Abstract
Choice of surgical approach in patients under clopidogrel treatment is controversial. Intertrochanteric fractures are common in the elderly, who also suffer from a number of comorbidities.
The aim of this study is to assess the prognosis of elderly patients with clopidogrel treatment after surgery for intertrochanteric fracture.
This was a cohort study of 238 elderly patients who underwent proximal femur intramedullary nailing for intertrochanteric fracture between January 2012 and December 2013 at the Geriatric Trauma Center of the Beijing Army General Hospital. The patients were divided into the clopidogrel (n = 32) and control (n = 206) groups according to their history of long-term clopidogrel treatment before surgery. Demographic and clinical characteristics, intraoperative parameters, postoperative complications, and 1-year survival were compared between the 2 groups.
Preoperative American Society of Anesthesiologists (ASA) grade and the frequency of arterial stenting were different between the 2 groups (P = .002 and P < .001, respectively). The rate of intraoperative blood transfusion, ICU stay, and hospital stay were higher in the clopidogrel group compared with the control group (all P < .001). Postoperative complications were similar in the 2 groups. The 1-year mortality rate after surgery was significantly higher in the clopidogrel group compared with the control group (37.5% vs 20.3%, P = .030).
Prognosis after surgery for intertrochanteric fracture was poorer in elderly patients with clopidogrel treatment; these patients had lower 1-year survival, more intraoperative blood transfusion, longer ICU stay, and longer hospital stay. ASA grade, arterial stenting, and anesthesia mode were prognostic factors.
Keywords: clopidogrel, intertrochanteric fractures, platelet aggregation inhibitors, postoperative complications, proximal femur intramedullary nailing
1. Introduction
Intertrochanteric hip fractures are among the main causes of orthopedic surgical admissions worldwide. Elderly people (>65 years old) are particularly vulnerable to this type of injury because of the higher prevalence of osteoporosis or osteopenia.[1] In addition, these patients often present with associated health disorders that can be fatal, such as coronary artery disease, hypertension, cerebrovascular disease, and atrial fibrillation.[2] Cardiovascular disease is one of the most frequent comorbidities in patients who have to be operated for hip fracture and are often treated with anticoagulants.[3]
Clopidogrel is an antiplatelet agent used extensively as secondary prophylaxis in cases with coronary artery disease, myocardial infarction, vascular diseases, and stroke. Clopidogrel interferes with the cell-signaling events involved in blood clotting, notably the activation of the glycoprotein GPIIb/IIa complex.[4] The active form of clopidogrel (2-oxo-clopidogrel) has a half-life of about 8 hours[5] and causes inactivation of platelets for 7 days.[6] As a result, patients have been reported to have a full recovery of platelet function in only 7 days after the last dose of clopidogrel, which might increase bleeding complications associated with emergency surgical procedures such as reduction of intertrochanteric hip fractures.[7]
Clopidogrel use in patients with hip fracture is controversial, as the increased risk of perioperative bleeding must be weighed against the benefits conferred by the accelerated surgical admission, and the continuous effects of the compound on platelets and the reduction in blood clotting.[8,9] Consequently, there is no consensus regarding the specific guidelines that should be followed in the perioperative use of clopidogrel in patients with acute femoral neck fractures. The British Orthopaedic Association recommends operation within 48 hours of admission for medically fit patients.[10] Recent studies have found no increased risk of bleeding or bleeding-related wound complications associated with clopidogrel continuation in patients undergoing surgery for hip fracture,[11–13] though increased mortality was associated with delayed surgery.[14]
Because of the lack of consensus regarding the management of patients treated with clopidogrel and undergoing surgery, the present study aimed to compare the prognosis of clopidogrel treatment following intertrochanteric fracture surgery in elderly patients.
