Abstract
Vertical human immunodeficiency virus (HIV) infection has decreased in industrialized countries in recent decades, but there are no studies on the mechanisms of HIV transmission among infected children in Spain. Our aim was to study the characteristics and trends of diagnoses of vertically HIV-infected children in Spain from 2004 to 2013.
Vertically HIV-infected children were selected if they were diagnosed from 2004 to 2013, were aged 0 to 18 years old, and were included in the Cohort of the Spanish Pediatric HIV Network (CoRISpe). Demographic, clinical, immunological, and virological data at diagnosis were obtained. The rate of diagnoses of vertically HIV-infected children was calculated as the number of cases per 100,000 inhabitants. Obstetric data of mothers of Spanish children and prophylaxis at childbirth and postpartum were obtained.
A total of 218 HIV-infected children were included in the study. Of this sample, 182 children (83.5%) were perinatally HIV infected, and 125 out of those 182 children (68.7%) were born in Spain. The vertically HIV-infected Spanish children were diagnosed earlier and were in better clinical and immunological condition at diagnosis than were foreign children. The rate of vertically HIV-infected children declined from 0.09 in 2004 to 0.03 in 2013 due to the decrease in the rate of children born in Spain (0.08 in 2004 vs 0.01 in 2013). A total of 60 out of 107 mothers (56.1%) of Spanish children were diagnosed at or after childbirth. However, this number declined between 2004 and 2013.
The rate of new HIV diagnoses of vertically HIV-infected children decreased significantly between 2004 and 2013 from 0.09 to 0.03 per 100,000 inhabitants.
Keywords: adolescents, children, HIV, new diagnoses, vertical transmission
1. Introduction
Approximately, 35 million people worldwide are living with human immunodeficiency virus (HIV), and 2.1 million new infections were reported in 2013.[1] Most individuals living with HIV live in sub-Saharan Africa and have been infected mainly through sexual contact. A total of 29,157 new HIV infections (3278 of which were in Spain) within 30 European Union/European Economic Association countries were reported in 2013.[2,3] Due to the prevention of mother-to-child transmission (PMTCT) beginning in 1994 and the use of combination antiretroviral therapy (cART) since 1996, vertical HIV infection has decreased to 1% to 2% in newborns in industrialized countries such as Spain.[4–11] In 2007, the Spanish Health Ministry recommended HIV screening for pregnant women, and in 2007 and 2013, the Spanish Clinical Guide recommended HIV screening for all pregnant women within the first 3 months of pregnancy and in the third trimester if the previous test was negative.[12,13] Thus, of the 3278 new diagnoses reported in Spain in 2013, only 10 newborns (0.3%) were vertically HIV infected.[3]
Epidemiological surveillance of HIV in Spain is based on 2 systems: the National Registry of AIDS, including all AIDS cases from the beginning of the HIV epidemic in Spain (1981) recorded in the AIDS Spanish Registry of Autonomous Communities (AACC), and the Information Systems on New HIV Diagnoses (SINIVIH) (2000), including the new HIV diagnoses recorded in the AACC Surveillance Systems.[3] The SINIVIH collects epidemiological, clinical, and immunological data of children at HIV diagnosis. However, this system does not have any data on trends describing the incidence of new diagnoses of vertically HIV-infected children.
In 2008, an open, multicenter, retrospective, and prospective study called Cohort of the Spanish Pediatric HIV Network (CoRISpe), which collaborates actively with the Spanish HIV HGM BioBank, was founded in accordance with Spanish law on the protection of personal data.[14–16] The availability of CoRISpe electronic data has been critical in the research of vertically HIV-infected children since the first years of the pandemic. CoRISpe has collected epidemiological, clinical, immunological, virological, analytical, and antiretroviral prospective data from HIV-infected children and adolescents, with follow-up in Spanish pediatric units since 2008, as well as retrospective data from children since 1995.[14] Its aim is to facilitate the conducting of high-quality research studies on HIV-infected children. Until December 2013, the CoRISpe has included data from 1079 HIV-infected children and adolescents from 56 hospitals of 16 AACC, representing almost the entire geographical territory of Spain. We have obtained interesting results from these data, as seen in several of our published articles. Moreover, CoRISpe has collaborated for many years with various European cohorts (e.g., the European Pregnancy and Pediatric HIV Cohort Collaboration, EPPICC; Collaboration of Observational HIV Epidemiological Research Europe, COHERE; and Pediatric European Network for Treatment of AIDS, PENTA).
Our main objectives were to describe the characteristics of the children included in the CoRISpe who were newly diagnosed with HIV infection, as well as the trends in the rate of new diagnoses of vertically HIV-infected children from 2004 to 2013. Our specific objectives were as follows: to describe the mechanism of transmission of new HIV diagnoses in children and adolescents in Spain; to describe epidemiological trends in the rates of vertically HIV-infected children diagnosed in Spain, total and stratified by origin of the children; to analyze clinical, immunological, and virological differences in mother-to-child HIV-infected children, by origin (children born in Spain vs children born in other countries); and to describe the features of the mothers of the children born in Spain in terms of pregnancy and prophylaxis at childbirth and in the newborns, stratifying by mothers’ origin (Spanish mothers vs foreign mothers).
