Abstract
This study aimed to evaluate the efficacy of escitalopram monotherapy in the treatment of major depressive disorder (MDD) on the basis of pooled data analysis of 4 Chinese clinical trials.
A total of 649 outpatients with MDD score of ≥18 at the 17-item Hamilton Depression Rating Scale (HAMD17) were included across 4 eligible studies. Patients were treated with 10 mg/day escitalopram for 2 weeks, and then 20 mg/day escitalopram was administered if the clinical response was poor.
The change in total HAMD17 score was significantly greater in moderate MDD group than in other subgroups (P < .001), but the proportion of responders and remission rate in moderate MDD group were markedly lower than in mild MDD group. As compared to patients with concomitant anxiety, anxiety free patients showed significant improvement in total HAMD17 score at days 14 and 28 (P < .05). However, there was no significant difference in the change of total HAMD17 score at day 7 and the end of study. According to clinical global impression (CGI) score, the total response rate (very much improved and much improved) was 86.7%. There were 479 adverse events (AEs), but serious AEs were not observed. A total of 3.39% (22/649) of patients withdrew from these studies due to AEs. The most common (incidence ≥2.0%) AEs were nausea, dry mouth, somnolence, dizziness, fatigue, dyspepsia, liver dysfunction, and loss of appetite.
Escitalopram monotherapy is effective and safe in the treatment of MDD in Chinese patients, and therapeutic efficacy is dependent on the severity of MDD. Further study is needed to identify better predictors of therapeutic responses.
Keywords: efficacy, major depressive disorder, pooled analysis, randomized controlled trial
1. Introduction
Major depressive disorder (MDD) is a chronic, recurrent mental disease with disability characterized by both symptomatic and functional impairment. The World Health Organization estimates that, by 2030, MDD will become the 2nd most common cause of disability and burden of disease.[1]
There are some evidence-based psychotherapies and pharmacotherapies for MDD. Antidepressants remain a mainstay of treatment for MDD, especially for those with moderate to severe depression. The new antidepressants such as selective serotonin reuptake inhibitors (SSRIs), serotonin noradrenaline reuptake inhibitors (SNRIs), and agents with new mechanism of action and fewer side effects have been developed for the treatment of MDD. At present, most clinical guidelines recommend the new generation antidepressants as the 1st-line treatment for MDD.[2,3]
However, the available findings are still conflicting on the effectiveness of antidepressants in head-to-head trials, which might be ascribed to the small sample size in available studies. Consequently, pooled analyses are increasingly used to examine the effectiveness of a specific medication in patients. Pooled analysis is a powerful technique that may increase the statistical power to detect the differences between active treatments. However, for pooled analysis, studies included should be similar in patient selection, study design, and outcome assessment.[4]
In randomized, controlled, multicentered clinical trials, the sample size is usually limited; there are significant variations in the demographics and the severity of disease among studies. For example, younger and milder depression patients are easily recruited in some trials, but not in others. Thus, a single study is often difficult to identify the generalized efficacy and safety profiles for a new antidepressant and more difficult to demonstrate the difference in the therapeutic efficacy between subgroups. These issues may be resolved by pooled analysis. Escitalopram, an S-enantiomer of racemic citalopram, is an SSRI and also has modulatory effect at an allosteric binding site of the serotonin transporter protein (SERT).[5,6] A variety of placebo controlled, randomized trials have shown that escitalopram is effective for MDD,[7,8] and its efficacy and tolerability are superior to other SSRIs and other antidepressants.[9–13]
Since 2006, several clinical trials on escitalopram have been conducted in China. However, results from a single study may not represent the actual efficacy and safety of the drug due to the small sample size and study design in a specific study. In this report, 4 studies conducted in our site (as principal investigator) were pooled for further analysis. They were sponsored by pharmaceutical enterprises A (CFDA approval No.:2004L04118), B (approval No.:2005L00109), C (approval No.:2004L00814), and D (approval No.:2005L00773).
2. Materials and methods
2.1. Study design
The 4 trials included in this pooled analysis were very similar in the methodology. They were as randomized, multicenter, double-blind, double dummy, flexible dosage, active control, and parallel group trials. This prospective randomized controlled trial was conducted between March 1, 2007 and December 1, 2009 at 6 sites in Shanghai, Hebei, Xi’an (2 sites), Nanjing, and Yunnan in China. The study was performed in accordance with the principles in the Declaration of Helsinki and Good Clinical Practice guidelines, and the whole study was approved by the local ethics committees. All patients provided written informed consent.