2. Methods
2.1. Study design
The present trial was a non-interventional retrospective cohort study conducted in accordance with the principles of the Declaration of Helsinki. The study protocol was approved by the ethics committee of Beijing Army General Hospital (approval #EC-KS-2015–026; approved in April 2015). The need for individual informed consent was waived by the committee because of the retrospective nature of the study.
2.2. Patients
During the study period (January 2012 to December 2013), patients ≥65 years old with a primary admission diagnosis of intertrochanteric fracture who underwent proximal femur intramedullary nailing at the Geriatric Trauma Center of the Beijing Army General Hospital (a specialized center at a tertiary hospital) were included. The patients were identified using the hospital clinical database. The charts were reviewed to determine if they received clopidogrel or not.
Inclusion criteria were as follows: >65 years old; intertrochanteric fracture; and indications for fracture reduction. Exclusion criteria were as follows: femoral neck fracture or fractures involving the pelvis, subtrochanteric region, or femoral shaft; pathological hip fractures and traumatic injuries other than hip fractures; any disease or syndrome affecting coagulation; hematological malignancies; active bleeding or gastrointestinal ulcers in the past 6 months; history of warfarin or dipyridamole therapy; or thrombocytopenia at admission (platelet count <150 × 109 L−1).
2.3. Grouping
A total of 238 patients were eligible for the study. The patients were divided into 2 groups according to their history of long-term clopidogrel use (>3 months) prior to surgery: clopidogrel (with clopidogrel treatment) and control (without clopidogrel usage) groups. In the present study, only patients who were maintained on clopidogrel treatment were included in the clopidogrel group. Patients on clopidogrel but for whom clopidogrel was stopped for surgery were excluded.
2.4. Surgery
General or epidural anesthesia was selected according to preoperative pulmonary complications, bleeding, and coagulation time. Prior to surgery, all patients were given low molecular weight heparin, and the d-dimer ratio and international normalized value were dynamically monitored. All surgeries were conducted by the same group of experienced orthopedists. Closed traction reduction and minimally invasive insertion of proximal femoral nail were performed under a C-arm x-ray monitor (InterTan, Smith & Nephew, Memphis, TN; or PFNA, Mathys, Güterstrasse, Switzerland). The patient was positioned supine on a radiolucent table with the fractured limb placed in boot traction. Closed reduction was attempted by manipulating the limb with the use of traction. If the fracture reduced easily, a standard closed nailing was performed. If it was evident that the proximal fragment would not easily reduce, the closed nailing was converted to a limited open nailing.
2.5. Follow-up
The patients were followed up for 12 months after surgery at 1, 2, 3, 4, 6, and 12 months, as is the routine in our institution, at the hospital's outpatient clinic; for patients unable to come to the hospital, follow-up was performed by phone calls to them or their respective families.
2.6. Data collection and analysis
One-year overall survival, intraoperative parameters, and the number of postoperative complications within 1 month were examined. The occurrence of adverse events was evaluated at each follow-up visit.
The demographic and clinical characteristics, including causes of fracture, AO/ASIF classification, comorbidities, and the American Society of Anesthesiologists (ASA) grade of patients, were assessed before surgery as exposure factors. Comorbidities included hypertension, arterial stenting (in the heart or head), diabetes, coronary disease, cerebrovascular accident, atrial fibrillation, chronic obstructive pulmonary disease, chronic renal failure (defined as creatinine clearance rate <80 mL/min, serum creatinine >133 μmol/L, or chronic kidney disease or systemic disease involving the kidney), tumors synchronous with hip fracture, Alzheimer disease, preoperative hemoglobin levels, and perioperative waiting time.
The intraoperative parameters were classified according to the anesthesia mode and internal fixation type. The anesthesia mode was classified into general and epidural anesthesia, whereas the internal fixation type included parameters such as surgery duration, intraoperative bleeding, drainage volume, ICU stay, hospital stay, postoperative hemoglobin, and intraoperative blood transfusion.
