Abstract
Background:
The aim of this systematic review was to evaluate the efficacy and safety of liraglutide versus sitagliptin both in combination with metformin in patients with type 2 diabetes and provide reference basis for rational use of clinical drugs.
Methods:
Several databases were searched, including Web of science, PubMed, Cochrane library, CNKI, and Wanfang database. Only randomized controlled trials (RCTs) of liraglutide versus sitagliptin both in combination with metformin up to 31 August 2016 were included. Data were extracted independently by 2 reviewers, and a fixed or random effects model were used to analyze outcomes that were expressed as odds ratio (OR) or mean difference (MD) and 95% confidence intervals (95% CIs) for different situations.
Results:
Five RCTs involving 1440 participants were included. Compared with sitagliptin combination with metformin group, participants’ treatment with 1.2 mg and 1.8 mg liraglutide with metformin could significantly lower the level of glycosylated hemoglobin (HbA1c) (P < .00001, MD = −0.35, 95% CI −0.51 to −0.20). Moreover, patients with 1.8 mg liraglutide group had significant body weight loss (P < .00001, MD = −1.12, 95% CI −1.54 to −0.70). However, there were no obvious differences in cutting down the systolic blood pressure and diastolic blood pressure between liraglutide-metformin and sitagliptin-metformin groups. The incidence of gastrointestinal problems was significantly higher than sitagliptin with metformin groups.
Conclusion:
The results of this meta-analysis indicated that Liraglutide added on to metformin therapy could significantly lower the level of HbA1c and increase body weight loss. Meanwhile, the adverse reactions such as gastrointestinal problems were common in the liraglutide treatment group. Thus, this will provide an important reference for the treatment of patients with type 2 diabetes.
Keywords: diabetes mellitus, liraglutide, meta-analysis, metformin, randomized controlled trials, sitagliptin
1. Introduction
Diabetes mellitus (DM) is a chronic metabolic disease with main clinical etiologies of hyperglycemia and microvascular complications, and hyperglycemia primary resulting from defects in insulin secretion, insulin action, or both.[1] Globally, there are 415 million people with diabetes and the death toll of diabetes hit 5 million in 2015; moreover, the diabetes population is expected to rise to 642 million by 2040.[2] DM was divided into type 1and type 2 diabetes, and about 90% of DM patients are diagnosed with type 2 DM (T2DM). Good glycemic control plays an vital role in regulating blood glucose balance and reducing the occurrence of microvascular complications, including nephropathy, neuropathy, and retinopathy.[3] Unfortunately, the traditional therapeutic drugs for T2DM could not effectively control hyperglycemia, and frequently occurring side effects remain a big problem.
Metformin, a synthetic biguanide considered as the first-line oral hypoglycemic agent can be used for monotherapy and combination therapy for T2DM; this recommendation is based on metformin's glucose-lowering effects, lower level of side effects, and cost.[4] According to the prospective study of United Kingdom, metformin could reduce the risk of diabetes-related complications and mortality; metformin not only exercised strong control in blood glucose levels of obese patients with T2DM but also proved to be effective in normal-weight patients.[5] Initially, metformin monotherapy is often effective, although the effect of glucose control is limited after all, so a second agent is often required in most patients. Currently, metformin can be combined with insulin secretagogue, sulfonylureas, dipeptidyl peptidase-4 (DPP-4) inhibitors, and glucagon-like polypeptide-1 (GLP-1) agonist to reduce glycosylated hemoglobin (HbA1c) and achieve better effects on glucose control.[6,7]
Liraglutide is a human GLP-1 analogue approved in T2DM therapy in some countries. Recent scientific study showed that liraglutide had better glycemic control without major damages in renal function and increase in hypoglycemia risk.[8] Tanaka et al[9] assessed the efficacy and safety of liraglutide monotherapy compared with metformin monotherapy in Japanese obese patients with type 2 diabetes; the results showed that liraglutide and metformin monotherapy were similar in reducing the level of HbA1c, and no differences in weight gain and incidence of hypoglycemia were exhibited in the therapies. Later, another study found that liraglutide was very effective and well tolerated as a combination therapy with metformin in T2DM.[10]
Sitagliptin is a DPP-4 inhibitor that is effective in cutting down the level of HbA1c in patients with T2DM. In a 4-year clinic trial, final results showed that sitagliptin-metformin therapy could significantly lower the level of blood glucose without any serious adverse reactions or bad tolerance.[11] Recent studies on the efficacy of GLP-1 receptor agonist and DPP-4 inhibitors failed to draw rational conclusions in terms of concrete indicators such as body weight, blood pressure, and adverse reactions. Besides, liraglutide, and sitagliptin are the most commonly used GLP-1 receptor agonist and DPP-4 inhibitors for type 2 diabetes in China, and they add on to metformin achieving better treating effects on glucose control. Therefore, the purpose of our study was to comprehensively summarize the efficacy and safety of liraglutide versus sitagliptin both in combination with metformin in T2DM by retrieving data published.