2. Patients and methods
2.1. Children selection
HIV-infected children included in the CoRISpe, diagnosed in Spain from January 2004 to December 2013 between the ages of 0 and 18 years, were enrolled in our study. HIV-infected children were excluded if they were diagnosed in foreign countries before coming to Spain. Written informed consent was obtained from all patients, and the Ethics Committee of the participating hospitals approved the study.
2.2. Description of newly diagnosed HIV children and analysis of vertically HIV-infected children
The following data were collected from children at the time of HIV diagnosis: epidemiological data (birth country, sex, and birth and diagnosis information), clinical data (following the Centers for Diseases Control and Prevention [CDC] guides), immunological data (CD4+ T lymphocyte count and percentage), and virological data (viral load [VL] in copies/mL).
2.3. Calculating the pediatric HIV rate in the population
Vertically HIV-infected children and adolescents were selected from the children included in our study. Population projections in Spain were obtained from the National Statistics Institute on the first day of July every year to calculate rates, using the general population of each year as the denominator. The total rate of new diagnoses from 2004 to 2013 was calculated by dividing the number of vertically HIV-infected children by the sum of the corresponding population in the study period (i.e., number of cases/100,000 inhabitants). The rates by origin (children born in Spain vs foreign children) were also calculated, with the corresponding population in the study period as the denominator.
2.4. Characteristics of mothers of vertically HIV-infected children
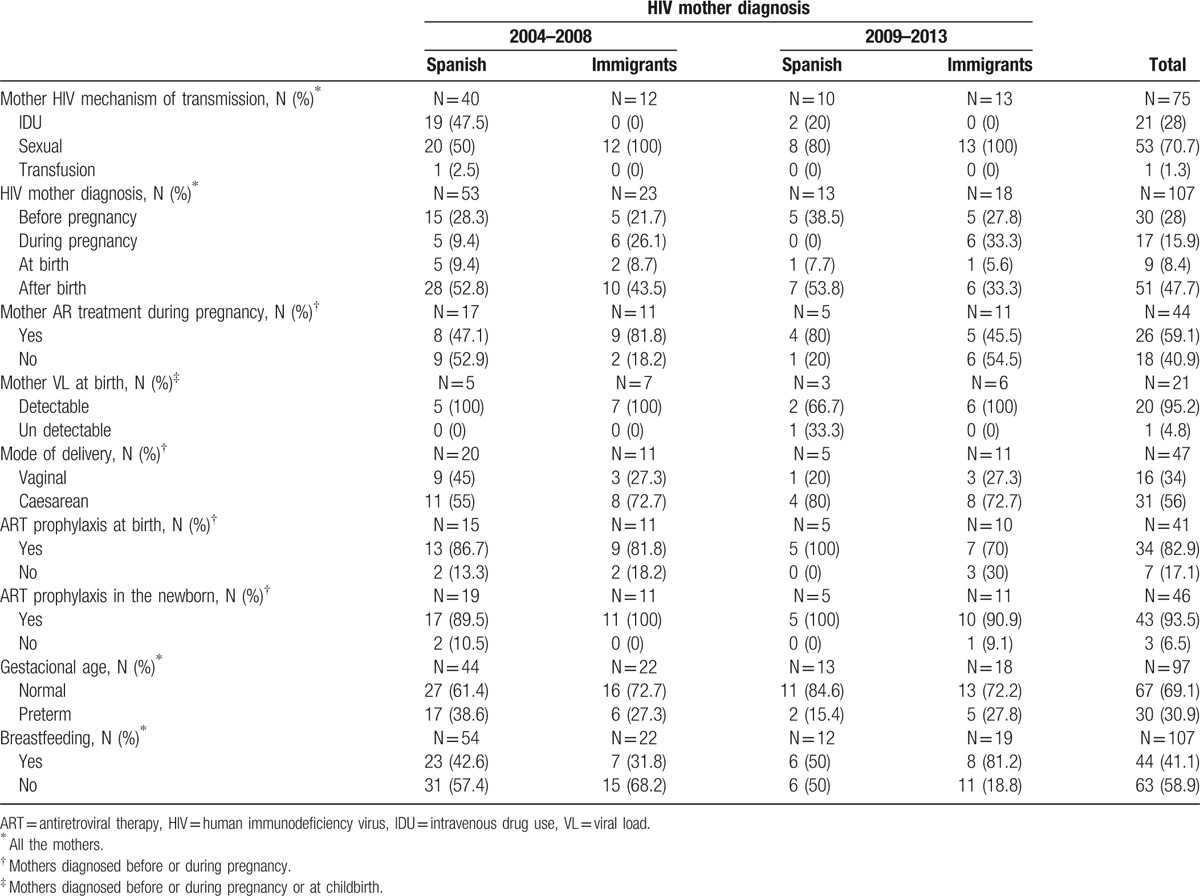
Data were analyzed from the mothers of the vertically HIV-infected children selected for the study sample. Children born in foreign countries were excluded from this analysis. The data collected from these mothers included the following: HIV mechanism of transmission, HIV diagnosis period (before and during pregnancy and childbirth), cART taken during pregnancy, VL at childbirth, mode of delivery, prophylaxis received at childbirth (mothers) and various gestational ages (newborn), and breastfeeding of the newborns by these mothers. Mothers with HIV diagnosed within 14 days before or after childbirth were considered to be late diagnoses. The results were stratified by mothers’ origin: mothers born in Spain versus mothers born in foreign countries (Fig. 1). VL below 50 copies/mL was considered undetectable.
