Abstract
Low-frequency variants showed that there is more power to detect risk variants than to detect protective variants in complex diseases. Aldosterone plays an important role in the renin–angiotensin–aldosterone system, and aldosterone synthase catalyzes the speed-controlled steps of aldosterone biosynthesis. Polymorphisms of the aldosterone synthase gene (CYP11B2) have been reported to be associated with essential hypertension (EH). CYP11B2 polymorphisms such as –344T/C, have been extensively reported, but others are less well known. This study aimed to assess the association between human CYP11B2 and EH using a haplotype-based case–control study. A total of 1024 EH patients and 956 normotensive controls, which consist of north Han population peasants, were enrolled. Seven single nucleotide polymorphisms (SNPs) (rs28659182, rs10087214, rs73715282, rs542092383, rs4543, rs28491316, and rs7463212) covering the entire human CYP11B2 gene were genotyped as markers using the MassARRAY system. The major allele G frequency of rs542092383 was found to be risk against hypertension [odds ratio (OR) 3.478, 95% confidence interval (95% CI) 1.407–8.597, P = .004]. The AG genotype frequency of SNP rs542092383 was significantly associated with an increased risk of hypertension (OR 4.513, 95% CI 1.426–14.287, P = .010). In the haplotype-based case–control analysis, the frequency of the T-G-T haplotype was higher for EH patients than for controls (OR 5.729, 95% CI 1.889–17.371, P = .000495). All |D′| values of the seven SNPs were >0.9, and r2 values for rs28659182- rs10087214-rs28491316-rs7463212 SNPs were >0.8 and showed strong linkage intensity. Haplotype T-G-T may therefore be a useful genetic marker for EH.
Keywords: CYP11B2, essential hypertension, genetics, haplotype, low-frequency variants
1. Introduction
Single nucleotide polymorphisms (SNPs) discovered by genome-wide association studies (GWAS), are significantly associated with many complex traits, yet account for only a small fraction of the genetic variation of complex traits in human populations. Where is the missing heritability? There is a evidence that the remaining heritability is due to incomplete linkage disequilibrium (LD) between causal variants and genotyped SNPs, exacerbated by causal variants having lower minor allele frequency (MAF) than the SNPs explored to date.[1]
Most complex diseases involve in a mix of genetic and environmental factors, and much of the heritability remains unaccounted for by common variants (≥5% frequency). It has been postulated that lower frequency variants contribute to the remaining heritability. Low-frequency (<5% frequency) variants showed that there is more power to detect risk variants than to detect protective variants.[2]
Essential hypertension (EH) is a kind of complex diseases and a major risk factor for diseases such as cardiovascular disease and kidney failure.[3–5] The renin–angiotensin–aldosterone system (RAAS) plays an important role in modulating blood pressure (BP) in EH by regulating sodium and intravascular volume homeostasis.[6,7] Aldosterone is a key component of the RAAS.[8,9] Aldosterone synthase, a member of the cytochrome P450 superfamily of enzymes, is encoded by CYP11B2, which is spread over 7 kb on chromosome 8q24.3, and contains 9 exons. It catalyzes the terminal steps of steroid biosynthesis, in which 11-deoxycorticosterone is converted to aldosterone.[10,11] Therefore, human CYP11B2 is a potential candidate gene for the development of EH.
Previous studies have shown that CYP11B2 may be involved in EH,[12,13] and a number of CYP11B2 polymorphisms have been identified such as C-344T, K173R, and intron 2 IC. Among them, C-344T in the promoter region has been widely studied, but the results are often inconsistent.[14,15] Studies on other CYP11B2 polymorphisms are rare, so this study aimed to investigate the association between novel CYP11B2 polymorphisms and susceptibility to EH in the Han population of northern China, and we found some low-frequency variants that may be a more powerful marker in EH.
2. Methods
2.1. Study population
All participants were recruited by way of health examination between July 2012 and July 2015. Informed consent was obtained from each participant and all protocols were previously approved by the Ethics Committee of Qiqihar University. All participants were of northern Han Chinese origin, within 3 generations, and currently reside in Lanxi County (46° 20′ 0″ N, 126° 16′ 0″ E) of Heilongjiang Province, in the north of China.