Citalopram was used as a control (except for trial A in which escitalopram [Lexapro] was used as a control), and the treatment last for 6 weeks. In these trials, patients were treated with 10 mg/day escitalopram or 20 mg/day citalopram for the 1st 2 weeks, and then 20 mg/day escitalopram or 40 mg/day citalopram was administered if a poor clinical response and good tolerability were observed.
2.2. Inclusion/exclusion criteria
Outpatients aged 18 to 65 years were recruited into these studies. Patients were diagnosed with MDD according to the diagnostic criteria from the Diagnostic and Statistical Manual for Mental Disorders-IV criteria and had total 17-item Hamilton Depression Rating Scale (HAMD17) score of ≥18 (≥20 in trial A).
All subjects were in good physical health as determined by medical history, physical examination, blood laboratory examination, electrocardiography, and urinalysis. Subjects were free of substance abuse or dependence for at least 3 months and had no serious suicide risk according to the clinical evaluation. Patients who had a history of epilepsy, no response or allergic reaction to citalopram or escitalopram were excluded from these studies.
2.3. Efficacy assessments
The primary outcome was the change in total HAMD17 score from baseline to the end of study. Response (prospectively defined as ≥50% decrease from baseline total HAMD17 score) and remission (prospectively defined as total HAMD17 score of ≤7) rates were also evaluated. The secondary outcomes were the score of the clinical global impression (CGI) and the change in total Hamilton Anxiety Rating Scale (HAMA) score from baseline to the end of study. In addition, post hoc analyses were employed to evaluate the patients with different severities of depression.
2.4. Statistical analysis
The therapeutic efficacy was evaluated with intent-to-treat (ITT) analysis, in which patients who received at least 1 dose of the study drug and had at least 1 valid evaluation of the primary efficacy scale. The missing data of HAMD17 score at the end of study in patients who prematurely discontinued treatment were analyzed using last observation carried forward (LOCF) method.
2.5. Post hoc analysis
Four RCT trials mentioned above were included for pooled analysis in which data of all patients treated with escitalopram were pooled for further analysis. In trial A, the control drug was escitalopram, and 260 patients in this trial were also enrolled into the pooled analysis. The severity of depression could be classified as mild (HAMD17 score 18–21), moderate (22–25), and moderate–severe (>26).[14]
In addition, patients were also divided into 3 subgroups: single depressive episode, recurrent MDD (defined as at least 1 depressive episode lasting no shorter than 2 weeks, and there was no depressive episode at least 2 months before the current episode), and chronic depression (MDD with chronic specification and so-called double depression [combined MDD and dysthymic disorder]).
The prospective primary outcome was a change in total HAMD17 score from baseline to week 6, which was analyzed using a likelihood-based mixed-effects model for repeated measures (MMRM) on the ITT and analysis of covariance. Post hoc and secondary analyses were retrospectively carried out to completely evaluate the primary results. The change in total HAMD17 score from baseline to week 6 in subgroups (different severities of depression) was analyzed using MMRM analysis.
Qualitative data are presented as number (%) and the differences among subgroups were tested using Pearson χ2 test. Quantitative data are presented as mean and standard deviation (SD), and the differences among subgroups were tested using analysis of variance. Bonferroni correction was used for multiple comparisons for post hoc testing. Statistical analysis was performed with the SAS9.3 software. A value of P < .05 was considered statistically significant.