Figure 1.
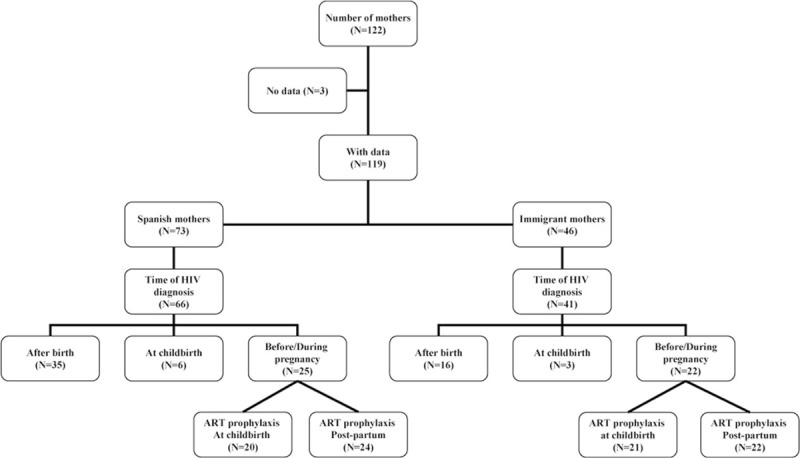
Characteristics of mothers of vertically HIV-infected children. HIV = human immunodeficiency virus.
2.5. Statistical analysis
Results involving qualitative variables were expressed as proportions, whereas results involving quantitative variables were expressed as medians and interquartile ranges. Hypothesis tests were not performed because the data collected for the study were population data.
3. Results
3.1. Description of new HIV children and adolescent diagnoses
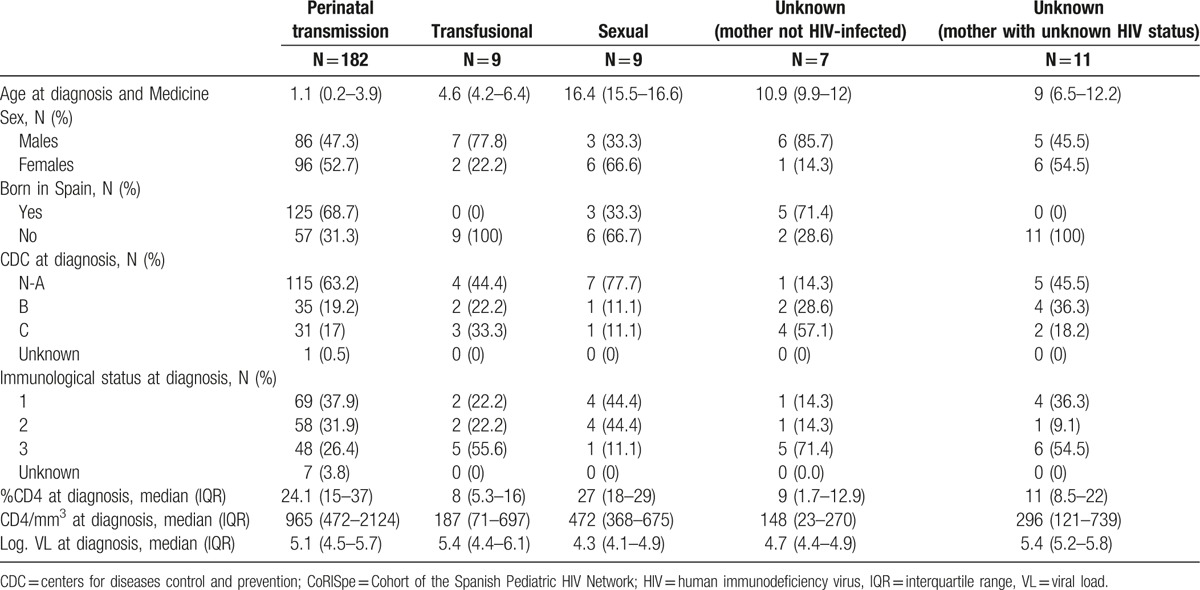
A total of 285 new cases of HIV-infected children and adolescents in CoRISpe hospitals were observed from 2004 to 2013. Of these cases, 51 children were excluded from the study because they were diagnosed with HIV infection in other countries before coming to Spain. Sixteen children were excluded because they were diagnosed in AACC or provinces with no hospitals participating in the CoRISpe or were first followed in hospitals out of the CoRISpe. Of the remaining children included in the study, 182 out of 218 (83.5%) were vertically HIV infected, 9 out of 218 (4.1%) acquired HIV infection through blood transfusion, 9 out of 218 (4.1%) were HIV infected by sexual transmission, and 18 out of 218 (7.4%) had an unknown route of transmission. Seven out of 18 mothers (3.2%) were HIV negative. In 11 out of 18 cases (5%), the HIV status of the mother was unknown. Vertically HIV-infected children were diagnosed at an earlier age than were children infected by other routes. Children infected with HIV through blood transfusion and those with an unknown mechanism of infection (and with unknown HIV status of mother) were born in foreign countries, mainly in sub-Saharan Africa (8 out of 9 [88.9%] and 9 out of 11 [81.8%], respectively), and Latin America (1 out of 9 [11.1%] and 1 out of 11 [9.1%], respectively). Children and adolescents infected with HIV through sexual contact mainly came from Latin America (6 out of 9, or 66.7%). Vertically HIV-infected children had higher CD4+ T lymphocyte counts and percentage values than did children infected through other routes of transmission (Table 1). Four out of 218 children died, 3 who died at 1, 5, and 7 months after being diagnosed