A total of 1024 unrelated patients (509 males and 515 females) with EH and 956 normotensive subjects (296 males and 660 females) were included in this investigation if they met the following inclusion criteria. Subjects who consistently had a systolic BP (SBP) ≥140 mm Hg and/or diastolic BP (DBP) ≥100 mm Hg or with a history of hypertension were diagnosed as hypertensives. The control group was composed of 956 subjects with SBP <120 mm Hg and DBP < 80 mm Hg, without a history of hypertension, and minor illness patients without hypertension, hyperlipidemia, diabetes mellitus, tumors, or a history of family hypertension in their previous records. Patients with secondary hypertension or pregnancy were excluded from this study.
BP was measured using a Riva–Rocci sphygmomanometer. Subjects rested for 10 minutes, and then their BP was measured 3 times, 5 minutes apart, on the right arm by experienced doctors according to standard protocols recommended by the American Heart Association.[16] The 3 consecutive values were averaged to match inclusion criteria and analysis. Body mass index was calculated by measuring height and weight without shoes and wearing light clothing.
2.2. SNP selection and genotyping
Selection of human CYP11B2 gene SNP was based on previous results of our team and the data come from National Center for Biotechnology Information SNP database (https://www.ncbi.nlm.nih.gov/snp). In the current study, number of choosing SNPs in CYP11B2 gene always rarely in 1 study, no polymorphisms covering the whole CYP11B2 gene. Seven SNPs were identified for typing: SNP1 (rs28659182), SNP2 (rs10087214), SNP3 (rs73715282), SNP4 (rs542092383), SNP5 (rs4543), SNP6 (rs28491316), and SNP7 (rs7463212). These cover the entire CYP11B2 gene for the first time (Fig. 1).
Figure 1.
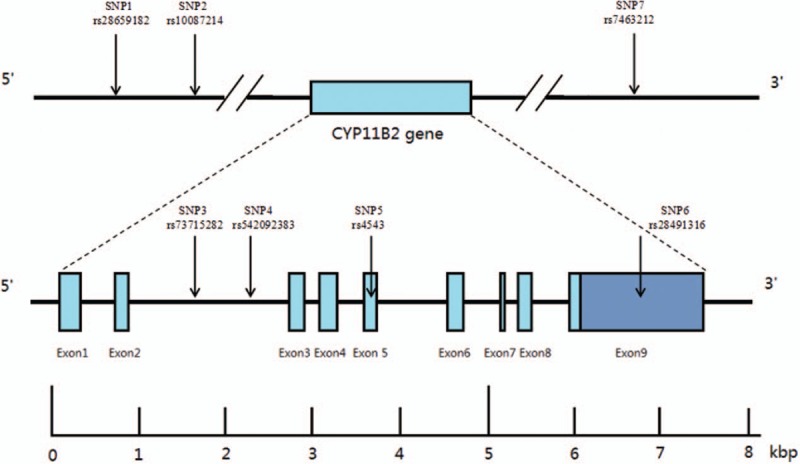
Structure of human CYP11B2. The gene consists of 9 exons (boxes) separated by 8 introns (lines). Arrows indicate the location of single nucleotide polymorphisms. kbp = kilobase pairs.
Blood samples were collected from all participants and anticoagulated with 2% ethylenediaminetetraacetic acid. Genomic DNA was extracted from peripheral blood leucocytes using the DNA extraction kit (ComWin Biotech Company, Beijing, China) and stored at –80°C. Genotyping was performed using the TaqMan SNP genotyping assay (Sequenom Company). Primers and probes were chosen from information available at the ABI website.
2.3. Statistical analysis
The Statistical Program for Social Science (SPSS version 17.0, Chicago, IL) was used to carry out statistical analysis. Differences in continuous data were compared by the t test. The Chi-square test was used to calculate categorical variables. Haplotypes data were analyzed using SHEsis online software (http://analysis.bio-x.cn/SHEsisMain.htm).[17] Genotype distributions for each SNP in the control group were analyzed using the Chi-square test to see if they deviated from Hardy–Weinberg equilibrium (HWE). SNPs were excluded from further analysis if they showed evidence of deviation. Logistic regression models were used to analyze the independent effect of each genetic variant on the risk of EH. P values <.05 were considered to be statistically significant. In the haplotype-based case–control analysis, haplotypes with frequencies <0.01 were excluded.
