Abstract
Limited evidence is available regarding the association between serum uric acid (SUA) and nonalcoholic fatty liver disease (NAFLD), especially in gender difference. Therefore, this study aimed to evaluate gender difference on the association between SUA, hyperuricemia, and NAFLD in the Chinese population. A cross-sectional study was carried out in a group of 1006 Chinese adults aged between 45 and 59 years old, in the city of Hangzhou, Zhejiang Province who were attending their annual health examination in the period between July 2015 and March 2017. Face-to-face interviews were conducted using a written questionnaire. Multivariate logistic regression analysis was performed to examine the associations between SUA, hyperuricemia, and NAFLD with adjustment of potential confounding variables. Wald tests were used to for heterogeneity between males and females. Body mass index (BMI), waist circumference (WC), waist–hip ratio (WHR), fasting plasma glucose (FPG), systolic blood pressure (SBP), diastolic blood pressure (DBP), triglycerides (TG), SUA, alanine aminotransferase (ALT), asparagine aminotransferase (AST), and the prevalence of hypertension, hyperuricemia, and NAFLD were significantly higher in male than in female (P < .05). Females had the significantly higher levels of total cholesterol and high-density lipoprotein–cholesterol (HDL-C). Simple correlation analysis showed that SUA was positively associated with BMI, WC, WHR, TG, ALT, AST and inversely associated with age and HDL-C. After adjusting for confounders, hyperuricemia was associated with an increased risk of NAFLD in both genders, with odds ratio (95%confidence interval) of 2.645 (1.213–5.768), 1.962 (1.051–3.661), respectively. There was a significant association in NAFLD found in males, compared with females (Wald = 118.589, df = 1, P < .0001).
Our findings indicated that the association of SUA with NAFLD was much more closely related in males than in females. Males with hyperuricemia had the higher risk of NAFLD. Further longitudinal studies are needed to confirm these findings.
Keywords: gender, hyperuricemia, nonalcoholic fatty liver disease, serum uric acid
1. Introduction
Nonalcoholic fatty liver disease (NAFLD) representing a spectrum of conditions from simple steatosis, nonalcoholic steatohepatitis to cirrhosis, is the most common liver disease worldwide.[1] In China, NAFLD has been recognized as a major health burden and the prevalence is increasing year by year.[2,3] In western countries, NAFLD has also become one of the most liver diseases, affecting 20% to 40% of the general population.[4] To our knowledge, NAFLD is considered as a multifactorial chronic disease that are associated with genetic, environmental, and metabolic factors.[5,6]
Hyperuricemia is defined as a serum urate concentration of more than 420 μmol/L for men, and more than 360 μmol/L for women.[7] In past several decades, the incidence of hyperuricemia appears to be increasing tread among the Chinese population.[8] The growing body of evidence has demonstrated that uric acid is an independent risk factor for cardiovascular disease, and the pathological processes included insulin resistance, oxidative stress, and systemic inflammation, which are all considered as important risk factors for the development or progression of NAFLD.[9–11] Recent studies showed that elevated SUA was associated with the development or progression of NAFLD.[12,13] However, to the best of our knowledge, data on the association between SUA and NAFLD in the Chinese population are limited,[14–16] and more importantly, no paper has evaluated the effect of hyperuricemia on progression of NAFLD, especially from gender impact.
Therefore, the present study aimed to investigate the gender difference on the association between SUA, hyperuricemia, and NAFLD in the Chinese population aged 45 to 59 years.
2. Subjects and methods
2.1. Study population
A total 1376 eligible participants aged 45 to 59 years (617 male, 759 female), who were recruited to attend their annual health check-up from July 2015 to March 2017 in the Medical Center for Physical Examination, Zhejiang Hospital. Firstly, 30 participants with missing or incomplete information in their questionnaires were excluded. In addition, we further excluded 340 participants self-reported a history of usage of drugs, drinking, or hereditary diseases. Finally, 1006 participants were included in this cross-sectional study. These participants were face to face interviewed by a trained interviewer using a written questionnaire. This study was approved by the institutional review and ethics committee of Zhejiang Hospital, and written informed consent was obtained from all participants.
