Abstract
Rationale:
Among the nontuberculous mycobacteria, Mycobacterium abscessus is a common cause of skin, soft tissue, and bone infections. However, disseminated M. abscessus infection that mimics cancer metastasis with an underlying relatively immunocompetent condition has rarely been reported.
Patient concerns:
A nonsmoking 73-year-old man with an underlying relatively immunocompetent condition reported a 2-month history of a mass in the region of his right parotid gland that had been steadily increasing in size.
Diagnoses:
The head and neck computed tomography showed an avidly enhancing tumor with central necrosis in the right parotid region and lymphadenopathy bilaterally at neck levels II–V (<6 cm) with a necrotic core. The radiologist and otolaryngologist both suspected a diagnosis of right parotid gland cancer with metastasis.
Interventions:
The necrotic tissue was removed surgically, and Mycobacterium culture showed M. abscessus. We collected a blood sample and detected anti-interferon-γ autoantibody.
Outcomes:
After 6 months of anti-M. abscessus treatment, physical examination showed remission of the parotid tumor, and axillary and supraclavicular lymphadenopathy.
Lessons:
We report a case of disseminated M. abscessus infection, which involved parotid glands with multiple lymphadenopathies in a person with an underlying relatively immunocompetent condition. Possible underlying mechanisms such as anti-interferon-γ autoantibody-associated immunodeficiency should be considered in a patient with disseminated M. abscessus infection without a known immunocompromised condition.
Keywords: anti-interferon-γ autoantibody, Mycobacterium abscessus, parotid gland cancer
1. Introduction
Nontuberculous mycobacteria (NTM) are common in the environment and are considered less virulent than Mycobacterium tuberculosis to humans.[1–3] Among the NTM, Mycobacterium abscessus is a common cause of skin, soft tissue, and bone infections.[4] Most clinical NTM infections are localized, but under certain conditions, these infections can be disseminated. A majority of disseminated NTM (dNTM) infections occur in patients with a compromised immune status, often due to a malignancy or infection with human immunodeficiency virus (HIV).[5] However, disseminated M abscessus infection that mimics cancer metastasis with an underlying relatively immunocompetent condition has rarely been reported. Here, we report a 73-year-old man with disseminated M abscessus infection mimicking parotid cancer with multiple metastases. Considering the underlying immunocompetent status of the patient, we collected a blood sample to detect anti-interferon-γ autoantibody, which is a recently recognized mechanism of dNTM infection.[6]
2. Case presentation
The Research Ethics Committees of the National Taiwan University Hospital approved this study and the informed consent was obtained.
A nonsmoking 73-year-old man reported a 2-month history of a mass in the region of his right parotid gland that had been steadily increasing in size, and left upper limb swelling with an armpit mass was also noted 5 months before admission. His associated symptoms were fevers and fatigue. On physical examination, there was bilateral axillary and supraclavicular lymphadenopathy. There was also a tender, reddish, and enlarged (5 cm in diameter) right parotid gland with pus and a fibrin coating (Fig. 1A). The chest computed tomography (CT) showed lymphadenopathy bilaterally over the axillary regions, mediastinum, and right hilum (Fig. 1B). CT-guided lymph node biopsy of left axillary lymphadenopathy revealed only necrosis and a tiny noncaseating granuloma. The head and neck CT showed an avidly enhancing tumor with central necrosis in the right parotid region and lymphadenopathy bilaterally at neck levels II-V (<6 cm) with a necrotic core. The radiologist and otolaryngologist both suspected a diagnosis of right parotid gland cancer with metastasis (Fig. 1C). Initially, the patient underwent a core needle biopsy of the right parotid gland. Pathology showed an abscess with focal tissue necrosis. Numerous acid-fast positive bacilli were revealed. Because the head and neck CT was highly suspicious for parotid cancer, incisional drainage and biopsy were performed. The necrotic tissue was removed surgically without complications.
Figure 1.
