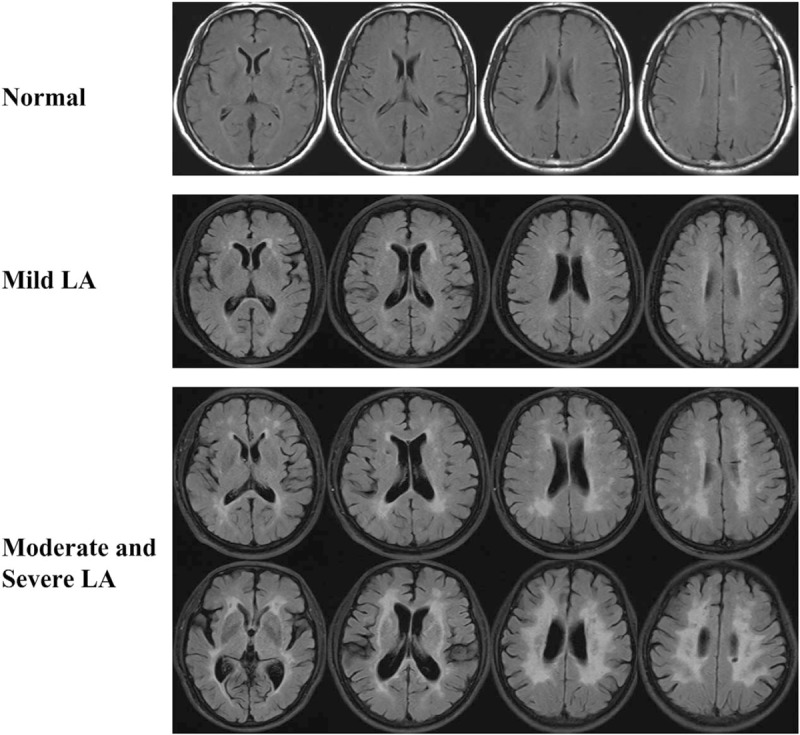
White matter changes on several sections of T2-weighted FLAIR MRI scans. LA-related neuroimaging was classified as normal, mild LA, moderate, and severe LA, according to the modified Fazekas scale. LA = Leukoaraiosis.
The enrolled patients were then classified into “normal,” “mild LA,” and “moderate and severe LA” groups. The last group included those patients whose severity of LA ranged from “moderate” to “severe.” All 3 groups met the following criteria: ≥40 years of age and no changes or any degree of changes in cerebral subcortical white matter on the FLAIR MRI scans of the brain.
2.4. Statistical analyses
The categorical variables were expressed in numbers and percentages, and the continuous variables were expressed in mean values ± standard deviation (standard deviation) unless otherwise specified. We first used Pearson χ2 test to compare the differences between the normal and the LA groups regarding sex, age, hypertension, diabetes, smoking, drinking, and LDL-C and HCY levels. Then, we used a multivariate logistic regression model to estimate odds ratio (OR) and corresponding 95% confidence intervals (CIs) for the association of the above factors with the overall LA risk, LA occurrence, and LA progression, respectively. Furthermore, the correlations of these variables were measured. All of the statistical analyses were performed using SPSS software version 17.0. All P values were calculated using a 2-sided test and a P value <.05 was considered statistically significant.