3. Results
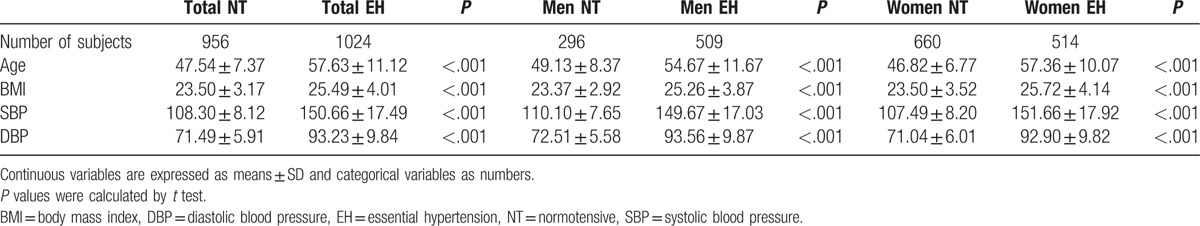
Table 1 summarizes the demographic characteristics of subjects. For all characteristics, significant differences were observed between normotensive controls and patients with EH. There was no deviation from HWE for the 7 SNPs in the normotensive group.
Table 1.
Demographic characteristics between normotensive and patients with essential hypertension.
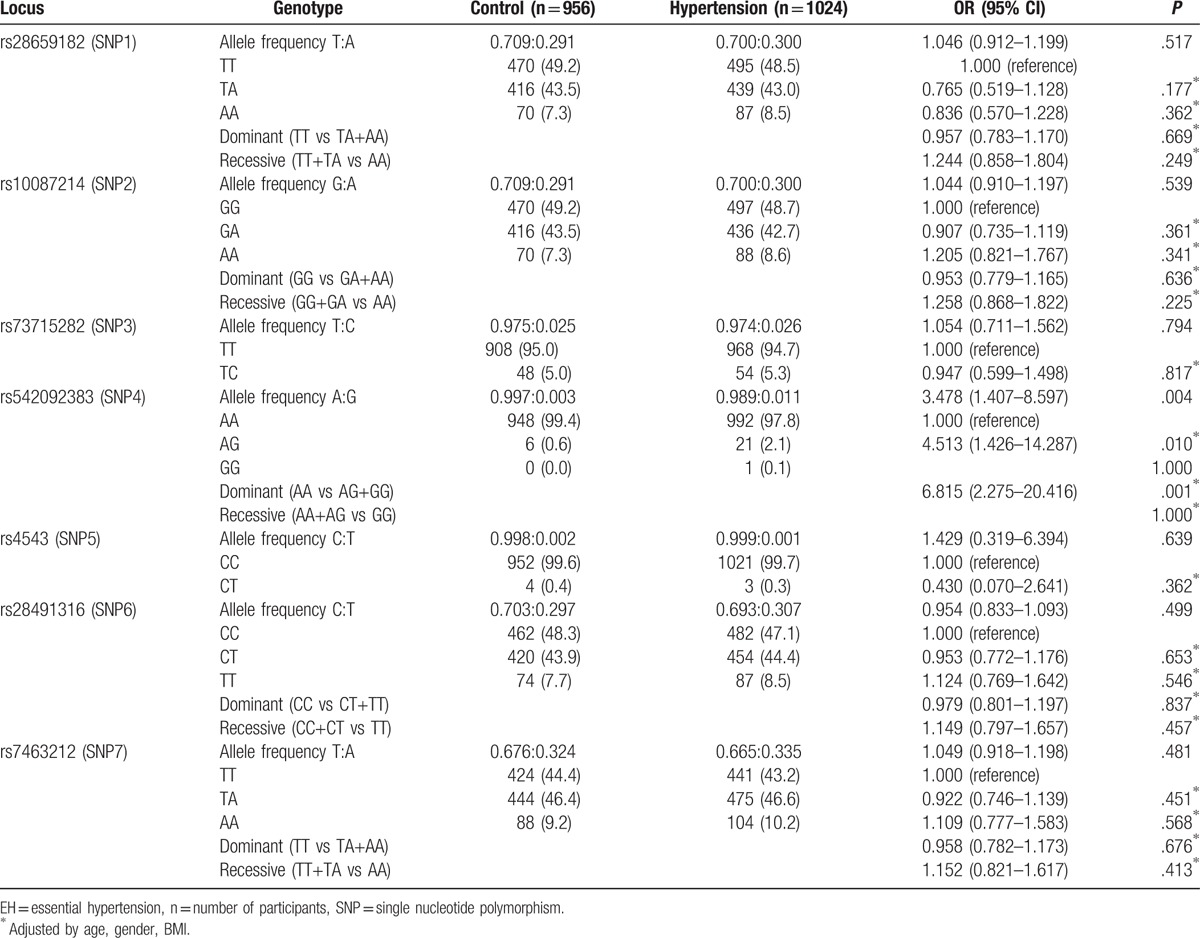
Single-locus analyses of genotype and allele distributions in normotensive and EH patients for these SNPs are summarized in Table 2. CYP11B2 SNP MAFs were 29.08%, 29.08%, 2.51%, 0.31%, 0.21%, 7.74%, and 32.43% in normotensive controls and 30.02%, 29.97%, 2.64%, 1.13%, 0.15%, 8.50%, and 33.48% in hypertensive patients for rs28659182, rs10087214, rs73715282, rs542092383, rs4543, rs28491316, and rs7463212, respectively. All genotype frequencies of these SNPs were in HWE (P > .05). The major allele G frequency of rs542092383 was found to be risk against hypertension [odds ratio (OR) 3.478, 95% confidence interval (95% CI) 1.407–8.597, P = .004]. The AG genotype frequency of rs542092383 was significantly associated with an increased risk of hypertension (OR 4.513, 95% CI 1.426–14.287, P = .010). The A allele frequency of rs542092383 was 0.9969 in normotensive controls and 0.9887 in patients with EH. The differences