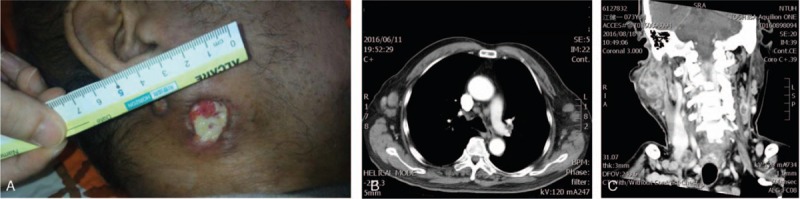
(A) Gross finding with focal tissue necrosis in a 2 × 2 cm defect. (B) Chest CT with contrast; lymphadenopathy bilaterally over the axillary regions, mediastinum, and right hilum. (C) Head and neck CT with contrast; a tumor with central necrosis in the right parotid region, and lymphadenopathy bilaterally at neck levels II-V (<6 cm). CT = computed tomography.
Final pathological findings demonstrated an abscess with focal tissue necrosis. Microscopically, the biopsy showed numerous acid-fast bacilli and necrotic debris (Fig. 2A and B). No pathogen was identified in periodic acid-Schiff staining or Gömöri methenamine stain. The excised necrotic tissue was sent for mycobacterial culture. The specimen was spread onto Lowenstein-Jensen slopes and tested using a fluorometric BACTEC system (BACTEC Mycobacterium Growth Indicator Tube 960 system; Becton, Dickinson and Company). Mycobacteria were identified to the species level using conventional biochemical methods.[7]Mycobacterium growth was noted after 6 days of culture in a Mycobacterium Growth Indicator Tube. Species level identification showed M abscessus. Considering the underlying immunocompetent status of the patient, we collected a blood sample and detected anti-interferon-γ autoantibody. After 6 months of anti-M abscessus treatment, which included 1 month of azithromycin, imipenem, and doxycycline, and 5 months of azithromycin, doxycycline, and levofloxacin, physical examination showed remission of the parotid tumor, and axillary and supraclavicular lymphadenopathy.
Figure 2.
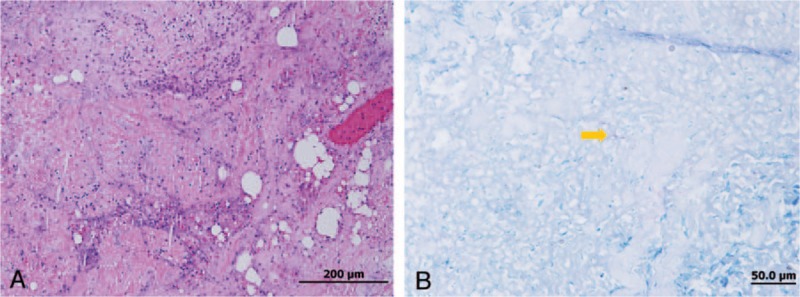
(A) Hematoxylin and eosin staining (H&E) (×20) and (B) acid-fast staining (AFS) (×1000); an abscess with focal tissue necrosis and numerous acid-fast bacilli (arrow).
