Supplemental Digital Content is available in the text
Keywords: fracture, fracture healing, meta-analysis, ultrasonic therapy
Abstract
Background:
Low-intensity pulsed ultrasonography (LIPUS) is a form of mechanical stimulation that is delivered via a special device to the fracture site for the acceleration of fracture healing. We conducted a meta-analysis to assess the effect of LIPUS for fresh fractures in adults.
Methods:
MEDLINE, EMBASE and the Cochrane Library searched between Jan 1980 and Nov 2016. Studies should be quasi-randomized and randomized controlled trials (RCTs) comparing treatment with LIPUS to placebo or no treatment in adults with fresh fractures, reporting outcomes such as function; time to union; delayed union or non-union. Summary standard mean difference (SMD) and the risk ratio (RR) with their 95% confidence interval (CI) calculated with a random effects model. I2 statistic was used to assess the heterogeneity. Risk of bias was assessed by the Cochrane risk-of-bias tool. The GRADE system was used to evaluate the evidence quality.
Results:
A total of 12 trials with 1099 patients were included. The pooled results showed that LIPUS significantly reduced the time to fracture union (SMD: 0.65, 95% CI: 1.13 to 0.17), improved the quality of life (SMD: 0.20, 95% CI: 0.03–0.37) without affecting the time to full weight bearing (SMD: 0.76, 95% CI: 1.92 to 0.4), the time to return to work (SMD: 0.06, 95% CI: 0.14 to 0.27), or the incidence rate of delayed union and nonunion (RR: 1.02, 95% CI: 0.60–1.74).
Conclusions:
Moderate-to-high quality evidence shows that LIPUS treatment reduces the time to fracture union and improves the quality of life without affecting functional recovery and incident rate of delayed union and nonunion, suggesting that LIPUS treatment may be a good treatment modality for adults with fresh fractures. However, there are some methodological limitations in the eligible trials, further studies are needed to determine the clinical circumstances under which LIPUS is truly valid and to examine the optimal approach for the use of this adjunctive therapy.
1. Introduction
Fractures are common. In the United States, approximately 5.6 million fractures occur each year.[1] In England, the fracture incidence for all age groups is 3.6% every year.[2] Moreover, it is suggested that >33% of people will experience a fracture in their life time.[3] Meanwhile, 5% to 10% of these fractures show delayed healing or nonunion.[4] The delay unions or nonunions often require further intervention and may cause serious complications, such as pain and functional limitations.[5] Reoperation is usually necessary to promote bone healing, and it is invasive and expensive. To reduce the substantial risk of disability and the socioeconomic costs, the development of a method for accelerating fracture healing is becoming more and more important.[6,7]
Low-intensity pulsed ultrasonography (LIPUS) is a safe and effective noninvasive adjunctive therapy used to promote the bone healing process.[8–10] Although the underlying mechanism of LIPUS treatment on fracture healing remains unclear,[11] it is suggested that LIPUS accelerates fracture healing through multiple avenues,[12] such as stimulating signal transduction, blood flow, angiogenesis, and osteogenic gene expression.[13] Now, we do know that LIPUS can cause pressure waves at the fracture site, and this mechanical signal converts to a biochemical signal inside the cells, through the expression of cyclooxygenase-2 gene,[14] induces the production of cyclooxygenase-2.[15] The enhanced cyclooxygenase-2 level further induces the production of prostaglandin E2.[16] Accordingly, this drives the expression of osteogenic genes, such as c-fos[17] and aggrecan.[18] These osteogenic genes help to heal the fracture by enhancing endochondral ossification. The US Food and Drug Administration (FDA) approved the use of LIPUS for accelerating healing of fresh fractures in 1994.[19] Since then, several clinical trials have been designed to investigate the effect of LIPUS on bone healing. However, its effectiveness is controversial. Recently, several meta-analyses published on this topic determined that that LIPUS reduces the time to fracture healing.[20–25] However, none of these meta-analyses offered definitive conclusions. All these meta-analyses identified the need for additional trials. Moreover, a number of the prior meta-analyses focusing on the effectiveness of LIPUS on fracture treatment were limited by loose inclusion criteria, inappropriate evaluation techniques, and a focus only on radiographic healing over other patient-important outcomes, such as functional recovery and the incident rate of delayed union and nonunion.
To provide a high-quality evidence for the use of LIPUS in clinical practice, we conducted a meta-analysis of quasi-randomized and randomized controlled trials (RCTs), comparing the different effects between LIPUS treatment and placebo (sham LIPUS or none), for fresh fractures in adults, with the time to fracture union, functional recovery, the incidence rate of delayed union and nonunion, the time to full weight bearing, and the time to return to work as the outcomes.
2. Methods
This study did not involve human or animal experiments, and thus, ethical approval was not necessary. Two authors (SHL and HCL) independently performed the meta-analysis. Any disagreements were resolved through discussion and sometimes by seeking an independent third author (LCZ).
2.1. Protocol and registration
This meta-analysis was performed according to the recommendations of the Cochrane Handbook, was reported on the basis of the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines[26] and was registered on the PROSPERO International prospective register of systematic reviews (CRD42016042068).
2.2. Eligibility criteria
2.2.1. Participants
Adults with all types of fresh fractures were included. Fresh fractures were fractures within 2 weeks. Patients with post-corticotomy (eg, osteotomy and distraction osteogenesis) were excluded.
2.2.2. Interventions
The intervention was LIPUS with a precise definition, and the LIPUS had to meet the following conditions[27]: frequency: 1.5 MHz; form: pulsed; impulse length: 200 μs; signal repetition frequency: 1 kHz; and intensity: 30 mW/cm2. We also included trials where LIPUS was used as an adjunctive therapy to nonsurgical or surgical treatments.
2.2.3. Comparators
LIPUS could be the only treatment or an adjunctive therapy, and the comparison was a placebo (sham ultrasound) or no additional treatment. Trials comparing LIPUS with other interventions were excluded.
2.2.4. Outcomes
The outcomes included the time to fracture union (days), functional recovery (score), the incidence rate of delayed union (%) and nonunion (%), the time to full weight bearing, and the time to return to work (days).
2.2.5. Study design
All of the relevant RCTs and quasi-RCTs were included. The quasi-RCTs were trials using a quasi-random method (eg, allocation by date of birth, day of the week, medical record number, or month of the year) of allocating the participants to the different interventions.
2.2.6. Time
Studies of any duration conducted at any time were included.