2. Methods
2.1. Search strategy
We searched the electronic databases, including PubMed, Cochrane library, CNKI, Web of science, and Wanfang database without language restriction. We used the following words and subject terms in this search: “glucagon like polypeptide-1or liraglutide” and “dipeptidyl peptidase-4 inhibitors or sitagliptin” and “metformin” and “diabetes or diabetes mellitus or type 2 diabetes mellitus”; and we set the publication deadline on August 31, 2016, before conducting the literature search.
2.2. Study selection criteria
Inclusion criteria are as follows. First, all participants were aged 18 to 80 years old, and they were diagnosed with T2DM based on the standards criteria of American Diabetes Association (ADA).[12] Second, randomized controlled trials (RCTs) were taken to compare the efficacy and safety of liraglutide versus sitagliptin both in combination with metformin in patients with T2DM. Third, the patients had HbA1c of 7.5% to 10.0% and body mass index (BMI) ≤45 kg/m2, and had been treated with metformin monotherapy (≥1500 mg daily) for 3 months or longer. Fourth, all patients had minimum treatment duration of 16 weeks and availability of a full text publication. Fifth, the outcomes should contain the changes of HbA1c, body weight, systolic blood pressure (SBP), diastolic blood pressure (DBP), and side effects. Articles with significant shortcomings in study protocol or data analysis were excluded.
2.3. Data extraction
Two investigators independently extracted data using a standardized tool. On the basis of the inclusion criteria, the reviewers (ML and YY) carefully scrutinized the baseline characteristics of participants, study design, daily dose of liraglutide and sitagliptin, and subsequent outcomes.
2.4. Quality assessment
The methodological quality of all included studies was evaluated according to the Cochrane risk of bias tool.[13] Each study was scored on a scale of 0 to 7. Any disagreements during the process of data extraction and quality assessment were resolved by consultation. For this type of study, ethical approval and informed consent are not required.
2.5. Statistical analysis
We performed traditional meta-analysis to analyze the outcomes using RevMan software 5.3. (The software was released by the cochrane Collaboration, the Nordic Cochrane Centre, Copenhagen, Denmark) For dichotomous data, the outcomes were expressed as the odds ratio (OR) and 95% confidence intervals (95% CIs). For continuous outcomes, we used mean difference (MD) with 95% CI. The Chi-square test based Q-statistic and I2 statistic were used to assess heterogeneity. Substantial heterogeneity were considered as I2 values of 50% or more, and significant heterogeneity difference showed when P < .05. The fixed-effects model was used when I2 values were less than 50%, otherwise the random-effects model should be applied instead. All included studies were evaluated in terms of the risk of bias using the Cochrane risk of bias tool, and sensitivity analysis was conducted by excluding the mixed studies that lead to potential bias. All P values are 2-tailed.
3. Results
3.1. Description of the studies
After primary search from the databases, 445 references in English and 8 references in Chinese were sorted out. Finally, 5 studies[14–18] met the inclusion criteria and were selected for our study (Fig. 1). The main characteristics of the studies are summarized in Table 1. A total of 1440 participants were included in 5 studies and 829 participants were randomized and injected subcutaneously with liraglutide and 611 with sitagliptin. The daily dosages of liraglutide were 1.2 or 1.8 mg, 100 mg sitagliptin was taken orally once daily, and 1500 mg metformin was input orally on a daily basis. All RCTs were carried out more than 16 weeks. All included studies were evaluated in terms of the risk of bias using the Cochrane risk of bias tool; the results are shown in Fig. 2. For allocation concealment, blinding of participants, and personnel and blinding of outcome assessment, only 3 of the 5 studies provided details. The largest risk of bias was the selection bias. All studies had a Jadad score, and only 1 of the 5 studies scored more than 5; the other 4 got scores of less than or equal to 4.
Figure 1.