2.2. Anthropometric measurements
The subjects who wear light clothes and stand without shoes were measured to the nearest 0.1 kg in height and 0.1 cm in height. Body mass index (BMI) was calculated as weight (kg)/height (m)2. Waist circumstance (WC) was measured at the end of normal expiration in duplicate on bare skin midway between the lowest rib and the superior border of the iliac crest.[17]
2.3. Assessment of biomarker
All participants were invited for blood collection after fasting overnight (12 hours). The samples were analyzed in the Medical Center for Physical Examination, Zhejiang Hospital for fasting plasma glucose (FPG), triglyceride (TG), total cholesterol (TC), high-density lipoprotein–cholesterol (HDL-C), low-density lipoprotein–cholesterol (LDL-C), serum uric acid (SUA), alanine aminotransferase (ALT), and asparagine aminotransferase (AST) using the Hitachi 7180 autoanalyzer (Hitachi, Tokyo, Japan).
2.4. Blood pressure measurement
Blood pressure was measured by using an automated sphygmomanometer with the subjects in a quite environment and sitting position. A trained nurse measured the blood pressure 3 times, and thereafter the mean of 3 measurements was considered as the subject's blood pressure.
2.5. Definitions
Hyperuricemia was defined as SUA > 420 μmol/L in men, and >360 μmol/L in women.[7] NAFLD was defined as the absence of excessive alcohol use (>20 g/day in men and 10 g/day in women), no use of steatogenic medications within the past 6 months, no exposure to hepatotoxins, no history of bariatric surgery, and the presence of moderate–severe hepatic steatosis (by B-ultrasonic examination).[18] Obesity was defined by BMI ≥ 28 kg/m2 and abdominal adiposity was defined as (male: WC ≥ 85 cm; female: WC ≥ 80 cm).[19] Hypertension was defined as SBP ≥ 140 mm Hg or/and diastolic blood pressure (DBP) ≥ 90 mm Hg.[20]
2.6. Statistical analysis
Data were generally analyzed by gender. Data were expressed as the mean and standard deviation (SD) for continuous variables and as sum (proportions) for categorical variables. The Chi-square test was used for categorical variables, while the independent samples t test or Mann–Whitney U test was used for continuous variables. Pearson bivariate correlation was performed to analyze the relation between SUA levels and other variables. Multivariable-adjusted logistic regression analysis was used to determine the effect of SUA and hyperuricemia on progression of NAFLD. The statistical significance of NAFLD for gender was evaluated using the Wald test.
Statistical analyses were performed using the SPSS software package version 20.0 for Windows (SPSS, Inc., Chicago, IL). Two-sided P-values <.05 were considered statistically significant.
3. Results
Of the 1006 enrolled participants, 348 (247 males and 101 females) fulfilled the diagnostic criteria for NAFLD. Total prevalence of hyperuricemia in this study population was 10.6%, with male was 14.93% and female was 6.89%. The demographic and clinical characteristics of subjects by genders are shown in Table 1. BMI, WC, WHR, SBP, DBP, fasting glucose (FG), TG, SUA, ALT, AST and the prevalence of hypertension, hyperuricemia, and NAFLD were significantly higher in males than in females (P < .05). In addition, males had significantly lower levels of TC and HDL-C (5.05 ± 1.11 vs 5.23 ± 0.89, 1.22 ± 0.28 vs 1.51 ± 0.37, P < .01, respectively) than females.
Table 1.
Demographic and clinical characteristics of subjects by gender.