2.2.7. Other
Unpublished and published studies that were written in any language were included. For our study, unpublished data means data not published as an article. The unpublished data include meeting abstracts, supplementary materials, and data published on ClinicalTrials.gov.
2.3. Search strategy
We systematically searched MEDLINE, Embase, and the Cochrane Library from January 1, 1980 until November 1, 2016, with no language restrictions. We also searched Google scholar (www.scholar.google.com), ClinicalTrials.gov registry, (www.clinicaltrials.gov) and screened the references of both retrieved articles and relevant reviews to further identify potentially eligible trials. The search strategies were developed using text words as well as medical subject headings (MeSH) associated with the terms relevant to “fracture healing,” “fracture,” “ultrasonic therapy,” and “ultrasonography” together with “randomized control trial.” The full search strategies used in the MEDLINE, Embase, and the Cochrane Library databases are provided in Supplemental Digital Content (SDC) 1.
2.4. Study selection
Our search records were imported into ENDNOTE X7 reference management software, and the duplicate records were removed both electronically and manually. After excluding the duplicate and apparently irrelevant articles, the remaining studies were further reviewed by reading the full text to assess the eligibility for inclusion. After completion, both of the authors met and reviewed their selections for agreement.
2.5. Data extraction
A standard data extraction form was created using Microsoft Excel 2016 to collect the data of interest. The major categories of the variables to be coded were as follows: study characteristics, participant characteristics, and outcome characteristics.
2.6. Risk of bias assessment
The risk of bias of the individual studies was assessed by the Cochrane risk-of-bias tool.[28] The Cochrane risk-of-bias tool is an evaluation scale that is recommended by the Cochrane handbook for RCTs, and it assesses the bias across the following 7 aspects: random-sequence generation, allocation concealment, blinding of the participants and personnel, blinding of the outcome assessment, incomplete outcome data, selective reporting, and other biases. Each aspect could further be classified as a low, high, or unclear risk. For the study design, we assessed random-sequence generation, allocation concealment, blinding of the participants, and outcome reporting. For each outcome, we assessed blinding of the outcome assessors and loss to follow-up.
2.7. Outcomes
The outcomes of this study included the time to fracture union, quality of life, functional recovery, and the incidence rate of delayed union and nonunion. For the outcome of the time to fracture union, both the radiographic and the clinical union were used to define a healed fracture, which are the widely acceptable definitions in the literature. In our study, fracture union[29] was defined as follows: a callus is present bridging at least 3 of 4 cortices on orthogonal radiographs (radiographic union) or there is no pain, tenderness, or movement at the fracture site (clinical union). The outcome of quality of life was measured by the short form-36 (SF-36) physical component summary scores.[30–33] The outcome of function recovery was measured by the time to return to work and the time to full weight bearing. The time to return to work was defined as the time to return to work without limitations (return to the level before their injury). The time to full weight bearing was defined as when the patients were able to place all their weight on the operated extremity. The incidence rate of the delayed union and nonunion was diagnosed according to the guidelines of the different fractures.
2.8. Synthesis of the results
We pooled the treatment effects of LIPUS with same outcomes across the included trials. The continuous outcomes are expressed as the standardized mean differences (SMD) and the 95% confidence interval (CI), using the generic inverse variance methods. The dichotomous outcomes are expressed as the risk ratios (RRs) and the 95% CI, using the Mantel-Haenszel method.
For the dichotomous outcomes, we extracted the original data regarding the events (ne) and the total number (nt) in both the experimental group and the control group. For the continuous outcomes, we extracted the original data, including the mean, standard deviation (SD), and the total number (n) in both the experimental group and the control group. If the original data was not available, we calculated the data through the available coefficients. For example, we computed the mean from the median and the SD for the standard error (SE), the interquartile range (IQR) or the P values, according to the methods described in the Cochrane Handbook.
The meta-analysis was performed using a random-effects model, which provided more conservative estimated effects. Cochrane Q statistic, the I2 statistic (I2 >50% as a threshold indicates significant heterogeneity), and P values (P < .10 as a threshold indicates significant heterogeneity) were used to assess the heterogeneity.[34] Review Manager (version 5.3) and Comprehensive Meta-Analysis (version 2.0) were used for the statistical analysis.
As different fracture types were pooled for analysis in this study, we further performed preplanned subgroup analyses to explore the sources of heterogeneity according to the following categories: upper versus lower limb fractures, operative versus conservative management, and radiographic versus clinical union. Subgroup analyses were also used to evaluate the relationship between the duration of the treatment and the outcomes. Sensitivity analyses were performed to examine the robustness of our analysis by omitting specific trials from the overall analysis. Publication bias was assessed by funnel plots and the Egger test,[35] when sufficient trials (no less than 10) were identified.
The quality of the evidence for the outcomes was assessed according to the guidelines of the Grading of Recommendations Assessment, Development and Evaluation (GRADE)[36] for risk of bias, inconsistency, indirectness, imprecision, and publication bias. Each assessment result was rated as very low, low, moderate, or high. Summary tables were constructed using the GRADE Profiler (version 3.6).
3. Results
3.1. Search results
A total of 925 articles were obtained through electronic and hand searches. After 59 duplicates were removed, the titles and abstracts of 866 records were reviewed, 844 records were excluded for not meeting the inclusion criteria, and thus, the remaining 22 articles were retrieved for further assessment. Two trials[37,38] were excluded because the treatment was not LIPUS but was high-intensity focused ultrasound. Two trials[39,40] were excluded because the fracture was a stress fracture and not a fresh fracture. The other 6 trials[41–46] were excluded because these trials reported the same data. Finally, 12 trials[47–58] fulfilled our inclusion criteria and were included in our meta-analysis (Fig. 1).
Figure 1.
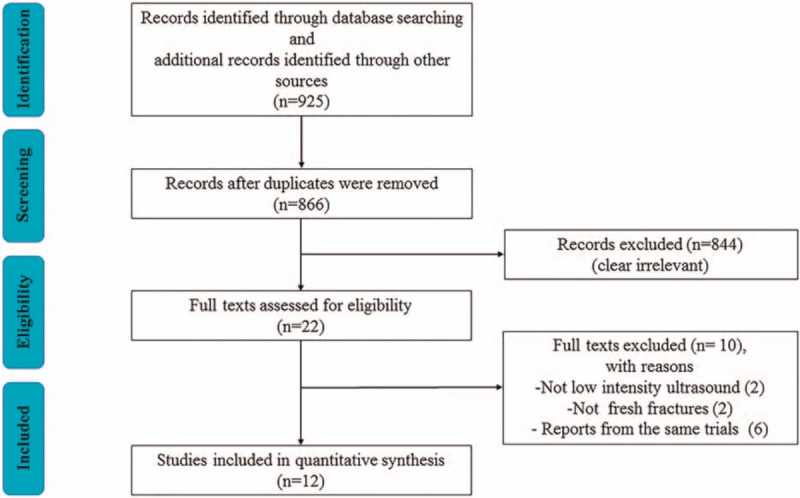
Flow diagram showing the process of literature selection.