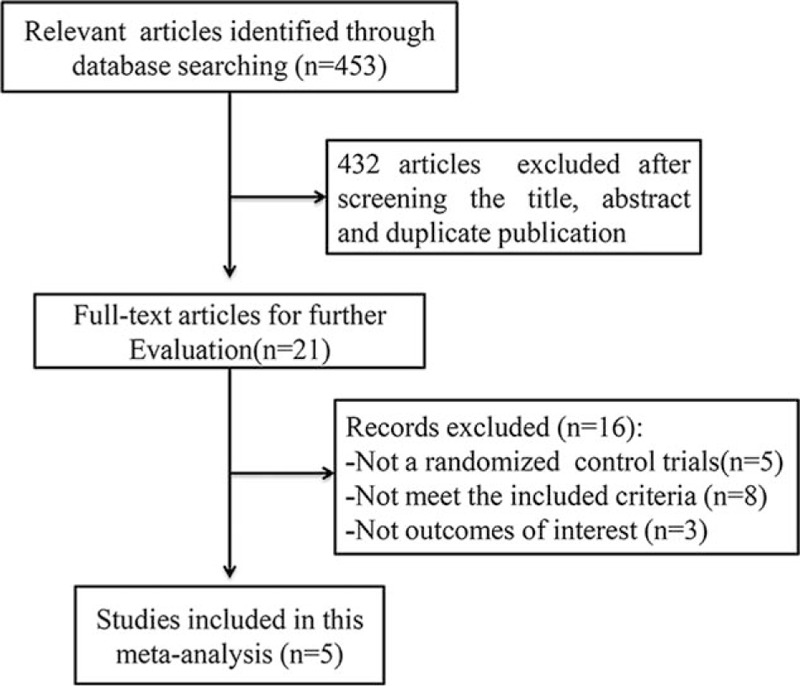
Flow diagram for identification of studies in meta-analysis.
Table 1.
Characteristics of RCTs included in the meta-analysis.
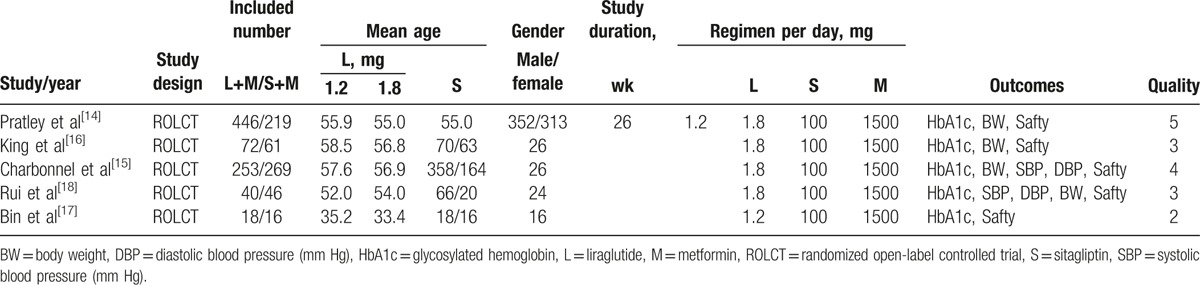
Figure 2.
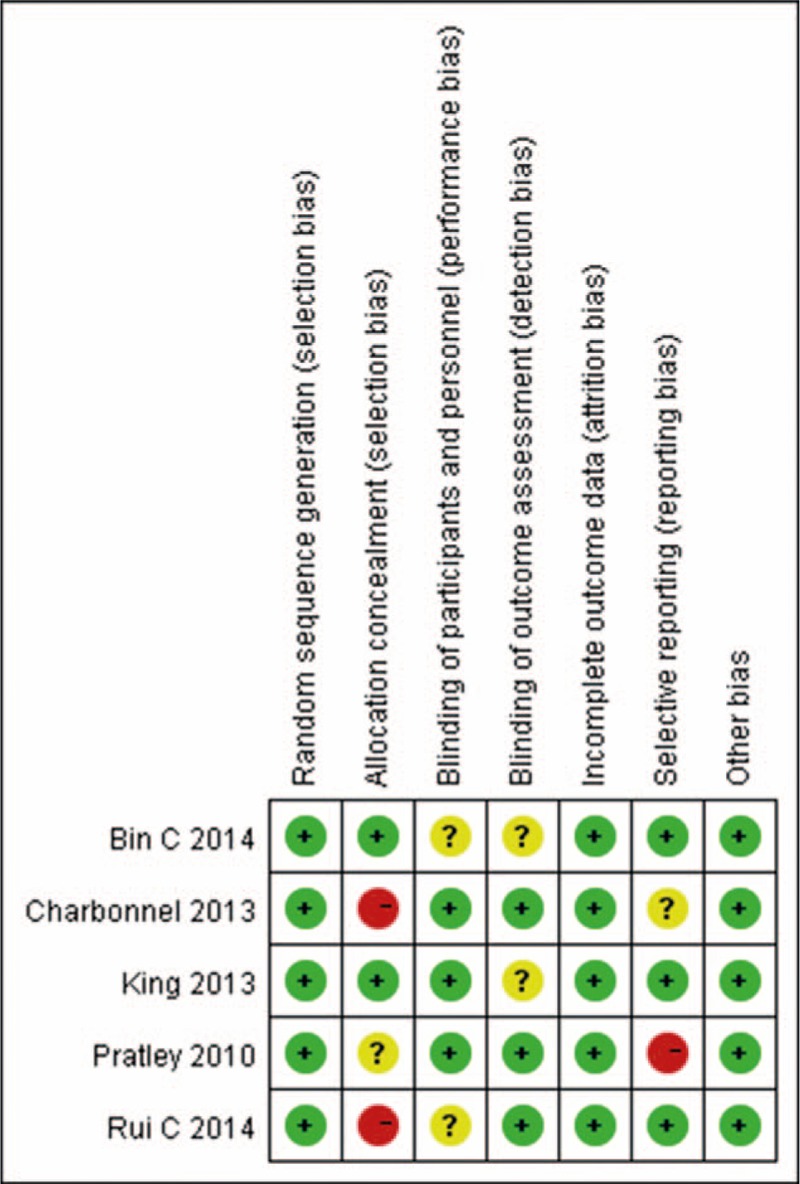
Risk of bias summary. Red (-), high risk of bias; yellow (?), unclear risk of bias; green (+), low risk of bias.