with HIV, and 1 who died at 7 months and was diagnosed postmortem. Of these 4 children who died, 3 were vertically HIV infected, while the mechanism of transmission of the remaining child was unknown. The cause of death of the 3 vertically HIV-infected children was Pneumocystis jirovecii pneumonia, whereas the other child passed away from secondary pulmonary hypertension.
Table 1.
Demographic, clinical, immunological, and virological profile of the HIV-infected children diagnosed from 2004 to 2013 in the CoRISpe, by transmission category.
3.2. Calculating the pediatric HIV rate in the population
A decrease was observed in the rate of new diagnoses by vertical transmission per 100,000 inhabitants, from 0.09 in 2004 to 0.03 in 2013. After stratifying the rates by origin, we observed that the rate of children from Spain decreased from 0.08 in 2004 to 0.01 in 2013, whereas the rate of foreign children was stable, from 0.01 in 2004 to 0.02 in 2013. In fact, the rate of foreign children was higher than the rate of Spanish children for the first time in 2013 (Fig. 2).
Figure 2.
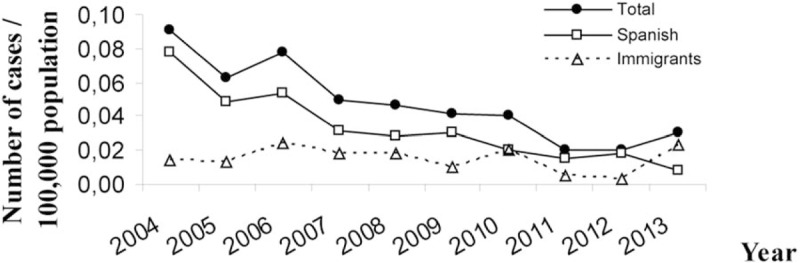
Rates of new HIV diagnoses by vertical transmission in Spain per year of diagnosis. HIV = human immunodeficiency virus.
3.3. Analysis of vertically HIV-infected children
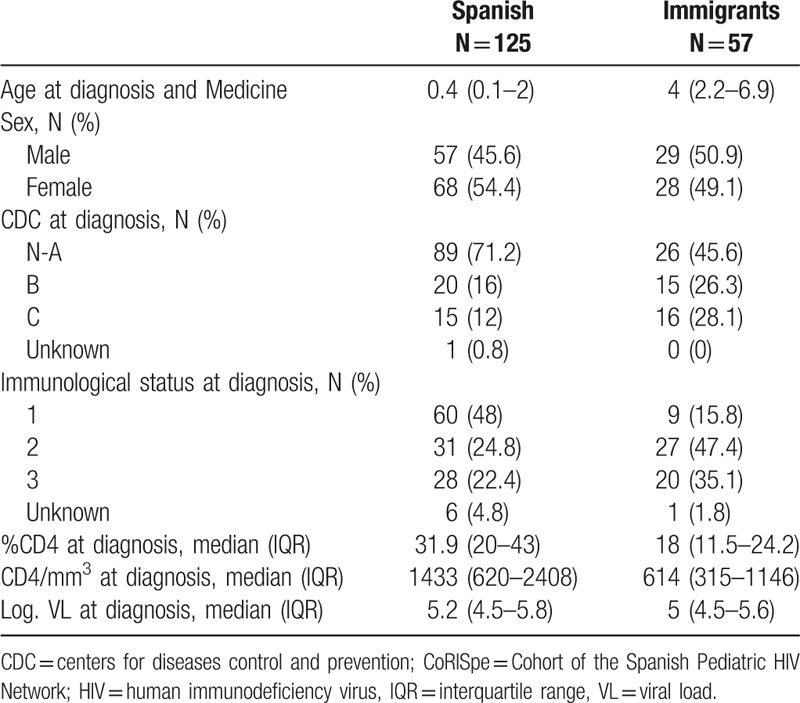
A total of 125 out of 182 children (68.7%) were born in Spain, while 57 out of those 182 children (31.3%) were born in foreign countries. Although the majority of the foreign children came from sub-Saharan Africa (41 out of 57, or 71.9%), 6 out of 57 (10.5%) came from Europe, 5 out of 57 (8.8%) from Latin America, 3 out of 57 (5.3%) from North Africa, and 2 out of 57 (3.5%) from Asia. Children born in foreign countries were diagnosed later than children born in Spain (median age 4 years vs 0.4 years, respectively), had lower CD4+ T lymphocyte counts and percentage values (614 [18%] CD4+ T lymphocytes vs 1433 [31.9%] CD4+ T lymphocytes, respectively) and were in worse clinical situations (54.4% vs 28%, respectively, with CDC B and C status) and immunological situations (82.5% vs 47.2%, respectively, with immunological status 2 and 3). However, no differences in VL between groups were observed (Table 2).