3. Results
3.1. Demographics and baseline characteristics
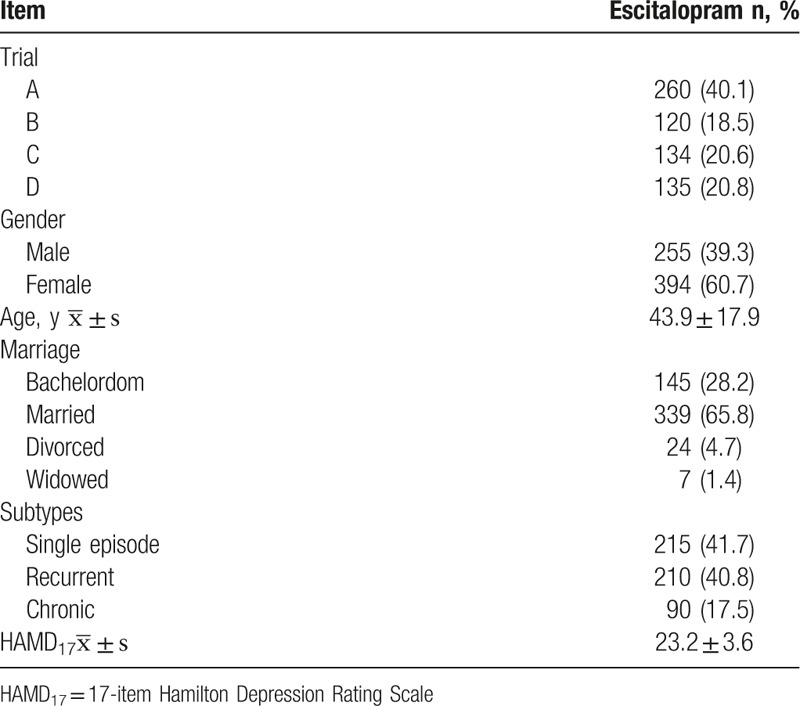
A total of 649 patients meeting the inclusion criteria were included these 4 studies. The average total HAMD17 score was 23.2 ± 3.6 at baseline. At the end of study, the average dosage of escitalopram was 14.2 ± 4.9 mg/day. The escitalopram dose in 42.4% (275/649) of patients was titrated to 20 mg/day, and 57.6% (374/649) of patients were treated with 10 mg/day escitalopram.
The demographics and baseline characteristics of patients treated with escitalopram are presented in Table 1.
Table 1.
Demographics and baseline characteristics of patients recruited.
3.2. Therapeutic efficacy of escitalopram in MDD
Table 2 shows the change in total HAMD17 score from baseline to the end of study. The average change in total HAMD17 score was 12.4 ± 5.0, 12.9 ± 6.8, and 15.0 ± 7.9 in mild, moderate, and moderate–severe subgroups, respectively. The change in total HAMD17 score from baseline to day 42 was greater in the moderate–severe subgroup than in other subgroups (P < .001) Table 2 (Fig. 1).
Table 2.
Change in total 17-item Hamilton Depression Rating Scale (HAMD17) score from baseline to the endpoint.
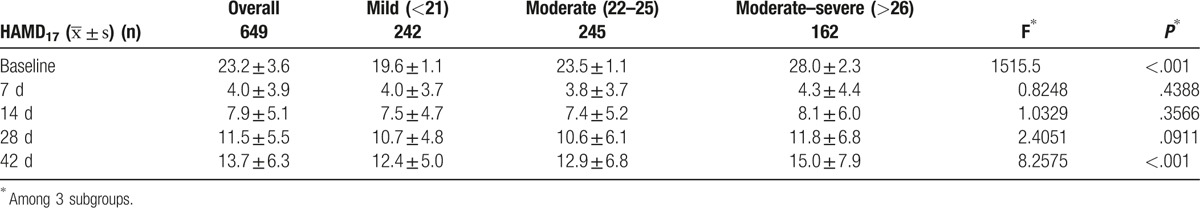
Figure 1.
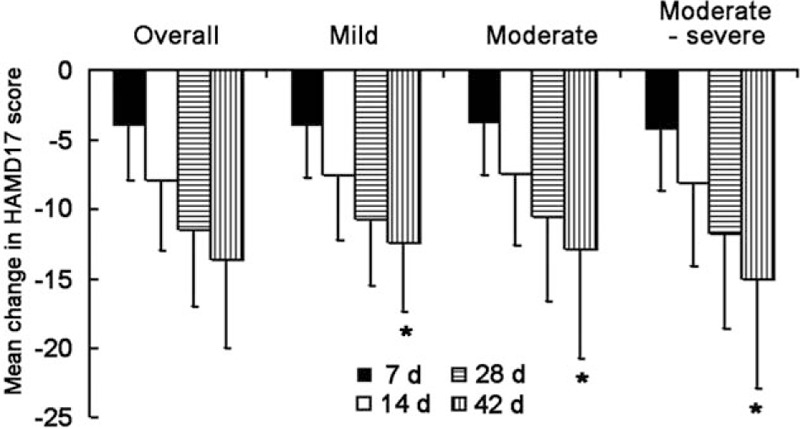
Mean change in 17-item Hamilton Depression Rating Scale (HAMD17) score from baseline to the end of study. ∗P < .05 among three groups.
