Abstract
Background:
This study aimed to assess the effectiveness and safety of percutaneous electrical nerve stimulation (PENS) in migraine treatment.
Methods:
Sixty-two patients with at least 2 migration attacks each month were recruited and randomly divided into a verum PENS group and a sham PENS group in a ratio of 1:1. All patients received verum or sham PENS 30 minutes daily, 5 times weekly for 12 weeks. The primary outcomes were change in monthly migraine days (MMD) and the 50% responder rate (RR). Secondary outcomes were evaluated using the monthly migraine attacks (MMA), monthly headache days (MHD), and monthly acute antimigraine drug intake (MAADI). All outcome measurements were performed at treatment initiation to establish a baseline and again after 12 weeks of treatment.
Results:
At the end of the 12 weeks, the group receiving verum PENS exhibited statistically significant decrease in the mean MMD compared with the group receiving sham PENS intervention (P < .05). Additionally, the 50% RR was significantly higher in the verum PENS group than that in the sham PENS group (P < .05). Furthermore, the MMA, MHD, and MAADI were also significantly lower in the verum PENS group that those in the sham PENS group (P < .05).
Conclusion:
The results of this study demonstrated that verum PENS is more effective and safe than Sham PENS for the treatment of migraine.
Keywords: effectiveness, migraine, percutaneous electrical nerve stimulation, randomized controlled trial, safety
1. Introduction
Migraine is one of the most common pain problems. According to the reported study of recent epidemiologic data, it is a highly prevalent primary headache disorder.[1] In addition, it is also one of the most disabling diseases worldwide.[1] Although a wide variety of antimigraine drug therapies have been used in the treatment of migraine, those drugs are of limited effectiveness in relieving migraine symptoms and many of them are associated with cumbersome side effects.[2–5] It is reported that only 28.3% and 44.8% of patients with episodic and chronic types of migraine, respectively,[6] take preventive medication.[7] Additionally, hardly any novel migraine preventive drug has been marketed since the last decade. Thus, the new preventive therapies with similar or better clinical effectiveness and fewer treatment-related side effects are urgently needed in clinic. Nonpharmacological interventions, including relaxation, hypnosis,[8–10] and physical therapy[11] have also been used in the past few years. However, few well-designed randomized controlled trials have been conducted to evaluate their effectiveness.
Presently, peripheral nerve stimulation (PNS) has become increasingly popular as alternative and complementary therapies, showing positive preventive properties in acute and chronic migraine.[12] PNS has been reported to be effective in the management of migraine symptoms by using percutaneous electrical nerve stimulation (PENS); indeed, it also has been used for the prevention of migraine.[13] This kind of intervention often involves the insertion of needle probes, like the acupuncture needles, into the soft tissues through the skin with different depths corresponding to the migraine symptoms location and the electrical current application.
In this study, we hypothesized that verum PENS therapy for migraine treatment after 12 weeks of treatment would be superior to the effectiveness of sham PENS intervention. Thus, we designed this 2-arm, double-blinded, randomized, sham-controlled trial to assess the effectiveness of verum PENS therapy for migraine treatment.
2. Methods/design
2.1. Study design
This study was approved by the ethics committee of The People's Hospital of Yan’an and it was also conducted at the same hospital from January 2013 to December 2016. Sixty-two eligible patients were included and were randomly divided into a verum PENS group and a sham PENS group in a 1:1 allocation ratio. The participants in the verum PENS group were given verum PENS treatment, while the patients in the sham PENS group received sham PENS intervention. Interventions were administered 30 minutes daily, 5 times weekly for 12 weeks in both groups. Written informed consent was obtained from all patients in both groups.
2.2. Patients
Patients inclusion criteria dictated that all included patients must meet the International Classification of Headache Disorders (ICHD)-II code 1.2.1 or 1.1[14]; be 18 to 70 years old; have a history of migraine longer than 3 months; and have at least 2 attacks each month. Exclusion criteria were previous history of having undergone PENS, acupuncture, electroacupuncture therapies, or other related treatment during the past 3 months before this study. In addition, patients with failure on more than 3 well-conducted preventive drug treatments, tension-type headache, other severe neurologic or psychiatric disorders, cancer or severe mental disorders, and pregnancy were also excluded.