3.2. Study characteristics
The main characteristics of the included trials are summarized in Table 1. These trials were published between 1994 and 2016. The sample sizes ranged from 20 to 501, with a total of 1099 patients.
Table 1.
Characteristics of the included RCTs.
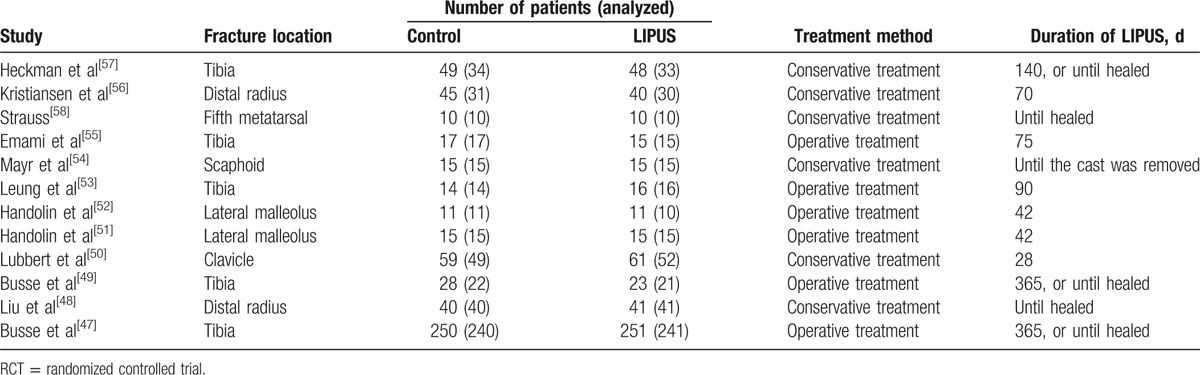
The duration of the LIPUS treatment lasted from 28 to 365 days. The LIPUS treatment was used for 20 minutes a day except in 2 trials.[48,58] In one of these trials,[48] the LIPUS treatment was used for 15 minutes every day, and in the other one,[58] the LIPUS treatment was used 2 times a day for 20 minutes each. All of the included studies demonstrated good compliance with the LIPUS treatment. No trials reported complications or adverse events associated with the LIPUS treatment. The start time of LIPUS treatment varied in the different studies. Most of the included trails did not report the specific time, whereas in clinical practice, most patients received LIPUS within 7 days. Moreover, previous studies have proved that the effect of LIPUS showed no significant differences within 6 weeks.[59]
For the included trials, 5 trials[48,50,54,56,57] used the conservative treatment, and the conservative treatment for these 5 trials used plaster of Paris to maintain reduction. The plaster was not removed when LIPUS was used. A cast window was created in the dorsal area of the cast over the site of the fracture, and an ultrasonic probe was applied on the fracture site though the window. The fracture could remain stable during the LIPUS treatment. For operative cases, the LIPUS device was applied to the area of the fracture using a probe directly on the skin.
3.3. Risk of bias assessment
Figure 2 summarizes the details of the risk of bias. One trial[58] was published only as a structured abstract with no sufficient information to assess the risk of bias, and thus, most of the 7 domains were classified as an unclear risk. For the other 11 trials,[47–57] we carefully assessed the risk of bias. Random sequence generation was reported in all of the trials except 1[53] that was a quasi-RCT. One trial[53] had a high risk of allocation concealment because of the quasi-RCT design, and 2 trials[48,54] did not adequately report the allocation concealment. The treatment was not blinded to the participants[48,54] and was not adequately reported by 1 trial,[53] which might lead to a potential performance bias. However, whether or not the participants were blinded had a limited impact on the time to fracture union. Blinding of the outcome assessment was adequately reported in all of the 8 trials except 1.[53] Three trials[51,56,57] had a high risk of attrition bias because of a high loss of follow-up (over 20%). Only two trials[47,49] had a low risk of reporting bias, because most of the included trials did not have protocols. Most of the included trials[48,50–54,56–58] had a potential risk of other biases because of the fact that the baseline age, the gender, or the smoking status were not clearly reported.
Figure 2.
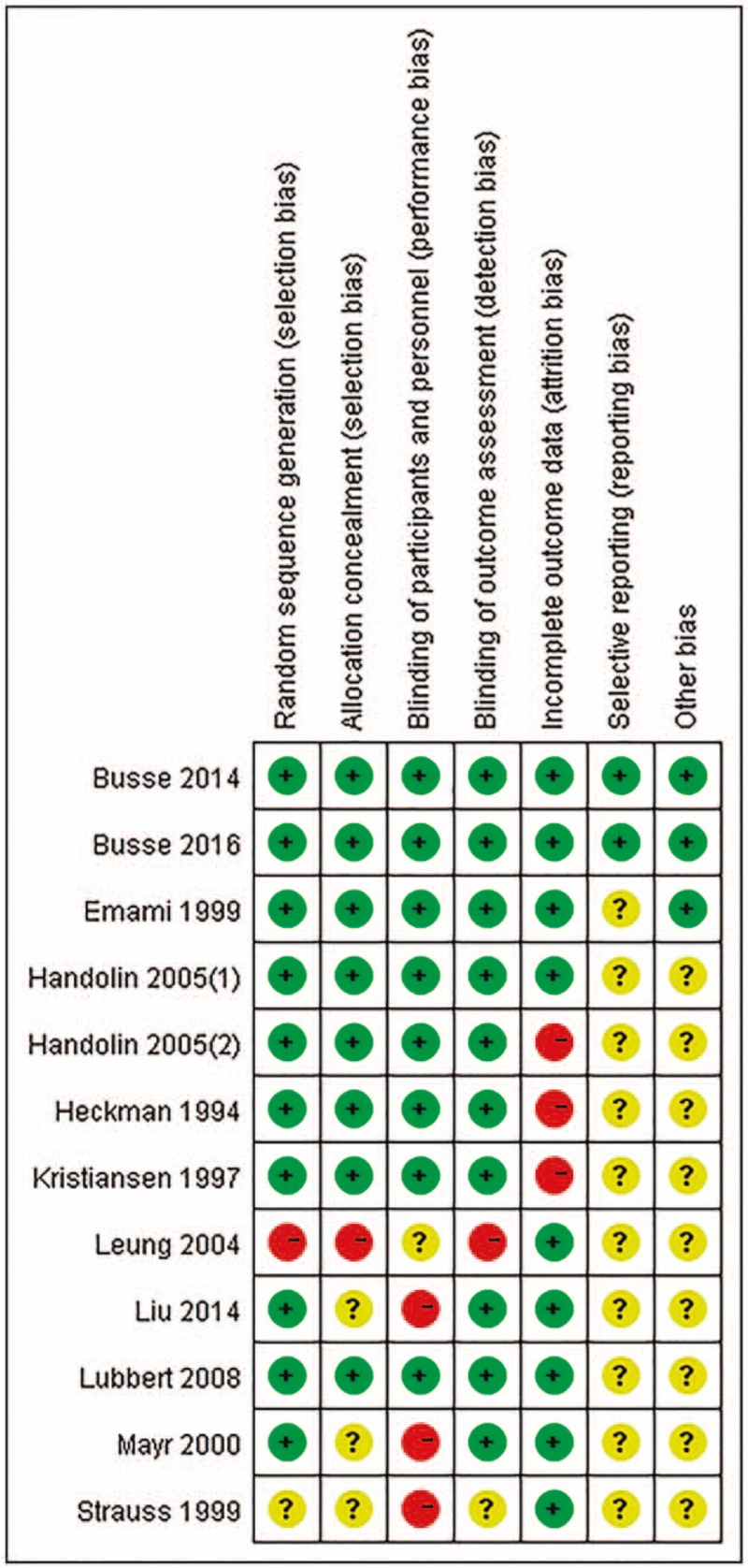
Risk of bias graph. Risk of bias summary. “+” means low risk; “?” means unclear risk and “-” means high risk.