Table 2.
Demographic, clinical, immunological, and virological profile of the vertically HIV-infected children diagnosed from 2004 to 2013 in the CoRISpe, by origin.
3.4. Characteristics of mothers of vertically HIV-infected children
We analyzed a sample of 122 mothers who had a total of 125 children born in Spain (Fig. 1). Data related to the mothers’ pregnancy, childbirth, and prophylaxis were obtained for 119 out of the 122 mothers, but several cases had incomplete information. A total of 73 out of the 119 mothers (61.3%) were born in Spain, and 46 out of the 119 (38.7%) were immigrants. Of these 46 immigrant mothers, 25 were from sub-Saharan Africa (54.3%), 9 were from Latin America (19.6%), 5 were from East Europe (10.9%), 4 were from North Africa (8.7%), and 3 were from western Europe (6.5%). The proportion of mothers from Spain was higher in the 2004 to 2008 period (59 out of 85 mothers, or 69.4%) than in the 2009 to 2013 period (14 out of 34 mothers, or 41.2%).
The major routes of transmission of HIV, sexual contact, and intravenous drug use (IDU), were the routes of transmission for 75 out of 119 mothers (63%), 50 of whom were from Spain and 25 of whom were from other countries. Of the 50 mothers from Spain, 28 (56%) were infected by sexual transmission and 21 (42%) by IDU. A higher proportion of mothers infected by IDU (19 out of 21, or 90.5%) were observed during the 2004 to 2008 period, compared with 2 out of 21 (9.5%) observed during the 2009 to 2013 period. All immigrant mothers were infected by sexual transmission.
Data at the time of diagnosis were collected for 107 of the 119 (89.9%) mothers, 66 of whom were from Spain and 41 of whom were from foreign countries. A total of 30 out of 107 mothers (28%) were diagnosed with HIV before pregnancy, 17 out of 107 (15.9%) were diagnosed during pregnancy, 9 out of 107 (8.4%) were diagnosed at childbirth, and 51 out of 107 (47.7%) were diagnosed after childbirth. A higher proportion of late diagnoses were observed in Spanish mothers compared with foreign mothers (62.1% vs 46.3%, respectively). The number of late diagnoses was higher (45 out of 60, or 75%) during the 2004 to 2008 period, most of which were among mothers from Spain (33 out of 45, or 73.3%), compared with the 2009 to 2013 period (15 out of 60 late diagnoses, or 25%).
Two out of 9 mothers diagnosed at childbirth (22.2%) had a caesarean section, 2 out of 9 mothers (22.2%) received prophylaxis at childbirth, and 7 out of 9 newborns (77.8%) received zidovudine (AZT), lamivudine (3TC), and nevirapine (NVP) prophylaxis postpartum.
Data related to cART were collected in 44 out of 47 mothers diagnosed before or during pregnancy, and 26 out of these 44 mothers (59.1%) received cART. In 41 out of 47 mothers, data of prophylaxis at childbirth were available, and 34 out of these 41 mothers (82.9%) received cART prophylaxis. In the 47 mothers diagnosed before or during pregnancy, the mode of delivery was known, and 31 out of the 47 (56%) had a caesarean section. Finally, data on whether newborns received prophylaxis postpartum were available for 46 out of the 47 mothers, and 43 out of the 46 newborns (93.5%) received prophylaxis postpartum. Regarding the children, 13 out of 43 (30.2%) received AZT prophylaxis, and 28 out of 43 (65.1%) received AZT and 3TC or AZT and NVP. Data on the type of prophylaxis were not available for 2 of the children.
Data on VL near delivery were available for 21 out of 56 mothers, and only 1 out of 21 newborns had undetectable values of VL. For these newborns, a ventouse suction cup was used, and the newborn received postpartum prophylaxis with zidovudine; however, although some VL tests were negative, the child was diagnosed with HIV. No differences between mothers from Spain and foreign countries were observed with respect to mode of delivery, prophylaxis received at childbirth (mother) and various gestational ages (newborn), or breastfeeding in newborns (Table 3).