3. Results
3.1. Characteristics of the study subjects
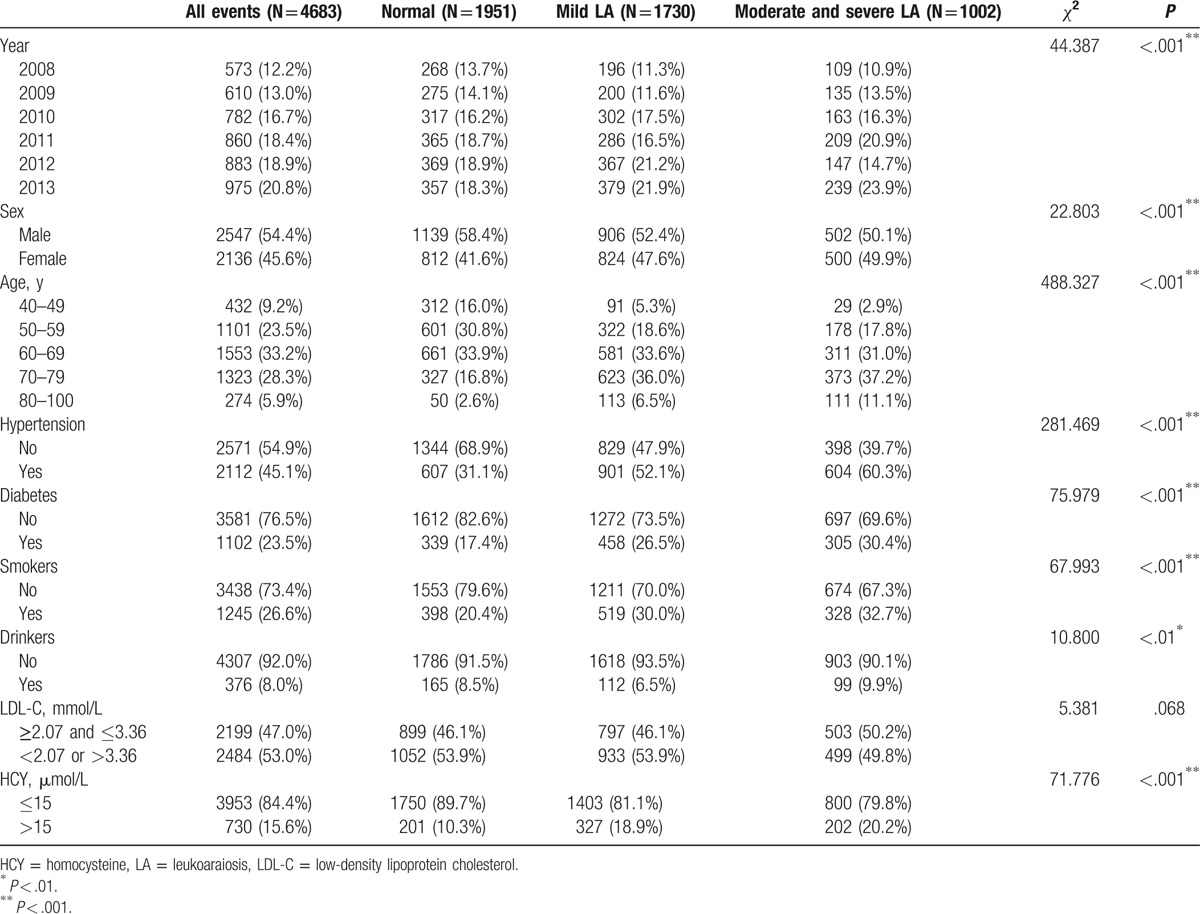
The demographic characteristics are shown in Table 1. During the 6 years of the study, the study population grew from 573 patients in 2008 to 975 in 2013, and the detectable rate of LA patients significantly increased from 53.2% to 63.4% (P < .001). Amongst those subjects, 2547 (54.4%) were male and 3977 (84.9%) were 50 to 79 years old (23.5%, 33.2%, and 28.3% were 50–59, 60–69, and 70–79 years old, respectively). The mean age was 64.3 ± 9.5 years (62.1 ± 9.8 years for men and 66.9 ± 8.3 years for women). The majority of the subjects did not have a history of diabetes (76.5%), smoking (73.4%), or drinking (92.0%). Amongst all of the subjects, 2112 (45.1%) suffered from hypertension, and 2484 (53.0%) showed abnormal LDL-C levels (53.0%). However, only 730 (15.6%) showed abnormal HCY levels. In addition, as shown in Table 1, 41 7% were normal (58.4% were men, 41.6% were women), 36.9% had mild LA (52.4% were men, 47.9% were women) and 21.4% had moderate and severe LA (50.1% were men, 49.9% were women).
Table 1.
Baseline characteristics of the study population.