3.4. Time to fracture union
Eleven trials,[47–57] including 887 patients, reported the time to fracture union. Both radiographic and clinical unions were used to define the fracture union. Nine trials[47,49,51–57] reported the time to radiographic union, and the radiographic union was measured by radiography[47,51,53,55–57] or computed tomography (CT).[52,54] Two trials[48,50] reported the time to clinical union. There were significant differences in the time to fracture union between the LIPUS and placebo groups (SMD: −0.65, 95% CI: −1.13 to −0.17, P < .01, I2 = 89%; Fig. 3). Sensitivity analyses were performed to examine the robustness of our analysis by omitting each study in turn, and the pooled SMD was not affected (Fig. S1).
Figure 3.
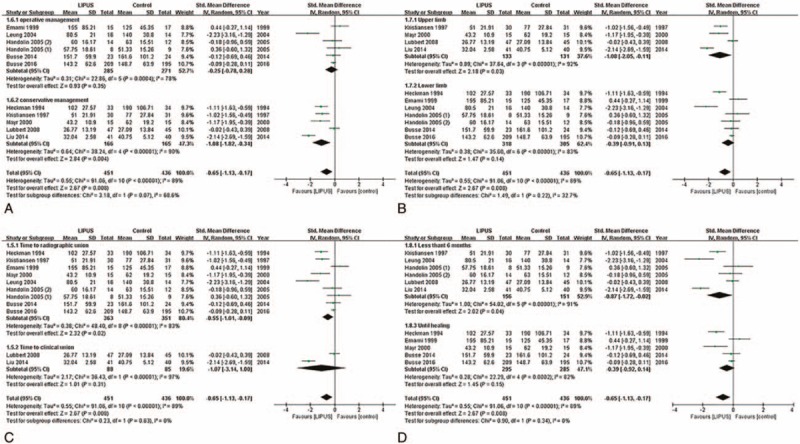
Forest plot for the time to fracture union. (A) Operative and conservative management; (B) upper and lower limb; (C) radiological union and clinical union; (D) time less than 6 mo and time until healing.
The first subgroup analysis was performed using the different treatments (Fig. 3A). The LIPUS treatment was effective for fractures treated with conservative management (SMD: −1.08, 95% CI: −1.82 to −0.34, P < .01, I2 = 90%) but not for those treated with operative management (SMD: −0.25, 95% CI: −0.78 to 0.28, P = .35, I2 = 78%). However, the tests for the subgroup differences did not indicate that the results of conservatively managed fractures and operatively managed fractures were statistically significantly different from each other (P = .07 for interaction).
The second subgroup analysis was performed based on the upper limb and lower limb (Fig. 3B). The results suggested that LIPUS treatment was effective on upper limb fractures (SMD: −1.08, 95% CI: −2.05 to −0.11, P = .03, I2 = 92%), but not for lower limb fractures (SMD: −0.39, 95% CI: −0.91 to 0.13, P = .14, I2 = 83%). However, the test for subgroup differences indicated that the findings from the upper and lower limb subgroups were not statistically significantly different from each other (P = .22 for interaction).
Moreover, a subgroup analysis was performed to determine whether the different definitions of fracture union could affect the results (Fig. 3C). Although the effect of the LIPUS treatment was different between the time to radiographic union (SMD: −0.55, 95% CI: −1.01 to −0.09, P = .02, I2 = 83%) and the time to clinical union (SMD: −1.07, 95% CI: −3.14 to 1, P = .31, I2 = 97%), the test for subgroup differences did not indicate that the results were statistically significantly different from each other (P = 0.63 for interaction).
Finally, we performed a subgroup analysis to evaluate the relationship between the duration of the LIPUS treatment and the time to fracture union (Fig. 3D). The results demonstrated that when the duration was <6 months, the LIPUS treatment reduced the time to fracture union (SMD: −0.87, 95% CI: −1.72 to −0.02, P = .04, I2 = 91%). However, when the duration assessed was the time until healing, the effect of the LIPUS treatment was not statistically significant (SMD: −0.39, 95% CI: −0.92 to 0.14, P = .15, I2 = 82%). In addition, a test for subgroup differences indicated that the duration of treatment did not affect the effect of LIPUS (P = .34 for interaction).
3.5. Quality of life (SF-36 physical component summary scores)
Two trials[47,49] reported the functional recovery by the SF-36 physical component summary scores. Compared with placebo, LIPUS enhanced the SF-36 physical component summary scores (SMD: 0.2, 95% CI: 0.37–0.02, P = .02, I2 = 0%, Fig. 4A).
Figure 4.

Forest plot of the quality of life, the time to full weight bearing and the time to return to work. (A) Quality of life; (B) time to full weight bearing; and (C) time to return to work.