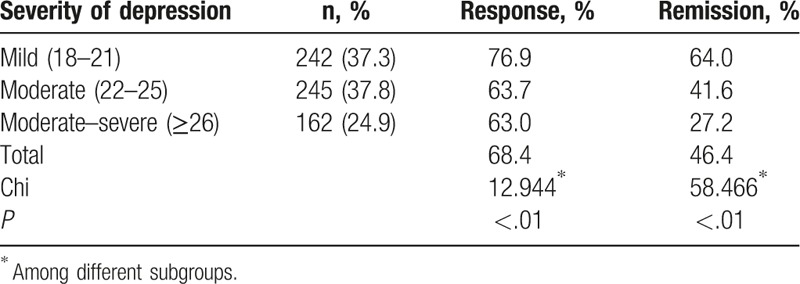
The overall response and remission rates after escitalopram monotherapy were 68.4% and 46.4%, respectively. The response rate was 76.9%, 63.7%, and 63.0%, in mild, moderate, and moderate–severe subgroups, respectively, showing significant difference among them (P < .01). The remission rate was 64.0%, 41.6%, and 27.2% in mild, moderate, and moderate–severe subgroups, respectively, showing significant difference among them (P < .01) (Table 3).
Table 3.
Response and remission rates in different subgroups after treatment.
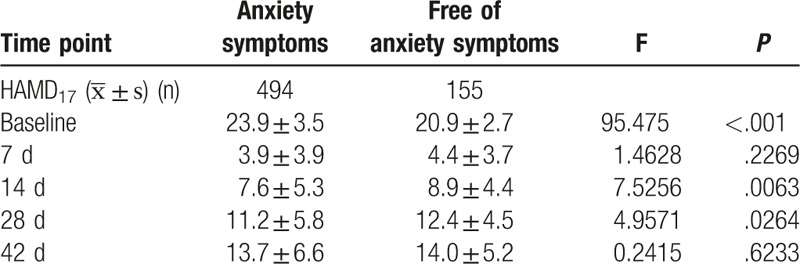
3.3. Efficacy in patients with concomitant anxiety
Among 649 patients, 76.1% (494/649) had concomitant anxiety symptoms (HAMA ≥ 14) which was more severe than in those without anxiety at baseline, and significant difference was observed in total HAMD17 score between them (P < .001). When compared with patients with concomitant anxiety, anxiety free patients showed significant improvement in total HAMD17 score at days 14 and 28, but not on day 7 and at the end of study (Table 4).
Table 4.
Change in total 17-item Hamilton Depression Rating Scale (HAMD17) score from baseline to the endpoint in patients with and without concomitant anxiety symptoms.
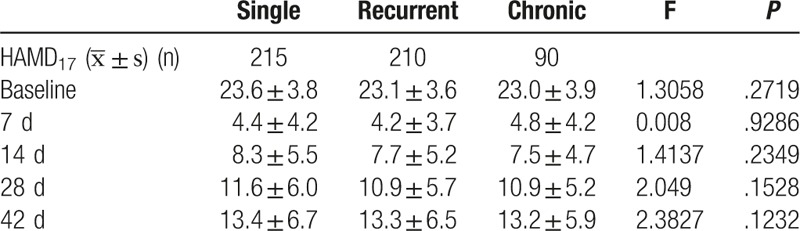
3.4. Efficacy in different episode subgroups
There were 215, 210, and 90 patients in single, recurrent, and chronic MDD subgroups. No significant difference was observed in the change of total HAMD17 score among these subgroups (P > .05) (Table 5).
Table 5.
Change in total 17-item Hamilton Depression Rating Scale (HAMD17) score from baseline to the endpoint in different episode subgroups.