3.2. Incidence of LA in males and females
The overall incidence of LA was 58.3% in the total subjects (2732 of 4683 cases), 55.3% in men (1408 of 2547 cases) and 62.0% in women (1324 of 2136 cases). Of all the LA subjects, 1730 (63.3%) had mild LA, and 1002 (36.7%) had moderate and severe LA. Similar to that of total LA, both the overall incidence of mild LA and the incidence of moderate and severe LA in women were higher than those in men (38.6% vs 35.6% for mild LA, 23.4% vs 19.7% for moderate and severe LA) (Fig. 3). The age-specific incidence of LA in men and women is shown in Figure 3A. The incidence of LA increased with age amongst men and women and peaked in the age group 80 to 100 years for both men and women. The incidence of LA in men was 33.4% in those aged 40 to 49 years, 56.6% in those aged 50 to 59 years, 55.9% in those aged 60 to 69 years, 62.4% in those aged 70 to 79 years, and 80.7% in men older than 80 years; in women, the prevalence was 3.7% for ages 40 to 49 years, 9.2% for ages 50 to 59 years, 59.3% for ages 60 to 69 years, 80.0% for ages 70 to 79 years, and 82.7% for individuals older than 80 years. As shown in Figure 3A, the specific incidence of LA was higher in women than in men after the age of 60 years, when the incidence increased significantly amongst women while decreasing slightly amongst men.
Figure 3.
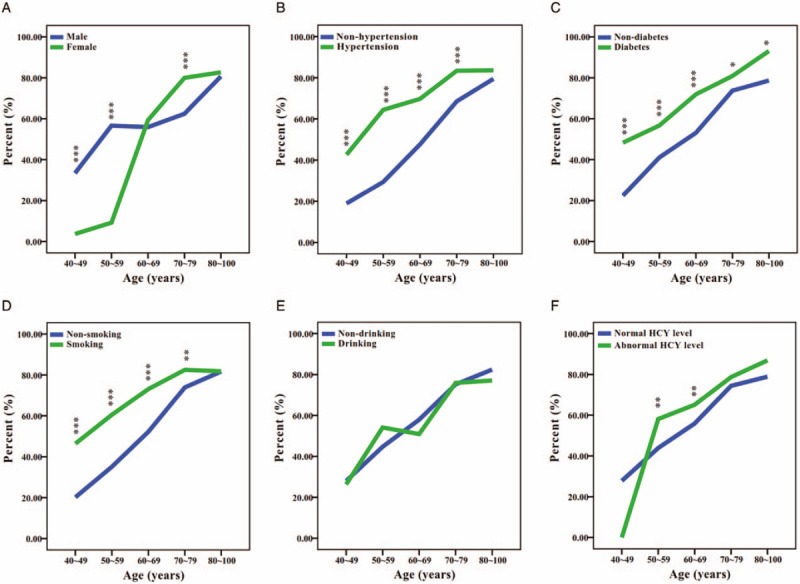
Comparison of the age-specific incidence of leukoaraiosis for 6 risk factors (sex, hypertension, diabetes, smoking, drinking, and HCY levels) among Chinese hospitalized patients. ∗P < .05, ∗∗P < .01, and ∗∗∗P < .001.
3.3. Risk factors for LA
To identify the risk factors for LA, we first evaluated the associations of sex, age, hypertension, diabetes, smoking, drinking, and LDL-C and HCY levels with LA risk using the χ2 test. The results are displayed in Table 1 and Supplemental Figure 1. Except for LDL-C levels (P = .118), all of the variables were significantly associated with LA (P < .001). The significant risk factors were age (P < .001) and hypertension (P < .001). As shown in Supplemental Figure 2, the incidences of overall LA, mild LA, and moderate and severe LA all increased with age, indicating that age not only acts as a crucial risk factor for LA but also has an important effect on the severity of LA. Furthermore, we stratified by age for those categories of the 6 significantly associated factors described above. As expected, the age-specific incidence of overall LA increased with increasing age within each category, as shown in Figure 3. We showed that the age-specific incidence of LA differed significantly according to categories of sex, hypertension, diabetes, smoking, and HCY level (Fig. 3A–D, F), but it did not differ significantly according to drinking category (Fig. 3E). Furthermore, we found that the age-specific incidences of LA were significantly higher in hypertension, diabetes, and smoking groups in which subjects were 40 to 79 years old (P < .001 for 40–69 years, P < .05 for 70–79 years) (Fig. 3B–D), whereas this was true primarily in subjects with abnormal HCY levels who were 50 to 69 years old (P < .01) (Fig. 3F).