3.2. Glycosylated hemoglobin (HbA1c)
In this meta-analysis, we examined the HbA1c changes of all the 1419 individuals involved. Random effect models were used to analyze this outcome because of the moderate heterogeneity between the 2 groups (P = .05, I2 = 56%). Subgroup analyses were performed based on the dosages of liraglutide. As shown in Fig. 3, 1.2 and 1.8 mg liraglutide combination with metformin displayed better efficacy to control the level of HbA1c than sitagliptin with metformin (P < .00001, MD = −0.35, 95% CI −0.51 to −0.20).
Figure 3.
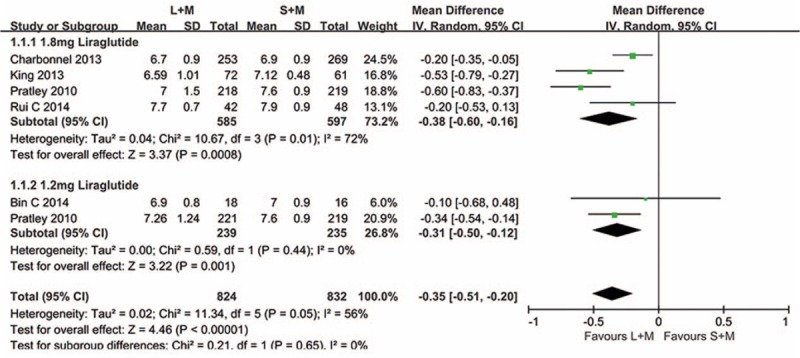
Comparison the level of HbA1c of 1.2 and 1.8 mg liraglutide with sitagliptin when added on to metformin. L = liraglutide, M = metformin, S = sitagliptin.
3.3. Body weight
Four studies involving 1182 patients investigated the changes of body weight. The results are shown in Fig. 4, and fixed effect models were used to analyze the data, as heterogeneity between the 2 groups was low (P = .18, I2 = 38%). Compared with sitagliptin combination with metformin therapy, 1.8 mg liraglutide with metformin could significantly control weight gain (P < .00001, MD = −1.12, 95% CI −1.54 to −0.70).
Figure 4.
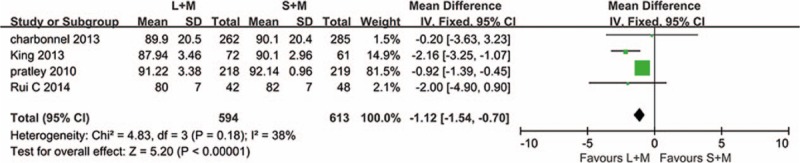
Effects of 1.8 mg liraglutide versus sitagliptin on the body weight when added on to metformin. L = liraglutide, M = metformin, S = sitagliptin.
3.4. SBP and DBP
Two trials with a total of 637 patients measured the SBP and DBP changes in this meta-analysis. Fixed effect models were used to analyze this outcome, as there is no heterogeneity between the 2 groups (SBP, P = .69, I2 = 0%; DBP, P = .83, I2 = 0%) in Fig. 5. Compared with sitagliptin combination with metformin group, the therapy of 1.8 mg liraglutide with metformin showed no significant difference in lowering the SBP and DBP (SBP, P = .12, MD = −1.35, 95% CI −0.35 to 0.36; DBP, P = .88, MD = −0.07, 95% CI −0.92 to 0.79).
Figure 5.
