Abstract
Background:
Metformin is effective for the treatment of polycystic ovary syndrome (PCOS), but conflicting results regarding its impact on serum levels of C-reactive protein (CRP) and interleukin-6 (IL-6) in women with PCOS have been reported. To provide high-quality evidence about the effect of treatment with metformin on CRP and IL-6 in PCOS, relevant studies that assessed the serum levels of CRP and IL-6 in women with PCOS receiving metformin treatment were reviewed and analyzed.
Methods:
A literature search was conducted in the Science Citation Index, PubMed, Embase, and Cochrane Library databases, and personal contact was made with the authors. Random-effects model was used to estimate the standardized mean differences (SMDs) with 95% confidence intervals (95% CIs). To ensure synthesis of the best available evidence, subgroup analysis, sensitivity analysis, meta-regression analysis, and publication bias were performed.
Results:
Of 216 studies identified, 20 were included in the meta-analysis (7 prospective, nonrandomized studies, and 13 randomized control trials). Data suggest that serum levels of CRP were decreased after metformin treatment in PCOS patients with an SMD (95% CI) of −0.86 [−1.24 to −0.48] and P = .000 (random-effects). However, significant heterogeneity was detected across studies (I2 = 84.6% and P = .000). Unfortunately, the sources of heterogeneity were not found by subgroup analysis and meta-regression analysis. Serum IL-6 concentrations were not significantly changed after metformin treatment in PCOS with an SMD (95% CI) of −0.48 [−1.26 to 0.31] and P > .05 (random-effects). Significant heterogeneity was also detected across studies (I2 = 90.9% and P = .000). The subgroup analysis suggested that treatment-related reductions in serum IL-6 levels were significantly correlated with BMI, whereas the sources of heterogeneity were not found. In addition, we noticed that metformin treatment could decrease BMI in the CRP and IL-6 related studies (SMD = −0.45, 95% CI: −0.68 to −0.23; SMD = −0.44, 95% CI: −0.73 to −0.16).
Conclusion:
This meta-analysis showed a significant decrease of serum CRP levels, especially in obese women, but no significant changes in IL-6 levels after metformin treatment in women with PCOS. In general, the data support that early metformin therapy may ameliorate the state of chronic inflammation in women with PCOS. Considering the obvious heterogeneity reported in the literature, further well-designed investigations with larger samples are needed to ascertain the long-term effects of metformin on chronic inflammation in PCOS.
Keywords: C-reactive protein, interleukin-6, meta-analysis, metformin, polycystic ovary syndrome
1. Introduction
Polycystic ovary syndrome (PCOS) is a common endocrine disorder in women that is characterized by hyperandrogenism, ovulatory dysfunction, and polycystic ovarian morphology.[1] It is frequently accompanied by insulin resistance and obesity, resulting in a high risk for type 2 diabetes and cardiovascular disease.[2] It is now clear that PCOS is a proinflammatory state, and emerging data suggest that chronic low-grade inflammation seems to play an essential role in the pathogenesis of PCOS, and might be the precursor to the development of insulin resistance and metabolic consequences.[3–5] Significant elevations in serum levels of inflammatory factors including C-reactive protein (CRP) and interleukin-6 (IL-6) were observed in women with PCOS compared with the control women.[6–8] IL-6 may be an early low-grade chronic inflammatory marker in PCOS,[9] a finding that is consistent with other evidence that IL-6 may be a key mediator of low-grade chronic inflammation in PCOS.[10] CRP, secreted in response to cytokines, may not only be a maker of inflammatory disease but also amplify the inflammation process by further activation of monocytes and endothelial cells.[11] A meta-analysis revealed that CRP is the most reliable circulating marker of chronic low-grade inflammation in PCOS.[12]
There are accumulated evidences that insulin resistance played a crucial role in the pathogenesis of PCOS.[13–15] Chronic low-grade inflammation induces insulin resistance both directly and indirectly via activation of serine kinase and mobilization of free fatty acids.[16,17] Moreover, metformin not only improves chronic inflammation through the improvement of metabolic parameters such as hyperglycemia and insulin resistance but also has a direct anti-inflammatory action. Recent studies have suggested that metformin has a direct anti-inflammatory action by inhibition of nuclear factor κB via adenosine monophosphate-activated protein kinase dependent and independent pathways.[18,19] Thus, metformin has been widely used in women with PCOS to improve insulin sensitivity and induce ovulation.
However, the impact of metformin on serum inflammation makers (CRP and IL-6) in PCOS remains controversial. Some studies reported positive results,[9,20–25] whereas others found no significant changes in CRP and IL-6 levels after metformin administration.[26–38] The aim of the present study was to systematically review the literature and to meta-analyze the best evidence on the effects of metformin treatment on serum levels of CRP and IL-6 in women with PCOS.
2. Methods
2.1. Search strategy
The following databases were searched for prospective studies and randomized clinical trials (RCTs) published until October 2016: Science Citation Index, PubMed, Embase, and Cochrane Library. No limits were set on publication date or language. The following search terms were included:
-
(1)
[(C-reactive protein OR CRP) AND metformin] AND (PCOS OR polycystic ovary syndrome)
-
(2)
[(interleukin-6 OR IL-6) AND metformin] AND (PCOS OR polycystic ovary syndrome)
2.2. Inclusion and exclusion criteria
Studies that met the following criteria were included: measuring serum CRP and IL-6 levels in women with PCOS receiving metformin therapy in prospective studies and RCTs; reporting CRP and IL-6 means and standard deviation (SD) or sufficient information that can calculate CRP and IL-6 means and SD; Rotterdam[39] or National Institute of Health (NIH) criteria[40] were used as diagnosis criteria of PCOS.
Studies were excluded if they were publications from letters, reviews, animal studies, case reports, and studies without controls. If the studies based on the same study population published more than one article, we chose the most recent published date or the one with the largest sample size.
2.3. Data extraction and quality assessment
Information from each study was extracted independently by 2 researchers (Wang and Zhu) by using a standardized data extraction form. The general characteristics, body mass index (BMI), and outcomes (both the total CRP/IL-6 means and SDs before and after metformin administration) of each study were recorded, where available, and double-checked. Where appropriate, the data set was completed through communication with the authors. Disagreements were resolved by consensus (Xu). The quality of the information accessed in RCTs was classified as low, unclear, or high by evaluating the following 7 components: random sequence generation, allocation concealment, blinding of outcome assessment, blinding of participants and personnel, incomplete outcome data, selective outcome reporting, and ”other bias” according to the guidelines of the Cochrane Handbook for Systematic Reviews of Interventions.
2.4. Statistical analysis
Standard mean differences (SMDs) and 95% confidence intervals (95% CIs) of the CRP and IL-6 levels were calculated for all eligible studies and combined by using appropriate fixed or random effects models. The statistical heterogeneity was assessed using the X2-based Q-statistic and the I2-statistic. P value less than .05 for Q-statistic or I2 larger than 50% was considered as having significant heterogeneity, which existed between the studies. The random-effects model was applied if heterogeneity was detected; otherwise, the fixed-effects model was used. Subsequently, to identify the sources of heterogeneity, subgroup analysis was carried out, including age, country, BMI, therapy duration, and dose. Furthermore, restricted maximum likelihood-based random effects meta-regression analysis was performed to evaluate the aforementioned potential heterogeneity factors. Univariate meta-regression analysis was carried out first, after which the variables that were significant at the 0.1 level were entered into the multivariable model. A sensitivity analyses was performed to evaluate the stability of the meta-analysis results. Potential publication bias was investigated by using the Begg test. All statistical analyses were performed using Stata (version12; StataCorp, College Station, TX).
