Abstract
Background:
Nonalcoholic fatty liver disease (NAFLD) is emerging as a public health issue worldwide and is highly prevalent in patients with type 2 diabetes mellitus (T2DM). However, there was a great disparity across studies in the estimated prevalence of NAFLD in T2DM patients. This meta-analysis, therefore, aimed to estimate the pooled prevalence of NAFLD in T2DM patients.
Methods:
Electronic databases of PubMed, Web of Science, Embase, Chinese National Knowledge Infrastructure, and Wanfang were searched using MeSH terms to identify relevant studies. Eligibility assessment and data extraction were conducted independently by 2 investigators and a meta-analysis was performed to synthesize the data. Heterogeneity was evaluated using the Cochran Q test and quantified using the I2 statistic. Publication bias was assessed using both the Begg and Egger tests. Subgroup analyses were performed to identify the possible sources of heterogeneity.
Results:
Twenty-four studies involving 35,599 T2DM patients were included in this meta-analysis, of which 20,264 were identified with NAFLD. A high degree of heterogeneity (I2 = 99.0%, P < .001) was observed among the eligible studies, with the reported prevalence ranging from 29.6% to 87.1%. The pooled prevalence of NAFLD in T2DM patients, by a random-effects model, was 59.67% (95% confidence interval: 54.31–64.92%). Sensitivity was low and both the Begg test and Egger test showed low possibility of publication bias. Subgroup analyses indicated that the prevalence of NAFLD in T2DM patients differed by gender, obesity, hypertension, dyslipidemia, coronary heart disease, and chronic kidney disease.
Conclusions:
The high pooled prevalence of NAFLD in T2DM patients found in this study significantly underscores the need for early assessment of NAFLD and the importance of strengthening the management of NAFLD in T2DM patients.
Keywords: meta-analysis, nonalcoholic fatty liver disease, prevalence, type 2 diabetes mellitus
1. Introduction
Nonalcoholic fatty liver disease (NAFLD), defined as the presence of hepatic steatosis in the absence of secondary causes, is emerging as a public health issue worldwide, with a global pooled prevalence, by imaging, of 25.24% (95% confidence interval [CI]: 22.10–28.65%) among general population.[1] NAFLD includes a spectrum of diseases ranging from simple steatosis to nonalcoholic steatohepatitis (NASH), and to advanced fibrosis and cirrhosis and hepatocellular carcinoma, causing considerable liver-related morbidity and mortality.[2] Accumulated evidence has indicated that NAFLD could be regarded as part of or, indeed, a hepatic manifestation of metabolic syndrome associated with metabolic risk factors such as obesity, diabetes mellitus, and dyslipidemia.[3,4]
The association between NAFLD and type 2 diabetes mellitus (T2DM) has been well established, which could be explained by the insulin-resistance and compensatory hyperinsulinemia progressing to defective lipid metabolism and hepatic triglyceride (TG) accumulation in NAFLD or to β-cell dysfunction in T2DM.[5] Compared with nondiabetic subjects, patients with T2DM appear to have an increased risk of developing NAFLD and certainly have a heightened risk of developing advanced liver diseases, such as fibrosis, cirrhosis, and hepatocellular carcinoma.[6–8] Furthermore, NAFLD in T2DM may lead to a higher risk of developing cardiovascular disease and diabetic vascular complications, independently of other known risk factors.[9,10] In this regard, an accurate and reliable estimate of the prevalence of NAFLD in T2DM patients is important as it can help service providers to predict the number of subjects who may develop NAFLD, and hence implement intervention strategies to cope with the problem.[11,12]
Notably, the prevalence of T2DM has been increasing rapidly over the past 2 decades worldwide. For example, it increased from 10.6% in 1989 to 32.1% in 2009 among the Saudi population,[13] which stimulated a growing research interest in NAFLD among T2DM population. However, there was a great disparity across studies in the estimated prevalence of NAFLD in T2DM patients, ranging from 45% to 80%.[14,15] This variation may be associated with the sample characteristics and the techniques used to make the diagnosis of NAFLD.[16] Therefore, this meta-analysis aimed to explore the pooled prevalence of NAFLD in T2DM patients by synthesizing the reported data.
2. Methods
2.1. Ethical approval
Ethical approval was not required for this study, given that this was a meta-analysis, which utilized published data.
2.2. Search strategy
This meta-analysis was carried out based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. A literature search of the electronic databases of PubMed, Web of Science, Embase, Chinese National Knowledge Infrastructure (CNKI), and Wanfang was carried out from their inception to July 2017. MeSH terms were used to identify relevant studies. Specifically, for the databases of PubMed and Web of Science, search term was: (“Non-alcoholic Fatty Liver Disease” [MeSH]) AND “Diabetes Mellitus, Type 2” [MeSH]; for the database of Embase, search term was: “nonalcoholic fatty liver”/mj AND “noninsulin dependent diabetes mellitus”/mj; and for the Chinese databases of CNKI and Wanfang, a combination of the MeSH terms of  and
and  was used. The reference lists of full articles were also reviewed.
was used. The reference lists of full articles were also reviewed.