Table 3.
Risk factors and obstetric characteristics of mothers of vertically HIV-infected children born in Spain, by HIV diagnosis year of the children and origin of the mothers.
4. Discussion
This is the first study that describes the characteristics of children in Spain newly diagnosed with HIV. In our study, which includes data from as early as 2004, 125 out of 182 (68.7%) vertically HIV-infected children were born in Spain, but this number decreased twice—first from 2004 to 2008 and second from 2009 to 2013. Consistent with research conducted in other European cohorts describing an increase in the number of foreign vertically HIV-infected children,[17] we found that 57 of the 182 (31.3%) vertically HIV-infected children were born in other countries but diagnosed in Spain. The vertically HIV-infected children had higher CD4+ T lymphocyte counts at diagnosis than children infected with HIV by other routes of transmission. This phenomenon occurs because once a child is diagnosed with HIV, he or she begins to take cART, depending on his or her clinical, immunological and virological status.[18,19,20] However, the HIV-infected children born in foreign countries in this study sample were diagnosed later than the children born in Spain and therefore had more advanced disease with lower CD4+ counts. Immigrant and Spanish children with unknown diagnosis clinically progressed according to the natural course of the HIV infection, with gradual decreases in CD4+ T lymphocytes.[18] In conclusion, it is essential to diagnose the children as soon as possible and begin cART according to clinical guidelines.[3]
We observed a decrease in the diagnosis rate of vertically HIV-infected children from 2004 to 2013 in Spain. This finding may be explained by the decrease in the birth rate in Spain. However, the diagnosis rate of vertically HIV-infected foreign children remained stable for this period of study. The rate of vertical HIV infection among foreign children was lower than the rate among Spanish children, except in 2013, when the rate among foreign children was higher. Children from foreign, high-burden HIV countries should be offered testing upon or soon after arrival into the country to avoid delays in diagnosis and inevitable disease progression.[1]
Of the 75 total mothers whose HIV mechanism of transmission was known, 21 out of 50 (42%) mothers from Spain were infected by IDU, and 28 were infected by sexual contact; in contrast, all 25 foreign mothers were infected by sexual transmission. Trends in the mechanism of transmission changed from 2004 to 2013 among Spanish mothers with HIV. During the 2004 to 2008 period, more mothers were infected with HIV by IDU than in the 2009 to 2013 periods, during which more mothers were infected by sexual contact. Although a downward trend in new diagnoses of HIV by IDU has been observed in Spain during the last 10 years, the mothers in our study sample were infected before this downward trend occurred.[3]
Interestingly, more than the 50% of the mothers knew their HIV status at or after childbirth, according to data from 2004 to 2008. It is important to consider that until 2007, physicians did not request routine HIV testing for pregnant women. Therefore, it was not possible to know the HIV status of all pregnant women. Currently, it is mandatory to test all pregnant women for HIV at the beginning of and during their pregnancy.[21–25] In this way, the risk of mother-to-child transmission of new HIV infection has decreased due to cART and detectable VL during early pregnancy.[26–30]
There are important limitations to our study. First, some hospitals and HIV pediatric units out of the CoRISpe could not send data for our study. Some pediatricians of HIV-infected children who were diagnosed and followed in hospitals were not contacted. However, due to the number of AACC participants (16 out of 17) in our study, the children excluded from hospitals not participating in CoRISpe did not affect the results of our study. The CoRISpe keeps data from 2004 to 2013 of approximately 90% of all HIV-infected children in Spain. Second, there are no Spanish cohorts of HIV-infected mothers. Therefore, we do not know if the decrease in the rate of children born in Spain is related to a decrease in the number of pregnancies among Spanish women infected with HIV from 2004 to 2013. However, we observed a decrease in the number of mothers diagnosed at or after childbirth throughout the study period. This decrease is probably due to the administration of HIV testing to all pregnant women, according to the recommendations of the Spanish Health Ministry and Clinical Guidelines. HIV-infected mothers had access to complete PMTCT, and subsequently, new vertical HIV infections have been prevented. Third, data were not available for some of the mothers of vertically HIV-infected children born in Spain, specifically the VL of mothers at birth or near birth, because some children were born in other hospitals before being diagnosed with HIV. However, of the 21 mothers with available data, only 1 mother (4.8%) had an undetectable VL at birth or near birth. Although this child could have been infected with HIV during pregnancy, the infection was probably caused at birth due to the use of a ventouse suction cup during the delivery. It is important to emphasize that assisted birth must be avoided even though control of VL is optimal. In this case, prophylaxis in the newborn with more than 1 drug must be used, as established by protocol in the case of mothers with detectable VLs during pregnancy.
In conclusion, the rate of new HIV diagnoses of vertically HIV-infected children decreased significantly from 2004 to 2013, from 0.09 to 0.03 per 100,000 inhabitants. Improved access to PMTCT measures has resulted in a decline in new infections in Spanish children. Immigrant children, especially from high-burden HIV countries, should be offered HIV testing so that infected children may be identified at less advanced stages of disease.