2.5. Ethical approval
The ethical approval was not necessary for the reason that our study was a meta-analysis belonging to secondary analysis.
3. Results
3.1. Flow chart of study selection
Figure 1 provides details of the study selection. The primary search identified 216 articles. After title and abstract screening, 30 potentially eligible studies were retrieved for full text review. Of these 30 articles, 10 were excluded: 5 did not offer sufficient data, 3 did not meet the inclusion criteria, and 2 measured other proteins in women with PCOS receiving metformin therapy. Therefore, 20 studies were included in the meta-analysis: 7 prospective, nonrandomized studies comparing the use of metformin in PCOS women and healthy controls[9,21,22,25,27,28,36] and 13 RCTs [3 studies comparing metformin with ethinyl estradiol cyproterone acetate (EE-CA) in PCOS women, 3 comparing metformin with rosiglitazone in PCOS women, 2 comparing metformin with rimonabant in PCOS women, 1 comparing metformin with flutamide levonorgestrel ethinyl estradiol in PCOS women, 1 comparing metformin with exenatide in PCOS women, 1 comparing metformin with rosuvastatin in PCOS women, 1 comparing metformin with hypocaloric diet in PCOS women, and 1 comparing metformin with placebo in PCOS women].[20,23,24,26,29–35,37,38]
Figure 1.
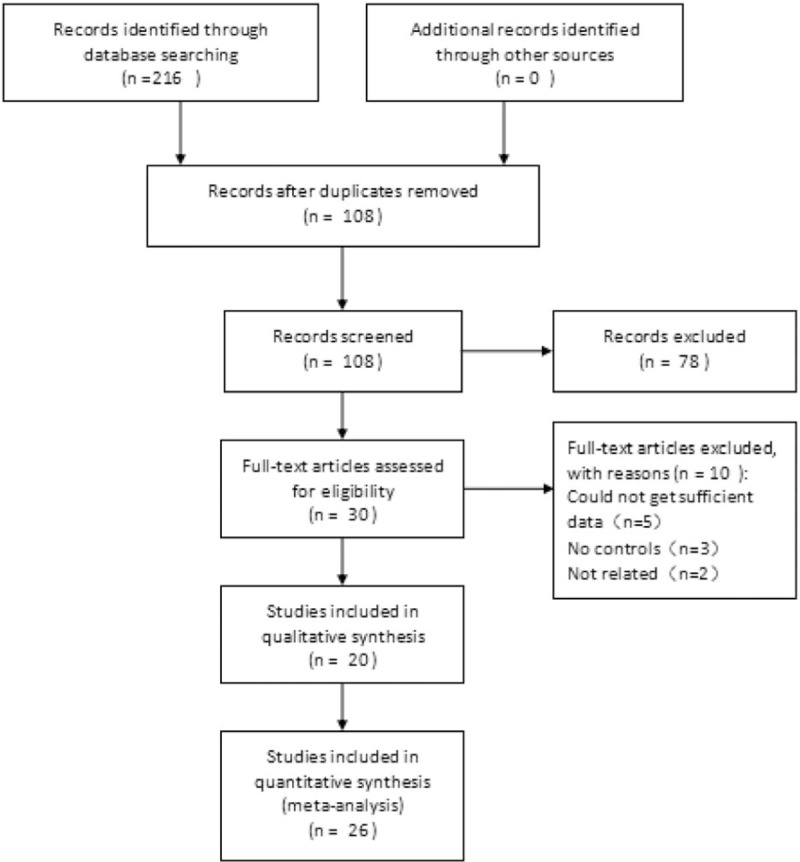
The search flow diagram.
3.2. Characteristics of included studies
Table 1 summarizes the characteristics of the 20 studies included in the meta-analysis. Seven studies focused on eastern women[9,23,29,32,33,35,37] and 13 on western women. Sixteen of these studies employed Rotterdam criteria for the diagnosis of PCOS and 4 used NIH criteria.[29,30,33,37] One study (comparing metformin vs rosiglitazone) did not report the mean age for each group separately, which included young women,[32] and 7 included PCOS women over 28 years old.[20,21,24,28,31,37,38] Study duration ranged from 3 to 6 months. Two 6-month studies also reported 3-month data.[20,33] Study dose ranged from 1000 to 2550 mg per day.
Table 1.
Characteristics of the 20 studies in the meta-analysis.
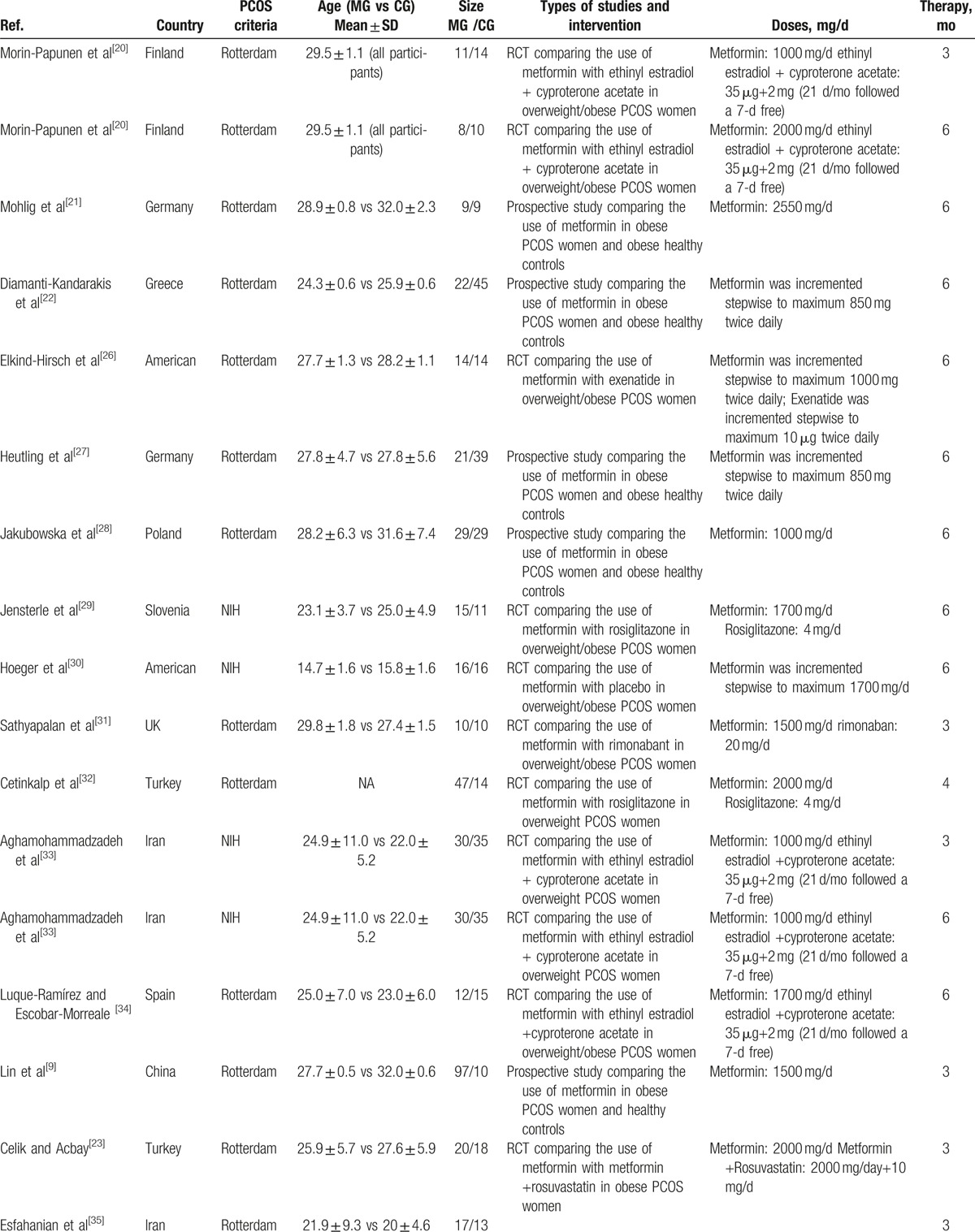
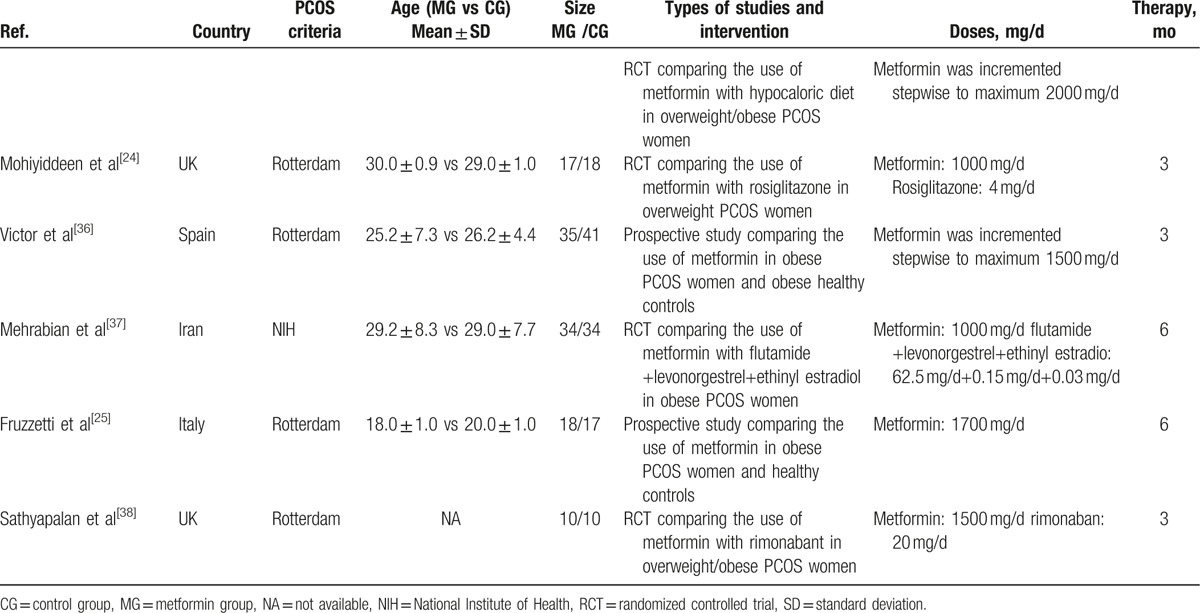