for other SNPs between these groups were not significant. The dominant (AA vs AG+GG) distribution of rs542092383 revealed a significant association (OR 6.815, 95% CI 2.275–20.416, P = .001) with EH risk.
Table 2.
Genotypes of the 7 CYP11B2 SNPs and the risk of EH.
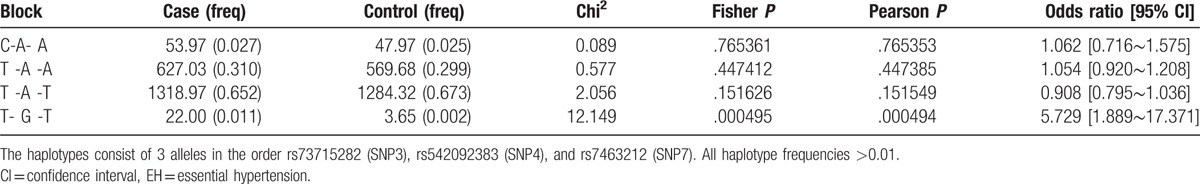
The level of LD between the 7 SNPs was denoted by |D¢| and r2 values, which are presented in Table 3. In general, r2 is similar to D′ and it became more powerful even if one or both alleles are in low frequency.[18] The findings indicate that all 7 SNPs are located in 1 haplotype block because all |D¢| values exceeded 0.9. r2 values for SNP1–SNP2, SNP1–SNP6, SNP1–SNP7, SNP2–SNP6, SNP2–SNP7, and SNP6–SNP7 were >0.8, indicating a strong linkage intensity. This also suggests that SNP1, SNP2, SNP6, and SNP7 could not be used to simultaneously construct the haplotype, and this analysis has previously been used in a similar study.[19] Therefore, the haplotype was constructed using SNP7, which showed a larger MAF than SNP1, SNP2, or SNP6. The minor genotype frequency of SNP5 is too small, so we constructed a haplotype consisting of SNP3, SNP4, and SNP7. In the haplotype-based case–control analysis, the frequency of the T-G-T haplotype was significantly higher for EH patients than for controls (OR 5.729, 95% CI 1.889–17.371, P = .000495) in Table 4.