Footnotes
Abbreviations: 3TC = lamivudine, AACC = Autonomous Communities, AZT = zidovudine, cART = combination antiretroviral therapy, CDC = Centers for Diseases Control and Prevention, COHERE = Collaboration of Observational HIV Epidemiological Research Europe, CoRISpe = Cohort of the Spanish Pediatric HIV Network, EPPICC = The European Pregnancy and Pediatric HIV Cohort Collaboration, HIV = human immunodeficiency virus, IDU = intravenous drug use, NVP = nevirapine, PENTA = Pediatric European Network for Treatment of AIDS, PMTCT = prevention of mother-to-child transmission, SINIVIH = information systems on new HIV diagnoses.
This work has been partially funded by the Red Temática de Investigación en SIDA (RED RIS); supported by the Instituto de Salud Carlos III (ISCIII; RD12/0017/0035, RD12/0017/0037, RD16/0025/0019) project as part of the Plan R+D+I; and co-financed by ISCIII-Subdirección General de Evaluación and Fondo Europeo de Desarrollo Regional (FEDER), project Plataforma Red Nacional de BioBancos PT13/0010/0028 supported by ISCIII, RIS-EPICLIN-19/2015, Fondo para la Investigación Sanitaria of the Spanish Ministry of Science and Innovation (FIS PI16/01863, FIS PI13/02016, PI13/00422), Translational Research Network in Pediatric Infectious Diseases (RITIP), EPIICAL project, and RED TEMÁTICA Iberoamericano de Ciencia y Tecnología para el Desarrollo CYTED (214RT0482).
The authors have no conflicts of interest to disclose.
Contributor Information
Collaborators: Working groups of CoRISpe
References
- [1].World AIDS Day Report 2014 (UNAIDS web site); 2015. Available at: http://www.unaids.org/sites/default/files/media_asset/JC2686_WAD2014report_en.pdf. Accessed September 15, 2015. [Google Scholar]
- [2].HIV/AIDS surveillance in Europe (ECDC web site); 2014. Available at: http://ecdc.europa.eu/en/publications/Publications/hiv-aids-surveillance-report-Europe-2013.pdf. Accessed September 15, 2015. [Google Scholar]
- [3].Epidemiological surveillance of HIV/AIDS in Spain. Update 30 June 2014; Ministry of Health, Social Services and Equality website. Available at: https://www.msssi.gob.es/ciudadanos/enfLesiones/enfTransmisibles/sida/vigilancia/home.htm. Accessed June 15, 2015. [Google Scholar]
- [4].Antiretroviral Therapy of HIV Infection in Infants and Children: Towards Universal Access (Organization WH web site); 2010. Available at: http://whqlibdoc.who.int/publications/2010/9789241599801.eng.pdf. Accessed June 15, 2015. [Google Scholar]
- [5].Luzuriaga K. Mother-to-child transmission of HIV: a global perspective. Curr Infect Dis Rep 2007;9:511–7. [DOI] [PubMed] [Google Scholar]
- [6].Palladino C, Bellon JM, Perez-Hoyos S, et al. Spatial pattern of HIV-1 mother-to-child-transmission in Madrid (Spain) from 1980 till now: demographic and socioeconomic factors. AIDS 2008;22:2199–205. [DOI] [PubMed] [Google Scholar]
- [7].Bellon Cano JM, Sánchez-Ramon S, Ciria L, et al. The effects on infants of potent antiretroviral therapy during pregnancy: a report from Spain. Med Sci Monit 2004;10:CR179–84. [PubMed] [Google Scholar]
- [8].Resino S, Larrú B, Maria Bellón J, et al. Effects of highly active antiretroviral therapy with nelfinavir in vertically HIV-1 infected children: 3 years of follow-up. Long-term response to nelfinavir in children. BMC Infect Dis 2006;6:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Chadwick EG, Pinto J, Yogev R, et al. Early initiation of lopinavir/ritonavir in infants less than 6 weeks of age: pharmacokinetics and 24-week safety and efficacy. International Maternal Pediatric Adolescent Clinical Trials Group (IMPAACT) P1030 Team. Pediatr Infect Dis J 2009;28:215–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hawkins D, Blott M, Clayden P, et al. Guidelines for the management of HIV infection in pregnant women and the prevention of mother-to-child transmission of HIV. HIV Med 2005;6(Suppl):107–48. [DOI] [PubMed] [Google Scholar]
- [11].Bamford A, Turkova A, Lyall H, et al. Paediatric European Network for Treatment of AIDS (PENTA) guidelines for treatment of paediatric HIV-1 infection 2015: optimizing health in preparation for adult life. HIV Med 2015;doi: 10.1111/hiv.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Recommendations of the Secretariat of the National AIDS Plan (SNAP), the Study Group of Aids SGA), the Spanish Society of Gynecology and Obstetrics (SSGO) and the Spanish Association of Pediatrics (SAP) for monitoring infection by HIV in relation to the reproduction, pregnancy and the prevention of vertical transmission. Ministry of Health, Social Services and Equality website. Available at: https://www.msssi.gob.es/ciudadanos/enfLesiones/enfTransmisibles/sida/vigilancia/home.htm. Accessed June 15, 2015. [Google Scholar]