The 3 RCTs included in the meta-analysis, comparing metformin versus EE-CA in PCOS women, lasted 3 to 6 months. Two studies used the same dosages for both drugs[20,33] and 1 used a higher dosage of metformin.[34] The sample size ranged from 10 to 35 women for both the PCOS group treated with metformin and the PCOS group treated with EE-CA. Three studies compared metformin with rosiglitazone in PCOS women and used the same dosages for rosiglitazone.[24,29,32] Two studies compared metformin with rimonabant in overweight/obese women with PCOS for 3 months reporting CRP and IL-6 parameters, respectively.[31,38] The rest RCTs compared metformin with flutamide levonorgestrel ethinyl estradiol or exenatide or rosuvastatin or hypocaloric diet or placebo.[23,26,30,35,37]
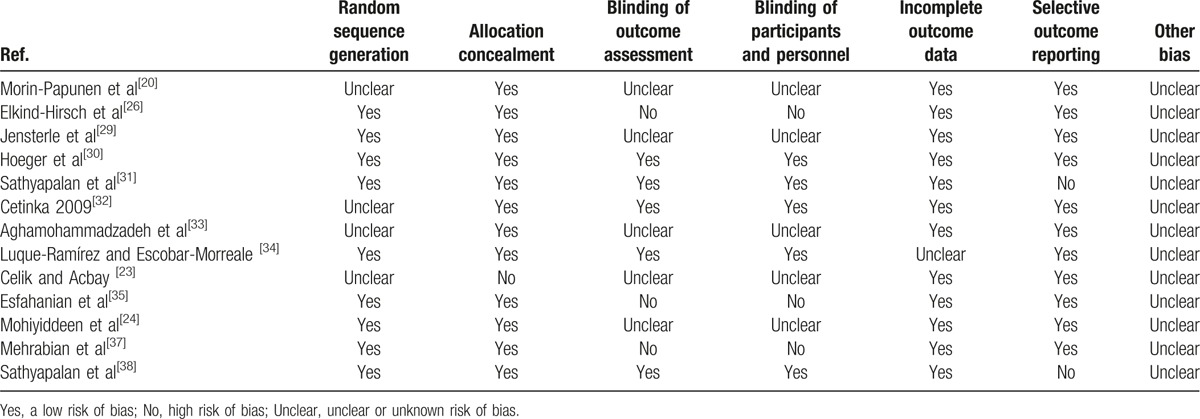
The quality assessment of randomized trials included in our study is summarized in Table 2. Randomization was performed according to a computer-generated random list or by means of a randomly generated number pattern in a majority of the trials.[24,26,29–31,34,35,37,38] Most randomized trials included in our study were characterized by a low risk of incomplete outcome data and selective outcome reporting.[20,23,24,26,29,30,32,33,35,37] Eight randomized trials included in our study were characterized by a high risk of blinding of participants and personnel and outcome assessment.[20,23,24,26,29,33,35,37] Moreover, all randomized trials were with an unclear risk of other bias. In conclusion, the quality of these studies was low to moderate.
Table 1 (Continued).
Characteristics of the 20 studies in the meta-analysis.
3.3. CRP
3.3.1. Selection of studies
The characteristics of the included studies for the CRP levels are provided in Table 3.
Table 2.
The risk of bias of randomized trials.
3.3.2. Pooled analysis
Seventeen studies, including 403 women, were eligible for the meta-analysis. The I2 value was 84.6%, indicating high heterogeneity among the included studies. Therefore, we used the random-effects model to combine effect size. The meta-analysis revealed that serum CRP concentrations were decreased after metformin treatment in PCOS with an SMD (95% CI) of −0.86 [−1.24 to −0.48] and P = .000 (Fig. 2). We also performed a meta-analysis regarding the impact of metformin administration on BMI in patients with PCOS. The result showed that the total SMD for BMI was −0.45 units (95% CI, −0.68 to −0.23, P < .05) (Fig. 3). These results suggested that metformin therapy was associated with a significant decrease in BMI in patients with PCOS.
Figure 2.