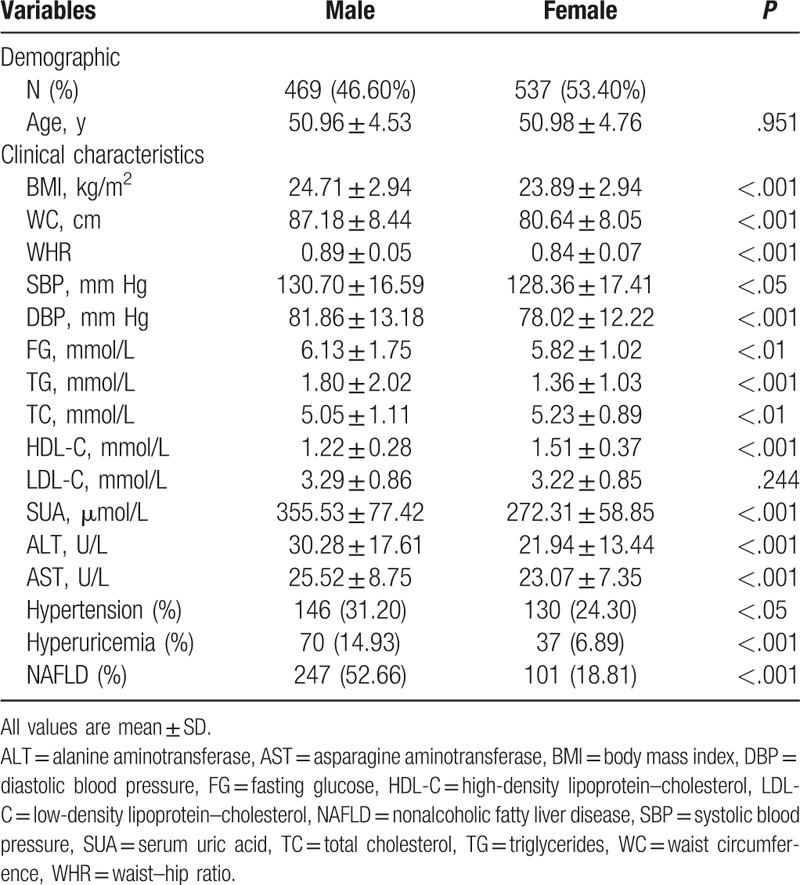
Clinical and biochemical variables are shown and compared by the cut-off value of hyperuricemia (Table 2), which indicated significantly higher levels of weight, BMI, WC, TG, SUA, and AST, and significantly lower level of HDL-C, in both genders among participants with hyperuricemia than nonhyperuricemia. There were significantly higher levels of WHR, TC, and ALT in male among participants with hyperuricemia than nonhyperuricemia. Moreover, age, height, and blood pressure did not impact the association between hyperuricemia and NAFLD in this study.
Table 2.
Variables’ characteristics of the study population by cutoff value of hyperuricemia (mean ± SD).
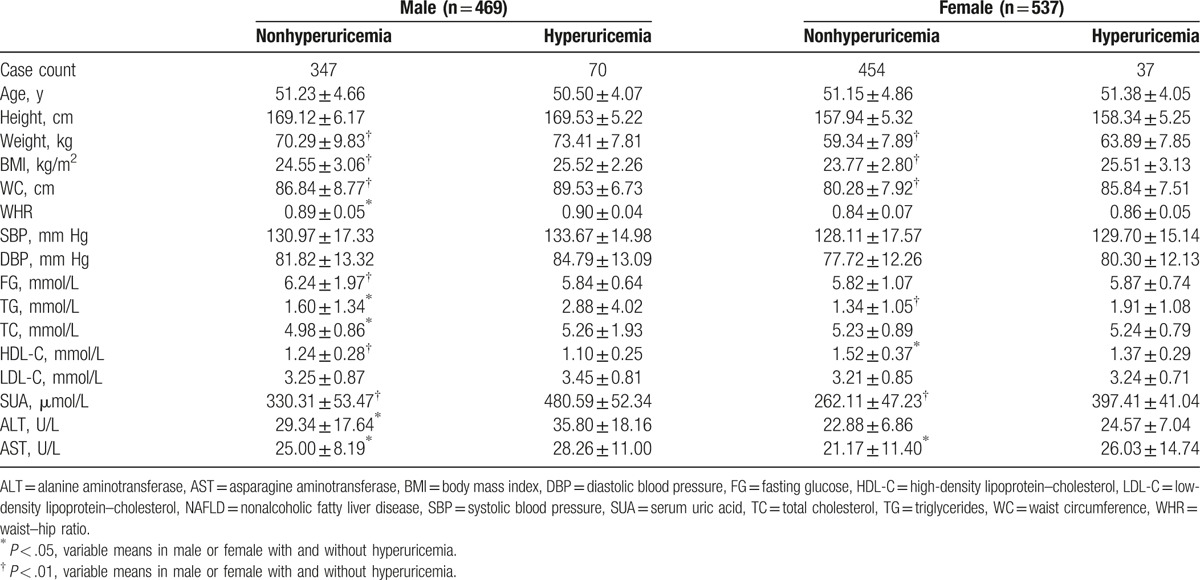
The correlation coefficients between SUA and other variables were calculated to identify whether any relationship between them (Table 3). The top 4 correlation coefficients belonged to TG (r = 0.227, P < .001), ALT (r = 0.214, P < .001), weight (r = 0.202, P < .001), and WC (r = 0.184, P < .001) in male, and WC (r = 0.258, P < .001), weight (r = 0.224, P < .001), BMI (r = 0.209, P < .001), and ALT (r = 0.174, P < .001) in female. Moreover, HDL-C was inversely associated with SUA in both genders, and age and FG were inversely associated with SUA in males.