2.3. Randomization and blinding
All eligible participants entered a run-in phase of 1 month. After this baseline period, patients should still meet the inclusion criteria of at least 2 migraine attacks each month. Subsequently, patients were randomly assigned to the verum PENS group or sham PENS group in a 1:1 ratio. Randomization schedule was performed by using a computerized number generator with SAS package 8.1 (SAS Institute, Inc., Cary, NC). Allocation information containing the randomized group assignments of all patients was concealed in sequentially numbered, opaque, sealed envelopes. All participants, physicians, outcome assessors, and data analysts were blinded to the treatment allocation information.
2.4. Intervention
Patients received either verum PENS or sham PENS at bilateral Taiyang (EX-HN 5) acupoints. Two gauge stainless steel probes (0.32 mm × 40 mm) similar to acupuncture needles were inserted into bilateral acupoints EX-HN 5 at a 20-mm depth. An LH202H Han Electrostimulator (Jinghua Wei Industry Development Company, Beijing, China), connected with the positive pole at the healthy side of EX-HN 5 and negative pole at the attacked side. Treatment was administered in pulses of disperse-dense wave for 30 minutes at a frequency of 2/100 Hz, 5 times weekly for 12 weeks. Sham PENS intervention was applied by the same electrostimulator at the same bilateral acupoints EX-HN 5 without electrical power.
2.5. Outcome measurements
Primary outcome measurements included the change in monthly migraine days (MMD) and the 50% responder rate (RR). MMD was defined as the change in MMD between the run-in month and the third month of treatment. RR was defined as the percentage of “responders” who had at least 50% reduction of MMD between run-in and third month of treatment.
Secondary outcomes were evaluated using the monthly migraine attacks (MMA), monthly headache days (MHD), and monthly acute antimigraine drug intake (MAADI). Of these, MMA was defined as the change in MMA frequency. MAADI was defined as the change in monthly acute antimigraine drug use between run-in and third month of treatment.
2.6. Statistical analysis
The data analysis was conducted by using Statistical Package for the Social Sciences (SPSS) software v.17.0. The sample size was calculated based on the responder rates (RRs) with 15% for sham PENS and 55% for verum PENS.[15] The minimum size of each group was estimated at 26 participants with α = 0.5, β = 0.8. Assuming a 20% drop-out rate, the required sample size of this study was therefore estimated to be 62 participants, with 31 assigned to each group. The mean change from baseline (with a 95% confidence interval [CI]) was evaluated by intervention and the difference (with a 95% CI) between verum PENS and sham PENS in order to evaluate the effectiveness of verum PENS for treating migraine. All data were analyzed by intention-to-treat (ITT). The Mann–Whitney U test was used to compare the primary and secondary outcome measurements. Fisher 2-tailed exact test was used to analyze the RRs. The statistical significance level was set at P < .05.