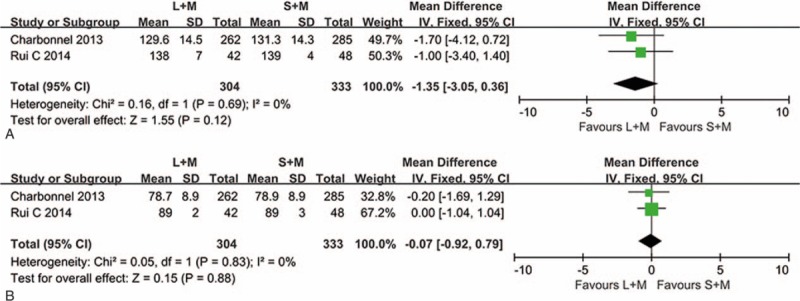
Effects of 1.8 mg liraglutide versus sitagliptin on the SBP and DBP when added on to metformin. (A) For SBP (B) For DBP. DBP = diastolic blood pressure, L = liraglutide, M = metformin, S = sitagliptin, SBP = systolic blood pressure.
3.5. Safety
In the treatment process, gastrointestinal complaints were the most common adverse effects in liraglutide combination with metformin group and sitagliptin with metformin group. The main side effects included dyspepsia, nausea, diarrhea, and vomiting. The results are shown in Figs. 6 and 7; subgroup analyses and fixed effect models were used to assess the adverse reactions. As expected, in the cases of 1.8 mg liraglutide, the incidents of dyspepsia, diarrhea, and vomiting were significantly higher than sitagliptin with metformin group (P < .0001, OR = 3.81, 95% CI 1.97–7.38; P < .00001, OR = 3.43, 95% CI 2.09–5.64; P < .0001, OR = 3.20, 95% CI 1.82–5.63). In addition, more patients showed symptom of nausea in the therapies of 1.2 and 1.8 mg liraglutide (P < .00001, OR = 6.39, 95% CI 4.41–9.25).
Figure 6.
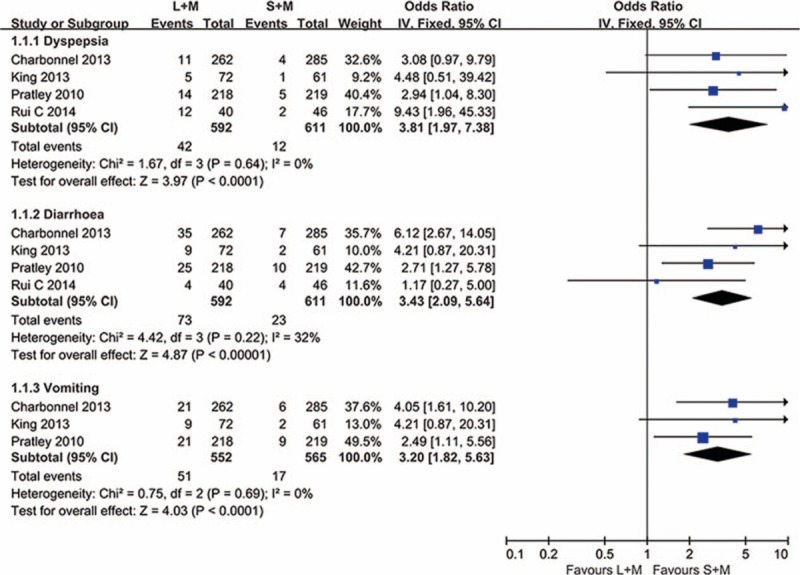
The summary of the occurrence of dyspepsia, diarrhea, and vomiting in this meta-analysis. L = liraglutide, M = metformin, S = sitagliptin.
Figure 7.
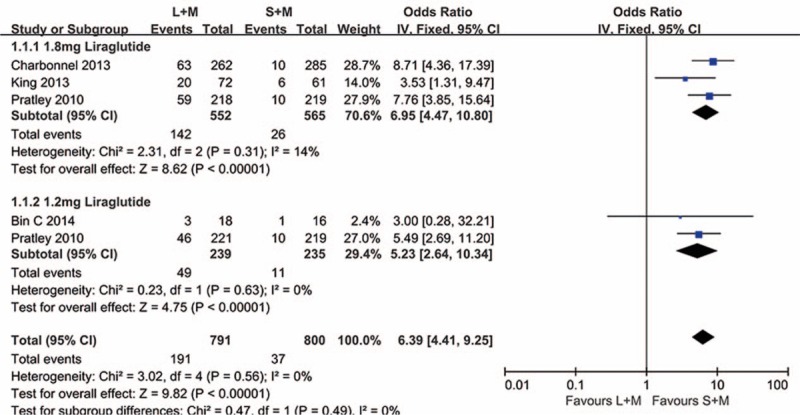
The summary of the occurrence of nausea in 1.2 and 1.8 mg liraglutide versus sitagliptin when added on to metformin. L = liraglutide, M = metformin, S = sitagliptin.