We next performed a multivariable logistic analysis to estimate the OR and corresponding 95% CI for the associations of sex, age, hypertension, diabetes, smoking, drinking, and HCY and LDL-C levels with the risk of overall LA. Table 2 showed that age (OR = 1.071, 95% CI: 1.063–1.080), sex (OR = 1.394, 95% CI: 1.204–1.613), hypertension (OR = 2.425, 95% CI: 2.125–2.766), diabetes (OR = 1.635, 95% CI: 1.396–1.914), smoking (OR = 2.051, 95% CI: 1.736–2.423), and HCY levels (OR = 1.434, 95% CI: 1.188–1.731) were all significantly associated with an increased risk of LA (P < .001). However, neither dinking (OR = 0.950, 95% CI: 0.747–1.209, P = .676) nor LDL-C levels (OR = 0.891, 95% CI: 0.784–1.013, P = .079) showed significant associations with LA risk. Taken together, those results suggest that age, sex, hypertension, diabetes, smoking, and HCY levels are risk factors for LA.
Table 2.
Odds ratio and 95% confidence intervals for overall LA, early onset, and late progression of LA in the multivariate logistic regression.
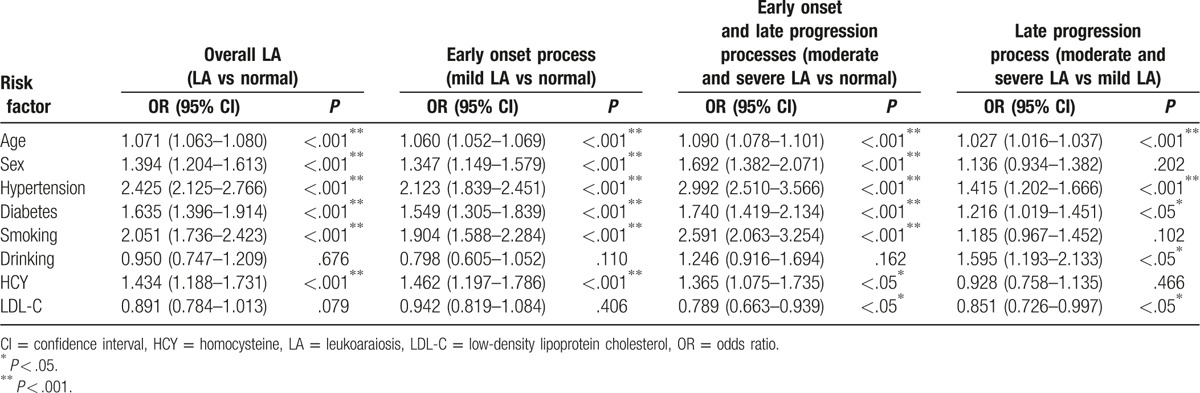
3.4. Differences of risk factors for early onset and late progression of LA
To decipher the different risk factors between LA occurrence and development, we considered the process from “normal” to “mild LA” and that from “mild LA” to “moderate and severe LA” as the early onset and late progression of LA, respectively; furthermore, we performed a multivariable logistic analysis on “mild LA” vs “normal” and “moderate and severe LA” vs “mild LA”, respectively. Unexpectedly, not all of the risk factors for LA described above were shown to be associated with both processes. Surprisingly, we found that both smoking (OR = 1.904, 95% CI: 1.588–2.284, P < .001) and HCY levels (OR = 1.462, 95% CI: 1.197–1.786, P < .001) were only shown to be significantly associated with the early onset of LA. In addition, both drinking (OR = 1.595, 95% CI: 1.193–2.133, P < .05) and LDL-C levels (OR = 0.851, 95% CI: 0.726–0.997, P < .05) were only shown to be significantly associated with the late progression (Table 2). In addition, age, hypertension, and diabetes all were associated with both early onset (OR = 1.060, 95% CI: 1.052–1.069, P < .001 for age; OR = 2.123, 95% CI: 1.839–2.451, P < .001 for hypertension; OR = 1.549, 95% CI: 1.305–1.839, P < .001 for diabetes) and late progression (OR = 1.027, 95% CI: 1.016–1.037, P < .001 for age; OR = 1.415, 95% CI: 1.202–1.666, P < .001 for hypertension; OR = 1.216, 95% CI: 1.019–1.451, P < .05 for diabetes) (Table 2). These results suggest that there is a difference between the risk factors for the early onset of LA and those contributing to the late progression.