2.3. Eligibility criteria
Studies were included in this meta-analysis if they were observational studies focusing on T2DM patients; provided a definition for the diagnosis of NAFLD; reported screening for alcohol excess and excluding other causes of liver diseases, such as viral hepatitis B or C; provided information about the sample size and the prevalence of NAFLD in T2DM; and were written in English or Chinese. In addition, if duplicated data were observed across studies, the earlier publication was included and, for longitudinal studies, baseline NAFLD prevalence in T2DM was included. Reviews, case-reports, comments, or book chapters were excluded from this meta-analysis. Besides, consistent with previous meta-analyses aimed at estimating the pooled prevalence, studies with a sample size of <300 were excluded since such sample size may lead to liable prevalence.[17–19]
2.4. Data extraction
Two investigators (WD and LY) independently assessed the eligibility of articles and extracted data from eligible articles. Any discrepancies between them were resolved by consensus. In particular, the following data were extracted: first author, year of publication, region, study design, sample source (facility-based or population-based), mean (standard deviation [SD]) age of the whole T2DM sample (if mean [SD] age of the whole T2DM sample was not reported, then mean [SD] age of the NAFLD patients in the whole T2DM sample was presented), diagnostic criteria of NAFLD, number of T2DM patients with NAFLD, sample size, prevalence of NAFLD in T2DM, and study quality. Furthermore, if available, data on gender, obesity, hypertension, dyslipidemia, diabetic retinopathy (DR), coronary heart disease (CHD), chronic kidney disease (CKD), and chronic obstructive pulmonary disease (COPD) were extracted for performing subgroup analyses. Full data for this study are available upon request to the corresponding author.
2.5. Quality assessment
In accordance with previous meta-analyses focusing on observational studies, the Agency for Healthcare Research and Quality (AHRQ) was used to evaluate the quality of eligible studies.[20,21] AHRQ is an 11-item instrument with response options Yes/No/Unclear for each item. According to the scoring guideline, the response of “Yes” is scored “1,” and the response of “No” or “Unclear” is scored “0.” Thus, the total score for this instrument ranges from 0 to 11, with a total score from 0 to 3, 4 to 7, and 8 to 11 indicating low quality, moderate quality, and high quality, respectively.
2.6. Statistical analyses
Statistical analyses for this meta-analysis were performed using the R statistical software version 3.4.1. Heterogeneity was evaluated using the Cochran Q test and quantified using the I2 statistic.[22] The pooled prevalence of NAFLD in T2DM patients, presented as percentage with corresponding 95% CI, was estimated using Freeman-Tukey double arcsine method by a random-effects model when significant heterogeneity was observed (P ≤ .10 and I2 > 50%). Otherwise, a fixed-effects model was used.[23] Sensitivity was evaluated by the effect of excluding low-quality articles on the stability of the pooled prevalence.[24,25] Publication bias was assessed using both Begg test and Egger test, and an Egger funnel plot for asymmetry was presented.[17,26] Subgroup analyses, defined as differences in gender, obesity, hypertension, dyslipidemia, DR, CHD, CKD, and COPD, were performed to identify the possible sources of heterogeneity. Chi-square (χ2) tests were used to assess the differences across subgroups.[24,27] All tests were 2-sided and a P value of <.05 was considered significant.
3. Results
3.1. Search results
A total of 3341 articles were initially searched in this study. Of these, 124 full articles were shortlisted for eligibility assessment. Among the 124 articles, 48 were excluded for not reporting the prevalence of NAFLD in T2DM patients, 28 were excluded due to a sample size of <300, 18 were excluded for neither reporting excluding other causes of liver diseases nor reporting screening for alcohol excess, and 6 were excluded for repeated data. Finally, 24 eligible articles were included in this study (Fig. 1).
Figure 1.
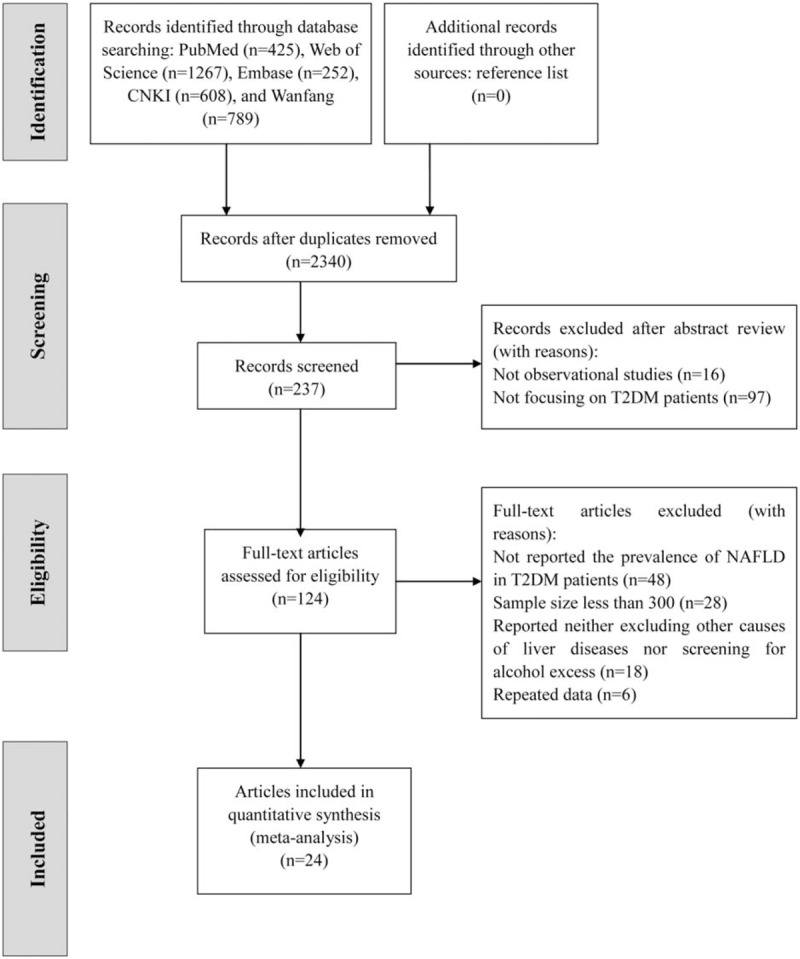
Flow chart of study identification and selection.