3.6. Functional recovery
3.6.1. Time to full weight bearing
Three trials[47,53,55] reported the time to full weight bearing. The results showed that LIPUS did not reduce the time to full weight bearing (SMD: −0.76, 95% CI: −1.92 to 0.4, P = .2, I2 = 91%; Fig. 4B).
3.6.2. Time to return to work
Two trials[47,50] reported the time to return to work. LIPUS treatment did not reduce the time to return to work (SMD: 0.06, 95% CI: −0.14 to 0.27, P = .56, I2 = 0%; Fig. 4C).
3.7. Incident rate of delayed union and nonunion
Eight trials,[47,49–53,55,58] consisting of 773 patients, provided the available data about the incident rate of delayed union and nonunion. The results showed that LIPUS did not reduce the incident rate of delayed union and nonunion (RR: 1.02, 95% CI: 0.60–1.74, P = .94, I2 = 14%; Fig. 5). Sensitivity analyses were performed to examine the robustness of our analysis by omitting each study in turn, and the data showed that the pooled RR was not affected (Fig. S2).
Figure 5.
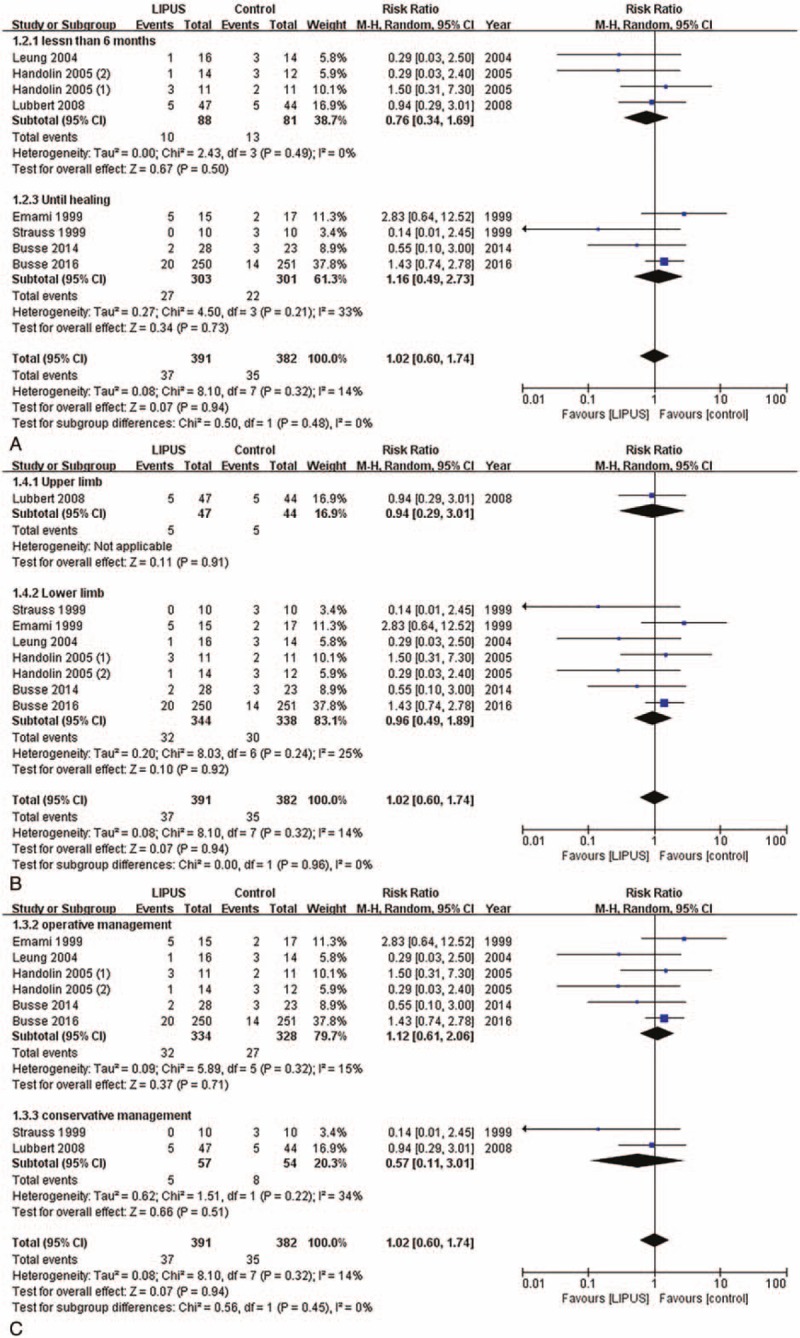
Incident rate of delayed union and nonunion. (A) Time less than 6 mo and time until healing; (B) operative and conservative management; and (C) upper and lower limb.
Based on the results of the subgroup analyses, there were 2 different groups of treatment based on the duration, including <6 months (RR: 0.76, 95% CI: 0.34–1.69, P = .5, I2 = 0%) and time until healing (RR: 1.16, 95% CI: 0.49–2.73, P = .73, I2 = 33%) and that the duration of the treatment did not affect the effect of the LIPUS treatment (P = .48 for interaction; Fig. 5A).
Meanwhile, the subgroup differences did not indicate that the findings from the upper (RR: 0.94, 95% CI: 0.29–3.01, P = .91, I2 = 0%) and lower limb subgroups (RR: 0.96, 95% CI: 0.49–1.89, P = .92, I2 = 25%) were statistically significantly different from each other (P = .96 for interaction; Fig. 5B).
Similarly, we found that there were no significant differences between the operative (RR: 1.12, 95% CI: 0.61–2.06, P = .71, I2 = 15%) and conservative management (RR: 0.57, 95% CI: 0.11–3.01, P = .51, I2 = 34%; P = .45 for interaction; Fig. 5C).
3.8. Publication bias
For the time to fracture union outcome, the publication bias was assessed through a visual inspection of the funnel plots and the Egger test. Both the funnel plot and the Egger test suggested there was no significant publication bias (P = .18 for Egger test; Fig. S3). For the incident rate of delayed union and nonunion outcome, the funnel plot was symmetrical, suggesting that there was no significant publication bias (Fig. S4).