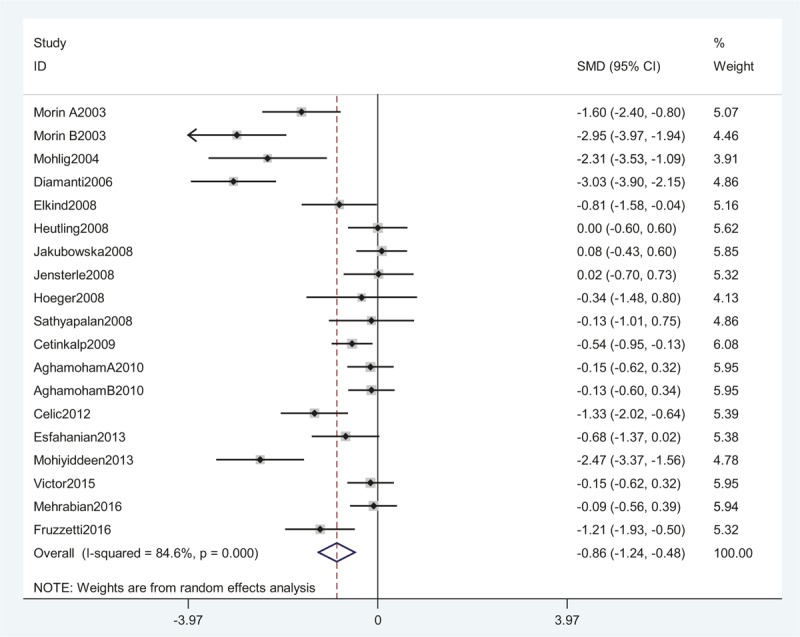
A meta-analysis of data about serum CRP levels in the women with PCOS before and after metformin treatment from 17 studies using a random-effect model. CI = confidence interval, CRP = C-reactive protein, PCOS = polycystic ovary syndrome, SMD = standard mean differences.
Figure 3.
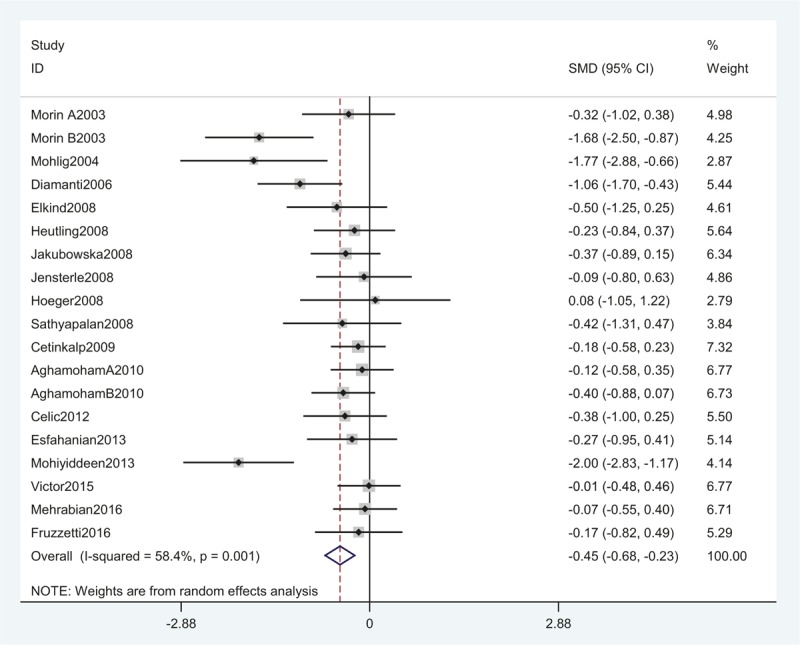
A meta-analysis of data about comparison of BMI before and after metformin treatment in the CRP-related studies using a random-effect model. BMI = body mass index, CI = confidence interval, CRP = C-reactive protein, SMD = standard mean differences.
3.3.3. Subgroup analysis and meta-regression analysis
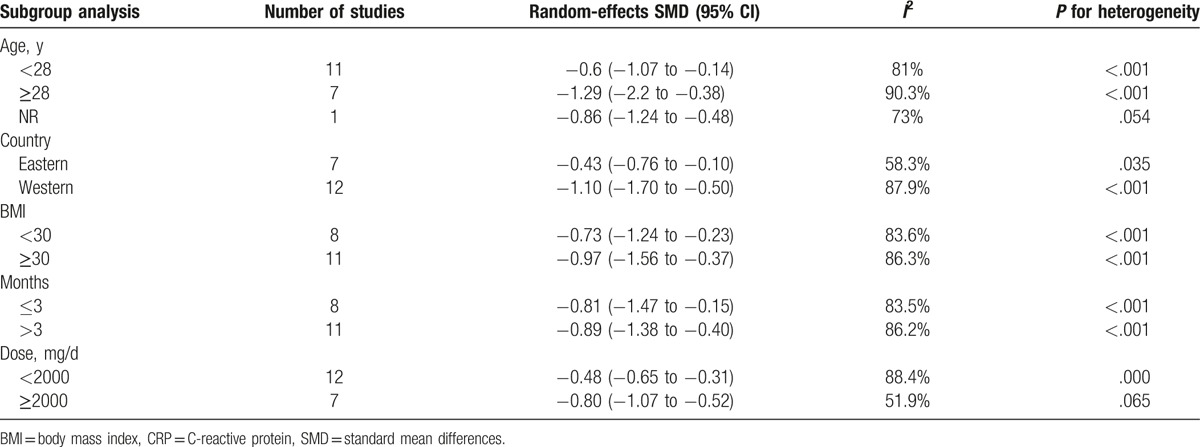
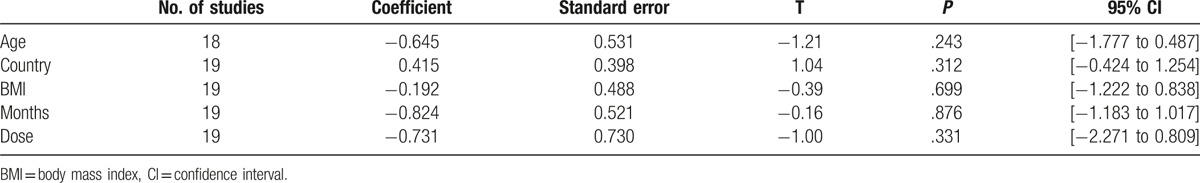
Subgroup analysis was performed according to age (<28, ≥28 years, or not available), country (Eastern or Western), BMI (<30 or ≥30 kg/m2), therapy duration (≤3 or >3 months), and dose (<2000 or ≥2000 mg/day). In the subgroup analysis, the overall pattern of pooled effect did not vary substantially by the potential sources of heterogeneity, including age, region, BMI, therapy duration, and dose (Table 4). Unfortunately, subgroup analysis showed obvious heterogeneity, and these variables were not found to be the main source of heterogeneity in all studies. To further investigate the impact of the characteristics mentioned above, we carried out a meta-regression analysis. A univariate meta-regression analysis revealed that the regression coefficients of aforementioned variables were insignificant (P > .1) (Table 5) and multivariate meta-regression was not carried out further. Therefore, the source of heterogeneity still was not found.
Table 3.
Characteristics of the 17 included studies on CRP.