3.2. Study characteristics
Table 1 displays the characteristics of the 24 eligible studies conducted in 6 countries. A total of 35,599 T2DM patients were involved, of which 20,264 were identified with NAFLD. Also, among the 24 eligible studies, 3 were longitudinal and 21 were cross-sectional; 1 was community-based and 23 were facility-based; and 1 used the aminotransferase level from blood sample to make the diagnosis of NAFLD and 23 used ultrasound imaging. Moreover, according to the AHRQ quality assessment, 9 were considered low quality, 13 moderate quality, and 2 high quality.
Table 1.
Characteristics of eligible studies.
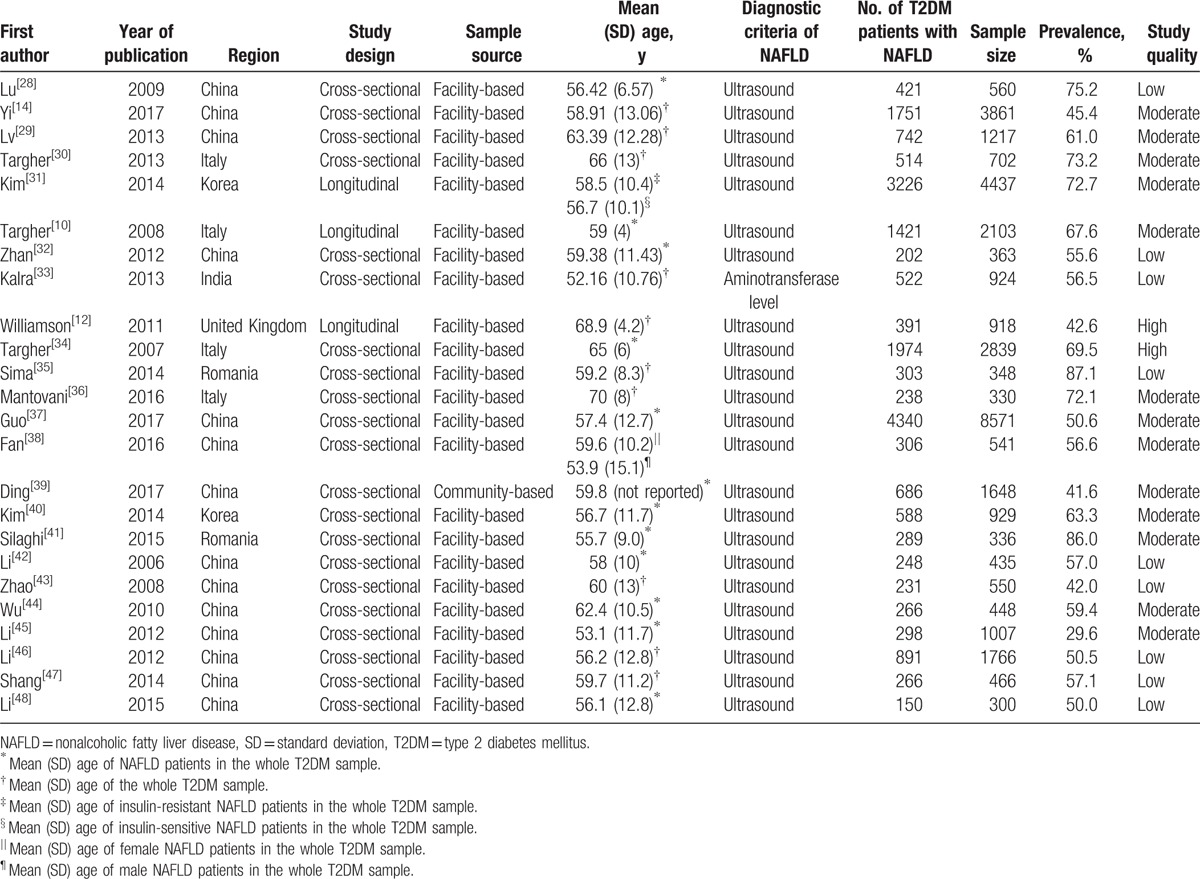
3.3. Pooled prevalence of NAFLD in T2DM patients
The reported prevalence of NAFLD in T2DM patients among the eligible studies ranged from 29.6%[45] to 87.1%.[35] Because significant heterogeneity (I2 = 99.0%, P < .001) was observed among the eligible studies, a random-effects model was used to estimate the pooled prevalence. Thus, the pooled prevalence of NAFLD in T2DM patients was 59.67% (95% CI: 54.31–64.92%). Figure 2 presents the details.
Figure 2.
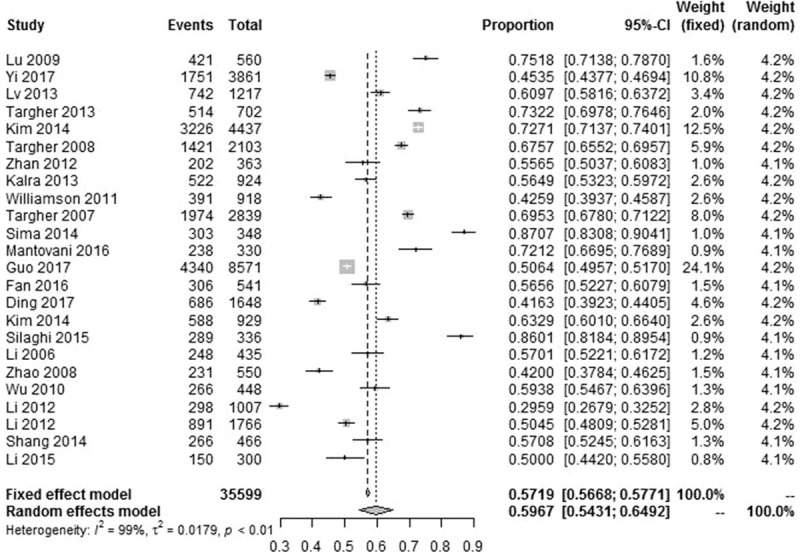
Forest plot presenting the prevalence of NAFLD in T2DM patients. NAFLD = nonalcoholic fatty liver disease, T2DM = type 2 diabetes mellitus.