Postoperative complications were recorded, and categorized accordingly: local complications such as local hematoma and effusion, incomplete healing of skin and subcutaneous tissues, and incision infection; systemic complications such as pulmonary embolism, cardiovascular events (hypertensive heart disease, coronary atherosclerotic heart disease, acute coronary syndrome, arrhythmia, and heart failure), acute cerebral stroke, cerebrovascular accident, stent arterial thrombosis, transient ischemic attack, acute renal failure, respiratory failure, and gastrointestinal bleeding; and complications related to prolonged immobilization such as pneumonia, urinary tract infection, deep vein thrombosis, and/or pressure sores.
The data of postoperative complication examination were collected within 1 month. Overall survival was assessed at 1, 2, 3, 4, 6, and 12 months following surgery.
2.7. Statistical analysis
Statistical analyses were performed with SPSS 20.0 (IBM, Armonk, NY). Continuous variables were presented as mean ± standard deviation, and categorical variables as frequency and percentage. Differences between the 2 groups were tested by Student t test for continuous variables, and the chi-square or Fisher exact test for categorical variables. One-year survival in the 2 groups was analyzed and compared by the Kaplan-Meier method, with the log-rank test. To evaluate the associations of clopidogrel treatment with various variables during the follow-up period, 95% confidence intervals (CIs) were applied. A 2-tailed P value <.05 was considered statistically significant.
3. Results
3.1. Characteristics of the patients
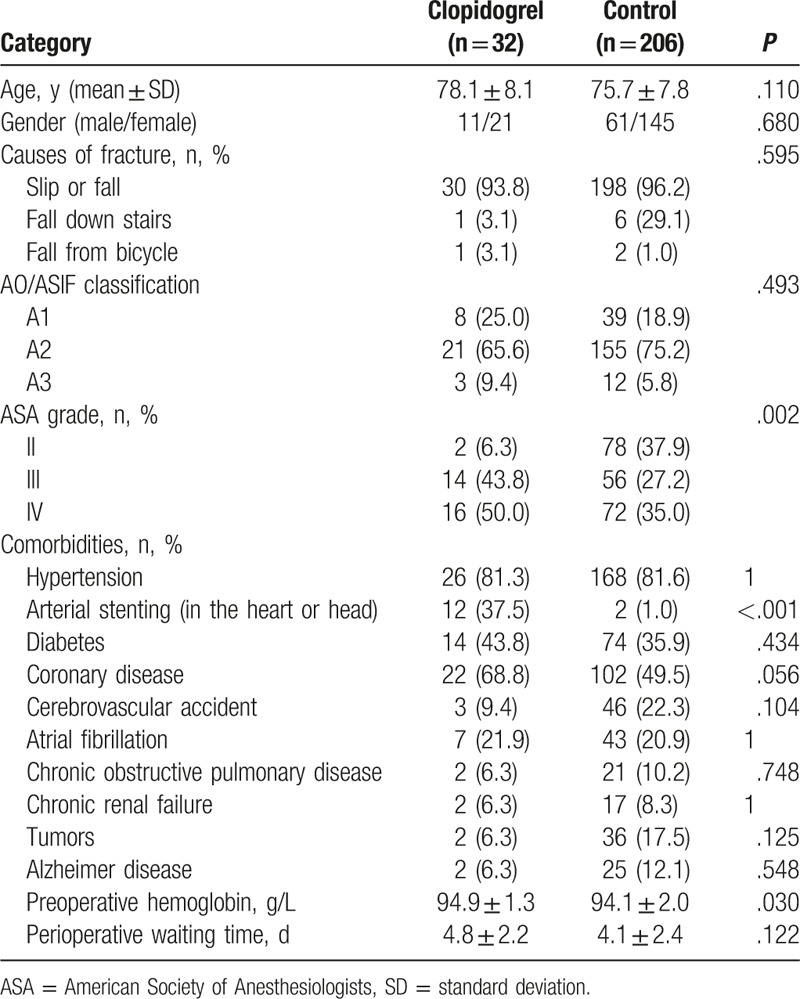
A total of 238 patients were included in the study (Fig. 1); 32 patients were included in the clopidogrel group (11 males and 21 females) and 206 patients in the control group (61 males and 145 females). The characteristics of all patients are presented in Table 1. There were no differences between the 2 groups in age, gender, the cause of fracture, preoperative hemoglobin, and perioperative waiting time (Table 1). The rate of arterial stenting in the clopidogrel group was significantly higher compared with that of the control group (P < .001), whereas a trend of higher incidence of coronary disease was observed in the same group (P = .056). Preoperative hemoglobin was significantly different between the 2 groups (94.9 ± 1.3 vs 94.1 ± 2.0 g/L, P = .030).