3.6. Sensitivity analysis
We implemented a sensitivity analysis using the method of combined data (random or fixed effect models). The value of MD or OR was close in random or fixed effect models, and the overall effect was similar. Meanwhile, we also performed a sensitivity analysis by excluding the mixed and potential biased studies, and the results exhibited no significant difference.
4. Discussion
DM is a syndrome characterized by cutting down insulin production or increasing insulin resistance, and patients with DM often suffer from hyperglycaemia.[19] DM-related complications include neuropathy, nephropathy, retinopathy, cerebrovascular, and cardiovascular diseases, all of which would undermine life quality and increase the burden on individuals.[20–22] Currently, the ADA and the European Association for the Study of Diabetes (EASD) suggested that the management of patients with T2DM should be focused on better control of blood glucose level to ensure HbA1c ≤7%, lifestyle change of patients, including smoking cessation, controlling blood pressure, and lipid levels in a suitable range.[23]
Metformin stands out as an old first-line oral hypoglycemic drug; the drug not only reduces appetite and intestinal absorption of carbohydrate but also inhibits hepatic gluconeogenesis and increases glucose uptake, leading to a lower level of blood glucose and weight decline, thus playing an important role in treating endothelial dysfunction and cardiovascular outcomes.[24,25] Many clinical trials proved that metformin could be combined with other anti-diabetes agents in a safe and efficacious manner to keep Hb1Ac within a normal range and control body weight in treating patients with T2DM.[26] Furthermore, the combinations of metformin and other drugs may have an additive effect on insulin secretory and insulin-sensitizing model.
Liraglutide is a GLP-1 analogue with a 97% structural homology to native GLP-1; the half-life was about 13 hours.[27,28] It can bound with β cells specific receptors through activation of adenosine enzyme, enhance the secretion of glucose-dependent insulin, and inhibit the secretion of glucagon, reduce appetite, and restrain gastric emptying, so as to regulate blood glucose level and control weight.[9] Some trials had demonstrated that Liraglutide was beneficial to glycemic control, dose-dependent weight loss, lowered SBP, and enhanced β cells function.[29,30] Therefore, it provides an effective option for T2DM and obesity. Sitagliptin is a DDP-4 inhibitor in that it could increase the levels of GLP-1 and glucose-dependent insulinotropic polypeptide, and promoted the secretion of insulin and glycemic control.[31] Many studies had evaluated the efficacy and safety of sitagliptin monotherapy versus combination with metformin for T2DM; the results showed that sitagliptin has a low risk of hypoglycemia and neutral weight; it could promote glucose-dependent insulin secretion and increase the level of GLP-1, offering an attractive initial combination therapy for T2DM.[32]
In our meta-analysis, the results showed that liraglutide combination with metformin was more effective in reducing the level of HbA1c than sitagliptin with metformin (P < .00001). We performed a subgroup analysis to evaluate this result based on the dosages of liraglutide. The results showed that 1.2 and 1.8 mg liraglutide significantly lowered HbA1c by 0.31% and 0.38%, respectively. For body weight loss, the results indicated that liraglutide added on to metformin reduced bodyweight by 1.12 kg, which is likely to be associated with an increased stimulation of GLP-1 receptor by liraglutide. A meta-analysis into 9 RCTs of 2981 patients receiving liraglutide showed that liraglutide in all dosages added on to metformin significantly lowered body weight, while the effect of exercising control is less desirable.[33] Other clinical trials found that DDP-4 inhibitor sitagliptin had a negligible effect.[34] As obesity is one of the leading factors in the development of diabetes, the anti-diabetes agents should be effective on weight loss; thus, it could reduce the occurrence of cardiovascular and cerebrovascular disease and cut down mortality.
With regard to blood pressure, our results found that there were no significant declines in SBP and DBP in the 2 groups. Pratley et al[35] also demonstrated that no significant decline in SBP was found and 1.8 mg liraglutide could reduce the DBP. As for the side effects, we found that most of them were moderate and mild; gastrointestinal problems, dyspepsia, nausea, diarrhea, and vomiting reaction, in particular, were more frequent with liraglutide combination with metformin group than the sitagliptin group. Some studies have also found that the frequency of nausea was higher with liraglutide in the early stage of medication, and then the occurrence of nausea was similar to that with sitagliptin in later weeks.[36,37]
However, there were some limitations in this meta-analysis that should be taken into accounts. First, 5 RCTs were included, while 3 of them had small sample sizes, so the results might be overestimated. Second, data in this meta-analysis only came from published literature, and some negative data were difficult to extract, resulting in the publication bias. Last but not the least, heterogeneity existed in the selected trials; we performed the subgroup analysis based on the dosages of liraglutide to determine the potential heterogeneity. Therefore, larger sample size and more sufficient data are needed to assess the efficacy and safety of the therapies in the future.