3.4. Sensitivity analysis and publication bias
After excluding 9 articles with low quality, the pooled prevalence of NAFLD in T2DM increased slightly from 59.67% (95% CI: 54.31–64.92%) to 59.75% (95% CI: 52.76–66.54%), indicating low sensitivity of this meta-analysis. Besides, both the Begg test (z = 0.744, P = .457) and Egger test (t = 0.774, P = .447) showed low possibility of publication bias, and an Egger funnel plot for asymmetry was presented (Fig. 3).
Figure 3.
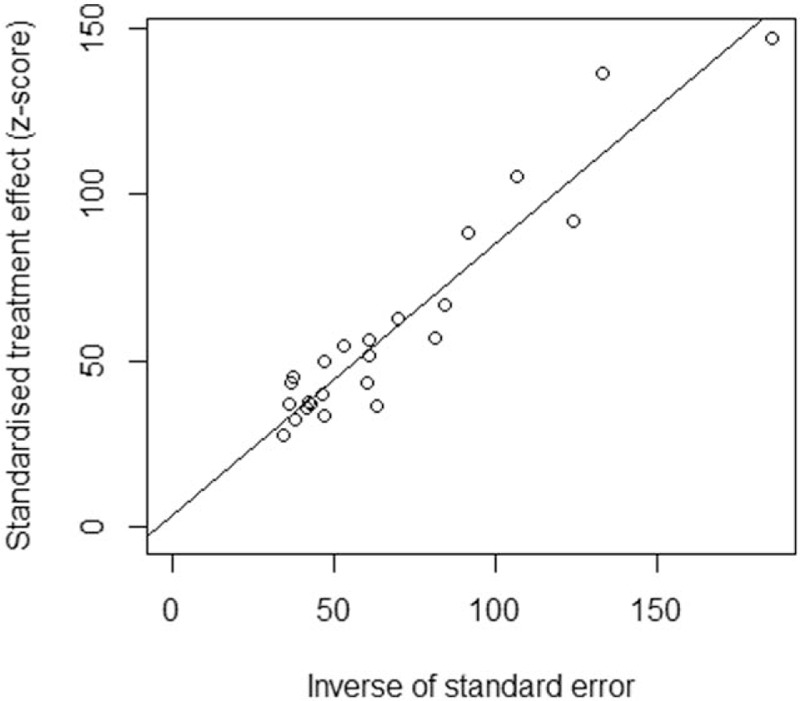
Egger funnel plot of the 24 eligible studies for this meta-analysis.
3.5. Subgroup analyses
Table 2 shows the results of subgroup analyses. The pooled prevalence of NAFLD in male and female T2DM patients was 60.11% (95% CI: 53.63–66.41%) and 59.35% (95% CI: 53.28–65.28%), respectively. The pooled prevalence of NAFLD in T2DM patients with and without obesity was 77.87% (95% CI: 65.51–88.14%) and 55.74% (95% CI: 30.35–79.63%), respectively. Furthermore, the pooled prevalence of NAFLD in T2DM patients with and without hypertension was 66.50% (95% CI: 57.63–74.82%) and 55.78% (95% CI: 49.06–62.39%), respectively.
Table 2.
Subgroup analyses of NAFLD in T2DM patients.
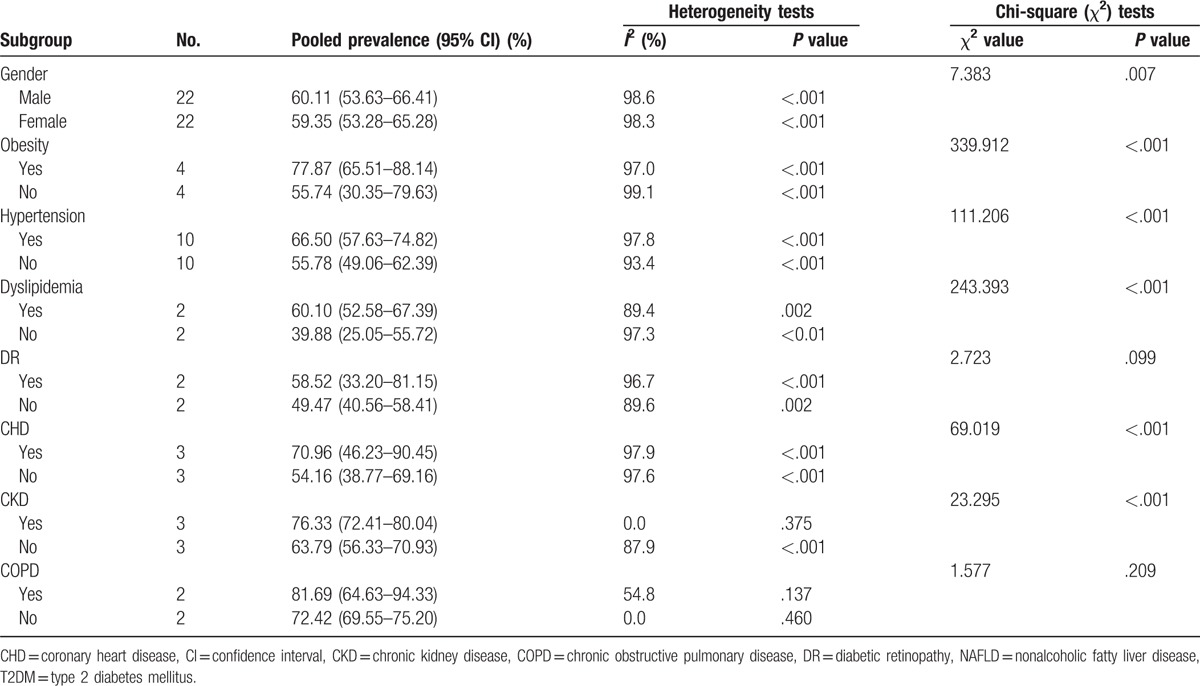
Subgroup analyses also indicated that the prevalence of NAFLD in T2DM differed by gender, obesity, hypertension, dyslipidemia, CHD, and CKD (P < .05). Specifically, the prevalence of NAFLD was significantly higher in T2DM patients with male gender (vs female gender), obesity (vs without obesity), hypertension (vs without hypertension), dyslipidemia (vs without dyslipidemia), CHD (vs without CHD), and CKD (vs without CKD). Besides, the heterogeneity was high in the whole population and most subgroups. However, the heterogeneity was quite low when estimating the pooled prevalence of NAFLD in T2DM patients with CKD (I2 = 0.0, P = .375) and without COPD (I2 = 0.0, P = .460).