Figure 1.
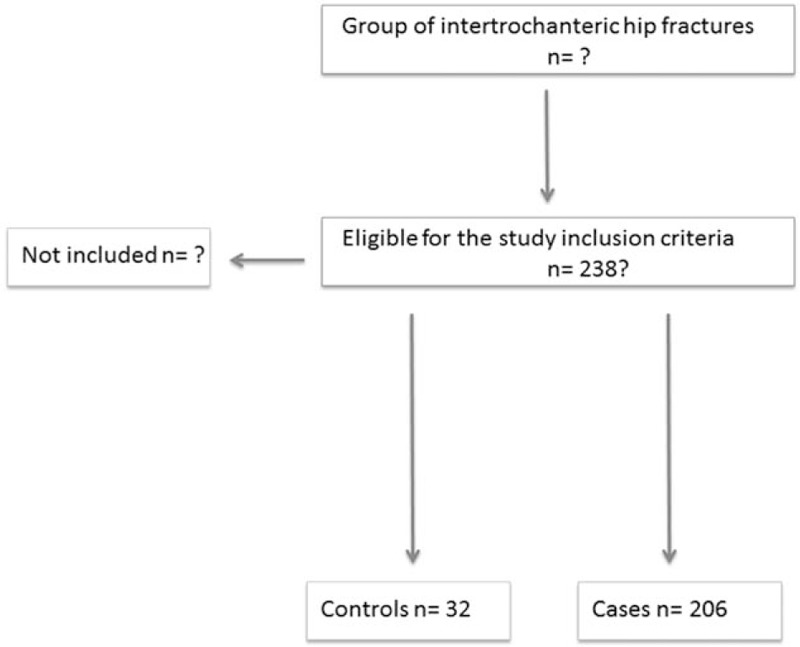
Study flowchart.
Table 1.
Demographic and clinical characteristics of the patients.
3.2. Intraoperative parameters
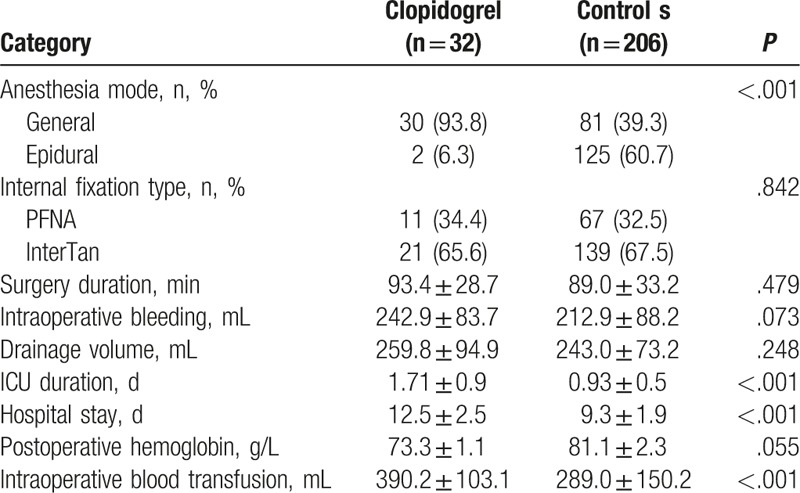
The distribution of anesthesia mode in the clopidogrel group was significantly different from that of the control group (P < .001) (Table 2). ICU stay and hospital stay in the clopidogrel group were significantly longer compared with control values (both P < .001) (Table 2). Intraoperative blood transfusion in the clopidogrel group was significantly higher compared with that of the control group (390.2 ± 103.1 vs 289.0 ± 150.2 mL, P < .001, Table 2).