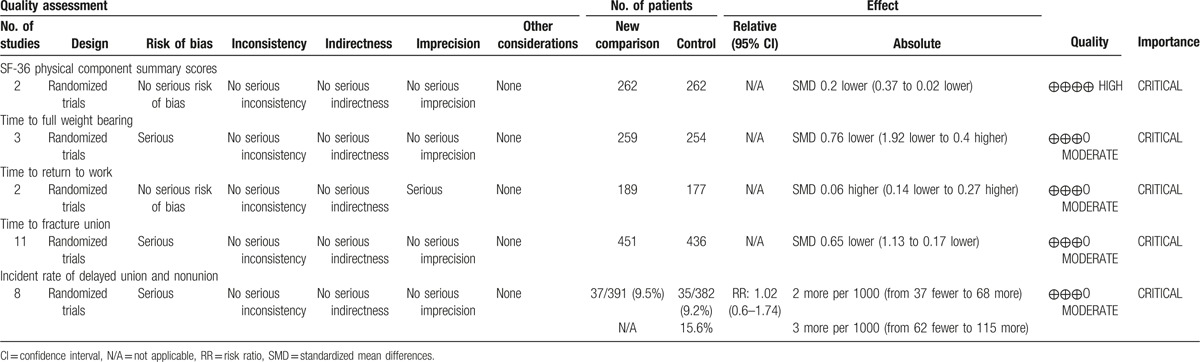
3.9. GRADE profile evidence
GRADE evidence profiles for each outcome are shown in Table 2. All of the included trials were quasi-RCTs or RCTs and had no serious inconsistencies, indirectness, or significant publication bias. A risk of bias existed in each outcome except for the outcome from the SF-36 physical component summary scores and the time to return to work. The selection bias, the performance bias, and the attrition bias were major causes of risk of bias. Imprecision existed in the outcome of the time to return to work. Although the included RCTs were considered as high quality evidence, the quality was down rated by the aforesaid limitations. Therefore, the strength of the evidence was limited, and the available evidence of each outcome was high to moderate.
Table 2.
The GRADE evidence quality for each outcome.
4. Discussion
4.1. Main findings
Our meta-analysis provides moderate to high quality evidence that LIPUS treatment reduces the time to fracture union and improves the quality of life, which was measured by the SF-36 physical component summary scores. We also determine that LIPUS treatment does not affect functional recovery, which was measured by the time to return to work and the time to full weight bearing. In addition, LIPUS treatment dose not increase the incident rate of delayed union and nonunion.
4.2. Comparison with other systematic reviews
Our results are consistent with other meta-analyses in the fact that LIPUS treatment significantly reduced the time to fracture union.[23,25,60,61] This meta-analysis, however, was different from previous meta-analyses in several important aspects. First, this meta-analysis adds to the existing literature by not only assessing the outcome of the time to fracture union, but also assessing the outcomes about the quality of life, functional recovery, and the incident rate of delayed union and nonunion, which are also very important for patients. Second, heterogeneity was well explored by subgroup analyses and sensitivity analyses, and the overall quality of the evidence was also assessed by GRADE approach. Third, this study included a greater number of eligible trials for the pooled analysis.
4.3. Implications for clinical practice
Currently, many surgeons use LIPUS as part of their management of fractures.[62] However, LIPUS is a high-energy wave that generates heat energy[9] and the increased heat energy may further aggravate the thermal osteonecrosis caused by bone drilling during the operation.[63] Thus, the situation under which LIPUS is truly valid should be noted. There are important clinical implications to this study's findings. LIPUS treatment might be more suitable for fractures with conservative treatment rather than those with operative treatment. And it might be more suitable for fractures of the upper limb rather than those of the lower limb. In addition, for fresh fractures with potential risks of delayed union or nonunion, LIPUS treatment should not be expected as a method to reduce the incident rate of delayed union and nonunion.
4.4. Limitations
This study has limitations. First, there were some methodological limitations in the included trials, which included the quasi-random method, an inadequate concealment of treatment allocation, a high loss of follow-up, the unclear age baseline and the gender or smoking status. Second, although subgroup analyses and sensitivity analyses were used to explore the heterogeneity, obvious heterogeneity still existed in some of the outcomes. There were too few studies to use subgroup analysis methods to test for variables possibly associated with heterogeneity. We are not sure whether the effectiveness of LIPUS could be affected by treatment methods, fracture location, treatment duration, and other variables. Third, although our study suggested that LIPUS treatment could reduce the time to fracture union and improve the quality of life, the minimum clinically important difference for these outcomes has not been well established, raising questions about the clinical significance of LIPUS. Thus, results of this meta-analysis should be interpreted cautiously.
4.5. Implications for future research
To gain a comprehensive understanding and full evaluation of LIPUS treatment, future trials are still needed. The design of the future trials should pay attention to the following points: Trials should use LIPUS treatment with the same duration and frequency. For example, 20 minutes once a day was the most widely used in the literature. Trials should pay attention to the methodological limitations, such as blindness, and a placebo should be used in the design of trials. And trials should report more outcomes, such as functional recovery, pain, and cost-effectiveness.
5. Conclusions
In conclusion, moderate-to-high quality evidence shows that LIPUS treatment reduces the time to fracture union and improves the quality of life without affecting functional recovery and incident rate of delayed union and nonunion. Thus, LIPUS treatment may be a good treatment modality for adults with fresh fractures. However, there is still a need for further studies with large numbers of patients to determine the clinical circumstances under which LIPUS is truly valid and the optimal approach to the use of this therapy.
Supplementary Material
Footnotes
Abbreviations: CI = confidence interval, CT = computed tomography, FDA = US Food and Drug Administration, GRADE = Grading of Recommendations Assessment, Development and Evaluation, LIPUS = low-intensity pulsed ultrasonography, RCT = randomized controlled trial, RR = risk ratio, SDC = supplemental digital content, SF-36 = short form-36, SMD = standard mean difference.
SL and HL contributed equally to this work. PT conceived and designed the experiments, and reviewed the draft. SL and HL performed the experiments, analyzed the data, wrote the paper, prepared the figures and tables, and reviewed the draft. ZL and LZ contributed to the design of the search strategy, prepared the figures and tables, and reviewed the draft.
This meta-analysis received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
The authors report no conflicts of interest.
Supplemental Digital Content is available for this article.