3. Results
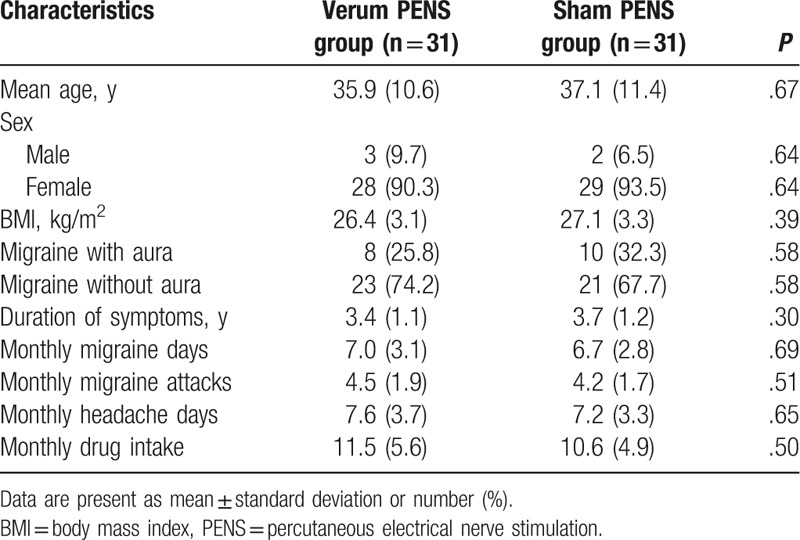
In total, 91 patients were admitted to the run-in phase and entered the study (Fig. 1). Of these, 29 did not meet the inclusion criteria. Seven patients withdrew from the study, mainly because of the consent withdrawal, and were lost to follow-up (Fig. 1). The characteristics of all included patients are listed in Table 1.
Figure 1.

Flow of participants selection.
Table 1.
Patients characteristics of 2 groups at baseline.
An analysis of the primary and secondary outcomes is presented in Table 2. At the end of the 12-week treatment, a significant reduction of the MMD was noted in verum PENS group, when compared to that in the sham PENS group with difference of −1.5 (−2.7, −0.6) (P < .05). In addition, there were significant differences of 50% RR with 25.6 (8.9, 38.7) (P < .05), and 25% RR with 22.2 (11.7, 33.6) (P < .05) between 2 groups. Furthermore, significant differences were also found between 2 groups in MMA (difference, −1.0 (−2.0, −0.4), P < .05), MHD (difference, −2.2 (−3.1, −1.2), P < .05), and MAADI (difference, −3.8 (−5.7, −2.5), P < .05).
Table 2.
Outcome measurements at the end of 12-week intervention (change from baseline).

During the 12-week intervention period, no adverse events or side effects such as discomfort related to verum PENS or sham PENS therapy occurred in either of the 2 groups.
4. Discussion
The results of the present study confirmed our hypothesis that verum PENS therapy contributed to better treatment outcomes against migraine after 12-week treatment compared with sham PENS intervention. It aimed to evaluate verum PENS as an alternative therapy to treat migraine. Our findings demonstrated the promising effectiveness of PENS therapy for treating the occurrence of migraine in patients with frequent migraine episodes.
Previous study has explored the effect of PENS for the treatment of patients with chronic headache.[16] It utilized visual analog scales tool for the assessment of pain, physical activity, and quality of sleep. The results showed that PENS as a complementary therapy was more effective in short-term management for patients with recurrent headache symptoms.[16] The conclusions of the present study are consistent with the previously study,[16] although they used different outcome measurements tools.
In this study, the primary outcome was measured by the change in MMD and the 50% RR. Secondary outcomes were measured by the MMA, MHD, and MAADI. At the end of the 12-week treatment, verum PENS showed statistically significant decrease in the change of mean MMD (P < .05), but the improvement of 50% RR (P < .05), compared with the sham PENS. Additionally, the results of MMA, MHD, and MAADI were also better in the verum PENS group than those in the sham PENS group. Significant differences of MMA, MHD, and MAADI were found between 2 groups (P < .05). These results indicate the promising effectiveness of verum PENS for treating the symptoms of migraine. Furthermore, the treatment also appears encouraging for improving the RR in patients with migraine.
The present study has several limitations. First, it was impossible for the patients to discontinue their acute antimigraine drugs during the treatment period. Therefore, the observed effectiveness of the treatment may be the combined results of PENS plus medications, rather than PENS alone, although the baseline medication was similar between the 2 groups. Second, another shortcoming of this study was the missing follow-up assessment. Indeed, follow-up regarding both short and long-term effectiveness of PENS for treatment of migraine still needs to be assessed. Finally, we did not evaluate the comprehensive conditions of the participants did, because this study did not assess the quality of life of the included patients.
5. Conclusions
The results of this study demonstrated that verum PENS can treat migraine effectively. Future studies with long-term treatment and follow-up assessment are still needed to warrant the present study.