Table 2.
Intraoperative parameters of the patients.
3.3. Postoperative complications
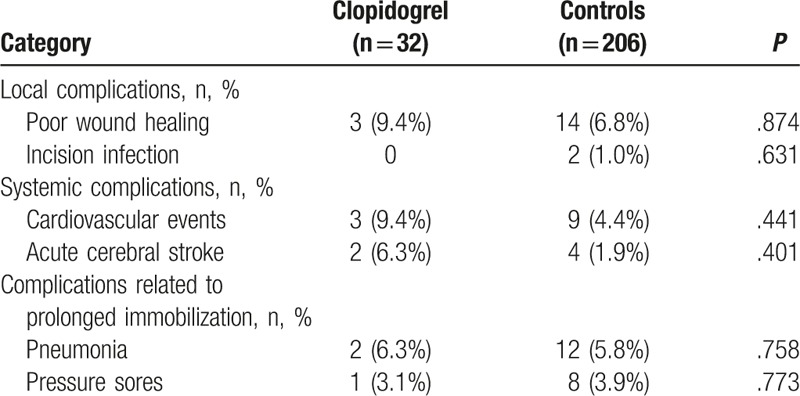
The number of patients with local complications in the clopidogrel group was similar to that of the control group (P > .05) (Table 3). No significant differences in cardiovascular events and acute cerebral stroke were found between the 2 groups (both P > .05) (Table 3). The complications related to prolonged immobilization that were observed in this study were pneumonia and pressure sores, but without differences between the 2 groups (P > .05) (Table 3).
Table 3.
Postoperative complications of the patients.
3.4. One-year overall survival
The 1-year overall survival rate was significantly higher in the control group compared with the clopidogrel group (P = .030, Fig. 2). A mortality rate of 37.5% was observed in the clopidogrel group (12 patients), higher compared with 20.3% found in the control group.
Figure 2.
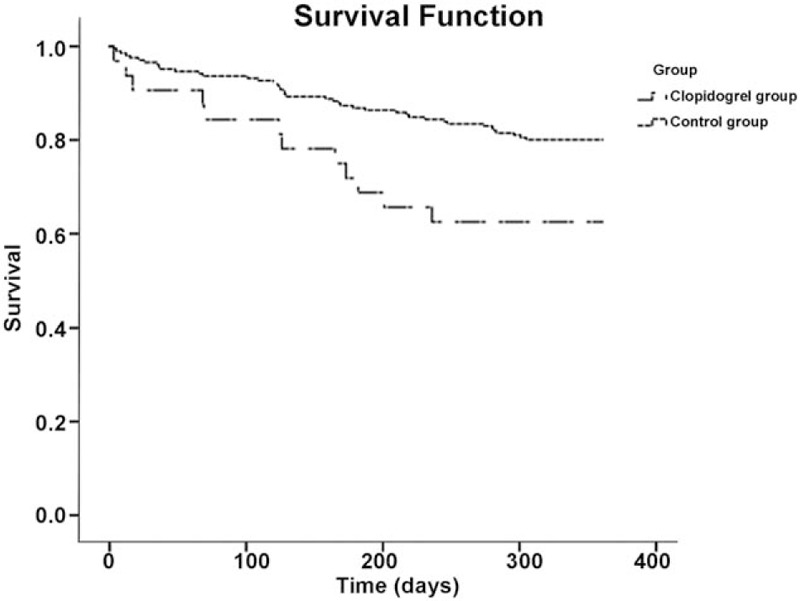
Comparison of 1-y overall survival rates in patients with and without clopidogrel at the time of surgery for intertrochanteric fracture.