Table 3.
Pair linkage disequilibrium for 7 SNPs in the study subjects.
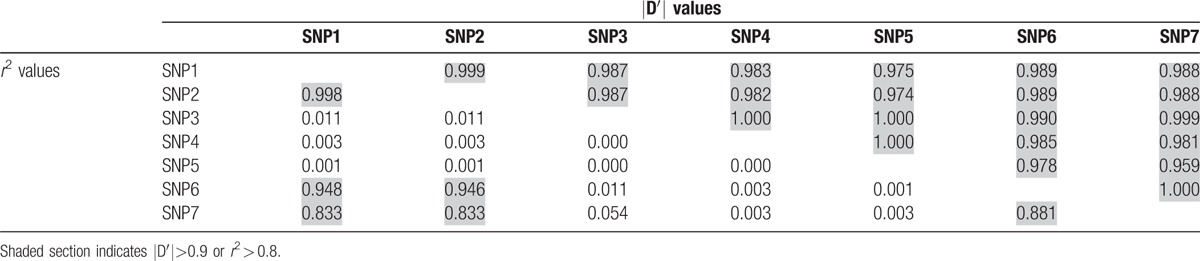
Table 4.
Haplotype frequencies of the 3 CYP11B2 SNPs and the risk of EH.
4. Discussion
EH is a complex disease influenced by genetic and environmental factors and does not follow Mendelian genetic law. Risk factors include age, gender, family history of hypertension, being overweight, diabetes mellitus, excessive consumption of sodium, and smoking.[20] Hypertension is responsible for most deaths caused by heart disease and stroke,[21] suggesting that candidate genes for EH may also influence these other diseases.
Although genome-wide studies have provided valuable insights into the genetic basis of human complex diseases, SNPs discovered by GWAS account for only a small part of human complex diseases, and there is a question where is the “missing heritability.”[22] Most of the heritability in complex diseases is not missing but has not previously been detected because the individual effects are too small to pass stringent significant tests, and causal variants have lower MAF than the SNPs explored.[23] So, there is an advantage in the results of low-frequency variants over common variants.
In the candidate gene era, many SNPs within RAAS have been reported to be significantly associated with EH; however, the unbiased GWAS rarely identified that the SNPs within RAAS were associated with hypertension or BP traits, and the effect of RAAS polymorphisms may have been overestimated.[24]
Many studies have previously revealed that the RAAS has a key function in regulating human BP. Human cytochrome P450 family 11 subfamily B member 2 (CYP11B2) within RAAS is an essential enzyme in steroid hormone biosynthesis, catalyzing the last 3 reaction steps of aldosterone synthesis, and is an important player in the development of hypertension.[25] Previously, studies suggested that several CYP11B2 variations associate with hypertension, including C-344T, K173R, rs3802230, and rs10086846.[26–28] The C-344T polymorphism of CYP11B2 has been extensively studied in various populations, but the results of these studies are often inconsistent.[13,29] Even there is no evidence to confirm that CYP11B2 (-344C/T) polymorphism within RAAS is associated with susceptibility of EH.[30] Sequencing is the primary approach for the detection of low-frequency variants in complex diseases. It is conceivable that genetic and environmental factors, population variation, small sample sizes, patient and control selection, and limited genetic alleles contribute to the conflicting or even contradictory results.
Although GWAS has the advantage of analyzing the genetics of complex traits, the candidate gene approach is an important alternative strategy when the selected population is sufficiently large and relatively homogeneous.[31]
Our sample of 1024 EH patients and 956 normotensive controls gave sufficient capacity to use a candidate gene approach to obtain a stronger biological hint. Moreover, this study population represents an ethnically homogeneous community, which was entirely made up of northern Han Chinese living in Lanxi County, Heilongjiang Province. In addition, the results of HWE supported the selection of this study population.