- [13].The Ministry of Health, Social Services and Equality. Consensus document of Gesida/Secretariat of the National AIDS Plan with respect to antiretroviral therapy in adults infected with human immunodeficiency virus. (Ministry of Health, Social Services and Equality web site); 2013. Available at: https://www.msssi.gob.es/ciudadanos/enfLesiones/enfTransmisibles/sida/publicaciones/profSanitarios/docTARGesidaPNS2013Def.pdf. Accessed June 15, 2015. [Google Scholar]
- [14].de Jose MI, Jiménez de Ory S, Espiau M, et al. A new tool for the paediatric HIV research: general data from the Cohort of the Spanish Paediatric HIV Network (CoRISpe). BMC Infect Dis 2013;13:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].García-Merino I, de Las Cuevas N, Jiménez JL, et al. Spanish HIV bioBank. Retrovirology 2009;6:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].García-Merino I, de Las Cuevas N, Jiménez JL, et al. Pediatric HIV BioBank: a new role of the Spanish HIV BioBank in pediatric HIV research. AIDS Res Hum Retroviruses 2010;26:241–4. [DOI] [PubMed] [Google Scholar]
- [17].Bertie E, Thorne C, Noguera-Julian A, et al. The new face of the pediatric HIV epidemic in Western countries: demographic characteristics, morbidity and mortality of the pediatric HIV-infected population. Pediatr Infect Dis J 2015;34(Suppl):S7–13. [DOI] [PubMed] [Google Scholar]
- [18].Violari A, Cotton MF, Gibb DM, et al. Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med 2008;359:2233–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Faye A, Le Chenadec J, Dollfus C, et al. Early versus deferred antiretroviral multidrug therapy in infants infected with HIV type 1. Clin Infect Dis 2004;39:1692. [DOI] [PubMed] [Google Scholar]
- [20].Prendergast A, Mphatswe W, Tudor-Williams G, et al. Early virological suppression with three-class antiretroviral therapy in HIV-infected African infants. AIDS 2008;22:1333–43. [DOI] [PubMed] [Google Scholar]
- [21].Townsend CL, Cortina-Borja M, Peckham CS, et al. Trends in management and outcome of pregnancies in HIV-infected women in the UK and Ireland, 1990–2006. BJOG 2008;115:1078–86. [DOI] [PubMed] [Google Scholar]
- [22].Gazzard B, Clumeck N, d’Arminio Monforte A, et al. Indicator disease-guided testing for HIV—the next step for Europe? HIV Med 2008;9(Suppl):34–40. [DOI] [PubMed] [Google Scholar]
- [23].Ross CE, Tao G, Patton M, et al. Screening for human immunodeficiency virus and other sexually transmitted diseases among U.S. women with prenatal care. Obstet Gynecol 2015;125:1211–6. [DOI] [PubMed] [Google Scholar]
- [24].Kissin DM, Akatova N, Rakhmanova AG, et al. Rapid HIV testing and prevention of perinatal HIV transmission in high-risk maternity hospitals in St. Petersburg, Russia. Am J Obstet Gynecol 2008;198:183.e1–7. [DOI] [PubMed] [Google Scholar]
- [25].Johnson M, Afonina L, Haanyama O. The challenges of testing for HIV in women: experience from the UK and other European countries. Antivir Ther 2013;18(Suppl):19–25. [DOI] [PubMed] [Google Scholar]
- [26].Navér L, Albert J, Böttiger Y, et al. Prophylaxis and treatment of HIV-1 infection in pregnancy: Swedish recommendations 2013. Scand J Infect Dis 2014;46:401–11. [DOI] [PubMed] [Google Scholar]
- [27].Short CE, Taylor GP. Antiretroviral therapy and preterm birth in HIV-infected women. Expert Rev Anti Infect Ther 2014;12:293–306. [DOI] [PubMed] [Google Scholar]
- [28].Aebi-Popp K, Mulcahy F, Rudin C, et al. National Guidelines for the prevention of mother-to-child transmission of HIV across Europe—how do countries differ? Eur J Public Health 2013;23:1053–8. [DOI] [PubMed] [Google Scholar]
- [29].Prieto LM, González-Tomé MI, Muñoz E, et al. Low rates of mother-to-child transmission of HIV-1 and risk factors for infection in Spain: 2000–2007. Pediatr Infect Dis J 2012;31:1053–8. [DOI] [PubMed] [Google Scholar]
- [30].Tariq S, Townsend CL, Cortina-Borja M, et al. European Collaborative Study; National Study of HIV in Pregnancy Childhood. Use of zidovudine-sparing HAART in pregnant HIV-infected women in Europe: 2000–2009. J Acquir Immune Defic Syndr 2011;57:3. [DOI] [PMC free article] [PubMed] [Google Scholar]