References
- [1].Einhorn TA. Enhancement of fracture-healing. J Bone Joint Surg Am 1995;77:940–56. [DOI] [PubMed] [Google Scholar]
- [2].Donaldson LJ, Reckless IP, Scholes S, et al. The epidemiology of fractures in England. J Epidemiol Community Health 2008;62:174–80. [DOI] [PubMed] [Google Scholar]
- [3].Poolman RW, Agoritsas T, Siemieniuk RA, et al. Low intensity pulsed ultrasound (LIPUS) for bone healing: a clinical practice guideline. BMJ 2017;356:j576. [DOI] [PubMed] [Google Scholar]
- [4].Zura R, Xiong Z, Einhorn T, et al. Epidemiology of fracture nonunion in 18 human bones. JAMA Surg 2016;151:e162775. [DOI] [PubMed] [Google Scholar]
- [5].Cook JJ, Summers NJ, Cook EA. Healing in the new millennium: bone stimulators: an overview of where we’ve been and where we may be heading. Clin Podiatr Med Surg 2015;32:45–59. [DOI] [PubMed] [Google Scholar]
- [6].Ebrahim S, Mollon B, Bance S, et al. Low-intensity pulsed ultrasonography versus electrical stimulation for fracture healing: a systematic review and network meta-analysis. Can J Surg 2014;57:E105–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Busse JW, Bhandari M. Therapeutic ultrasound and fracture healing: a survey of beliefs and practices. Arch Phys Med Rehabil 2004;85:1653–6. [DOI] [PubMed] [Google Scholar]
- [8].Siska PA, Gruen GS, Pape HC. External adjuncts to enhance fracture healing: what is the role of ultrasound. Injury 2008;39:1095–105. [DOI] [PubMed] [Google Scholar]
- [9].Mundi R, Petis S, Kaloty R, et al. Low-intensity pulsed ultrasound: fracture healing. Indian J Orthop 2009;43:132–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Watanabe Y, Matsushita T, Bhandari M, et al. Ultrasound for fracture healing: current evidence. J Orthop Trauma 2010;24(suppl 1):S56–61. [DOI] [PubMed] [Google Scholar]
- [11].Claes L, Willie B. The enhancement of bone regeneration by ultrasound. Prog Biophys Mol Biol 2007;93:384–98. [DOI] [PubMed] [Google Scholar]
- [12].Harrison A, Lin S, Pounder N, et al. Mode and mechanism of low intensity pulsed ultrasound (LIPUS) in fracture repair. Ultrasonics 2016;70:45–52. [DOI] [PubMed] [Google Scholar]
- [13].Khan Y, Laurencin CT. Fracture repair with ultrasound: clinical and cell-based evaluation. J Bone Joint Surg Am 2008;90(suppl 1):138–44. [DOI] [PubMed] [Google Scholar]
- [14].Wadhwa S, Godwin SL, Peterson DR, et al. Fluid flow induction of cyclo-oxygenase 2 gene expression in osteoblasts is dependent on an extracellular signal-regulated kinase signaling pathway. J Bone Miner Res 2002;17:266–74. [DOI] [PubMed] [Google Scholar]
- [15].Reher P, Harris M, Whiteman M, et al. Ultrasound stimulates nitric oxide and prostaglandin E2 production by human osteoblasts. Bone 2002;31:236–41. [DOI] [PubMed] [Google Scholar]
- [16].Saini V, Yadav S, McCormick S. Low-intensity pulsed ultrasound modulates shear stress induced PGHS-2 expression and PGE2 synthesis in MLO-Y4 osteocyte-like cells. Ann Biomed Eng 2011;39:378–93. [DOI] [PubMed] [Google Scholar]
- [17].Warden SJ, Favaloro JM, Bennell KL, et al. Low-intensity pulsed ultrasound stimulates a bone-forming response in UMR-106 cells. Biochem Biophys Res Commun 2001;286:443–50. [DOI] [PubMed] [Google Scholar]
- [18].Yang KH, Parvizi J, Wang SJ, et al. Exposure to low-intensity ultrasound increases aggrecan gene expression in a rat femur fracture model. J Orthop Res 1996;14:802–9. [DOI] [PubMed] [Google Scholar]
- [19].Rubin C, Bolander M, Ryaby JP, et al. The use of low-intensity ultrasound to accelerate the healing of fractures. J Bone Joint Surg Am 2001;83-A:259–70. [DOI] [PubMed] [Google Scholar]
- [20].Snyder BM, Conley J, Koval KJ. Does low-intensity pulsed ultrasound reduce time to fracture healing? A meta-analysis. Am J Orthop 2012;41:E12–9. [PubMed] [Google Scholar]
- [21].Bashardoust TS, Houghton P, MacDermid JC, et al. Effects of low-intensity pulsed ultrasound therapy on fracture healing: a systematic review and meta-analysis. Am J Phys Med Rehabil 2012;91:349–67. [DOI] [PubMed] [Google Scholar]
- [22].Busse JW, Kaur J, Mollon B, et al. Low intensity pulsed ultrasonography for fractures: systematic review of randomised controlled trials. BMJ 2009;338:b351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Hannemann PF, Mommers EH, Schots JP, et al. The effects of low-intensity pulsed ultrasound and pulsed electromagnetic fields bone growth stimulation in acute fractures: a systematic review and meta-analysis of randomized controlled trials. Arch Orthop Trauma Surg 2014;134:1093–106. [DOI] [PubMed] [Google Scholar]
- [24].Griffin XL, Costello I, Costa ML. The role of low intensity pulsed ultrasound therapy in the management of acute fractures: a systematic review. J Trauma 2008;65:1446–52. [DOI] [PubMed] [Google Scholar]
- [25].Griffin XL, Parsons N, Costa ML, et al. Ultrasound and shockwave therapy for acute fractures in adults. Cochrane Database Syst Rev 2014;6:CD008579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Khanna A, Nelmes RT, Gougoulias N, et al. The effects of LIPUS on soft-tissue healing: a review of literature. Br Med Bull 2009;89:169–82. [DOI] [PubMed] [Google Scholar]
- [28].Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Morshed S. Current options for determining fracture union. Adv Med 2014;2014:708574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 1992;30:473–83. [PubMed] [Google Scholar]
- [31].Busse JW, Bhandari M, Guyatt GH, et al. Use of both Short Musculoskeletal Function Assessment questionnaire and Short Form-36 among tibial-fracture patients was redundant. J Clin Epidemiol 2009;62:1210–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].McHorney CA, Ware JE, Raczek AE. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care 1993;31:247–63. [DOI] [PubMed] [Google Scholar]
- [33].Bhandari M, Sprague S, Hanson B, et al. Health-related quality of life following operative treatment of unstable ankle fractures: a prospective observational study. J Orthop Trauma 2004;18:338–45. [DOI] [PubMed] [Google Scholar]