This study is the first to report on the genetics of 7 CYP11B2 polymorphisms in the north Chinese Han population. These SNPs are distributed from near the 3′ end of the gene (downstream) to near its 5′ end (upstream), and cover the entire gene. We found that the allelic frequencies of rs542092383 differed between controls and EH patients. The AG genotype of rs542092383 could therefore be used as a biomarker associated with an increased risk of hypertension. Moreover, the frequency of the T-G-T haplotype established by SNP3–SNP4–SNP7 was significantly lower for control subjects than for EH patients. These results come from G allele of rs542092383, which is a risk factor of EH, even if its frequency is low. Low-frequency variants showed that there is more power to detect risk variants than to detect protective variants in complex diseases. We got a larger OR value in dominant model than other genetic model.
LD is the nonrandom association of alleles at different loci and plays an important role in several aspects of human genetics.[32] Understanding the structure and extent of LD is therefore very important in association mapping[33] where the extent of the LD block is influenced by SNPs chosen by investigators. As summarized in Table 3, all 7 SNPs chosen in this study were located in the same haplotype block, and SNP1–SNP2–SNP6–SNP7 had a stronger linkage intensity than other SNPs; similar results were obtained for both male and female subjects.
There is increasing evidence that most recombination occurs in or around genes. Haplotype structure strongly affects recombination in maize,[34] and LD spans larger segments in the rat and mouse than the human structure, which is characterized by much smaller blocks. Guryev et al[35] found that recombination events were enriched within gene sequences, indicating that the probability of recombination in or around CYP11B2 is greater than outside the gene. The map of the haplotype block can reflect the linkage intensity between some SNPs, but the actual recombination unit may possess a 3-dimensional structure. Synapsis occurs during prophase of meiosis I, which facilitates subsequent recombination events.[36] It is inferred that differences in SNP linkage intensity may be caused by the synaptonemal complex, which is a proteinaceous structure formed by transverse filaments and lateral elements. During prophase of meiosis I, the connection between 3′ near gene region and 5′ near gene region may be short in space. The block of this investigation is merely a part of 1 larger human block, but self-similar phenomenon exists extensively in biology. The biological hints may be gained from results of this study.
5. Conclusion
We got results from the low-frequency variants that are more powerful to detect risk than to detect protective variants in EH. We used a candidate association approach to report an association between the AG genotype of CYP11B2 rs542092383 with an increased risk of hypertension in the Han population of northern China. The T-G-T haplotype of CYP11B2 was also associated with hypertension susceptibility. The intensity of linkage between the 3′ downstream region (SNP1-SNP2) to the 5′ upstream region (SNP6-SNP7) of CYP11B2 was stronger than that at other locations of this gene.
Acknowledgments
We thank the subjects for participating in our study. We gratefully acknowledge the assistance of clinical field and laboratory staff.
Footnotes
Abbreviations: BMI = body mass index, CI = confidence interval, CYP11B2 = cytochrome P450 family 11 subfamily B member 2, DBP = diastolic blood pressure, EH = essential hypertension, GWAS = genome-wide association studies, LD = linkage disequilibrium, MAF = minor allele frequency, OR = odds ratio, RAAS = renin–angiotensin–aldosterone system, SBP = systolic blood pressure, SNPs = single nucleotide polymorphisms.
Authorship: C-CQ and M-YC conceived the study and helped to edit the manuscript. HZ drafted the manuscript and performed the statistical analyses. J-PL and K-YZ participated in the design. X-YL, LZ, QZ, MJ, and NW collected the data and the samples. C-CQ and M-YC made the same contributions to this study.
Funding/support: This work was supported by grants from the National Natural Science Foundation of China (31171146, 31371208, 31440054), and the Scientific Research Foundation of Education Bureau of Heilongjiang Province, China Scientific (2016-KYYWF-0864).
The authors declare no conflict of interest.