4. Discussion
In the present study, we reported a high incidence of LA. The incidence of LA in our subjects (58.3%) was higher than that previously reported in Chinese hospitalized patients (36.5% rate),[18] but it was lower than those observed in healthy populations from the Netherlands,[5] Australia,[21] and Europe,[7] respectively. Age range, comorbid medical disorders, ethnicity, and sample sizes likely account for the between-study differences in LA incidence. In addition, our study showed that the overall incidence and severity of LA gradually increased with age, with a similar pattern in men and women, in accordance with previous studies. These results support age as the crucial risk factor for LA.
In accordance with the previous studies,[5,9,22–24] this present study showed that the incidences of overall LA, mild LA, and moderate and severe LA were higher in women than in men, suggesting that women, particularly older subjects (>60 years old), are predisposed to LA. However, we found that both sexes had similar contributions from factors such as hypertension, diabetes, and abnormal HCY levels in the association analysis. Physiological causes (such as hormone levels, brain morphology, etc) and/or genetic factors may contribute to the sex difference.[25,26]
In addition to the age and sex, the present study also confirmed the close association between hypertension, diabetes, smoking, and HCY levels and LA through both single-factor analysis and multivariate logistic analysis. Consistent with previous results,[27–29] our findings identified hypertension as the most important risk factor for LA. Although the roles of hypertension in LA remain unclear, we think that they may be associated with the pathology of LA, such as widespread perivascular rarefaction of myelin, patchy demyelination, partial loss of axons and oligodendroglial cells, mild reactive astrocytic gliosis, and sparsely distributed macrophages and the disruption of ependymal.[4,30]
In accord with the significant association of hypertension with overall LA, we found that the incidence of LA in the group with diabetes was significantly higher than that in the group without diabetes at each age category. Consistent with the recent finding,[31] this suggests that diabetes is an important risk factor for LA.
In the multivariate logistic analysis, both smoking and HCY levels were shown to be risk factors for LA occurrence only. However, drinking and LDL-C levels may not contribute to the occurrence of LA, but they may contribute to its progression. We thought that the lack of a significant association between drinking and LDL-C with the overall LA observed in both the single-factor analysis and the multivariate analysis was mainly a result of the different effects of risk factors on LA onset and development. Moreover, these novel findings suggested that controlling smoking and HCY levels could prevent the occurrence of LA, but it could not attenuate the progression of LA. Conversely, controlling drinking and LDL-C levels could not trigger LA onset but could slow down or stop previously existing LA changes. However, it is urgent to perform longitudinal population-based studies to investigate the roles of those risk factors in all the processes of LA. Once the association of the risk factors with LA is confirmed, this information will be helpful for clinicians to manage the risk factors for LA and to generate effective strategies for its prevention and treatment.
Taken together, the above results indicate that LA is not a typical type of neurodegenerative disease but is an avoidable disease, and controlling certain vascular risk factors could be helpful for patients to prevent LA occurrence and slow down LA progression, or even reverse already existing LA lesions. To date, the effect of antihypertensive medication on LA progression has been confirmed by at least 3 large prospective population-based studies.[27–29] Those reports showed that antihypertension treatment was associated with a smaller increase in WML volume than no hypertensive treatment. Moreover, participants with poorly controlled hypertension had a higher risk of WMLs than those with successfully treated hypertension. Those findings suggested that the effective treatment of hypertension could reduce the risk of LA and prevent its progression. In addition to antihypertensive treatment, large longitudinal population-based studies are necessary to assess the role of antidiabetic treatment, smoking cessation, abstinent alcoholics, and controlling blood lipid levels with statins in the management of LA.