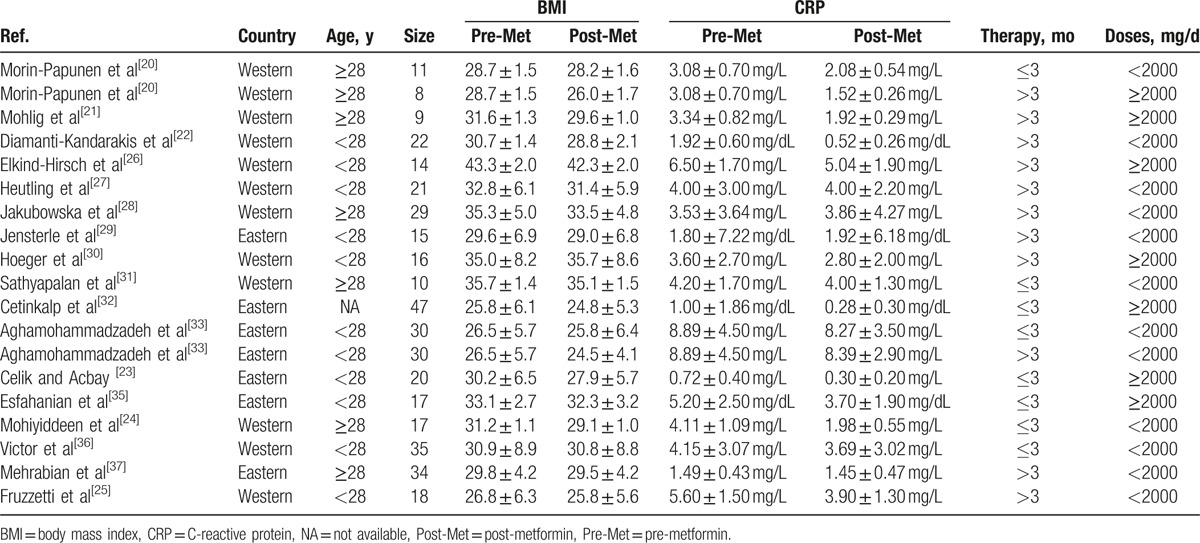
Table 4.
Subgroup meta-analysis of the included studies for CRP.
3.3.4. Sensitivity analysis and publication bias
Sensitivity analysis revealed that removal of any study from the analysis did not subvert the result of the present pooled analysis (data not shown). Therefore, this pooled analysis outcome could be regarded with a higher degree of certainty. Furthermore, no publication bias was identified by Begg test (Fig. 4).
Figure 4.
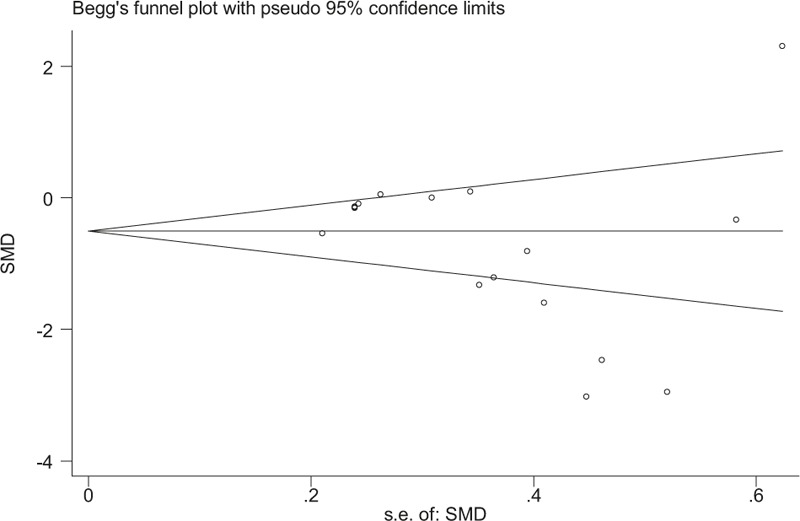
A funnel plot of studies evaluating the association between CRP and metformin in PCOS with P = .149 (Begg regression asymmetry test). CRP = C-reactive protein, PCOS = polycystic ovary syndrome, SMD = standard mean differences.
3.4. IL-6
3.4.1. Selection of studies
The characteristics of the included studies for the IL-6 levels are provided in Table 6.
Table 5.
Meta-regression analysis for the variables between studies.
3.4.2. Pooled analysis
Seven studies, including 192 women, were eligible for the meta-analysis. The I2 value was 90.9%, indicating high heterogeneity among the included studies. Therefore, we used the random-effects model to combine effect size. The meta-analysis revealed that serum IL-6 concentrations were not significantly changed after metformin treatment in PCOS with an SMD (95% CI) of −0.48 [−1.26 to 0.31] and P > .05 (Fig. 5). We also performed a meta-analysis regarding the impact of metformin administration on BMI in patients with PCOS. The result showed that the total SMD for BMI was −0.44 units (95% CI, −0.73 to −0.16, P < .05) (Fig. 6). These results suggested that metformin therapy could decrease BMI in patients with PCOS.
Figure 5.
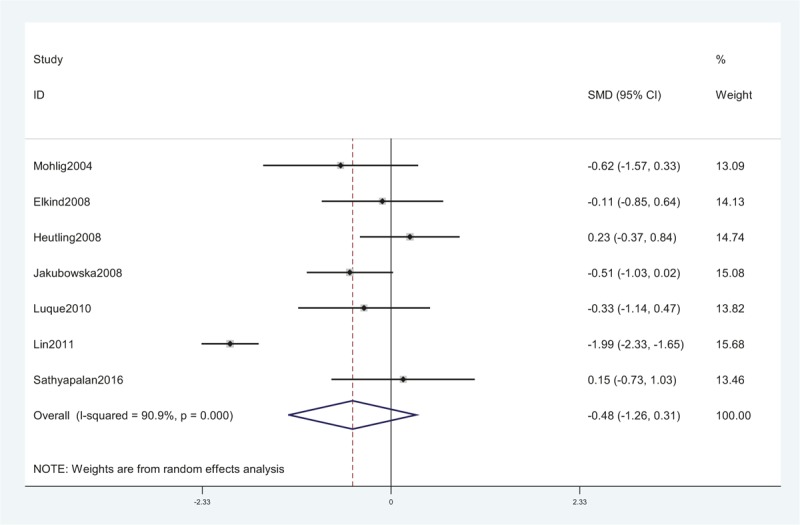
A meta-analysis of data about serum IL-6 levels in the women with PCOS before and after metformin treatment from 7 studies using a random-effect model. CI = confidence interval, IL-6 = interleukin-6, PCOS = polycystic ovary syndrome, SMD = standard mean differences.
Figure 6.
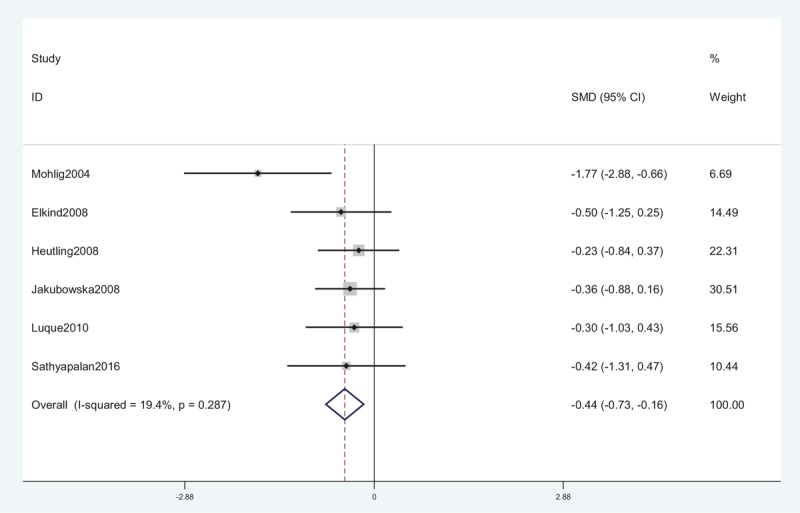
A meta-analysis of data about comparison of BMI before and after metformin treatment in the IL-6 related studies using a random-effect model. BMI = body mass index, CI = confidence interval, IL-6 = interleukin-6, SMD = standard mean differences.