Table 3.
Pearson bivariate correlations between serum uric acid and other variables in male and female, respectively.
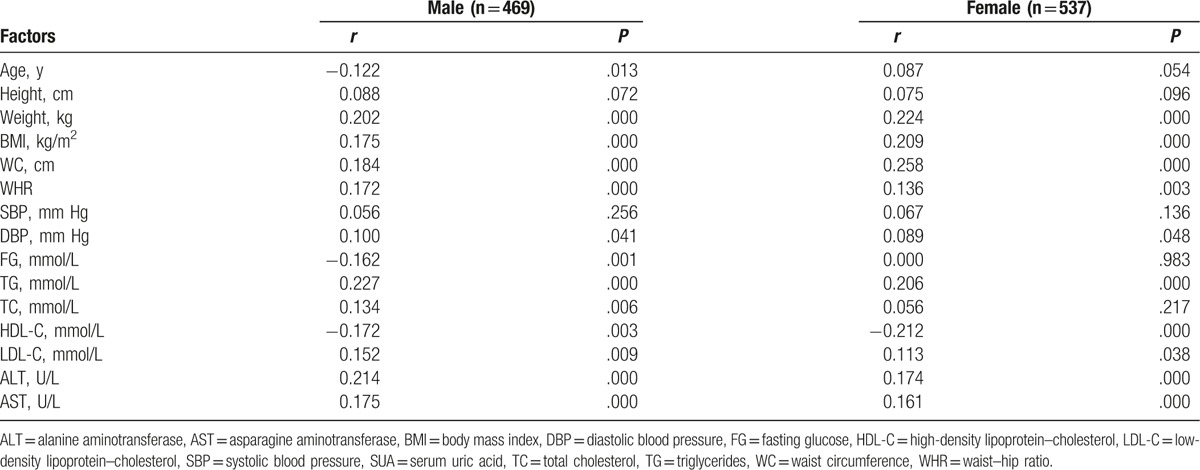
Multivariable-adjusted OR (95% CI) of hyperuricemia and SUA for NAFLD stratified by gender are shown in Table 4. In Model 1, males were more likely to suffer from NAFLD compared to females (OR = 3.912, 95% CI (1.930–7.930); OR = 2.640, 95% CI (1.494–4.663), P < .001, respectively). In Model 2 and Model 3, the results showed that the odds of having NAFLD was significantly higher in males (OR = 2.917, 95% CI: 1.364–6.273; OR = 2.236, 95% CI: 1.219–4.102, P < .01, respectively) compared with females (OR = 2.236, 95% CI: 1.219–4.102; OR = 1.962, 95% CI: 1.051–3.661, P < .05, respectively). Moreover, the SUA level was classified into quartiles. The results showed that subjects with quartile 4 had higher odds of NAFLD, when compared to subjects with quartile 1, and this relationship was more was significantly stronger in males than in females.
Table 4.
Multivariable-adjusted odds ratios (OR) and 95% confidence intervals (CI) of SUA and hyperuricemia for NAFLD stratified by gender.
A binary logistic regression model was used to examine the association between gender and NAFLD. The significant association was observed (χ2 = 126.8, df = 1, P < .001). In the Wald test, the results indicated that there was a significant association in NAFLD found in males, compared with in females (Wald = 118.589, df = 1, P < .0001). The Exp (B)/OR value was 4.833 (95% CI: 3.640–6.418).
4. Discussion
In this cross-sectional study, we found that the prevalence of NAFLD was significantly higher in males than that in females, and SUA and hyperuricemia were associated with an increased risk of NAFLD, adjusting for potential confounders. In addition, the results also showed the association of hyperuricemia with NAFLD in males was significantly stronger than in females. To our knowledge, this is the first published study reporting the association between hyperuricemia and NAFLD, with a special focuses on the gender differences in the Chinese population aged 45 to 59 years.