Although multiple putative mechanisms have been reported to be involved in the pathogenesis of LA, such as abnormal cerebral perfusion, dysfunction of the blood-brain barrier, ischemia damage, disturbances in cerebrospinal fluid circulation, diffuse cerebral microangiopathy, inflammatory response, and genetic changes,[32–41] an efficient management strategy has not been established to prevent the LA occurrence, slow down the disease progression or reverse already existing WMLs.[42] This study for the first time revealed the difference of risk factors between LA onset and progression processes, and it could provide an epidemiological evidence for making several specific interventions for the patients with different severity degree of LA
The major strengths of our study are the large sample size and broad age range. In addition, patients with several LA-associated small vessel diseases for which LA may be a causal or predictive factor were excluded in this study, and thus avoiding the possibility that risk factors for those LA-associated disorders interfered the effects of those factors for LA. However, we should acknowledge that there are some limitations in this study. The major shortcoming is that this is a hospitalized cross-sectional study in which there is almost certainly a selection bias. Therefore, the incidence in this study may not represent the overall incidence of LA in the general population. Moreover, we are unable to make inferences as to causality. Another potential limitation was the use of visual rating scales which may not only make the results of the study incomparable but may also result in an inexact assessment of LA incidence and progression. Therefore, an automated WMLs volumetric/quantitative analysis is suggested to be applied in the epidemiology study of LA in future. In addition, the association analysis of several recently reported risk factors, such as C-reactive protein, uric acid, thyroid function and vitamin B12, obesity index-body mass, and working and living conditions,[43–45] with the risk of LA were not conducted in this study since these parameters were not available for all of subjects. The last but not the least is the limitation that both the percent of subjects with stroke among this study cohort and the relationship of stroke and LA could not be assessed because the information is inaccessible for a large portion of subjects.
5. Conclusions
This study highlighted the high incidence of LA in hospitalized patients in China and confirmed age and hypertension as the most important risk factors for LA. Moreover, it suggested the sex difference that older women, particularly those aged 70 to 79 years, are predisposed to LA. In the present study, we revealed that LA was a complex illness that may be driven by multiple vascular factors, including diabetes. In addition, we found for the first time different patterns of risk factors between LA occurrence and progression, and we identified both smoking and high HCY levels as the risk factors in the early onset process of LA; we also recognized both drinking and abnormal LDL-C levels as the positive regulators in the late progression process of LA. The identification and further confirmation of those factors for overall LA risk, LA occurrence, and LA progression could provide effective information for clinicians and scientists to explore effective strategies for the prevention and treatment of LA.
Supplementary Material
Acknowledgments
The authors thank all the subjects for participating in this study. This study was approved by the Science and Technology Grant of Xiamen (No. 3502Z20164002), Fujian Provincial Sanitary Bureau for the middle-aged and young backbone (No. 2016-ZQN-81), and CTCTCT. This study was approved by the ethical committee of Xiamen University.
Footnotes
Abbreviations: CI = confidence interval, CNS = central nervous system, FLAIR = fluid-attenuated inversion recovery, LA = leukoaraiosis, LDL-C = low-density lipoprotein cholesterol, HCY = homocysteine, OR = odds ratio, SD = standard deviation, WMHs = white matter hyperintensities, WML = white matter lesion.
Current addresses: Translational Medicine Research Center (TMRC), School of Pharmaceutical Sciences, Xiamen University Xiang’an South Road, Xindian Town, Xiangan Dist, Xiamen, Fujian, China.
QL and W-QH have contributed equally to this work.
The authors report no conflicts of interest.
Supplemental Digital Content is available for this article.