References
- [1].Yang J, Benyamin B, McEvoy BP, et al. Common SNPs explain a large proportion of the heritability for human height. Nat Genet 2010;42:565–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Chan Y, Lim ET, Sandholm N, et al. An excess of risk-increasing low-frequency variants can be a signal of polygenic inheritance in complex diseases. Am J Hum Genet 2014;94:437–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Weder AB. Genetics and hypertension. J Clin Hypertens 2007;9:217–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Vasan RS, Larson MG, Leip EP, et al. Impact of high-normal blood pressure on the risk of cardiovascular disease. N Engl J Med 2001;345:1291–7. [DOI] [PubMed] [Google Scholar]
- [5].Singh M, Singh AK, Pandey P, et al. Molecular genetics of essential hypertension. Clin Exp Hypertens 2016;38:268–77. [DOI] [PubMed] [Google Scholar]
- [6].Steckelings UM, Rompe F, Kaschina E, et al. The evolving story of the RAAS in hypertension, diabetes and CV disease: moving from macrovascular to microvascular targets. Fundam Clin Pharmacol 2009;23:693–703. [DOI] [PubMed] [Google Scholar]
- [7].Tsai CT, Hwang JJ, Ritchie MD, et al. Renin-angiotensin system gene polymorphisms and coronary artery disease in a large angiographic cohort: detection of high order gene-gene interaction. Atherosclerosis 2007;195:172–80. [DOI] [PubMed] [Google Scholar]
- [8].Te Riet L, van Esch JH, Roks AJ, et al. Hypertension: renin-angiotensin-aldosterone system alterations. Circ Res 2015;116:960–75. [DOI] [PubMed] [Google Scholar]
- [9].Kawamoto T, Mitsuuchi Y, Toda K, et al. Role of steroid 11 beta-hydroxylase and steroid 18-hydroxylase in the biosynthesis of glucocorticoids and mineralocorticoids in humans. Proc Natl Acad Sci U S A 1992;89:1458–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Curnow KM, Tusie-Luna MT, Pascoe L, et al. The product of the CYP11B2 gene is required for aldosterone biosynthesis in the human adrenal cortex. Mol Endocrinol 1991;5:1513–22. [DOI] [PubMed] [Google Scholar]
- [11].Sookoian S, Gianotti TF, Gonzalez CD, et al. Association of the C-344T aldosterone synthase gene variant with essential hypertension: a meta-analysis. J Hypertens 2007;25:5–13. [DOI] [PubMed] [Google Scholar]
- [12].Munshi A, Sharma V, Kaul S, et al. Association of the -344C/T aldosterone synthase (CYP11B2) gene variant with hypertension and stroke. J Neurol Sci 2010;296:34–8. [DOI] [PubMed] [Google Scholar]
- [13].Alvarez-Madrazo S, Mackenzie SM, Davies E, et al. Common polymorphisms in the CYP11B1 and CYP11B2 genes: evidence for a digenic influence on hypertension. Hypertension 2013;61:232–9. [DOI] [PubMed] [Google Scholar]
- [14].Olcay A, Nisanci Y, Ekmekci CG, et al. Aldosterone synthase -344C/T and angiotensin-converting enzyme I/D polymorphisms in Turkish hypertensive patients with normal coronary arteries. Acta Cardiol 2006;61:29–34. [DOI] [PubMed] [Google Scholar]
- [15].Tanahashi H, Mune T, Takahashi Y, et al. Association of Lys173Arg polymorphism with CYP11B2 expression in normal adrenal glands and aldosterone-producing adenomas. J Clin Endocrinol Metab 2005;90:6226–31. [DOI] [PubMed] [Google Scholar]
- [16].Perloff D, Grim C, Flack J, et al. Human blood pressure determination by sphygmomanometry. Circulation 1993;88(5 Pt 1):2460–70. [DOI] [PubMed] [Google Scholar]
- [17].Shi YY, He L. SHEsis, a powerful software platform for analyses of linkage disequilibrium, haplotype construction, and genetic association at polymorphism loci. Cell Res 2005;15:97–8. [DOI] [PubMed] [Google Scholar]
- [18].Slatkin M. Linkage disequilibrium: understanding the evolutionary past and mapping the medical future. Nat Rev Genet 2008;9:477–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Pan S, Nakayama T, Sato N, et al. A haplotype of the GOSR2 gene is associated with essential hypertension in Japanese men. Clin Biochem 2013;46:760–5. [DOI] [PubMed] [Google Scholar]