Footnotes
Abbreviations: BMI = body mass index, CI = confidence interval, EX-HN 5 = Taiyang, ICHD = International Classification of Headache Disorders, ITT = intention-to-treat, MAADI = monthly acute antimigraine drug intake, MHD = monthly headache days, MMA = monthly migraine attacks, MMD = monthly migraine days, PENS = percutaneous electrical nerve stimulation, PNS = peripheral nerve stimulation, RR = responder rate, SPSS = Statistical Package for the Social Sciences.
The authors have no conflicts of interest to disclose.
References
- [1].Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2163–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Silberstein S, Latsko M, Schoenen J. Fernandez-de-las-Penas CCL, Schoenen J. Preventive Antimigraine Drugs. Contemporary Issues in Physical Therapy and Rehabilitation Medicine (ed) Multidisciplinary Management of Migraine. Burlington, USA: Jones & Bartlett Learning; 2012. 91–102. [Google Scholar]
- [3].Lipton RB, Newman LC, Solomon S. Over-the counter medication and the treatment of migraine. Headache 1994;34:547–8. [DOI] [PubMed] [Google Scholar]
- [4].Silberstein SD, Young WB. Analgesic rebound headache. How great is the problem and what can be done? Drug Saf 1995;13:133–44. [DOI] [PubMed] [Google Scholar]
- [5].Cranz H. Over-the-counter drugs. The issues. Drug Saf 1990;5:120–5. [DOI] [PubMed] [Google Scholar]
- [6].International Headache Society. The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia 2013;33:629–808. [DOI] [PubMed] [Google Scholar]
- [7].Blumenfeld AM, Bloudek LM, Becker WJ, et al. Patterns of use and reasons for discontinuation of prophylactic medications for episodic migraine and chronic migraine: results from the second international burden of migraine study (IBMS-II). Headache 2013;53:644–55. [DOI] [PubMed] [Google Scholar]
- [8].Smith WB. Biofeedback and relaxation training: the effect on headache and associated symptoms. Headache 1987;27:511–4. [DOI] [PubMed] [Google Scholar]
- [9].Diamond S, Montrose D. The value of biofeedback in the treatment of chronic headache: a four-year retrospective study. Headache 1984;24:5–18. [PubMed] [Google Scholar]
- [10].Cedercreutz C, Lahteenmaki R, Tulikoura J. Hypnotic treatment of headache and vertigo in skull injured patients. Int J Clin Exp Hypn 1976;24:195–201. [DOI] [PubMed] [Google Scholar]
- [11].Jay GW, Brunson J, Branson SJ. The effectiveness of physical therapy in the treatment of chronic daily headaches. Headache 1989;29:156–62. [DOI] [PubMed] [Google Scholar]
- [12].Magis D, Schoenen J. Advances and challenges in neurostimulation for headaches. Lancet Neurol 2012;11:708–19. [DOI] [PubMed] [Google Scholar]
- [13].Ghoname EA, Craig WF, White PF. Use of percutaneous electrical nerve stimulation (PENS) for treating ECT-induced headaches. Headache 1999;39:502–5. [DOI] [PubMed] [Google Scholar]
- [14].Headache Classification Subcommittee of the International Headache Society. The International Classification of Headache Disorders: 2nd edition. Cephalalgia 2004;24(suppl 1):9–160. [DOI] [PubMed] [Google Scholar]
- [15].Lipton RB, Dodick DW, Silberstein SD, et al. Single-pulse transcranial magnetic stimulation for acute treatment of migraine with aura: a randomised, double-blind, parallel group, sham-controlled trial. Lancet Neurol 2010;9:373–80. [DOI] [PubMed] [Google Scholar]
- [16].Ahmed HE, White PF, Craig WF, et al. Use of percutaneous electrical nerve stimulation (PENS) in the short-term management of headache. Headache 2000;40:311–5. [DOI] [PubMed] [Google Scholar]