3. Discussion
Among the NTM, M abscessus is a common cause of skin, soft tissue, and bone infections.[4] dNTM infection can mimic soft tissue malignancy, lymphoma, and cancer metastasis.[8–11] In contrast to NTM infection of the head and neck region in children,[12–16] NTM-caused cervical lymphadenitis is rare in adults.[12] Most of the reported NTM infection with parotitis in adults have underlying immunodeficiency, such as HIV infection or rheumatic disease with steroid use.[17–20] Here, we reported our unusual case of disseminated M abscessus infection that involved parotid glands with multiple lymphadenopathies in a person with an underlying relatively immunocompetent condition. Therefore, possible underlying mechanisms such as anti-interferon-γ autoantibody-associated immunodeficiency should be considered in a patient with disseminated M abscessus infection without a known immunocompromised condition. Anti-interferon-γ autoantibody-associated immunodeficiency is an emerging medical issue worldwide and plays an important role in mycobacterial infection.[21] However, the underlying mechanism that triggers anti-interferon-γ autoantibody production remains unclear. The clinical impact of anti-interferon-γ autoantibody is being increasingly recognized especially in people from East Asia, and in those of Asian descent.[6]
In Taiwan, anti-interferon-γ autoantibody has been recognized as a mechanism of dNTM infection.[6] In addition, identifying anti-interferon-γ autoantibody-associated immunodeficiency might be important because certain adjunct therapies such a rituximab or epitope-erased variant of interferon-γ might be used in the treatment of anti-interferon-γ autoantibody-associated refractory dNTM infection.[22–24]
The present study has some limitations. First, this is a single case study; not every dNTM infection patient was tested, nor proved to have anti-interferon-γ autoantibody. However, we emphasize that dNTM is a rare presentation of a patient without a known immunocompromised condition, and the underlying covert immunodeficiency warrants further study. Second, the trigger for the production of anti-interferon-γ autoantibody-associated immunodeficiency remains elusive. Some studies describe its pathophysiology with the blocking of production of downstream mediators of interferon-γ activity, including STAT1 phosphorylation, TNFα, and interleukin 12.[5,24] Despite these limitations, our case report offers valuable clinical differential diagnosis of a patient with dNTM infection and anti-interferon-γ autoantibody-associated immunodeficiency to physicians who might come across such cases.
In summary, we report a case of disseminated M abscessus infection that involved parotid glands with multiple lymphadenopathies in an elderly person with an underlying relatively immunocompetent condition. This case highlights the need for surgeons to be aware of the potential for clinical features similar to parotid malignancy. We recommend that disseminated M abscessus infection should be considered in the differential diagnosis of tumors arising around the parotid gland. These lesions of dNTM infection may be mistaken for lymphoma or metastasis radiologically; in particular, those in the head and neck may have radiological features similar to those of parotid or salivary gland cancers. Possible underlying mechanisms such as anti-interferon-γ autoantibody-associated immunodeficiency should be considered. In addition, further studies with large numbers of cases are needed for a better understanding of the relationship between NTM infections, autoantibodies to anti-interferon-γ, and parotid tumors.
Footnotes
Abbreviations: CT = computed tomography, dNTM = disseminated nontuberculous mycobacteria, HIV = human immunodeficiency virus, NTM = Nontuberculous mycobacteria.
This study was supported by National Taiwan University Hospital (NTUH.106-M3676).
The authors report no conflicts of interest.
References
- [1].Panesar J, Higgins K, Daya H, et al. Nontuberculous mycobacterial cervical adenitis: a ten-year retrospective review. Laryngoscope 2003;113:149–54. [DOI] [PubMed] [Google Scholar]
- [2].Rieu PN, van den Broek P, Pruszczynski M, et al. Atypical mycobacterial infection of the parotid gland. J Pediatr Surg 1990;25:483–6. [DOI] [PubMed] [Google Scholar]
- [3].Saggese D, Compadretti GC, Burnelli R. Nontuberculous mycobacterial adenitis in children: diagnostic and therapeutic management. Am J Otolaryngol 2003;24:79–84. [DOI] [PubMed] [Google Scholar]