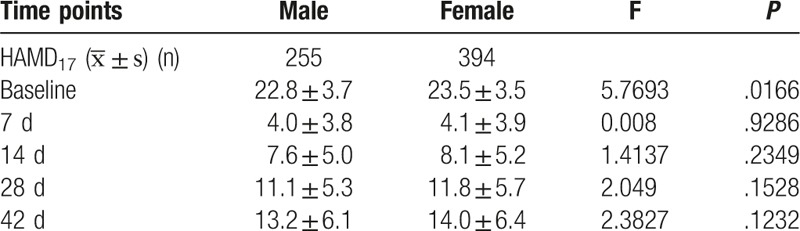
3.5. Relationship between therapeutic efficacy and gender
There were 255 males and 394 females in the pooled analysis. There was no significant difference in the change of total HAMD17 score from baseline to the end of study between males and females (P > .05) (Table 6).
Table 6.
Change in total 17-item Hamilton Depression Rating Scale (HAMD17) score from baseline to the endpoint in males and females.
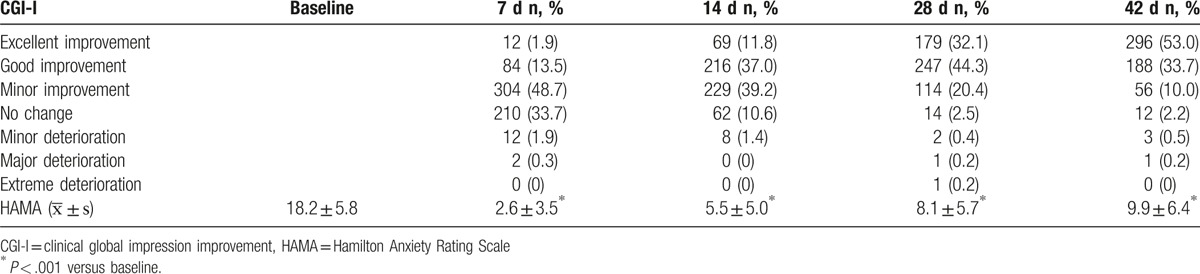
3.6. Secondary outcomes of therapeutic efficacy
According to the CGI improvement (CGI-I) at the end of study, 296 (53.0%) and 188 (33.7%) subjects achieved excellent improvement and good improvement, respectively. The total improvement rate (excellent improvement + good improvement) was 86.7%. The total HAMA score changed significantly on days 7, 14, 28, and 42 when compared with that at baseline (P < .001) (Table 7).
Table 7.
CGI-I and change in HAMA score in all the patients.
3.7. Safety and tolerance of escitalopram
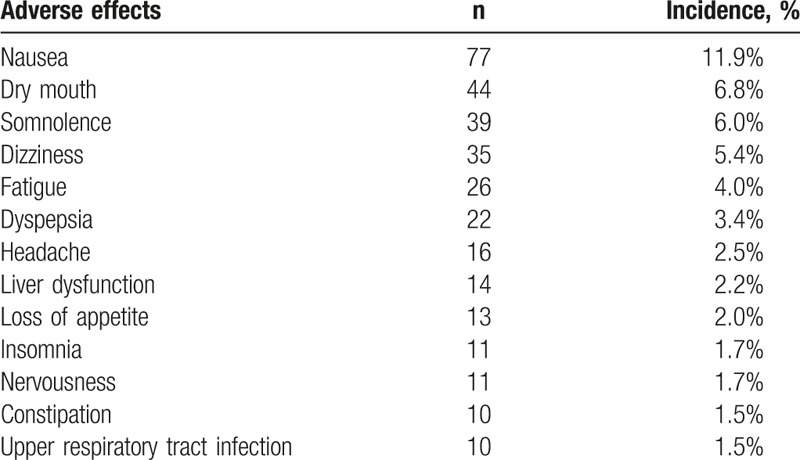
There were 479 adverse events (AEs) reported in these studies. The most common AEs were gastrointestinal symptoms (nausea, dry mouth, and dyspepsia). However, most of them were mild to moderate, and serious AEs were not observed. A total of 35 patients withdrew from this study (5.4%; 35/649), and 3.39% (22/649) withdrawn due to AEs such as liver dysfunction, nausea, suicide attempt, dizziness, and fatigue. The most common (incidence ≥2.0%) AEs were nausea, dry mouth, somnolence, dizziness, fatigue, dyspepsia, liver dysfunction, and loss of appetite (Table 8).