4. Discussion
This meta-analysis provides the first quantitatively pooled prevalence of NAFLD in T2DM patients. Twenty-four eligible studies with a total of 35,599 T2DM patients were included, of which 20,264 were identified with NAFLD. The reported prevalence of NAFLD in T2DM patients ranged from 29.6%[45] to 87.1%[35] among the eligible studies, and this meta-analysis indicated that pooled prevalence of NAFLD in T2DM patients was 59.67% (95% CI: 54.31–64.92%).
The pooled prevalence of NAFLD in T2DM patients was much higher than that of hypercortisolism (3.4%, 95% CI: 1.5–5.9%), Cushing syndrome (1.4%, 95% CI: 0.4–2.9%), moderate hypoglycemia (45%, 95% CI: 34–57%), severe hypoglycemia (6%, 95% CI: 5–7%), and depression (17.6%) in T2DM patients in previous meta-analyses,[49–51] indicating that NAFLD is highly prevalent in T2DM patients, which could be attributed to the shared metabolic risk factors between NAFLD and T2DM.[5] The rapidly increasing prevalence of T2DM indicated in previous studies[13,52] and the high pooled prevalence of NAFLD in T2DM found in this meta-analysis significantly underscore the need for strengthening the management of T2DM, as well as the importance of early assessment of NAFLD in T2DM patients.
Subgroup analyses indicated that the prevalence of NAFLD was significantly higher in male T2DM patients than female T2DM patients. This finding is consistent with many previous studies. For example, Yi et al found that female gender was an independent protective factor for NAFLD, with the prevalence of NAFLD being 48.0% and 42.9% in male and female T2DM patients, respectively.[14] Additionally, the prevalence of NAFLD was higher in men than women in the Third National Health and Nutrition Examination Survey of the United States, which enrolled 12,454 adults 20 to 74 years old from 1988 to 1994.[53] Gender difference in the prevalence of NAFLD in T2DM patients could be attributed to the gender differences in hormone levels and lipid levels. Specifically, female hormones may play a potentially protective role in NAFLD,[54] and the triglyceride/high density lipoprotein cholesterol (TG/HDLC) ratio appeared to be higher in men than women.[14]
The clinical associations of NAFLD with the element of metabolic syndrome, including obesity, hypertension, and dyslipidemia have been well established.[33] For example, Yi et al found that body mass index and dyslipidemia were independent risk factors for NAFLD in T2DM,[14] and Leite et al found that the occurrence of NAFLD in T2DM was associated with obesity and hypertriglyceridemia.[16] Additionally, Ding et al found that T2DM patients with NAFLD had significantly higher levels of systolic blood pressure and diastolic blood pressure than T2DM patients without NAFLD.[39] Based on these findings, it is deduced that NAFLD may be a hepatic manifestation of metabolic syndrome.[3,4] Consistently, this study found that the prevalence of NAFLD in T2DM patients differed significantly with differences in sample characteristics, including obesity, hypertension, and dyslipidemia. Insulin resistance, which is highly shared in the element of metabolic syndrome, could, to a large extent, account for the associations of obesity, dyslipidemia, and hypertension with NAFLD in T2DM.[44] In this regard, integrated assessment and treatment strategies targeting obesity, dyslipidemia, hypertension, and NAFLD in T2DM patients are warranted.
Among the eligible studies exploring the association between microvascular complications and NAFLD, Kim et al found that the occurrence of DR and NAFLD were negatively correlated in Korean T2DM patients,[40] whereas Wu et al found contradictory result in Chinese T2DM patients.[44] This meta-analysis did not observe a significant association between DR and NAFLD by pooling the data of these 2 studies. Given the limited number of the included studies, more relevant studies with different ethnic populations are needed. However, significant association between CHD, an important component of macrovascular complications, and NAFLD in T2DM was observed in this study. It is suggested by Lu et al that this association may be explained by alanine aminotransferase (ALT), since the occurrence of these 2 diseases were both significantly associated with elevated ALT levels in T2DM.[28]
Additionally, subgroup analyses indicated that the prevalence of NAFLD differed significantly in T2DM patients with and without CKD. Targher et al found that the association between CKD and NAFLD in T2DM was independent of a wide range of confounding factors.[10] However, the causal link between CKD and NAFLD remains unclear, and it is hypothesized by Targher et al that this association may be explained by the release of several pathogenic mediators from the liver, such as the elevated reactive oxygen species and increased advanced glycated end-products.[10] More multicenter prospective studies with large sample size are needed to clarify the association between NAFLD and CKD.