5. Conclusion
Our meta-analysis indicates that liraglutide added on to metformin has a better control of HbA1c level and body weight loss than the sitagliptin–metformin combination. Moreover, no serious adverse reactions were shown in these results. On the basis of these outcomes, we concluded that liraglutide is more effective than sitagliptin when added on to metformin for the treatment of T2DM, while gastrointestinal problems were a common side effect worth mentioning in the sitagliptin-metformin therapy. However, considering the poor methodological quality of the studies included and the potential limitation in this meta-analysis, future studies with larger sample and high-quality RCTs are needed to confirm these conclusions.
Footnotes
Abbreviations: CIs = confidence intervals, DM = diabetes mellitus, HbA1c = glycosylated hemoglobin, MD = mean difference, OR = odds ratio, RCTs = randomized controlled trials, T2DM = type 2 diabetes mellitus.
The authors report no conflicts of interest.
References
- [1].Associate AD. Diagnosis and classification of diabetes mellitus. Diabetes Care 2013;37:S81–90. [DOI] [PubMed] [Google Scholar]
- [2].Sharma SK, Panneerselvam A, Singh KP, et al. Teneligliptin in management of type 2 diabetes mellitus. Diabetes Metab Syndr Obes 2016;9:251–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ballav C, Gough S. Safety and efficacy of sitagliptin-metformin in fixed combination for the treatment of type 2 diabetes mellitus. Clin Med Insights Endocrinol Diabetes 2013;6:25–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Rojas LB, Gomes MB. Metformin: an old but still the best treatment for type 2 diabetes. Diabetol Metab Syndr 2013;1:6–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ito H, Ishida H, Takeuchi Y, et al. Long-term effect of metformin on blood glucose control in non-obese patients with type 2 diabetes mellitus. Nutr Metab (Lond) 2010;7:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Israel MK, Istvan E, Baron MA. Safety and efficacy of nateglinide/metformin combination therapy in the treatment of type 2 diabetes. Vasc Health Risk Manag 2008;6:1167–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Nicholson G, Hall GM. Diabetes mellitus: new drugs for a new epidemic. Br J Anaesth 2011;1:65–73. [DOI] [PubMed] [Google Scholar]
- [8].Davies MJ, Bain SC, Atkin SL, et al. Efficacy and safety of liraglutide versus placebo as add-on to glucose-lowering therapy in patients with type 2 diabetes and moderate renal impairment (LIRA-RENAL): a randomized clinical trial. Diabetes Care 2016;2:222–30. [DOI] [PubMed] [Google Scholar]
- [9].Tanaka K, Saisho Y, Kawai T, et al. Efficacy and safety of liraglutide monotherapy compared with metformin in Japanese overweight/obese patients with type 2 diabetes. Endocr J 2015;5:399–409. [DOI] [PubMed] [Google Scholar]
- [10].Kaku K, Kiyosue A, Ono Y, et al. Liraglutide is effective and well tolerated in combination with an oral antidiabetic drug in Japanese patients with type 2 diabetes: a randomized, 52-week, open-label, parallel-group trial. J Diabetes Investig 2016;1:76–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ku EJ, Jung KY, Kim YJ, et al. Four-year durability of initial combination therapy with sitagliptin and metformin in patients with type 2 diabetes in clinical practice; COSMIC study. PLoS One 2015;6:e129477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Gilor C, Niessen SJM, Furrow E, DiBartola SP. What's in a name? Classification of diabetes mellitus in veterinary medicine and why it matters. J Veter Internal Med 2016;4:927–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Higgins JPT, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Pratley RE, Nauck M, Bailey T, et al. Liraglutide versus sitagliptin for patients with type 2 diabetes who did not have adequate glycaemic control with metformin: a 26-week, randomised, parallel-group, open-label trial. Lancet 2010;9724:1447–56. [DOI] [PubMed] [Google Scholar]
- [15].Charbonnel B, Steinberg H, Eymard E, et al. Efficacy and safety over 26 weeks of an oral treatment strategy including sitagliptin compared with an injectable treatment strategy with liraglutide in patients with type 2 diabetes mellitus inadequately controlled on metformin: a randomised clinical trial. Diabetologia 2013;7:1503–11. [DOI] [PubMed] [Google Scholar]
- [16].King AB, Montanya E, Pratley RE, et al. Liraglutide achieves A1C targets more often than sitagliptin or exenatide when added to metformin in patients with type 2 diabetes and a baseline A1C <8.0%. Endocr Pract 2013;1:64–72. [DOI] [PubMed] [Google Scholar]