- [20].Chalmers J, MacMahon S, Mancia G, et al. 1999 World Health Organization-International Society of Hypertension Guidelines for the management of hypertension. Guidelines sub-committee of the World Health Organization. Clin Exp Hypertens 1999;21:1009–60. [DOI] [PubMed] [Google Scholar]
- [21].Lawes CM, Vander Hoorn S, Rodgers A. International Society of H. Global burden of blood-pressure-related disease, 2001. Lancet 2008;371:1513–8. [DOI] [PubMed] [Google Scholar]
- [22].Eichler EE, Flint J, Gibson G, et al. Missing heritability and strategies for finding the underlying causes of complex disease. Nat Rev Genet 2010;11:446–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Lin CY, Xing G, Ku HC, et al. Enhancing the power to detect low-frequency variants in genome-wide screens. Genetics 2014;196:1293–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ji LD, Li JY, Yao BB, et al. Are genetic polymorphisms in the renin-angiotensin-aldosterone system associated with essential hypertension? Evidence from genome-wide association studies. J Hum Hypertens 2017;[Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- [25].Williams GH. Aldosterone biosynthesis, regulation, and classical mechanism of action. Heart Fail Rev 2005;10:7–13. [DOI] [PubMed] [Google Scholar]
- [26].Rajan S, Ramu P, Umamaheswaran G, et al. Association of aldosterone synthase (CYP11B2 C-344T) gene polymorphism & susceptibility to essential hypertension in a south Indian Tamil population. Indian J Med Res 2010;132:379–85. [PubMed] [Google Scholar]
- [27].Chen B, Nie S, Yue Z, et al. Haplotype-based case-control study of the human CYP11B2 gene and essential hypertension in Yi and Hani minorities of China. Biochem Genet 2011;49:122–37. [DOI] [PubMed] [Google Scholar]
- [28].Kim YR, Kim SH, Kang SH, et al. Association of the k173r variant and haplotypes in the aldosterone synthase gene with essential hypertension. Genes Genom 2014;36:625–32. [Google Scholar]
- [29].Vamsi UM, Swapna N, Padma G, et al. Haplotype association and synergistic effect of human aldosterone synthase (CYP11B2) gene polymorphisms causing susceptibility to essential hypertension in Indian patients. Clin Exp Hypertens 2016;38:659–65. [DOI] [PubMed] [Google Scholar]
- [30].Chen JF, Jing J, Tan H, et al. Lack of association of CYP11B2-344C/T polymorphism with essential hypertension: a meta-analysis. Int J Clin Exp Med 2015;8:9162–7. [PMC free article] [PubMed] [Google Scholar]
- [31].Tabor HK, Risch NJ, Myers RM. Candidate-gene approaches for studying complex genetic traits: practical considerations. Nat Rev Genet 2002;3:391–7. [DOI] [PubMed] [Google Scholar]
- [32].Wall JD, Pritchard JK. Assessing the performance of the haplotype block model of linkage disequilibrium. Am J Hum Genet 2003;73:502–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Sun P, Zhang R, Jiang Y, et al. Assessing the patterns of linkage disequilibrium in genic regions of the human genome. FEBS J 2011;278:3748–55. [DOI] [PubMed] [Google Scholar]
- [34].He L, Dooner HK. Haplotype structure strongly affects recombination in a maize genetic interval polymorphic for Helitron and retrotransposon insertions. Proc Natl Acad Sci U S A 2009;106:8410–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Guryev V, Smits BM, van de Belt J, et al. Haplotype block structure is conserved across mammals. PLoS Genet 2006;2:e121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Fraune J, Schramm S, Alsheimer M, et al. The mammalian synaptonemal complex: protein components, assembly and role in meiotic recombination. Exp Cell Res 2012;318:1340–6. [DOI] [PubMed] [Google Scholar]