Although this meta-analysis included 24 eligible studies, some limitations still need to be acknowledged. First, the heterogeneity in the whole population and most subgroups was high. However, the inclusion criteria of a sample size of at least 300 would enhance the representability of the samples in the included studies and hence obtain a more reliable pooled prevalence by this meta-analysis. Second, the majority of the eligible studies made the diagnosis of NAFLD by ultrasound imaging. Though previous studies have shown that the specificity and sensitivity of ultrasound imaging in detecting NAFLD were high,[55] the diagnosis of NAFLD was not confirmed by liver biopsy in most original studies, which is the gold standard. Therefore, some incorrect classification of participants with and without NAFLD on the basis of ultrasound imaging remains a possibility. Third, the sample source for most eligible studies was facility-based (ie, hospitalized patients and diabetic clinic patients) rather than population-based, which may cause selection bias when estimating the prevalence of NAFLD in T2DM patients. Therefore, more community-based studies are warranted. Fourth, though several studies indicated that adolescents were also likely to suffer from NAFLD and T2DM,[56] and age was not a restriction for this study, it was worth noting here that all samples included in this meta-analysis appeared to consist of adults, with mean ages from 52 to 70. Therefore, whether the findings of this study could be replicated to adolescents remains unclear. Also, though several studies indicated that age may be related to the occurrence of NAFLD in T2DM,[28] subgroup analysis according to age was unable to perform, since very few eligible studies categorized age using consistent cutoff points.
5. Conclusions
The pooled prevalence of NAFLD in T2DM patients was 59.67% (95% CI: 54.31–64.92%). For male and female T2DM patients, the pooled prevalence of NAFLD was 60.11% (95% CI: 53.63–66.41%) and 59.35% (95% CI: 53.28–65.28%), respectively. The prevalence of NAFLD in T2DM patients differed by gender, obesity, hypertension, dyslipidemia, CHD, and CKD. The findings of this study significantly underline the need for early assessment of NAFLD and the importance of strengthening the management of NAFLD in T2DM patients. Furthermore, more population-based studies with diverse sample characteristics are warranted.
Acknowledgments
The authors are grateful to the authors of all the eligible studies included in this meta-analysis. The authors are also thankful to the Natural Science Foundation of Hunan Province (2017JJ3460) and the Finance Department of Hunan Province ([2016]62) for the support.
Footnotes
Abbreviations: AHRQ = Agency for Healthcare Research and Quality, ALT = alanine aminotransferase, CHD = coronary heart disease, CI = confidence interval, CKD = chronic kidney disease, CNKI = Chinese National Knowledge Infrastructure, COPD = chronic obstructive pulmonary disease, DR = diabetic retinopathy, NAFLD = nonalcoholic fatty liver disease, NASH = nonalcoholic steatohepatitis, PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analyses, SD = standard deviation, T2DM = type 2 diabetes mellitus, TG/HDLC = triglyceride/high density lipoprotein cholesterol.
This study is supported by the Natural Science Foundation of Hunan Province (2017JJ3460) and the Finance Department of Hunan Province ([2016]62).
The authors report no conflicts of interest.
References
- [1].Younossi ZM, Koenig AB, Abdelatif D, et al. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016;64:73–84. [DOI] [PubMed] [Google Scholar]
- [2].Adams LA, Lymp JF, St Sauver J, et al. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology 2005;129:113–21. [DOI] [PubMed] [Google Scholar]
- [3].Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology 2012;55:2005–23. [DOI] [PubMed] [Google Scholar]
- [4].Marchesini G, Brizi M, Bianchi G, et al. Nonalcoholic fatty liver disease: a feature of the metabolic syndrome. Diabetes 2001;50:1844–50. [DOI] [PubMed] [Google Scholar]
- [5].Forlani G, Giorda C, Manti R, et al. The burden of NAFLD and its characteristics in a nationwide population with type 2 diabetes. J Diabetes Res 2016;2016:2931985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Portillo-Sanchez P, Bril F, Maximos M, et al. High prevalence of nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus and normal plasma aminotransferase levels. J Clin Endocrinol Metab 2015;100:2231–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Leite NC, Villela-Nogueira CA, Pannain VL, et al. Histopathological stages of nonalcoholic fatty liver disease in type 2 diabetes: prevalences and correlated factors. Liver Int 2011;31:700–6. [DOI] [PubMed] [Google Scholar]
- [8].Younossi ZM, Gramlich T, Matteoni CA, et al. Nonalcoholic fatty liver disease in patients with type 2 diabetes. Clin Gastroenterol Hepatol 2004;2:262–5. [DOI] [PubMed] [Google Scholar]
- [9].Targher G, Bertolini L, Rodella S, et al. Nonalcoholic fatty liver disease is independently associated with an increased incidence of cardiovascular events in type 2 diabetic patients. Diabetes Care 2007;30:2119–21. [DOI] [PubMed] [Google Scholar]