- [17].Bin C, Xing-guang Z, Yan-ling L, et al. Effect observation of liraglutide and sitagliptin respectively combined with metformin in treatment of patients with type 2 diabetes mellitus. Med Pharm J Chin PLA 2014;9:44–6. [Google Scholar]
- [18].Rui C, Pei Y, Chunjun L, et al. Comparison of efficacy and safety between liraglutide and sitagliptin in overweight and obese type 2 diabetes patients. Chinese J Diabetes Mellitus 2014;3:157–62. [Google Scholar]
- [19].Tse G, Lai ETH, Tse V, Yeo JM. Molecular and electrophysiological mechanisms underlying cardiac arrhythmogenesis in diabetes mellitus. J Diabetes Res 2016;2016:2848759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].ASSOCIATION AD. Economic costs of diabetes in the U.S. in 2012. Diabetes Care 2013;4:1033–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Alva ML, Gray A, Mihaylova B, et al. The impact of diabetes-related complications on healthcare costs: new results from the UKPDS (UKPDS 84). Diabet Med 2015;4:459–66. [DOI] [PubMed] [Google Scholar]
- [22].Ha KH, Kim DJ. Current status of managing diabetes mellitus in Korea. Korean J Intern Med 2016;5:845–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Herman WH. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach. Diabetes Care 2015;9:e143. [DOI] [PubMed] [Google Scholar]
- [24].Bailey CJ. Metformin: a multitasking medication. Diab Vasc Dis Res 2008;3:156. [DOI] [PubMed] [Google Scholar]
- [25].Nathan DM, Buse JB, Davidson MB, et al. Medical management of hyperglycaemia in type 2 diabetes mellitus: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia 2009;1:17–30. [DOI] [PubMed] [Google Scholar]
- [26].Papanas N, Maltezos E, Mikhailidis DP. Metformin: diamonds are forever. Expert Opin Pharmacother 2009;15:2395–7. [DOI] [PubMed] [Google Scholar]
- [27].Baruah MP, Chaudhury T, Sethi BK, Dharmalingam M. Liraglutide in type 2 diabetes mellitus. J Indian Med Assoc 2012;5:335–8. [PubMed] [Google Scholar]
- [28].Agerso H, Jensen LB, Elbrond B, et al. The pharmacokinetics, pharmacodynamics, safety and tolerability of NN2211, a new long-acting GLP-1 derivative, in healthy men. Diabetologia 2002;2:195–202. [DOI] [PubMed] [Google Scholar]
- [29].Vilsboll T, Zdravkovic M, Le-Thi T, et al. Liraglutide, a long-acting human glucagon-like peptide-1 analog, given as monotherapy significantly improves glycemic control and lowers body weight without risk of hypoglycemia in patients with type 2 diabetes. Diabetes Care 2007;6:1608–10. [DOI] [PubMed] [Google Scholar]
- [30].Pi-Sunyer FX. The effects of pharmacologic agents for type 2 diabetes mellitus on body weight. Postgrad Med 2008;2:5–17. [DOI] [PubMed] [Google Scholar]
- [31].Golightly LK, Drayna CC, McDermott MT. Comparative clinical pharmacokinetics of dipeptidyl peptidase-4 inhibitors. Clin Pharmacokinet 2012;8:501–14. [DOI] [PubMed] [Google Scholar]
- [32].Chwieduk CM. Sitagliptin/metformin fixed-dose combination: in patients with type 2 diabetes mellitus. Drugs 2011;3:349–61. [DOI] [PubMed] [Google Scholar]
- [33].Gu J, Meng X, Guo Y, et al. The efficacy and safety of liraglutide added to metformin in patients with diabetes: a meta-analysis of randomized controlled trials. Sci Rep 2016;6:32714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Rhee EJ, Lee WY, Min KW, et al. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor gemigliptin compared with sitagliptin added to ongoing metformin therapy in patients with type 2 diabetes inadequately controlled with metformin alone. Diabetes Obes Metab 2013;6:523–30. [DOI] [PubMed] [Google Scholar]
- [35].Pratley R, Nauck M, Bailey T, et al. One year of liraglutide treatment offers sustained and more effective glycaemic control and weight reduction compared with sitagliptin, both in combination with metformin, in patients with type 2 diabetes: a randomised, parallel-group, open-label trial. Int J Clin Pract 2011;4:397–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Buse JB, Rosenstock J, Sesti G, et al. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6). Lancet 2009;9683:39–47. [DOI] [PubMed] [Google Scholar]
- [37].Nauck MA, Meininger G, Sheng D, et al. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, compared with the sulfonylurea, glipizide, in patients with type 2 diabetes inadequately controlled on metformin alone: a randomized, double-blind, non-inferiority trial. Diabetes Obes Metab 2007;2:194–205. [DOI] [PubMed] [Google Scholar]