3.4.3. Subgroup analysis
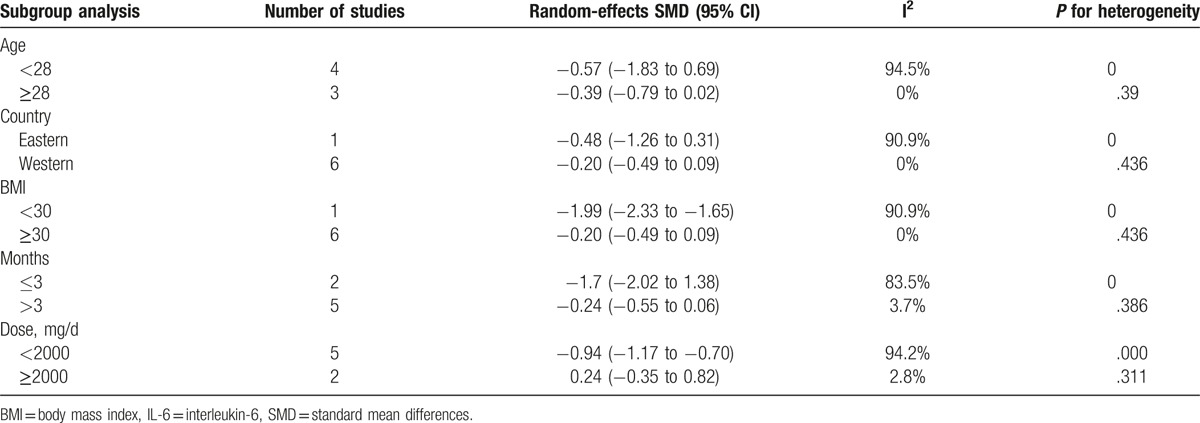
Subgroup analysis was also performed according to age (<28, ≥28 years, or not available), country (Eastern or Western), BMI (<30 or ≥30 kg/m2), therapy duration (≤3 or >3 months), and dose (<2000 or ≥2000 mg/day). In the subgroup analysis, the overall pattern of pooled effect did not vary substantially by the potential sources of heterogeneity, including age, region, BMI, therapy duration, and dose. Decreased levels of IL-6 after metformin treatment in PCOS were observed in the groups with BMI <30 [SMD (95% CI) of −1.99 (−2.33 to −1.65)]. However, there was no change of serum IL-6 concentrations in patients with BMI ≥30 after metformin treatment (Table 7). Unfortunately, subgroup analysis showed obvious heterogeneity, and these variables were not found to be the main source of heterogeneity in all studies.
Table 6.
Characteristics of the 7 included studies on IL-6.
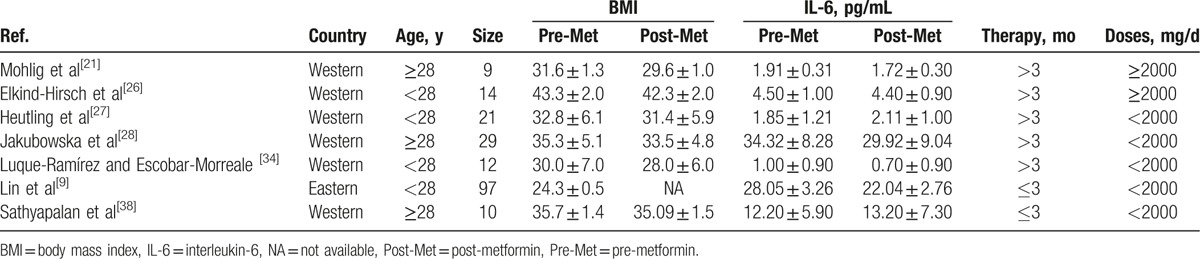
Table 7.
Subgroup meta-analysis of the included studies for IL-6.
4. Discussion
In the current meta-analysis, the results showed a significant decrease of serum CRP levels after metformin treatment in women with PCOS, especially in western obese women. In addition, we noticed that metformin treatment could decrease BMI in the CRP and IL-6 related studies. However, we found no significant changes in IL-6 levels during metformin treatment in the present study.
Similar to the findings of our meta-analysis, decreased levels of CRP have been observed in women with PCOS who were receiving 1000 to 2000 mg metformin daily for 6 months.[20,22,25] Insulin and glucose levels induced by activation of the AMP-kinase pathways have been shown to associate with CRP levels.[41,42] Decreased levels of CRP observed in women with PCOS after metformin administration may be the result of reduced insulin resistance and insulin levels. Elevated CRP levels are also associated with obesity in women with PCOS,[43] and serum CRP and IL-6 levels reduced after even moderate weight loss in obese subjects.[44] Interestingly, we observed a significant time and dose effect of metformin treatment on CRP levels in women with PCOS. Moreover, our study found that serum CRP levels decrease more in obese women with PCOS and their BMI reduced significantly after metformin treatment. In consistent with a study, it proved that higher dose metformin may be more efficient for BMI reduction in women with PCOS.[45] However, Chen et al[46] observed that CRP levels decreased more in the subgroup with a dose of <2000 mg/day than that of ≥2000 mg/day. The explanation could be that BMI, age, and clinical phenotypes influence the effect of metformin in PCOS. Thus, further investigations are needed to explore the optimal doses in PCOS women.
CRP is a classical marker of inflammation that is commonly used for cardiovascular disease risk stratification and improving cardiovascular risk prediction.[47,48] Large increases or sustained elevations in CRP over a 6-year period were associated with a subsequent increased risk of diabetes, cardiovascular events, and mortality.[49] On the basis of this meta-analysis and previous work, metformin treatment in women with PCOS was associated with a significant decrease in CRP levels. Therefore, it may be suspected that metformin administration is effective for preventing cardiovascular disease in women with PCOS.
In this meta-analysis, we found that serum IL-6 levels did not decrease significantly in women with PCOS after metformin treatment. In contrast with our results, 1 study[9] indicated that a significant change was observed in IL-6 concentration after treatment with 1500 mg of metformin daily for 3 months. Since the complexity of the factors that influence the effect of metformin on IL-6 levels, we could not identify the precise dose or duration of metformin therapy through our meta-analysis needed to maximize the decrease in IL-6 concentration in women with PCOS. IL-6, a major proinflammatory cytokine in chronic inflammation, has been shown to be closely associated with insulin resistance.[50] Luque-Ramírez and Escobar-Morreale[31] pointed out that serum IL-6 levels decreased during treatment with metformin in parallel to amelioration of insulin resistance. Meanwhile, another study suggested that metformin treatment response on low-grade chronic inflammatory markers in women with PCOS was related to insulin receptor substrate-2 (IRS-2) polymorphism.[9] Therefore, our results could be explained by the differences in race and severity of insulin resistance. Unfortunately, subgroup analysis according to country revealed that IL-6 levels did not change significantly after metformin treatment in women with PCOS.
We noticed that metformin treatment could reduce BMI in women with PCOS, and decreased IL-6 levels after metformin treatment in PCOS were observed in the groups with BMI <30 in our study. Multiple regression analysis found that IL-6 levels correlated significantly with BMI of PCOS patients.[51] Futhermore, short-term metformin therapy facilitated weight loss, and long-term therapy resulted in a reduction of IL-6 levels.[52] So, the explanation could be that metformin-associated reduction of weight before significant changes in IL-6 parameter perhaps involves different mechanisms of action.
Overall, the strength of this study is stronger than any single study, as the included primary studies are quite homogeneous. First, the criteria of diagnosis of PCOS were clearly defined. Besides, subgroup analysis and meta-regression analysis were carried out on the basis of several potential relevant factors. Although significant heterogeneity was detected, the sensitivity analysis did not show that a single study influenced the pooled results and no publication bias was detected. This would undoubtedly enhance the persuasiveness of the study.