4. Discussion
The present study aimed to assess the clinical and demographic characteristics, and intraoperative parameters of patients undergoing intertrochanteric fracture surgery who were on continuous clopidogrel treatment, compared with patients not receiving clopidogrel. The results showed that prognosis after surgery for intertrochanteric fracture was poorer in elderly patients with clopidogrel treatment; these patients had lower 1-year survival, more intraoperative blood transfusion, longer ICU stay, and longer hospital stay. ASA grade, arterial stenting, and anesthesia mode were prognosis factors.
The management of clopidogrel use in the elderly population that experiences hip fractures remains controversial because of the associated benefit/risk ratio of clopidogrel in relation to comorbidities and overall survival. Several studies have suggested that delayed surgery increases the risk of mortality and morbidity in these patients.[12,13,15–19] Recent studies have demonstrated no significant difference between bleeding parameters associated with clopidogrel treatment, notably hematoma, transfusion requirements, and perioperative blood loss of patients with or without clopidogrel.[11–13] Harty et al[20] showed that the ASA grades and duration of hospital stay are higher in the clopidogrel group (n = 21) compared with control values (n = 159), corroborating the present study. Moreover, Wallace et al[21] reported similar findings in 52 patients with clopidogrel compared with 58 controls. In addition, Collinge et al[18] revealed an increased ASA score and an increased length of stay in 74 out of 1056 patients receiving clopidogrel after early hip fractures, suggesting that this may affect the postoperative outcomes. Maheshwori et al[22] reported that the 1-year mortality rate of 31 patients on clopidogrel presenting with proximal femur fractures is related to postoperative blood transfusion and postoperative complications, again in agreement with the present study. In contrast to these observations, Wordsworth et al[13] examined retrospectively the effect of clopidogrel on mortality and comorbidities from hip fractures, and observed no significant differences in ASA grade, 1-year mortality, and/or intraoperative blood loss. Similar findings with respect to mortality have been documented by other studies.[17–19] Despite these discrepancies, there is a common consensus that prolonged hospitalization contributes to an increased risk of hip fracture-associated comorbidities. In the present study, the 1-year survival rate in the clopidogrel group was lower compared with that of the control group. This conclusion may seem to contradict previously published reports proposing that clopidogrel is safe and does not contribute to an increased mortality risk of patients with hip fracture and undergoing surgery. A possible explanation for this paradoxical observation is the small sample size of the treatment group, as opposed to the control group. Of note, 9 patients in the control group died in the first month compared with 3 in the clopidogrel group. Similar findings were observed by Harty et al[20] where a lower 1-month mortality rate was observed for the clopidogrel group (n = 21) compared with the control group (N = 159).
In addition, it is important to note that in the present study, the patients in the clopidogrel group exhibited poorer clinical characteristics regarding ASA scores, arterial stenting, and coronary disease (borderline significance) compared with the control group. These characteristics contribute to a poor baseline prognosis of the patients. Moreover, the longer hospital stay following surgery and longer ICU stay in the clopidogrel group may cause the progression of the disease with regard to surgery-associated comorbidities, which in turn may affect patient survival. Several studies have reported that surgery should be performed as soon as possible, and ASA scores can be affected by elevated postoperative length of stay in patients receiving clopidogrel, even though clopidogrel may not necessarily produce bleeding complications.[13,18,19] The data reported in the present study are in agreement with this hypothesis.
It has to be underlined that the majority of published results regarding clopidogrel use and hip fractures originate from retrospective and non-randomized studies, thus leading to weak conclusions.[2] More importantly, the paucity of case-control studies with approximately equal numbers of patients in the 2 groups is a significant limitation of the conclusions of previously published results.[2] In addition, most studies have focused on the controversy between the efficacy status of clopidogrel discontinuation for a certain period of time and early surgery occurring within 48 hours or sooner.[15,17,19,23] The studies that have examined the continuous effects of clopidogrel in hip fracture patients are very limited. To our knowledge, this is the first study that examined the effects of continuous clopidogrel treatment in intertrochanteric hip fracture patients.