References
- [1].Hachinski VC, Potter P, Merskey H. Leuko-araiosis. Arch Neurol 1987;44:21–3. [DOI] [PubMed] [Google Scholar]
- [2].O'Sullivan M. Leukoaraiosis. Pract Neurol 2008;8:26–38. [DOI] [PubMed] [Google Scholar]
- [3].Fazekas F, Barkhof F, Wahlund LO, et al. CT and MRI rating of white matter lesions. Cerebrovasc Dis 2002;13(suppl 2):31–6. [DOI] [PubMed] [Google Scholar]
- [4].Lin Q, Huang WQ, Tzeng CM. Genetic associations of leukoaraiosis indicate pathophysiological mechanisms in white matter lesions etiology. Rev Neurosci 2015;26:343–58. [DOI] [PubMed] [Google Scholar]
- [5].De Leeuw FE, De Groot JC, Achten E, et al. Prevalence of cerebral white matter lesions in elderly people: a population based magnetic resonance imaging study. The Rotterdam Scan Study. J Neurol Neurosurg Psychiatry 2001;70:9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Wen W, Sachdev PS, Li JJ, et al. White matter hyperintensities in the forties: their prevalence and topography in an epidemiological sample aged 44-48. Human Brain Mapp 2009;30:1155–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Launer LJ, Berger K, Breteler MM, et al. Regional variability in the prevalence of cerebral white matter lesions: an MRI study in 9 European countries (CASCADE). Neuroepidemiology 2006;26:23–9. [DOI] [PubMed] [Google Scholar]
- [8].Longstreth WT, Jr, Manolio TA, Arnold A, et al. Clinical correlates of white matter findings on cranial magnetic resonance imaging of 3301 elderly people. Stroke 1996;27:1274–82. [DOI] [PubMed] [Google Scholar]
- [9].Liao D, Cooper L, Cai J, et al. Presence and severity of cerebral white matter lesions and hypertension, its treatment, and its control. The ARIC Study. Atherosclerosis Risk in Communities Study. Stroke 1996;27:2262–70. [DOI] [PubMed] [Google Scholar]
- [10].Launer LJ. Epidemiology of white matter lesions. Top Magn Reson Imaging 2004;15:365–7. [DOI] [PubMed] [Google Scholar]
- [11].Xiong YY, Mok V. Age-related white matter changes. J Aging Res 2011;2011:617927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Debette S, Markus HS. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ 2010;341:c3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kim KW, MacFall JR, Payne ME. Classification of white matter lesions on magnetic resonance imaging in elderly persons. Biol Psychiatry 2008;64:273–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lambert C, Benjamin P, Zeestraten E, et al. Longitudinal patterns of leukoaraiosis and brain atrophy in symptomatic small vessel disease. Brain 2016;139(pt 4):1136–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Baezner H, Blahak C, Poggesi A, et al. Association of gait and balance disorders with age-related white matter changes: the LADIS study. Neurology 2008;70:935–42. [DOI] [PubMed] [Google Scholar]
- [16].Ryu WS, Woo SH, Schellingerhout D, et al. Stroke outcomes are worse with larger leukoaraiosis volumes. Brain 2017;140(pt 1):158–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Benjamin P, Zeestraten E, Lambert C, et al. Progression of MRI markers in cerebral small vessel disease: Sample size considerations for clinical trials. J Cereb Blood Flow Metab 2016;36:228–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Zhang S, Kang X. Investigation of the risk factors for leukoaraiosis (LA). Asia Pac J Public Health 2013;25(4 suppl):64S–71S. [DOI] [PubMed] [Google Scholar]
- [19].1999 World Health Organization-International Society of Hypertension Guidelines for the Management of Hypertension. Guidelines Subcommittee. J Hypertens 1999;17:151–83. [PubMed] [Google Scholar]
- [20].Alberti KG, Zimmet PZ. Definition diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med 1998;15:539–53. [DOI] [PubMed] [Google Scholar]
- [21].Caporaso GL, Bibb JA, Snyder GL, et al. Drugs of abuse modulate the phosphorylation of ARPP-21, a cyclic AMP-regulated phosphoprotein enriched in the basal ganglia. Neuropharmacology 2000;39:1637–44. [DOI] [PubMed] [Google Scholar]
- [22].Mukamal KJ, Longstreth WT, Jr, Mittleman MA, et al. Alcohol consumption and subclinical findings on magnetic resonance imaging of the brain in older adults: the cardiovascular health study. Stroke 2001;32:1939–46. [DOI] [PubMed] [Google Scholar]