In our analyses, the results showed that SUA and hyperuricemia were significantly related to NAFLD. Our results were in agreement with previous studies, which demonstrated the significant relationship between SUA and NAFLD.[14,16] There are several potential mechanisms explaining the significant association between hyperuricemia and NAFLD. The first explanation is insulin resistance, which has been identified as the critical factor for the development of NAFLD. Previous studies have found that hyperuricemia is closely associated with hyperinsulinemia and insulin resistance, which are involved in the development and progression of NAFLD.[21,22] In addition, some studies have also shown that insulin resistance not only increased uric acid synthesis,[23] but also decreased uric acid excretion.[24] The second is the oxidative stress and the possible inflammation, which have been reported to be associated with NAFLD.[25,26] It is well-known that NAFLD is associated with the increased risk of oxidative stress and hazardous cytokine, and the decreased risk of antiatherogenic factor-like adiponectin.[27,28] As aforementioned, these changes can provoke the inflammatory response in the arterial inside to deteriorate the arteriosclerosis. An experimental study has shown that uric acid may stimulate the secretion of proinflammatory cytokine (e.g., interleukin-1, interleukin-6, and tumor necrosis factor-α), all of which are proinflammatory molecules and stimulate production of CRP in the liver.[29,30] Ruggiero et al[31] have also found that uric acid is significantly associated with inflammatory markers in Italian men. Furthermore, some studies have confirmed that SUA is significantly associated with hypertension, obesity, hypertriglyceridemia, and hyperglycemia, which may increase the risk of MetS.[32] In fact, the MetS has been considered as an important role in the development or progression of NAFLD. Above all, uric acid could play an important role in the development of NAFLD.
The uniqueness of this study is we focused attention on the association between hyperuricemia and NAFLD in terms of gender. We found a much closer relationship between hyperuricemia and NAFLD in males than in females. The effect of gender differences on the progression of NAFLD has been reported[30]; however, less is known about gender differences in respect to the effect of hyperuricemia on NAFLD. In fact, although many studies[12–14] have provided information on the significant association of hyperuricemia with NAFLD, evidence is scarce on gender difference of their associations. Li et al[15] found that SUA is significantly associated with NAFLD, and elevated SUA level is an independent risk factor for NAFLD. To the best of our knowledge, no paper has yet evaluated the effect of hyperuricemia on progression of NAFLD in a middle-aged Chinese population, emphasizing the impact from the gender difference. Until July 2017, we retrieved only 1 epidemiological study and 1 meta-analysis from the published literatures[33,34] emphasizing partially in gender perspective. In a Chinese study, Yang et al[33] demonstrated that females with hyperuricemia seem to have higher NAFLD risk than males. Moreover, recent a meta-analysis[34] comprising 117,712 subjects also reported that increased NAFLD risk is closely associated with hyperuricemia in both men (RR = 2.01, 95% CI: 1.58–2.56, P < .001) and women (RR = 1.26, 95% CI: 1.15–1.37, P < .001). Similarly, our results also showed a more intimate association between hyperuricemia and NAFLD in males than in females, due to higher r values between SUA and NAFLD components (Table 3) and approximately 1.5-fold as high ORs for the risk of NAFLD in males (Table 4). Several potential mechanisms underlying this difference have been discussed in other study. As previously known, SUA level has been suggested to be associated with the metabolic syndrome and cardiovascular disease, which play an important role in the development or progression of NAFLD.[8,35] Alternatively, hyperuricemia and gout affected males more commonly than females, and Zhou et al[36] in Chinese population have demonstrated that there was a uric acid difference of 30 to 120 μmol/L between males and females. To our knowledge, the gender difference in respect to SUA and NAFLD may lie in the fact that estrogen is a uricosuric agent, which can lower the level of SUA by promoting uric acid degradation and excretion.