Nevertheless, several limitations must be admitted when considering the generalizability of these data. First, the most important limitation is the scarcity of high-quality, multicenter, large sample standard RCTs that directly assess the efficacy of metformin treatment in women with PCOS. Second, there was a wide gap of the number of objects in the recruited trails, ranging from 9 to 97, which may weaken the strength of pooled studies. Third, most of the studies included in the meta-analysis contained small numbers of cases, which is probably the reason for significant heterogeneity. Furthermore, obvious heterogeneity across the included studies was not eliminated by the subgroup analyses and the meta-regression analysis also could not determine the source of heterogeneity; it might reflect clinical heterogeneity related to physical activity, PCOS phenotypes, concomitant subclinical inflammatory diseases, etc.
5. Conclusion
This meta-analysis showed a significant decrease of serum CRP levels, especially in obese women, but no significant changes in IL-6 levels after metformin treatment in women with PCOS. In general, the data support that early metformin therapy may ameliorate the state of chronic inflammation in women with PCOS. Further investigation is required to determine whether these findings may prove to be of clinical significance for PCOS patients. Considering the obvious heterogeneity reported in the literature, further well-designed investigations with larger samples are needed to ascertain the long-term effects of metformin on chronic inflammation in PCOS.
Footnotes
Abbreviations: BMI = body mass index, CIs = confidence intervals, CRP = C-reactive protein, EE-CA = ethinyl estradiol cyproterone acetate, IL-6 = interleukin-6, IRS-2 = insulin receptor substrate-2, NIH = National Institute of Health, PCOS = polycystic ovary syndrome, RCTs = randomized clinical trials, SD = standard deviation, SMD = standard mean differences.
JW and LZ contributed equally to this work.
Funding/support: The study was supported by grants from the National Natural Science Funds of China (nos. 81560154 and 81460018), Jiangxi Provincial Science Technology Foundation of China (No. 20151BBG70073), and Jiangxi Provincial Department of Education Scientific Research Funds of China (nos. GJJ13145 and GJJ13178).
The authors declare that “No conflicts of interest exist.”
References
- [1].Azziz R, Carmina E, Chen Z, et al. Polycystic ovary syndrome. Nat Rev Dis Primers 2016;2:16057. [DOI] [PubMed] [Google Scholar]
- [2].Norman RJ, Dewailly D, Legro RS, et al. Polycystic ovary syndrome. Lancet 2007;370:685–97. [DOI] [PubMed] [Google Scholar]
- [3].Repaci A, Gambineri A, Pasquali R. The role of low-grade inflammation in the polycystic ovary syndrome. Mol Cell Endocrinol 2011;335:30–41. [DOI] [PubMed] [Google Scholar]
- [4].Shorakae S, Teede H, Lambert G, et al. The emerging role of chronic low-grade inflammation in the pathophysiology of polycystic ovary syndrome. Semin Reprod Med 2015;33:257–69. [DOI] [PubMed] [Google Scholar]
- [5].Spritzer PM, Lecke SB, Satler F, et al. Adipose tissue dysfunction, adipokines, and low-grade chronic inflammation in polycystic ovary syndrome. Reproduction 2015;149:219–27. [DOI] [PubMed] [Google Scholar]
- [6].El-Mesallamy HO, Abd El-Razek RS, El-Refaie TA. Circulating high-sensitivity C-reactive protein and soluble CD40 ligand are inter-related in a cohort of women with polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol 2013;168:178–82. [DOI] [PubMed] [Google Scholar]
- [7].Nehir Aytan A, Bastu E, Demiral I, et al. Relationship between hyperandrogenism, obesity, inflammation and polycystic ovary syndrome. Gynecol Endocrinol 2016;32:709–13. [DOI] [PubMed] [Google Scholar]
- [8].Peng Z, Sun Y, Lv X, et al. Interleukin-6 levels in women with polycystic ovary syndrome: a systematic review and meta-analysis. PLoS One 2016;11:e0148531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Lin YS, Lin MW, Yang CT, et al. Interleukin-6 as an early chronic inflammatory marker in polycystic ovary syndrome with insulin receptor substrate-2 polymorphism. Am J Reprod Immunol 2011;66:527–33. [DOI] [PubMed] [Google Scholar]
- [10].Tumu VR, Govatati S, Guruvaiah P, et al. An interleukin-6 gene promoter polymorphism is associated with polycystic ovary syndrome in South Indian women. J Assist Reprod Genet 2013;30:1541–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ogita M, Miyauchi K. C-reactive protein and cardiovascular disease. J Cardiol 2016;68:179. [DOI] [PubMed] [Google Scholar]
- [12].Escobar-Morreale HF, Luque-Ramirez M, Gonzalez F. Circulating inflammatory markers in polycystic ovary syndrome: a systematic review and meta-analysis. Fertil Steril 2011;95:1048–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Csenteri OK, Sándor J, Kalina E, et al. The role of hyperinsulinemia as a cardiometabolic risk factor independent of obesity in polycystic ovary syndrome. Gynecol Endocrinol 2017;33:34–8. [DOI] [PubMed] [Google Scholar]
- [14].Polak K, Czyzyk A, Simoncini T, et al. New markers of insulin resistance in polycystic ovary syndrome. J Endocrinol Invest 2017;40:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Barber TM, Dimitriadis GK, Andreou A, et al. Polycystic ovary syndrome: insight into pathogenesis and a common association with insulin resistance. Clin Med (Lond) 2015;15:72–6. [DOI] [PubMed] [Google Scholar]
- [16].Hotamisligil GS. Inflammation and metabolic disorders. Nature 2006;444:860–7. [DOI] [PubMed] [Google Scholar]
- [17].Carmina E. Obesity, adipokines and metabolic syndrome in polycystic ovary syndrome. Front Horm Res 2013;40:40–50. [DOI] [PubMed] [Google Scholar]
- [18].Saisho Y. Metformin, inflammation: its potential beyond glucose-lowering effect. Endocr Metab Immune Disord Drug Targets 2015;15:196–205. [DOI] [PubMed] [Google Scholar]
- [19].An H, He L. Current understanding of metformin effect on the control of hyperglycemia in diabetes. J Endocrinol 2016;228:97–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Morin-Papunen L, Rautio K, Ruokonen K, et al. Metformin reduces serum C - reactive protein levels in women with polycystic ovary syndrome. J Clin Endocrinol Metab 2003;88:4649–54. [DOI] [PubMed] [Google Scholar]
- [21].Mohlig M, Spranger J, Osterhoff M, et al. The polycystic ovary syndrome per se is not associated with increased chronic inflammation. Eur J Endocrinol 2004;150:525–32. [DOI] [PubMed] [Google Scholar]
- [22].Diamanti-Kandarakis E, Paterakis T, Alexandraki K, et al. Indices of low-grade chronic inflammation in polycystic ovary syndrome and the beneficial effect of metformin. Hum Reprod 2006;21:1426–31. [DOI] [PubMed] [Google Scholar]
- [23].Celik O, Acbay O. Effects of metformin plus rosuvastatin on hyperandrogenism in polycystic ovary syndrome patients with hyperlipidemia and impaired glucose tolerance. J Obstet Gynaecol Res 2012;39:806–13. [DOI] [PubMed] [Google Scholar]
- [24].Mohiyiddeen L, Watson AJ, Apostolopoulos NV, et al. Effects of low-dose metformin and rosiglitazone on biochemical, clinical, metabolic and biophysical outcomes in polycystic ovary syndrome. J Obstet Gynaecol 2013;33:165–70. [DOI] [PubMed] [Google Scholar]