- [10].Targher G, Bertolini L, Rodella S, et al. Non-alcoholic fatty liver disease is independently associated with an increased prevalence of chronic kidney disease and proliferative/laser-treated retinopathy in type 2 diabetic patients. Diabetologia 2008;51:444–50. [DOI] [PubMed] [Google Scholar]
- [11].Almobarak AO, Barakat S, Suliman EA, et al. Prevalence of and predictive factors for nonalcoholic fatty liver disease in Sudanese individuals with type 2 diabetes: Is metabolic syndrome the culprit? Arab J Gastroenterol 2015;16:54–8. [DOI] [PubMed] [Google Scholar]
- [12].Williamson RM, Price JF, Glancy S, et al. Prevalence of and risk factors for hepatic steatosis and nonalcoholic fatty liver disease in people with type 2 diabetes: the Edinburgh Type 2 Diabetes Study. Diabetes Care 2011;34:1139–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Alharbi NS, Almutari R, Jones S, et al. Trends in the prevalence of type 2 diabetes mellitus and obesity in the Arabian Gulf States: systematic review and meta-analysis. Diabetes Res Clin Pract 2014;106:e30–3. [DOI] [PubMed] [Google Scholar]
- [14].Yi M, Chen RP, Yang R, et al. Increased prevalence and risk of non-alcoholic fatty liver disease in overweight and obese patients with Type 2 diabetes in South China. Diabetic Med 2017;34:505–13. [DOI] [PubMed] [Google Scholar]
- [15].Dvorak K, Hainer R, Petrtyl J, et al. The prevalence of nonalcoholic liver steatosis in patients with type 2 diabetes mellitus in the Czech Republic. Biomed Pap 2015;159:442–8. [DOI] [PubMed] [Google Scholar]
- [16].Leite NC, Salles GF, Araujo ALE, et al. Prevalence and associated factors of non-alcoholic fatty liver disease in patients with type-2 diabetes mellitus. Liver Int 2009;29:113–9. [DOI] [PubMed] [Google Scholar]
- [17].Chen X, Li L, Zhou T, et al. Prevalence of hypertension in rural areas of china: a meta-analysis of published studies. PloS One 2014;9:e115462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ho NS, Olds T, Schranz N, et al. Secular trends in the prevalence of childhood overweight and obesity across Australian states: A meta-analysis. J Sci Med Sport 2017;20:480–8. [DOI] [PubMed] [Google Scholar]
- [19].Tabatabaei-Malazy O, Qorbani M, Samavat T, et al. Prevalence of dyslipidemia in Iran: a systematic review and meta-analysis study. Int J Prev Med 2014;5:373–93. [PMC free article] [PubMed] [Google Scholar]
- [20].Yang LS, Zhang ZH, Sun L, et al. Prevalence of suicide attempts among college students in China: a meta-analysis. PloS One 2015;10:e0116303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Hu J, Dong Y, Chen X, et al. Prevalence of suicide attempts among Chinese adolescents: a meta-analysis of cross-sectional studies. Compr Psychiatr 2015;61:78–89. [DOI] [PubMed] [Google Scholar]
- [22].Ades AE, Lu G, Higgins JP. The interpretation of random-effects meta-analysis in decision models. Med Decis Making 2005;25:646–54. [DOI] [PubMed] [Google Scholar]
- [23].Li Y, Li Y, Cao J. Factors associated with suicidal behaviors in mainland China: a meta-analysis. BMC Public Health 2012;12:524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Han C, Zhang M, Luo X, et al. Secular trends in the prevalence of type 2 diabetes in adults in China from 1995 to 2014: a meta-analysis. J Diabetes 2017;9:450–61. [DOI] [PubMed] [Google Scholar]
- [25].Dai W, Chen L, Lai Z, et al. The incidence of post-traumatic stress disorder among survivors after earthquakes: a systematic review and meta-analysis. BMC Psychiatr 2016;16:188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088–101. [PubMed] [Google Scholar]
- [27].He Q, Peng WJ, Zhang JQ, et al. Prevalence of unprotected anal intercourse and unprotected vaginal intercourse among HIV-positive men who have sex with men in China: a meta-analysis. Sex Transm Infect 2012;88:229–33. [DOI] [PubMed] [Google Scholar]
- [28].Lu H, Zeng L, Liang B, et al. High prevalence of coronary heart disease in type 2 diabetic patients with non-alcoholic fatty liver disease. Arch Med Res 2009;40:571–5. [DOI] [PubMed] [Google Scholar]
- [29].Lv W-S, Sun R-X, Gao Y-Y, et al. Nonalcoholic fatty liver disease and microvascular complications in type 2 diabetes. World J Gastroenterol 2013;19:3134–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Targher G, Mantovani A, Pichiri I, et al. Non-alcoholic fatty liver disease is associated with an increased prevalence of atrial fibrillation in hospitalized patients with Type 2 diabetes. Clin Sci 2013;125:301–9. [DOI] [PubMed] [Google Scholar]
- [31].Kim S-K, Choi YJ, Huh BW, et al. Nonalcoholic fatty liver disease is associated with increased carotid intima-media thickness only in type 2 diabetic subjects with insulin resistance. J Clin Endocrinol Metab 2014;99:1879–84. [DOI] [PubMed] [Google Scholar]
- [32].Zhan Y-T, Zhang C, Li L, et al. Non-alcoholic fatty liver disease is not related to the incidence of diabetic nephropathy in type 2 diabetes. Int J Mol Sci 2012;13:14698–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Kalra S, Vithalani M, Gulati G, et al. Study of prevalence of nonalcoholic fatty liver disease (NAFLD) in type 2 diabetes patients in India (SPRINT). J Assoc Physicians India 2013;61:448–53. [PubMed] [Google Scholar]
