Abstract
Rationale:
Although trigger point injection is known as an easy and low-risk procedure, it is contraindicated to patients with hemorrhagic disorders or who regularly take anticoagulants/antiplatelets. However, taking clopidogrel is not a defined contraindication to this low-risk procedure.
Patient concerns:
The chief complaint of a 76-year old woman regularly taking clopidogrel was low back and left buttock pain which prolonged for several years.
Diagnoses:
The patient was diagnosed with L4-5 and L5-S1 spinal stenosis at the orthopedics department and was referred for lumbar spinal epidural steroid injection.
Intervention:
She was treated with trigger point injection.
Outcomes:
Three hours after the injection, she complained motor weakness and pain in the injection area. A hematoma on left gluteus medium muscle was detected with ultrasonography and ultrasound-guided needle aspiration was accomplished to relieve the symptom.
Lessons:
Trigger point injection for patients taking clopidogrel should be done with a caution to prevent such complication.
Keywords: clopidogrel, hematoma, trigger point injection
1. Introduction
Trigger point injection may induce complications such as vasovagal syncope, skin infection, hematoma, and pneumothorax, which depends on injection sites.[1,2] There is no complete statistics regarding the prevalence of these complications. A previous research which studied 12,134 admitted patients for complications after intramuscular injection revealed that abscess and hematoma were detected from 15 (0.12%) patients, and 8 (0.07%) individuals suffered bleeding.[3] Although low-risk procedures are known as easy and safe process, trigger point injection is contraindicated to patients with hemorrhagic disorders or who regularly take anticoagulants/antiplatelets.[4,5] However, it is not necessary to stop medicating cilostazol and clopidogrel before the procedure.[6] This case demonstrates a development of hematoma with motor weakness in a patient who was taking clopidogrel after trigger point injection.
2. Case presentation
2.1. Patient information
A 76-year-old Korean woman with a chief complaint of low back and left buttock pain prolonged for several years visited our clinic. She was diagnosed with L4-5 and L5-S1 spinal stenosis at the orthopedics department and was referred for lumbar spinal epidural steroid injection. She was taking 75 mg of clopidogrel and 80 mg of valsartan daily since after the diagnosis of hypertension 10 years ago. Besides hypertension and spinal stenosis, she was not diagnosed with other medical conditions. She used NSAID intermittently to control the pain, but it was not that affective. Four months before, she received trigger point injection in quadratus lumborum and gluteus medius at a local clinic, but it was not affective either. The informed consent was obtained from the patient after the objective of this paper was explained.
2.2. Clinical findings and timeline
The patient complained pain of the numerical rating scale (NRS) of 5, but there were no signs of weakness or sensory impairment. Based on physical examination (no signs of pain or radiating pain on both buttock detected by straight leg raising test), several pain points were observed in various sites of both quadratus lumborum and gluteus medius, and several trigger point injections were performed on that site. The patient was placed in a prone position, and trigger points on the left quadratus lumborum and gluteus medius were checked. A dose of 8 mL of 0.5% mepivacaine was injected 4 mL each with 25-G, 38-mm needle to the sites. To prevent bleeding, the injected sites were manually compressed for about 2 minutes. After confirming that there was no external bleeding, the patient was changed to a supine position and stayed still for 10 minutes. During this resting period, the patient told that the pain in the left lumbar and buttock was alleviated to NRS of 2. Additional 20 more minutes of resting, she returned home without specific findings. About 2 hours later, she came back to our clinic in a wheelchair with her guardian. The patient experienced pain, numbness, and weakness in the left buttock and lower legs about an hour after she left the hospital. Additionally, the pain was intensified to NRS of 7, and there was muscle weakness so that she could not maintain a straight posture while standing.
2.3. Diagnostic assessment
The patient was placed in a supine position and the muscle strength of left lower leg was checked. The muscle strength was normal when the knee joint and ankle joint were moved. However, voluntary abduction of hip joint was prohibited and she complained pain in the left buttock and posterior side of thigh on hip flexion and extension. There was no rash or bleeding on the left buttock area in a prone position, but she complained a severe tenderness. A 6 to 13 MHz linear transducer (Sonosite, Bothell, WA) under ultrasound-guided imaging was used to identify any anomalies in the site. Ultrasonographic images showed low echoic shadows of 18 × 9 mm in the left gluteus medius muscle (Fig. 1), which was suspected as a hematoma. When checked with the color Doppler image, there were no blood vessels located nearby.