- [25].Fruzzetti F, Ghiadoni L, Virais A, et al. Adolescents with classical polycystic ovary syndrome have alterations in the surrogate markers of cardiovascular disease but not in the endothelial function. The possible benefits of metformin. J Pediatr Adolesc Gynecol 2016;29:489–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Elkind-Hirsch K, Marriontaux O, Bhushan M, et al. Comparison of single and combined treatment with exenatide and metformin on menstrual cyclist in overweight women with polycystic ovary syndrome. J Clin Endocrinol Metab 2008;93:2670–8. [DOI] [PubMed] [Google Scholar]
- [27].Heutling D, Schulz H, Nickel I, et al. Asymmetrical dimethylarginine, inflammatory and metabolic parameters in women with polycystic ovary syndrome before and after metformin treatment. J Clin Endocrinol Metab 2008;93:82–90. [DOI] [PubMed] [Google Scholar]
- [28].Jakubowska J, Milewicz A, Szymczak J, et al. Plasma cytokines in obese women with polycystic ovary syndrome, before and after metformin treatment. Gynecol Endocrinol 2008;24:378–84. [DOI] [PubMed] [Google Scholar]
- [29].Jensterle M, Sebestjen M, Janez A, et al. Improvement of endothelial function with metformin and rosiglitazone treatment in women with polycystic ovary syndrome. Eur J Endocrinol 2008;159:399–406. [DOI] [PubMed] [Google Scholar]
- [30].Hoeger K, Davidson K, Kochman L, et al. The impact of metformin, oral contraceptives, and lifestyle modification on polycystic ovary syndrome in obese adolescent women in two randomized, placebo-controlled clinical trials. J Clin Endocrinol Metab 2008;93:4299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Sathyapalan T, Cho LW, Kilpatrickt ES, et al. A comparison between rimonabant and metformin in reducing biochemical hyperandrogenaemia and insulin resistance in patients with polycystic ovary syndrome (PCOS): a randomized open-label parallel study. Clin Endocrinol (Oxf) 2008;69:931–5. [DOI] [PubMed] [Google Scholar]
- [32].Cetinkalp S, Karadeniz M, Erdogan M, et al. The effects of rosiglitazone, metformin, and estradiol-cyproterone acetate on lean patients with polycystic ovary syndrome. Endocrinologist 2009;19:94–7. [Google Scholar]
- [33].Aghamohammadzadeh N, Aliasgarzadeh A, Baglar L, et al. Comparison of metformin and cyproteroneestrodiol compound effect on hs c-reactive protein and serum androgen levels in patients with poly cystic ovary syndrome. Pak J Med Sci 2010;26:347–51. [Google Scholar]
- [34].Luque-Ramírez M, Escobar-Morreale HF. Treatment of polycystic ovary syndrome (PCOS) with metformin ameliorates insulin resistance in parallel with the decrease of serum interleukin-6 concentrations. Horm Metab Res 2010;42:815–20. [DOI] [PubMed] [Google Scholar]
- [35].Esfahanian F, Zamani MM, Heshmat R, et al. Effect of Metformin compared with hypocaloric diet on serum C-reactive protein level and insulin resistance in obese and overweight women with polycystic ovary syndrome. J Obstet Gynaecol Res 2013;39:806–13. [DOI] [PubMed] [Google Scholar]
- [36].Victor VM, Rovira-Llopis S, Diaz-Morales N, et al. Effects of metformin on mitochondrial function of leukocytes from polycystic ovary syndrome patients with insulin resistance. Eur J Endocrinol 2015;173:683–91. [DOI] [PubMed] [Google Scholar]
- [37].Mehrabian F, Ghasemi-Tehrani H, Mohamadkhani M, et al. Comparison of the effects of metformin, flutamide plus oral contraceptives, and simvastatin on the metabolic consequences of polycystic ovary syndrome. J Res Med Sci 2016;21:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Sathyapalan T, Javed Z, Kilpatrickt ES, et al. Endocannabinoid receptor blockade increases vascular endothelial growth factor and inflammatory markers in obese women with polycystic ovary syndrome. Clin Endocrinol (Oxf) 2017;86:384–7. [DOI] [PubMed] [Google Scholar]
- [39].Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril 2004;81:19–25. [DOI] [PubMed] [Google Scholar]
- [40].Carmina E. Diagnosis of polycystic ovary syndrome: from NIH criteria to ESHRE-ASRM guidelines. Minerva Ginecol 2004;56:1–6. [PubMed] [Google Scholar]
- [41].Davis BJ, Xie Z, Viollet B, et al. Activation of the AMP-activated kinase by antidiabetes drug metformin stimulates nitric oxide synthesis in vivo by promoting the association of heat shock protein 90 and endothelial nitric oxide synthase. Diabetes 2006;55:496–505. [DOI] [PubMed] [Google Scholar]
- [42].Asemi Z, Esmaillzadeh A. DASH diet, insulin resistance, and serum hs-CRP in polycystic ovary syndrome: a randomized controlled clinical trial. Horm Metab Res 2015;47:232–8. [DOI] [PubMed] [Google Scholar]
- [43].Moradi S, Mollabashi M, Kerman SR. Relation between C-reactive protein and body mass index in patients with polycystic ovarian syndrome. Gynecol Endocrinol 2011;27:480–5. [DOI] [PubMed] [Google Scholar]
- [44].Möller K, Ostermann AI, Rund K, et al. Influence of weight reduction on blood levels of C-reactive protein, tumor necrosis factor-(, interleukin-6, and oxylipins in obese subjects. Prostaglandins Leukot Essent Fatty Acids 2016;106:39–49. [DOI] [PubMed] [Google Scholar]
- [45].Bruno RV, de Avila MA, Neves FB, et al. Comparison of two doses of metformin (2.5 and 1 5 g/day) for the treatment of polycystic ovary syndrome and their effect on body mass index and waist circumference. Fertil Steril 2007;88:510–2. [DOI] [PubMed] [Google Scholar]
- [46].Chen Y, Li M, Deng H, et al. Impact of metformin on C-reactive protein levels in women with polycystic ovary syndrome: a meta-analysis. Oncotarget 2017;8:35425–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Dregan A, Charlton J, Chowienczyk P, et al. Chronic inflammatory disorders and risk of type 2 diabetes mellitus, coronary heart disease, and stroke: a population-based cohort study. Circulation 2014;130:837–44. [DOI] [PubMed] [Google Scholar]
- [48].Bekwelem W, Lutsey PL, Loehr LR, et al. White blood cell count, C-reactive protein, and incident heart failure in the Atherosclerosis Risk in Communities (ARIC) Study. Ann Epidemiol 2011;21:739–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Parrinello CM, Lutsey PL, Ballantyne CM, et al. Six-year change in high-sensitivity C-reactive protein and risk of diabetes, cardiovascular disease, and mortality. Am Heart J 2015;170:380–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Kuo FC, Huang YH, Lin FH, et al. Circulating soluble IL-6 receptor concentration and visceral adipocyte size are related toinsulin resistance in Taiwanese adults with morbid obesity. Metab Syndr Relat Disord 2017;15:187–93. [DOI] [PubMed] [Google Scholar]
- [51].Samy N, Hashim M, Sayed M, et al. Clinical significance of inflammatory markers in polycystic ovary syndrome: their relationship to insulin resistance and body mass index. Dis Markers 2009;26:163–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Tsilchorozidou T, Mohamed-Ali V, Conway GS. Determinants of interleukin-6 and C-reactive protein vary in polycystic ovary syndrome, as do effects of short- and long-term metformin therapy. Horm Res 2009;71:148–54. [DOI] [PubMed] [Google Scholar]