Table 8.
Adverse events (AEs) with incidence higher than 1.5%.
4. Discussion
Since the middle 1980s, SSRIs have become the 1st-line treatment of depression with well-established efficacy and safety. Citalopram, one of SSRIs, is a racemic mixture of R-enantiomer and the S-enantiomer. The S-enantiomer is approximately 30 to 40 times more potent than the R-enantiomer in the ability to inhibit SERT.[5,6] The R-enantiomer is able to competitively bind to SERT and blocks the S-enantiomer. As a result, not only the efficacy will be impaired but also the risk for adverse effects associated with this drug might increase.[6] Escitalopram, the purified active S-enantiomer, is more selective to the reuptake of serotonin than other SSRIs with a low affinity to other receptors. Thus, it is presumed to be more effective and safer in the treatment of MDD.
In available studies, the overall efficacy and safety were reported, and subgroup analysis was rare; the raw data and unpublished studies are unavailable, which makes the high-quality meta-analysis difficult. This study pooled the 4 clinical trials in which our hospital participated in for further analysis. The influences of severity of depression, age, gender, and concomitant anxiety on the therapeutic efficacy of escitalopram, whether the therapeutic efficacy of escitalopram was achieved sooner and whether escitalopram was more effective for severe depressed patients were investigated, which may provide evidence on the clinical treatment of MDD with escitalopram.
The efficacy of escitalopram in the treatment of MDD in Chinese patients as demonstrated in this pooled analysis was consistent with that reported in other populations. According to the CGI, 86.7% of patients achieved good/excellent improvement at the end of study. The response rate (68.4%) as measured by HAMD17 was similar to that in other studies (73.0%, 63.7%, and 72.3% respectively).[15–19] The response and remission rates in this pooled analysis were lower than those in another 2 Chinese studies[18,19] (response rate: 78.4% and 79.8%, respectively; remission rate: 57.5% and 66.4%, respectively). To our knowledge, this was the 1st pooled analysis with comparatively large sample size that evaluated the efficacy of escitalopram in the treatment of MDD in Chinese patients.
In general, the more severe the depressive symptoms, the more difficult the treatment is. It has been demonstrated that escitalopram has better efficacy than other SSRIs[9,13,15,16,20] and is either better than[10,13] or equivalent[21,22] to SNRIs (such as duloxetine and venlafaxine) in the treatment of MDD. Studies have shown that the efficacy of escitalopram is better than that of citalopram and SNRIs for severely depressed patients.[14,15,17,23,24] This pooled analysis also confirmed that the change in total HAMD17 score was greater in moderate–severe subgroup than in other subgroups. These results suggest that escitalopram may be suitable for the treatment of severe depression. Although the HAMD17 score reduced significantly at different time points after treatment in severe depression patients, the response and remission rates in these patients were markedly lower than in mild depression patients after 6-week treatment. The remission rate (46.4%) was also lower than in other studies.[9,16] Moreover, 57.6% of patients were treated with 10 mg/day escitalopram at the end of study. This dose is lower than commonly used in clinical practice, especially in severe depression patients.
Patients with MDD often have concomitant anxiety, which might be ascribed to the similarities in the pathogenesis and symptoms between depression and anxiety. Furthermore, the majority of antidepressants can also alleviate anxiety symptoms. Our results showed that the proportion of MDD patients with concomitant anxiety was as high as 76.1%, and these patients had higher baseline HAMD17 score. At the end of study, the efficacy was similar between patients with and without anxiety. Therefore, escitalopram is also effective for anxiety symptoms associated with MDD and depressive symptoms as shown in available studies.[25–27] However, the existing anxiety may affect the therapeutic effectiveness of escitalopram. In this study, the changes in HAMD17 score from baseline to weeks 2 and 4 were significantly greater in patients without anxiety. However, the better efficacy of escitalopram for MDD patients with apparent anxiety as previously reported[28] was not observed in our analysis. Further studies with a more elegant design are needed to explore whether escitalopram is more effective for MDD with concomitant anxiety.