[37] Recently a study also found a lower level of SUA after hormone replacement therapy in postmenopausal women with hyperuricemia.[38] Besides, in the present study, there were significantly higher levels of BMI, WC, and WHR in men with hyperuricemia compared with women. A previous study has indicated that obesity is the most important risk factors for NAFLD.[39] Furthermore, elevation of ALT has been found to be frequently associated with obesity, insulin resistance, and hyperlipidemia,[40] which are the important risk for NAFLD. However, males with hyperuricemia had a higher ALT level than females in this study. Moreover, our findings also indicated that SUA level was positively associated with age in females, and inversely associated with age in males. Previous a study in USA has found that the level of SUA became higher at around age 50 years in females, and higher between 10 and 16 years in males.[41] The age of eligible participants was between 45 and 59 years in present study. Thus, a decline in estrogen level with age may be accompanied by a rise in the level of SUA in females. Furthermore, Ka et al[42] have found that exercise may decrease uric acid excretion and accelerate purine degradation. It is well known that women, especially retired women are more prone to comply with physician's advises to keep body fitness, while men tend to be more sedentary in China.[43]
4.1. Strengths and limitations
The present study holds its strengths and limitations. Firstly, to the best of our knowledge, this is the first study investigating the association between hyperuricemia and NAFLD among the Chinese population aged 45 to 59 years. Our findings further confirm the associations between hyperuricemia and NAFLD in terms of gender. Secondly, our findings are reliable because all these biochemical data were obtained from the Medical Center for Physical Examination, Zhejiang Hospital. Thirdly, we have adjusted for known confounding variables in our analyses. Nevertheless, several potential limitations in the present study need to be considered. Firstly, given the cross-sectional design, we are unable to make a causal inference based on our findings. Thus, future prospective studies are needed to confirm these findings. Secondly, the diagnosis of NAFLD was based on ultrasonographic examination, which is not sensitive enough to detect mild steatosis. Finally, the study participants of our analyses were restricted to the city of Hangzhou, Zhejiang Province, East China, and therefore these conclusions may not be generalized to the population in the whole China.
In conclusion, our results demonstrated the significant relationship between SUA level, hyperuricemia, and NAFLD, and this relationship was significantly stronger in males than in females among Chinese adults aged 45 to 59 years. Further studies are required to establish the role of uric acid in the pathogenesis of NAFLD.
Acknowledgments
We thank all participants from Department of Nutrition, Zhejiang Hospital for their assistance and support. We also acknowledge the Medical Center for Physical Examination, Zhejiang Hospital for their important contributions to collection of data in this study.
Footnotes
Abbreviations: ALT = alanine aminotransferase, AST = asparagine aminotransferase, BMI = body mass index, CI = confidence interval, DBP = diastolic blood pressure, FG = fasting glucose, HDL-C = high-density lipoprotein–cholesterol, LDL-C = low-density lipoprotein–cholesterol, NAFLD = nonalcoholic fatty liver disease, OR = odds ratio, SBP = systolic blood pressure, SUA = serum uric acid, TC = total cholesterol, TG = triglycerides, WC = waist circumference, WHR = waist–hip ratio.
Funding: This study was supported by the medical platform projects of Zhejiang Province (Grant No. 2016ZDA001) and Natural Science Foundation of Zhejiang (Grant No. Y17H030031).
The authors have no conflicts of interest to disclose.
References
- [1].Colak Y, Tuncer I, Senates E, et al. Nonalcoholic fatty liver disease: a nutritional approach. Metab Syndr Relat Disord 2012;10:161–6. [DOI] [PubMed] [Google Scholar]