Future studies must address the difficulties encountered with large-scale randomized clinical trials, to provide more robust and conclusive evidence regarding the effect of clopidogrel on the mortality of patients with hip fracture undergoing surgery. The data reported here support the finding that the use of clopidogrel in the elderly population with intertrochanteric fractures contributes to poor prognosis and postoperative comorbidities. Nevertheless, the generalizability of the results is limited by the selection of patients with a single type of femoral fracture, from a single institution. Additional studies are necessary to improve the generalizability of these results.
The main limitation of the present study is the small sample size of the clopidogrel group, which does not allow an accurate estimation of clinical and intraoperative parameters, as well as the 1-year survival rate. The present study was not randomized. The history of clopidogrel use is a bias in itself because this information was used for medical decisions that affected the parameters measured, for example, surgery duration, hospital stay, intraoperative bleeding, and ICU stay. These interventions can clearly influence the results obtained for the aforementioned parameters. Furthermore, future studies should address endpoints that contribute to survival associated with coronary heart disease, bone metabolism status (osteoporosis and osteopenia), myocardial infarction, edema, ulcers, and pulmonary embolism. Finally, the present study examined the intertrochanteric fractures but not the other types of hip fractures such as femoral neck fractures.
In conclusion, prognosis after surgery for intertrochanteric fracture was poorer in elderly patients with clopidogrel treatment. ASA grade, arterial stenting, and anesthesia mode prior to surgery were possible prognostic factors. It is suggested that patients on continuous clopidogrel treatment may require more intraoperative blood transfusion, which in turn can severely affect their rehospitalization and consequently survival.
Footnotes
Abbreviations: ASA = American Society of Anesthesiologists, CI = confidence intervals.
JZ and XC carried out the studies, participated in collecting data, and drafted the manuscript. JW and ZL performed the statistical analysis and participated in study design. XW, JR, and TS helped draft the manuscript. All authors read and approved the final manuscript.
This study was funded by the “Regulatory effect of COX-2 inhibitor on inflammatory response in elderly patients with hip fracture” and the Capital Training Program of Beijing Municipal Commission of Science and Technology (Z141100002114030).
The authors declare no conflicts of interest for this article.
References
- [1].Gupta RK, Gupta V, Gupta N. Outcomes of osteoporotic trochanteric fractures treated with cement-augmented dynamic hip screw. Indian J Orthop 2012;46:640–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Soo CG, Torre PK, Yolland TJ, et al. Clopidogrel and hip fractures, is it safe? A systematic review and meta-analysis. BMC Musculoskelet Disord 2016;17:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Fisher A, Srikusalanukul W, Davis M, et al. Cardiovascular diseases in older patients with osteoporotic hip fracture: prevalence, disturbances in mineral and bone metabolism, and bidirectional links. Clin Interv Aging 2013;8:239–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Dorsam RT, Kunapuli SP. Central role of the P2Y12 receptor in platelet activation. J Clin Invest 2004;113:340–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Dansette PM, Rosi J, Bertho G, et al. Paraoxonase-1 and clopidogrel efficacy. Nat Med 2011;17:1040–1. [DOI] [PubMed] [Google Scholar]