There were 479 AEs reported in 4 studies. The most common AEs were gastrointestinal symptoms, but most of them were mild to moderate. Serious AEs were not observed. This was consistent with previously reported. In addition, the incidence of AEs and the proportion of patients withdrawing from 4 studies were significantly lower than previously reported.[9,16]
There were some limitations in this study. First, the majority of patients treated with escitalopram in 4 RCTs had mild and moderate MDD, and only 24.9% of patients were diagnosed with moderate to severe MDD (HAMD17 > 26). Second, the analysis of therapeutic efficacy might be influenced by the selection of cut-off value of total HAMD17 score at baseline among 4 trials. Moreover, fewer data were available on the efficacy of escitalopram in the treatment of severe depression patients. Third, the pooled analysis was based on randomized controlled trials in which treatment last for 6 weeks. This might be short for some patients, especially for severe MDD patients with concomitant anxiety.
In summary, the pooled analysis of 4 clinical trials investigate the efficacy of escitalopram in the treatment of MDD in Chinese patients and results show that escitalopram is effective for the treatment of MDD in Chinese patients with favorable safety.
Acknowledgments
The authors thank the National Science and Technology Major Project for IND (investigational new drug) (No. 2017ZX09304-020) for the support.
Footnotes
Abbreviations: AE = adverse event, CGI = clinical global impression, HAMA = Hamilton Anxiety Rating Scale, HAMD17 = 17-item Hamilton Depression Rating Scale, MDD = major depressive disorder, SERT = serotonin transporter protein, SNRI = serotonin noradrenaline reuptake inhibitor, SSRI = selective serotonin reuptake inhibitor.
GL and YS contributed equally to this work.
Funding/support: This study was supported by the National Science and Technology Major Project for IND (investigational new drug) (No. 2017ZX09304-020).
The authors have no conflicts of interest to disclose.
References
- [1].World Health Organization, Mathers C, Fat DM, Boerma J. The Global Burden of Disease: 2004 Update. 2008. [Google Scholar]
- [2].Davidson J. Major depressive disorder treatment guidelines in America and Europe. J Clin Psychiatry 2010;71(E1):e04. [DOI] [PubMed] [Google Scholar]
- [3].Gelenberg A. A review of the current guidelines for depression treatment. J Clin Psychiatry 2010;71:e15–15. [DOI] [PubMed] [Google Scholar]
- [4].Lieberman JA, Greenhouse J, Hamer RM, et al. Comparing the effects of antidepressants: consensus guidelines for evaluating quantitative reviews of antidepressant efficacy. Neuropsychopharmacology 2005;30:445–60. [DOI] [PubMed] [Google Scholar]
- [5].Braestrup C, Sanchez C. Escitalopram: a unique mechanism of action. Int J Psychiatry Clin Pract 2004;8(s1):11–3. [DOI] [PubMed] [Google Scholar]
- [6].Zhong H, Haddjeri N, Sánchez C. Escitalopram, an antidepressant with an allosteric effect at the serotonin transporter – a review of current understanding of its mechanism of action. Psychopharmacology 2012;1–3. [DOI] [PubMed] [Google Scholar]
- [7].Baldwin DS. Escitalopram: efficacy and tolerability in the treatment of depression. Hosp Med 2002;63:668–71. [DOI] [PubMed] [Google Scholar]
- [8].Lepola UM, Loft H, Reines EH. Escitalopram (10–20 mg/day) is effective and well tolerated in a placebo-controlled study in depression in primary care. Int Clin Psychopharmacol 2003;18:211–7. [DOI] [PubMed] [Google Scholar]
- [9].Kasper S, Baldwin DS, Larsson Lonn S, et al. Superiority of escitalopram to paroxetine in the treatment of depression. Eur Neuropsychopharmacol 2009;19:229–37. [DOI] [PubMed] [Google Scholar]
- [10].Montgomery SA, Andersen HF. Escitalopram versus venlafaxine XR in the treatment of depression. Int Clin Psychopharmacol 2006;21:297–309. [DOI] [PubMed] [Google Scholar]