- [34].Targher G, Bertolini L, Padovani R, et al. Prevalence of nonalcoholic fatty liver disease and its association with cardiovascular disease among type 2 diabetic patients. Diabetes Care 2007;30:1212–8. [DOI] [PubMed] [Google Scholar]
- [35].Sima A, Timar R, Vlad A, et al. Nonalcoholic fatty liver disease: a frequent condition in type 2 diabetic patients. Wien Klin Wochenschr 2014;126:335–40. [DOI] [PubMed] [Google Scholar]
- [36].Mantovani A, Rigamonti A, Bonapace S, et al. Nonalcoholic fatty liver disease is associated with ventricular arrhythmias in patients with type 2 diabetes referred for clinically indicated 24-hour Holter monitoring. Diabetes Care 2016;39:1416–23. [DOI] [PubMed] [Google Scholar]
- [37].Guo K, Zhang L, Lu J, et al. Non-alcoholic fatty liver disease is associated with late but not early atherosclerotic lesions in Chinese inpatients with type 2 diabetes. J Diabetes Complications 2017;31:80–5. [DOI] [PubMed] [Google Scholar]
- [38].Fan N, Zhang L, Xia Z, et al. Sex-specific association between serum uric acid and nonalcoholic fatty liver disease in type 2 diabetic patients. J Diabetes Res 2016;2016:3805372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Ding X, Xu Y, Wang Y, et al. Nonalcoholic fatty liver disease and associated metabolic risks of hypertension in type 2 diabetes: a cross-sectional community-based study. Int J Endocrinol 2017;2017:526256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Kim BY, Jung CH, Mok JO, et al. Prevalences of diabetic retinopathy and nephropathy are lower in Korean type 2 diabetic patients with non-alcoholic fatty liver disease. J Diabetes Invest 2014;5:170–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Silaghi CA, Silaghi H, Craciun AE, et al. Age, abdominal obesity, and glycated hemoglobin are associated with carotid atherosclerosis in type 2 diabetes patients with nonalcoholic fatty liver disease. Med Ultrason 2015;17:300–7. [DOI] [PubMed] [Google Scholar]
- [42].Li W, Xiang H, Ding DWX, et al. The clinical analysis of nonalcoholic fatty liver and its correlation factors in type 2 diabetes mellitus. Chin J Diabetes 2006;14:35–6. [Google Scholar]
- [43].Zhao L, Shrestha D, Zhang X, et al. A retrospective clinical analysis of prevalence of non-alcoholic fatty liver disease in inpatients with type 2 diabetes mellitus. Chin J Diabetes 2008;16: 535, 562. [Google Scholar]
- [44].Wu JC, Li XH, Peng YD, et al. Non-alcoholic fatty liver disease is associated with chronic kidney disease and diabetic retinopathy in type 2 diabetic patients. Chin J Pract Intern Med 2010;30:41–3. [Google Scholar]
- [45].Li MZ, Hao JY, Sun LR. Relationship between serum uric acid and nonalcoholic fatty liver in patients with type 2 diabetes. Chin J Endocrinol Metab 2012;28:215–6. [Google Scholar]
- [46].Li YJ, Su HY, Li SQ. Prevalence and characteristics of non-alcoholic fatty liver disease in patients with type 2 diabetes mellitus. Guangxi Med J 2012;34:86–8. [Google Scholar]
- [47].Shang HX, Li H, Yang ZG, et al. Associations between nonalcoholic fatty liver disease and renal injury in patients with type 2 diabetes. J Med Forum 2014;35:10–2. [Google Scholar]
- [48].Li CX, Liu JF, Shi L, et al. Risk factors of non-alcoholic fatty liver disease in patients with incipient type 2 diabetes mellitus. Guangxi Med J 2015;37:973–5. [Google Scholar]
- [49].Steffensen C, Pereira AM, Dekkers OM, et al. Diagnosis of endocrine disease: prevalence of hypercortisolism in type 2 diabetes patients: a systematic review and meta-analysis. Eur J Endocrinol 2016;175:R247–53. [DOI] [PubMed] [Google Scholar]
- [50].Edridge CL, Dunkley AJ, Bodicoat DH, et al. Prevalence and incidence of hypoglycaemia in 532,542 people with type 2 diabetes on oral therapies and insulin: a systematic review and meta-analysis of population based studies. PloS One 2015;10:e0126427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Ali S, Stone MA, Peters JL, et al. The prevalence of co-morbid depression in adults with Type 2 diabetes: a systematic review and meta-analysis. Diabetic Med 2006;23:1165–73. [DOI] [PubMed] [Google Scholar]
- [52].Adeloye D, Ige JO, Aderemi AV, et al. Estimating the prevalence, hospitalisation and mortality from type 2 diabetes mellitus in Nigeria: a systematic review and meta-analysis. BMJ Open 2017;7:e015424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Lazo M, Hernaez R, Eberhardt MS, et al. Prevalence of nonalcoholic fatty liver disease in the United States: the Third National Health and Nutrition Examination Survey, 1988–1994. Am J Epidemiol 2013;178:38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Moon SS. Relationship between serum uric acid level and nonalcoholic fatty liver disease in pre- and postmenopausal women. Ann Nutr Metab 2013;62:158–63. [DOI] [PubMed] [Google Scholar]
- [55].Saverymuttu SH, Joseph AE, Maxwell JD. Ultrasound scanning in the detection of hepatic fibrosis and steatosis. Br Med J 1986;292:13–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Liu JQ, Zhang ZJ, Wang W, et al. Risk factors for non-alcoholic fatty liver disease combined with type 2 diabetes mellitus in adolescents. World Chin J Digestol 2015;23:1812–7. [Google Scholar]