- [2].Hou XH, Zhu YX, Lu HJ, et al. Non-alcoholic fatty liver disease's prevalence and impact on alanine aminotransferase associated with metabolic syndrome in the Chinese. J Gastroenterol Hepatol 2011;26:722–30. [DOI] [PubMed] [Google Scholar]
- [3].Lin YC, Chang PF, Chang MH, et al. Genetic variants in GCKR and PNPLA3 confer susceptibility to nonalcoholic fatty liver disease in obese individuals. Am J Clin Nutr 2014;99:869–74. [DOI] [PubMed] [Google Scholar]
- [4].Anstee QM, Targher G, Day CP. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat Rev Gastroenterol Hepatol 2013;10:330–44. [DOI] [PubMed] [Google Scholar]
- [5].Zelber-Sagi S, Lotan R, Shlomai A, et al. Predictors for incidence and remission of NAFLD in the general population during a seven-year prospective follow-up. J Hepatol 2012;56:1145–51. [DOI] [PubMed] [Google Scholar]
- [6].Marchesini G, Marzocchi R, Agostini F, et al. Nonalcoholic fatty liver disease and the metabolic syndrome. Curr Opin Lipidol 2005;16:421–7. [DOI] [PubMed] [Google Scholar]
- [7].Liu L, Lou S, Xu K, et al. Relationship between lifestyle choices and hyperuricemia in Chinese men and women. Clin Rheumatol 2013;32:233–9. [DOI] [PubMed] [Google Scholar]
- [8].Zhang Q, Lou S, Meng Z, et al. Gender and age impacts on the correlations between hyperuricemia and metabolic syndrome in Chinese. Clin Rheumatol 2011;30:777–87. [DOI] [PubMed] [Google Scholar]
- [9].Feig DI, Kang DH, Johnson RJ. Uric acid and cardiovascular risk. N Engl J Med 2008;359:1811–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Sautin YY, Nakagawa T, Zharikov S, et al. Adverse effects of the classic antioxidant uric acid in adipocytes: NADPH oxidase-mediated oxidative/nitrosative stress. Am J Physiol Cell Physiol 2007;293:C584–96. [DOI] [PubMed] [Google Scholar]
- [11].Powell EE, Jonsson JR, Clouston AD. Metabolic factors and non-alcoholic fatty liver disease as co-factors in other liver diseases. Dig Dis 2010;28:186–91. [DOI] [PubMed] [Google Scholar]
- [12].Xu C, Yu C, Xu L, et al. High serum uric acid increases the risk for nonalcoholic Fatty liver disease: a prospective observational study. PLoS ONE 2010;5:e11578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ryu S, Chang Y, Kim SG, et al. Serum uric acid levels predict incident nonalcoholic fatty liver disease in healthy Korean men. Metabolism 2011;60:860–6. [DOI] [PubMed] [Google Scholar]
- [14].Liang J, Pei Y, Gong Y, et al. Serum uric acid and non-alcoholic fatty liver disease in non-hypertensive Chinese adults: the Cardiometabolic Risk in Chinese (CRC) study. Eur Rev Med Pharmacol Sci 2015;19:305–11. [PubMed] [Google Scholar]
- [15].Li Y, Xu C, Yu C, et al. Association of serum uric acid level with non-alcoholic fatty liver disease: a cross-sectional study. J Hepatol 2009;50:1029–34. [DOI] [PubMed] [Google Scholar]
- [16].Xie Y, Wang M, Zhang Y, et al. Serum uric acid and non-alcoholic fatty liver disease in non-diabetic Chinese men. PLoS ONE 2013;8:e67152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Esmaillzadeh A, Kimiaqar M, Mehrabi Y, et al. Dietary patterns, insulin resistance, and prevalence of the metabolic syndrome in women. Am J Clin Nutr 2007;85:910–8. [DOI] [PubMed] [Google Scholar]
- [18].Nascimbeni F, Pais R, Bellentani S, et al. From NAFLD in clinical practice to answers from guidelines. J Hepatol 2013;59:859–71. [DOI] [PubMed] [Google Scholar]
- [19].Wang HJ, Wang ZH, Yu WT, et al. Changes of waist circumference distribution and the prevalence of adiposity among Chinese adults from 1993 to 2006. Zhonghua Liu Xing Bing Xue Za Zhi 2008;29:953–8. [PubMed] [Google Scholar]
- [20].Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003;289:2560–72. [DOI] [PubMed] [Google Scholar]
- [21].Park SH, Kim BI, Yun JW, et al. Insulin resistance and C-reactive protein as independent risk factors for non-alcoholic fatty liver disease in non-obese Asian men. J Gastroenterol Hepatol 2004;19:694–8. [DOI] [PubMed] [Google Scholar]
- [22].Sui X, Church TS, Meriwether RA, et al. Uric acid and the development of metabolic syndrome in women and men. Metabolism 2008;57:845–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Modan M, Halkin H, Karasik A, et al. Elevated serum uric acid-a facet of hyperinsulinaemia. Diabetologia 1987;30:713–8. [DOI] [PubMed] [Google Scholar]