- [11].Nierenberg AA, Greist JH, Mallinckrodt CH, et al. Duloxetine versus escitalopram and placebo in the treatment of patients with major depressive disorder: onset of antidepressant action, a non-inferiority study. Curr Med Res Opin 2007;23:401–16. [DOI] [PubMed] [Google Scholar]
- [12].Pigott TA, Prakash A, Arnold LM, et al. Duloxetine versus escitalopram and placebo: an 8-month, double-blind trial in patients with major depressive disorder. Curr Med Res Opin 2007;23:1303–18. [DOI] [PubMed] [Google Scholar]
- [13].Sicras-Mainar A, Navarro-Artieda R, Blanca-Tamayo M, et al. Comparison of escitalopram vs. citalopram and venlafaxine in the treatment of major depression in Spain: clinical and economic consequences. Curr Med Res Opin 2010;26:2757–64. [DOI] [PubMed] [Google Scholar]
- [14].Llorca PM, Azorin JM, Despiegel N, et al. Efficacy of escitalopram in patients with severe depression: a pooled analysis. Int J Clin Pract 2005;59:268–75. [DOI] [PubMed] [Google Scholar]
- [15].Kennedy SH, Andersen HF, Thase ME. Escitalopram in the treatment of major depressive disorder: a meta-analysis. Curr Med Res Opin 2009;25:161–75. [DOI] [PubMed] [Google Scholar]
- [16].Lam RW, Lonn SL, Despiegel N. Escitalopram versus serotonin noradrenaline reuptake inhibitors as second step treatment for patients with major depressive disorder: a pooled analysis. Int Clin Psychopharmacol 2010;25:199–203. [DOI] [PubMed] [Google Scholar]
- [17].Montgomery S, Hansen T, Kasper S. Efficacy of escitalopram compared to citalopram: a meta-analysis. Int J Neuropsychopharmacol 2011;14:261–8. [DOI] [PubMed] [Google Scholar]
- [18].Shen Y, Li H, Li J, et al. Comparison of escitalopram with citalopram in treatment of depression: a randomized, double-blind, multicenter study. Chin J New Drugs Clin Rem 2012;30:901–6. [Google Scholar]
- [19].Chen H, Li H, Kuang Z, et al. Randomized double-blind controlled trial of escitalopram versus citalopram in the treatment of depression. Shanghai Arch Psychiatry 2010;22:300–3. [Google Scholar]
- [20].Chauvet-Gelinier JC. [Efficacy of escitalopram vs paroxetine on severe depression with associated anxiety: data from the “Boulenger” study]. Encephale 2010;36:425–32. [DOI] [PubMed] [Google Scholar]
- [21].Nierenberg AA, Greist JH, Mallinckrodt CH, et al. Duloxetine versus escitalopram and placebo in the treatment of patients with major depressive disorder: onset of antidepressant action, a non-inferiority study. Curr Med Res Opin 2007;23:401–16. [DOI] [PubMed] [Google Scholar]
- [22].Pigott TA, Prakash A, Arnold LM, et al. Duloxetine versus escitalopram and placebo: an 8-month, double-blind trial in patients with major depressive disorder. Curr Med Res Opin 2007;23:1303–18. [DOI] [PubMed] [Google Scholar]
- [23].Bech P, Andersen HF, Wade A. Effective dose of escitalopram in moderate versus severe DSM-IV major depression. Pharmacopsychiatry 2006;39:128–34. [DOI] [PubMed] [Google Scholar]
- [24].Si T, Wang G, Yang F, et al. Efficacy and safety of escitalopram in treatment of severe depression in Chinese population. Metab Brain Dis 2017;32:891–901. [DOI] [PubMed] [Google Scholar]
- [25].Baldwin DS, Reines EH, Guiton C, et al. Escitalopram therapy for major depression and anxiety disorders. Ann Pharmacother 2007;41:1583–92. [DOI] [PubMed] [Google Scholar]
- [26].Bandelow B, Andersen HF, Dolberg OT. Escitalopram in the treatment of anxiety symptoms associated with depression. Depress Anxiety 2007;24:53–61. [DOI] [PubMed] [Google Scholar]
- [27].Jiang K, Li L, Wang X, et al. Efficacy and tolerability of escitalopram in treatment of major depressive disorder with anxiety symptoms: a 24-week, open-label, prospective study in Chinese population. Neuropsychiatr Dis Treat 2017;13:515–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Boulenger JP, Hermes A, Huusom AK, et al. Baseline anxiety effect on outcome of SSRI treatment in patients with severe depression: escitalopram vs paroxetine. Curr Med Res Opin 2010;26:605–14. [DOI] [PubMed] [Google Scholar]