- [4].Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med 2007;175:367–416. [DOI] [PubMed] [Google Scholar]
- [5].Chi CY, Lin CH, Ho MW, et al. Clinical manifestations, course, and outcome of patients with neutralizing anti-interferon-gamma autoantibodies and disseminated nontuberculous mycobacterial infections. Medicine 2016;95:e3927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Browne SK, Burbelo PD, Chetchotisakd P, et al. Adult-onset immunodeficiency in Thailand and Taiwan. N Engl J Med 2012;367:725–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lai CC, Tan CK, Chou CH, et al. Increasing incidence of nontuberculous mycobacteria. Taiwan, 2000–2008. Emerg Infect Dis 2010;16:294–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Chen YP, Yen YS, Chen TY, et al. Systemic Mycobacterium kansasii infection mimicking peripheral T-cell lymphoma. APMIS 2008;116:850–8. [DOI] [PubMed] [Google Scholar]
- [9].Yeh I, Evan G, Jokinen CH. Cutaneous mycobacterial spindle cell pseudotumor: a potential mimic of soft tissue neoplasms. Am J Dermatopathol 2011;33:e66–9. [DOI] [PubMed] [Google Scholar]
- [10].Lin KH, Wang JH, Peng NJ. Disseminated nontuberculous mycobacterial infection mimic metastases on PET/CT scan. Clin Nucl Med 2008;33:276–7. [DOI] [PubMed] [Google Scholar]
- [11].Lai J, Abbey BV, Jakubovic HR. Epithelioid histiocytic infiltrate caused by Mycobacterium scrofulaceum infection: a potential mimic of various neoplastic entities. Am J Dermatopathol 2013;35:266–9. [DOI] [PubMed] [Google Scholar]
- [12].Lai KK, Stottmeier KD, Sherman IH, et al. Mycobacterial cervical lymphadenopathy. Relation of etiologic agents to age. JAMA 1984;251:1286–8. [DOI] [PubMed] [Google Scholar]
- [13].Chen CC, Chen SY, Chen YS, et al. Mycobacterium fortuitum-induced persistent parotitis: successful therapy with clarithromycin and ciprofloxacin. Head Neck 2007;29:1061–4. [DOI] [PubMed] [Google Scholar]
- [14].Cox HJ, Brightwell AP, Riordan T. Non-tuberculous mycobacterial infections presenting as salivary gland masses in children: investigation and conservative management. J Laryngol Otol 1995;109:525–30. [DOI] [PubMed] [Google Scholar]
- [15].Lindeboom JA, Kuijper EJ, Bruijnesteijn van Coppenraet ES, et al. Surgical excision versus antibiotic treatment for nontuberculous mycobacterial cervicofacial lymphadenitis in children: a multicenter, randomized, controlled trial. Clin Infect Dis 2007;44:1057–64. [DOI] [PubMed] [Google Scholar]
- [16].Padovani D, Aimoni C, Grasso DL, et al. Non tuberculous mycobacteria infection of the parotid region: two familiar cases. Auris Nasus Larynx 2007;34:577–9. [DOI] [PubMed] [Google Scholar]
- [17].Benharrats I, Jacob L, Taulera O. Parotitis due to Mycobacterium and HIV infection. La Revue de medecine interne 1998;19:676–7. [DOI] [PubMed] [Google Scholar]
- [18].Gittinger FS, Raible A, Kempf VA. Non-tuberculous mycobacterial infection of the parotid gland in an immunosuppressed adult. J Med Microbiol 2008;57(part 4):536–9. [DOI] [PubMed] [Google Scholar]
- [19].Lawn SD, Checkley A, Wansbrough-Jones MH. Acute bilateral parotitis caused by Mycobacterium scrofulaceum: immune reconstitution disease in a patient with AIDS. Sex Trans Infect 2005;81:517–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Shaaban HS, Bishop SL, Menon L, et al. Mycobacterium chelonae infection of the parotid gland. J Global Infect Dis 2012;4:79–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Lake MA, Ambrose LR, Lipman MC, et al. “Why me, why now?” Using clinical immunology and epidemiology to explain who gets nontuberculous mycobacterial infection. BMC Med 2016;14:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Czaja CA, Merkel PA, Chan ED, et al. Rituximab as successful adjunct treatment in a patient with disseminated nontuberculous mycobacterial infection due to acquired anti-interferon-gamma autoantibody. Clin Infect Dis 2014;58:e115–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Browne SK, Zaman R, Sampaio EP, et al. Anti-CD20 (rituximab) therapy for anti-IFN-gamma autoantibody-associated nontuberculous mycobacterial infection. Blood 2012;119:3933–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Lin CH, Chi CY, Shih HP, et al. Identification of a major epitope by anti-interferon-gamma autoantibodies in patients with mycobacterial disease. Nat Med 2016;22:994–1001. [DOI] [PubMed] [Google Scholar]