Figure 1.
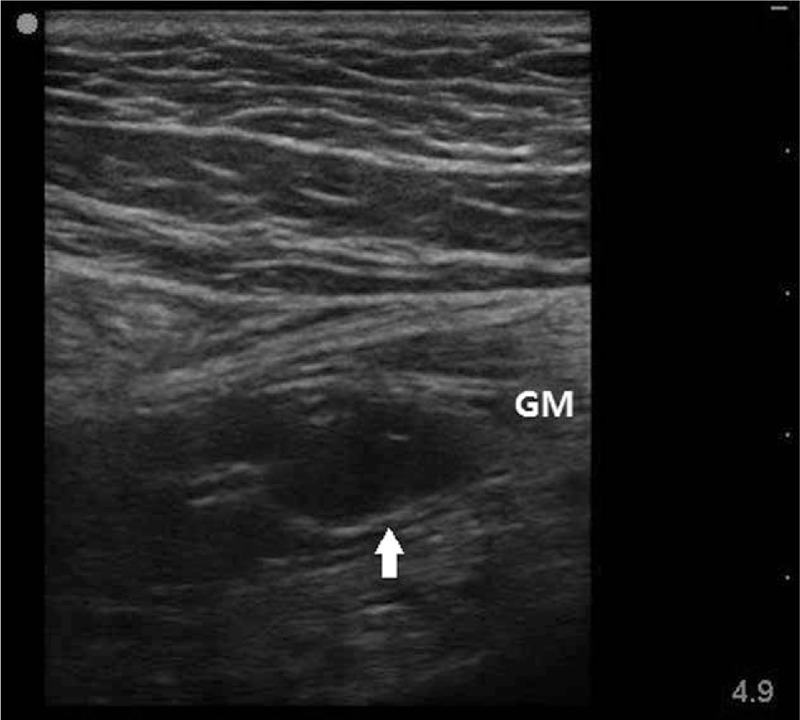
Gluteal sonogram obtained 3 hours after trigger point injection. This image shows hypoechoic oval-shaped intramuscular hematoma (18 × 9 mm) in gluteus medius muscle. Arrow represents hematoma. GM = gluteus medius.
2.4. Therapeutic intervention
With a 23-G needle, 0.7 mL of blood with a small volume of blood clots was aspirated from the hypoechoic site. The site of hematoma was manually compressed for about 10 minutes to prevent additional bleeding. The patient was placed in a supine position once again and rested for an hour. After resting, she could be able to stand still, and the pain and numbness in the area was relieved to NRS of 2. She still complained of a mild pain in the left buttock and leg, but the motor weakness improved so that she could be able to walk assisted or independently.
2.5. Follow-up and outcomes
Two days later, the patient visited again for follow-up. The pain on the left quadrates lumborum and gluteus medius muscles were decreased (NRS 1) and no other symptoms was detected except for a slight numbness. She was instructed to stop taking clopidogrel until the next follow-up, which was arranged after 1 week. The pain in the left buttock was increased to NRS 4 after a week and this time a blood test was performed before trigger point injection. Blood tests revealed normal values for prothrombin time (PT), activated partial thromboplastin time (aPTT), international normalized ratio (INR), bleeding time, and coagulation time. Ultrasound-guided trigger point injection was performed and no hematoma was detected during the procedure. After 5 minutes of manual compression, the pain was improved without any complication.