- [23].Liao D, Cooper L, Cai J, et al. The prevalence and severity of white matter lesions, their relationship with age, ethnicity, gender, and cardiovascular disease risk factors: the ARIC Study. Neuroepidemiology 1997;16:149–62. [DOI] [PubMed] [Google Scholar]
- [24].Tobias M, Yeh LC. How much does health care contribute to health gain and to health inequality?. Trends in amenable mortality in New Zealand 1981–2004. Aust N Z J Public Health 2009;33:70–8. [DOI] [PubMed] [Google Scholar]
- [25].Kanaan RA, Chaddock C, Allin M, et al. Gender influence on white matter microstructure: a tract-based spatial statistics analysis. PLoS One 2014;9:e91109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Atwood LD, Wolf PA, Heard-Costa NL, et al. Genetic variation in white matter hyperintensity volume in the Framingham Study. Stroke 2004;35:1609–13. [DOI] [PubMed] [Google Scholar]
- [27].Godin O, Tzourio C, Maillard P, et al. Antihypertensive treatment and change in blood pressure are associated with the progression of white matter lesion volumes: the Three-City (3C)-Dijon Magnetic Resonance Imaging Study. Circulation 2011;123:266–73. [DOI] [PubMed] [Google Scholar]
- [28].De Leeuw FE, De Groot JC, Oudkerk M, et al. Hypertension and cerebral white matter lesions in a prospective cohort study. Brain 2002;125(pt 4):765–72. [DOI] [PubMed] [Google Scholar]
- [29].Van Dijk EJ, Breteler MM, Schmidt R, et al. The association between blood pressure, hypertension, and cerebral white matter lesions: cardiovascular determinants of dementia study. Hypertension 2004;44:625–30. [DOI] [PubMed] [Google Scholar]
- [30].Fazekas F, Kleinert R, Offenbacher H, et al. Pathologic correlates of incidental MRI white matter signal hyperintensities. Neurology 1993;43:1683–9. [DOI] [PubMed] [Google Scholar]
- [31].Lucatelli P, Montisci R, Sanfilippo R, et al. Is there an association between leukoaraiosis volume and diabetes? J Neuroradiol 2016;43:273–9. [DOI] [PubMed] [Google Scholar]
- [32].Szolnoki Z. Pathomechanism of leukoaraiosis: a molecular bridge between the genetic, biochemical, and clinical processes (a mitochondrial hypothesis). Neuromolecular Med 2007;9:21–33. [DOI] [PubMed] [Google Scholar]
- [33].Pantoni L, Garcia JH. Pathogenesis of leukoaraiosis: a review. Stroke 1997;28:652–9. [DOI] [PubMed] [Google Scholar]
- [34].Pantoni L. Pathophysiology of age-related cerebral white matter changes. Cerebrovasc Dis 2002;13(suppl 2):7–10. [DOI] [PubMed] [Google Scholar]
- [35].Assareh A, Mather KA, Schofield PR, et al. The genetics of white matter lesions. CNS Neurosci Ther 2011;17:525–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Fornage M, Debette S, Bis JC, et al. Genome-wide association studies of cerebral white matter lesion burden: the CHARGE consortium. Ann Neurol 2011;69:928–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Verhaaren BF, Debette S, Bis JC, et al. Multiethnic genome-wide association study of cerebral white matter hyperintensities on MRI. Circ Cardiovasc Genet 2015;8:398–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Lin Q, Huang WQ, Tzeng CM. Genetic associations of leukoaraiosis indicate pathophysiological mechanisms in white matter lesions etiology. Rev Neurosci 2015;26:343–58. [DOI] [PubMed] [Google Scholar]
- [39].Sam K, Crawley AP, Poublanc J, et al. Vascular dysfunction in leukoaraiosis. Am J Neuroradiol 2016;37:2258–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Lin J, Wang D, Lan L, et al. Multiple factors involved in the pathogenesis of white matter lesions. BioMed Res Int 2017;2017:9372050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Huang WQ, Ye HM, Li FF, et al. Analysis of genetic polymorphisms associated with leukoaraiosis in the southern Chinese population: a case-control study. Medicine 2016;95:e3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Helenius J, Tatlisumak T. Treatment of leukoaraiosis: a futuristic view. Curr Drug Targets 2007;8:839–45. [DOI] [PubMed] [Google Scholar]
- [43].Li JJ, Huang YH, Lin YY, et al. The association of uric acid with leukoaraiosis. J Int Med Res 2017;45:75–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Grueter BE, Schulz UG. Age-related cerebral white matter disease (leukoaraiosis): a review. Postgrad Med J 2012;88:79–87. [DOI] [PubMed] [Google Scholar]
- [45].Guan J, Yan C, Gao Q, et al. Analysis of risk factors in patients with leukoaraiosis. Medicine 2017;96:e6153. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