- [6].Savi P, Combalbert J, Gaich C, et al. The antiaggregating activity of clopidogrel is due to a metabolic activation by the hepatic cytochrome P450-1A. Thromb Haemost 1994;72:313–7. [PubMed] [Google Scholar]
- [7].Inman DS, Michla Y, Partington PF. Perioperative management of trauma patients admitted on clopidogrel (Plavix). A survey of orthopaedic departments across the United Kingdom. Injury 2007;38:625–30. [DOI] [PubMed] [Google Scholar]
- [8].Rosencher N, Bonnet MP, Sessler DI. Selected new antithrombotic agents and neuraxial anaesthesia for major orthopaedic surgery: management strategies. Anaesthesia 2007;62:1154–60. [DOI] [PubMed] [Google Scholar]
- [9].Douketis JD, Berger PB, Dunn AS, et al. The perioperative management of antithrombotic therapy: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 2008;133:299s–339s. [DOI] [PubMed] [Google Scholar]
- [10].Howard-Alpe GM, de Bono J, Hudsmith L, et al. Coronary artery stents and non-cardiac surgery. Br J Anaesth 2007;98:560–74. [DOI] [PubMed] [Google Scholar]
- [11].Hossain FS, Rambani R, Ribee H, et al. Is discontinuation of clopidogrel necessary for intracapsular hip fracture surgery? Analysis of 102 hemiarthroplasties. J Orthop Traumatol 2013;14:171–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Manaqibwala MI, Butler KA, Sagebien CA. Complications of hip fracture surgery on patients receiving clopidogrel therapy. Arch Orthop Trauma Surg 2014;134:747–53. [DOI] [PubMed] [Google Scholar]
- [13].Wordsworth DR, Halsey T, Griffiths R, et al. Clopidogrel has no effect on mortality from hip fracture. Injury 2013;44:743–6. [DOI] [PubMed] [Google Scholar]
- [14].Shiga T, Wajima Z, Ohe Y. Is operative delay associated with increased mortality of hip fracture patients? Systematic review, meta-analysis, and meta-regression. Can J Anaesth 2008;55:146–54. [DOI] [PubMed] [Google Scholar]
- [15].Nwachuku IC, Jones M, Clough TM. Clopidogrel: is a surgical delay necessary in fractured neck of femur? Ann R Coll Surg Engl 2011;93:310–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Ginsel BL, Taher A, Whitehouse SL, et al. Effects of anticoagulants on outcome of femoral neck fracture surgery. J Orthop Surg 2015;23:29–32. [DOI] [PubMed] [Google Scholar]
- [17].Doleman B, Moppett IK. Is early hip fracture surgery safe for patients on clopidogrel? Systematic review, meta-analysis and meta-regression. Injury 2015;46:954–62. [DOI] [PubMed] [Google Scholar]
- [18].Collinge CA, Kelly KC, Little B, et al. The effects of clopidogrel (Plavix) and other oral anticoagulants on early hip fracture surgery. J Orthop Trauma 2012;26:568–73. [DOI] [PubMed] [Google Scholar]
- [19].Chechik O, Amar E, Khashan M, et al. In support of early surgery for hip fractures sustained by elderly patients taking clopidogrel: a retrospective study. Drugs Aging 2012;29:63–8. [DOI] [PubMed] [Google Scholar]
- [20].Harty JA, McKenna P, Moloney D, et al. Anti-platelet agents and surgical delay in elderly patients with hip fractures. J Orthop Surg 2007;15:270–2. [DOI] [PubMed] [Google Scholar]
- [21].Wallace HC, Probe RA, Chaput CD, et al. Operative treatment of hip fractures in patients on clopidogrel: a case-control study. Iowa Orthop J 2012;32:95–9. [PMC free article] [PubMed] [Google Scholar]
- [22].Maheshwari R, Acharya M, Monda M, et al. Factors influencing mortality in patients on antiplatelet agents presenting with proximal femoral fractures. J Orthop Surg 2011;19:314–6. [DOI] [PubMed] [Google Scholar]
- [23].Leonidou A, Cam NB, Chambers IR. Femoral neck fractures in patients on Clopidogrel. The effect of delaying surgery and the introduction of the new SIGN guidelines. Surgeon 2011;9:318–21. [DOI] [PubMed] [Google Scholar]