3. Discussion
Patients with spinal stenosis, as shown in the present case, are usually associated with secondary myofascial pain.[7,8] Trigger point injection is an effective intervention to control pain in lumbar and hip area because pain and referred pain are associated with trigger points.[4] Trigger point injection is an easy and simple procedure that does not require special instruments so that it is frequently performed in clinical practices than nerve blocks. Besides its versatile use in musculoskeletal diseases, it is contraindicated to patients with hemorrhagic disorders or local/systematic infection, or who use anticoagulants/antiplelets.[4,5] However, it is not always necessary to stop cilostazol or clopidogrel before application of low-risk procedures, including trigger point injection and nerve block.[6]
In the case presented, we performed trigger point injection instead of central nerve block based on the facts that the patient had prominent sites of pain and that she was regularly taking clopidogrel. Observed pain relief after the initial trigger point injection indicates the diagnosis and choice of intervention were appropriate for this patient. However, the increase in pain after several hours was probably attributed to delayed hemorrhage due to clotting problem induced by clopidogrel medication. The developed hematoma directly caused tenderness and referred pain that resulted in pain in the entire buttock. One possible cause of the combination of pain and weakness may be due to worsening of the lumbar spinal lesion. It is unlikely since there is no history of trauma after the procedure or abnormalities in the strength of the left knee joint and ankle joint, and the intensity of pain was gradually increased. The most possible explanation of the muscle weakness regarding the procedure site is the damage or block of superior gluteal nerve. The superior gluteal nerve runs between gluteus medius and gluteus minimus muscles, and controls the muscles. The damage of superior gluteal nerve induces relaxation of gluteus medius and minimus muscles, and affects abduction and flexion of the left lower limb like the patient in this case.
Three possibilities of the cause of the damage or block of superior gluteal nerve include the following: injection of mepivacaine close to the nerve that directly induced block of the nerve, direct damage by injection needle, and compression of superior gluteal nerve by intramuscular hematoma. Based on the facts that the observed hematoma in the present case was in the gluteus medius muscle and that the recovery after the aspiration of hematoma was rapid, the possibility of direct nerve block or damage is low. The blood observed after needle aspiration and fast recovery after aspiration supports the fact that the weakened muscle strength is most probably caused by compression of superior gluteal nerve by intramuscular hematoma.
Although the initial blood test observed at the time of the hematoma was not obtained, it is difficult to imagine that an underlying hemorrhagic disease is associated with the case because no specific finding was found in the blood test conducted a week later.
No previous study reported cases with development of hematoma without specific technical problem during the low-risk procedure associated with weakened muscle strength in a patient taking clopidogrel. Simple hematoma can occur after invasive procedures and it can be prevented by sufficient compression of soft tissue after the procedure. However, the present case indicated that a special attention and precautions are required even for low-risk procedures frequently performed in clinical practices for patients with medications.
4. Conclusions
Low-risk procedures, including trigger point injection, may cause unexpected complications like delayed bleeding and hematoma in patients with clopidogrel medication as observed in this case. Therefore, additional soft tissue compression and monitoring for a sufficient time are required.
Footnotes
Abbreviation: NRS = numerical rating scale.
The authors report no conflicts of interest.
References
- [1].Alvarez DJ, Rockwell PG. Trigger points: diagnosis and management. Am Fam Physician 2002;65:653–61. [PubMed] [Google Scholar]
- [2].Criscuolo CM. Interventional approaches to the management of myofascial pain syndrome. Curr Pain Headache Rep 2001;5:407–11. [DOI] [PubMed] [Google Scholar]
- [3].Greenblatt DJ, Allen MD. Intramuscular injection-site complications. JAMA 1978;240:542–4. [PubMed] [Google Scholar]
- [4].Simon DG, Travell JG. Myofascial Pain and Dysfunction: the Trigger Point Manual. 1999;Baltimore, MD: Williams & Wilkins, 94–173. [Google Scholar]
- [5].Ruoff GE. Technique of Trigger Point Injection. Procedures for Primary Care Physicians. 1994;St Louis, MO: Mosby, 164–167. [Google Scholar]
- [6].Narouze S, Benzon HT, Provenzano DA, et al. Interventional spine and pain procedures in patients on antiplatelet and anticoagulant medications: guidelines from the American Society of Regional Anesthesia and Pain Medicine, the European Society of Regional Anaesthesia and Pain Therapy, the American Academy of Pain Medicine, the International Neuromodulation Society, the North American Neuromodulation Society, and the World Institute of Pain. Reg Anesth Pain Med 2015;40:182–212. [DOI] [PubMed] [Google Scholar]
- [7].Chen CK, Nizar AJ. Myofascial pain syndrome in chronic back pain patients. Korean J Pain 2011;24:100–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Gerwin RD. Classification, epidemiology, and natural history of myofascial pain syndrome. Curr Pain Headache Rep 2001;5:412–20. [DOI] [PubMed] [Google Scholar]