- [34].Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Egger M, Davey SG, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Atkins D, Best D, Briss PA, et al. Grading quality of evidence and strength of recommendations. BMJ 2004;328:1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Yadav YK, Salgotra KR, Banerjee A. Role of ultrasound therapy in the healing of tibial stress fractures. Med J Armed Forces India 2008;64:234–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Wang CJ, Liu HC, Fu TH. The effects of extracorporeal shockwave on acute high-energy long bone fractures of the lower extremity. Arch Orthop Trauma Surg 2007;127:137–42. [DOI] [PubMed] [Google Scholar]
- [39].Rue JH, Armstrong DW, III, Frassica FJ, et al. The effect of pulsed ultrasound in the treatment of tibial stress fractures. Orthopedics 2004;27:1192–5. [DOI] [PubMed] [Google Scholar]
- [40].Gan TY, Kuah DE, Graham KS, et al. Low-intensity pulsed ultrasound in lower limb bone stress injuries: a randomized controlled trial. Clin J Sport Med 2014;24:457–60. [DOI] [PubMed] [Google Scholar]
- [41].Handolin L, Kiljunen V, Arnala I, et al. No long-term effects of ultrasound therapy on bioabsorbable screw-fixed lateral malleolar fracture. Scand J Surg 2005;94:239–42. [DOI] [PubMed] [Google Scholar]
- [42].Cook SD, Ryaby JP, McCabe J, et al. Acceleration of tibia and distal radius fracture healing in patients who smoke. Clin Orthop Relat Res 1997;337:198–207. [DOI] [PubMed] [Google Scholar]
- [43].Heckman JD, Sarasohn-Kahn J. The economics of treating tibia fractures. The cost of delayed unions. Bull Hosp Jt Dis 1997;56:63–72. [PubMed] [Google Scholar]
- [44].Mayr E, Rudzki M, Borchard B, et al. Accelerated healing of scaphoid fractures: a randomized study. J Orthop Trauma 1999;13:310. [Google Scholar]
- [45].Emami A, Larsson A, Petren-Mallmin M, et al. Serum bone markers after intramedullary fixed tibial fractures. Clin Orthop Relat Res 1999;368:220–9. [PubMed] [Google Scholar]
- [46].Kristiansen TK. The effect of low power specifically programmed ultrasound on the healing time of fresh fractures using a Colles’ Model. J Orthop Trauma 1990;4:227. [Google Scholar]
- [47].Busse JW, Bhandari M, Einhorn TA, et al. Re-evaluation of low intensity pulsed ultrasound in treatment of tibial fractures (TRUST): randomized clinical trial. BMJ 2016;355:i5351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Liu Y, Wei X, Kuang Y, et al. Ultrasound treatment for accelerating fracture healing of the distal radius. A control study. Acta Cir Bras 2014;29:765–70. [DOI] [PubMed] [Google Scholar]
- [49].Busse JW, Bhandari M, Einhorn TA, et al. Trial to re-evaluate ultrasound in the treatment of tibial fractures (TRUST): a multicenter randomized pilot study. Trials 2014;15:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Lubbert PH, van der Rijt RH, Hoorntje LE, et al. Low-intensity pulsed ultrasound (LIPUS) in fresh clavicle fractures: a multi-centre double blind randomised controlled trial. Injury 2008;39:1444–52. [DOI] [PubMed] [Google Scholar]
- [51].Handolin L, Kiljunen V, Arnala I, et al. The effect of low intensity ultrasound and bioabsorbable self-reinforced poly-l-lactide screw fixation on bone in lateral malleolar fractures. Arch Orthop Trauma Surg 2005;125:317–21. [DOI] [PubMed] [Google Scholar]
- [52].Handolin L, Kiljunen V, Arnala I, et al. Effect of ultrasound therapy on bone healing of lateral malleolar fractures of the ankle joint fixed with bioabsorbable screws. J Orthop Sci 2005;10:391–5. [DOI] [PubMed] [Google Scholar]
- [53].Leung KS, Lee WS, Tsui HF, et al. Complex tibial fracture outcomes following treatment with low-intensity pulsed ultrasound. Ultrasound Med Biol 2004;30:389–95. [DOI] [PubMed] [Google Scholar]
- [54].Mayr E, Rudzki MM, Rudzki M, et al. Does low intensity, pulsed ultrasound speed healing of scaphoid fractures? Handchir Mikrochir Plast Chir 2000;32:115–22. [DOI] [PubMed] [Google Scholar]
- [55].Emami A, Petrén-Mallmin M, Larsson S. No effect of low-intensity ultrasound on healing time of intramedullary fixed tibial fractures. J Orthop Trauma 1999;13:252–7. [DOI] [PubMed] [Google Scholar]
- [56].Kristiansen TK, Ryaby JP, McCabe J, et al. Accelerated healing of distal radial fractures with the use of specific, low-intensity ultrasound. A multicenter, prospective, randomized, double-blind, placebo-controlled study. J Bone Joint Surg Am 1997;79:961–73. [DOI] [PubMed] [Google Scholar]
- [57].Heckman JD, Ryaby JP, McCabe J, et al. Acceleration of tibial fracture-healing by non-invasive, low-intensity pulsed ultrasound. J Bone Joint Surg Am 1994;76:26–34. [DOI] [PubMed] [Google Scholar]
- [58].Strauss EJJ. Treatment of Jones’ fractures of the foot with adjunctive use of low-pulsed ultrasound stimulation. J Orthop Trauma 1999;13:310. [Google Scholar]
- [59].Zura R, Xu ZJ, Della RGJ, et al. When is a fracture not “fresh”? Aligning reimbursement with patient outcome after treatment with low-intensity pulsed ultrasound. J Orthop Trauma 2017;31:248–51. [DOI] [PubMed] [Google Scholar]
- [60].Griffin XL, Smith N, Parsons N, et al. Ultrasound and shockwave therapy for acute fractures in adults. Cochrane Database Syst Rev 2012;2:CD008579. [DOI] [PubMed] [Google Scholar]
- [61].Busse JW, Bhandari M, Kulkarni AV, et al. The effect of low-intensity pulsed ultrasound therapy on time to fracture healing: a meta-analysis. CMAJ 2002;166:437–41. [PMC free article] [PubMed] [Google Scholar]
- [62].Busse JW, Morton E, Lacchetti C, et al. Current management of tibial shaft fractures: a survey of 450 Canadian orthopedic trauma surgeons. Acta Orthop 2008;79:689–94. [DOI] [PubMed] [Google Scholar]
- [63].Augustin G, Zigman T, Davila S, et al. Cortical bone drilling and thermal osteonecrosis. Clin Biomech 2012;27:313–25. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