- [24].Quinones Galvan A, Natali A, Baldi S, et al. Effect of insulin on uric acid excretion in humans. Am J Physiol 1995;268:E1–5. [DOI] [PubMed] [Google Scholar]
- [25].Marchesini G, Brizi M, Bianchi G, et al. Nonalcoholic fatty liver disease: a feature of the metabolic syndrome. Diabetes 2001;50:1844–50. [DOI] [PubMed] [Google Scholar]
- [26].Yesilova Z, Yaman H, Oktenli C, et al. Systemic markers of lipid peroxidation and antioxidants in patients with nonalcoholic fatty liver disease. Am J Gastroenterol 2005;100:850–5. [DOI] [PubMed] [Google Scholar]
- [27].Matsuzawa Y, Funahashi T, Kihara S, et al. Adiponectin and metabolic syndrome. Arterioscler Thromb Vasc Biol 2004;24:29–33. [DOI] [PubMed] [Google Scholar]
- [28].Pischon T, Girman CJ, Hotamisligil GS, et al. Plasma adiponectin levels and risk of myocardial infarction in men. JAMA 2004;29:1730–7. [DOI] [PubMed] [Google Scholar]
- [29].Kanellis J, Watanabe S, Li JH, et al. Uric acid stimulates monocyte chemoattractant protein-1 production in vascular smooth muscle cells via mitogen-activated protein kinase and cyclooxygenase-2. Hypertension 2003;41:1287–93. [DOI] [PubMed] [Google Scholar]
- [30].Hotamisligil GS, Arner P, Caro JF, et al. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest 1995;95:2409–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Ruggiero C, Cherubini A, Ble A, et al. Uric acid and inflammatory markers. Eur Heart J 2006;27:1174–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Conen D, Wietlisbach V, Bovet P, et al. Prevalence of hyperuricemia and relation of serum uric acid with cardiovascular risk factors in a developing country. BMC Public Health 2004;4:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Yang C, Yang S, Xu W, et al. Association between the hyperuricemia and nonalcoholic fatty liver disease risk in a Chinese population: a retrospective cohort study. PLoS ONE 2017;12:e0177249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Gong S, Song J, Wang L, et al. Hyperuricemia and risk of nonalcoholic fatty liver disease: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol 2016;28:132–8. [DOI] [PubMed] [Google Scholar]
- [35].Tian Y, Chen K, Xie Z, et al. The association between serum uric acid levels, metabolic syndrome and cardiovascular disease in middle aged and elderly Chinese: results from the DYSlipidemia International Study. BMC Cardiovasc Disord 2015;15:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Zhou Y, Qi H, Zhao GM, et al. Relationship between hyperuricemia and chronic kidney disease in Pudong New Area of Shanghai. Zhonghua Liu Xing Bing Xue Za Zhi 2012;33:351–5. [PubMed] [Google Scholar]
- [37].Yahyaoui R, Esteva I, Haro-Mora JJ, et al. Effect of long-term administration of cross-sex hormone therapy on serum and urinary uric acid in transsexual persons. J Clin Endocrinol Metab 2008;93:2230–3. [DOI] [PubMed] [Google Scholar]
- [38].Hak AE, Choi HK. Menopause, postmenopausal hormone use and serum uric acid levels in US women—The Third National Health and Nutrition Examination Survey. Arthritis Res Ther 2008;10:R116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Holterman AX, Guzman G, Fantuzzi G, et al. Nonalcoholic fatty liver disease in severely obese adolescent and adult patients. Obesity (Silver Spring) 2013;21:591–7. [DOI] [PubMed] [Google Scholar]
- [40].Clark JM, Brancati FL, Diehl AM. The prevalence and etiology of elevated aminotransferase levels in the United States. Am J Gastroenterol 2003;98:960–7. [DOI] [PubMed] [Google Scholar]
- [41].Mikkelsen WM, Dodge HJ, Valkenburg H. The distribution of serum uric acid values in a population unselected as to gout or hyperuricemia: Tecumseh, Michigan 1959–1960. Am J Med 1965;39:242–51. [DOI] [PubMed] [Google Scholar]
- [42].Ka T, Yamamoto T, Moriwaki Y, et al. Effect of exercise and beer on the plasma concentration and urinary excretion of purine bases. J Rheumatol 2003;30:1036–42. [PubMed] [Google Scholar]
- [43].Lee SA, Xu WH, Zheng W, et al. Physical activity patterns and their correlates among Chinese men in Shanghai. Med Sci Sports Exerc 2007;39:1700–7. [DOI] [PubMed] [Google Scholar]


