ABSTRACT
Salmonella enterica serovar Typhimurium (S. Typhimurium) is a Gram-negative pathogen that causes various host-specific diseases. During their life cycle, Salmonellae survive frequent exposures to a variety of environmental stresses, e.g. carbon-source starvation. The virulence of this pathogen relies on its ability to establish a replicative niche, named Salmonella-containing vacuole, inside host cells. However, the microenvironment of the SCV and the bacterial metabolic pathways required during infection are largely undefined. In this work we developed different biological probes whose expression is modulated by the environment and the physiological state of the bacterium. We constructed transcriptional reporters by fusing promoter regions to the gfpmut3a gene to monitor the expression profile of genes involved in glucose utilization and lipid catabolism. The induction of these probes by a specific metabolic change was first tested in vitro, and then during different conditions of infection in macrophages. We were able to determine that Entner-Doudoroff is the main metabolic pathway utilized by Salmonella during infection in mouse macrophages. Furthermore, we found sub-populations of bacteria expressing genes involved in pathways for the utilization of different sources of carbon. These populations are modified in presence of different metabolizable substrates, suggesting the coexistence of Salmonella with diverse metabolic states during the infection.
KEYWORDS: bacteria adaptation, flow cytometric analysis, infection, metabolism, Salmonella, transcriptional reporters
Introduction
Salmonellais a Gram-negative enteric bacterial pathogen that can infect a variety of hosts including birds, reptiles and mammals. Salmonella entericasubsp. entericaserovar Typhimurium (S. Typhimurium) causes a self-limiting gastroenteritis in humans whereas the closely related serovar S. Typhi produces typhoid fever, a frequently fatal systemic disease. Salmonella is a major public health issue causing more than one billion new human infections each year that lead to more than three million deaths.1 The problem is greatly exacerbated by the emergence of multi-drug resistant strains.2 In addition, S. Typhimurium is studied because it induces in mice a systemic infection with some similarities to human typhoid fever. The virulence of Salmonella requires its intracellular replication within a membrane-bound compartment called the Salmonella-containing vacuole (SCV),3 and the expression of a Type 3 Secretion System (T3SS-2)4-9 that allows Salmonella to traslocate bacterial effectors into the infected cells.10-14 Salmonellae are capable of infecting and replicating in many cell types, but are thought to primarily replicate in macrophages.15-17 This is supported by observations that Salmonella strains that are defective for macrophage replication are avirulent in mouse models of infection.18
In order to survive and efficiently replicate in host cells, intracellular pathogens must adapt their metabolism to the available nutrients and physical conditions encountered (mainly pH, oxygen availability and osmotic pressure).20-23 There exist more than 100 suitable carbon substrates available, within the numerous niches that bacteria can find in vertebrate bodies, as well as various nitrogen, phosphorous and sulfur sources.23-25 Most of the existing studies related to the interactions between Salmonella and host cells have focused on microbial virulence factors. There are scarce reports related to the characterization of the in vivometabolism of Salmonella or other intracellular bacterial pathogens, due essentially to important methodological limitations.23,24,26 However, recent technical developments in this field, as high-throughput methods (microarrays, proteomics), reporter systems, studies with mutant strains and 13C-isotopologue profiling assays are beginning to provide insights about this important aspect of pathogenesis.
There exists evidence that glucose is the main source of carbon during Salmonella infection in different models.25,27-32 However, there is no consensus regarding the metabolic pathways that this pathogen privileges to catabolize glucose. The Entner-Doudoroff and the glycolysis pathways have been proposed as the most probable routes of glucose consumption.25-27 Bowden et al. showed that mutants deficient in glycolysis and glucose transport are severely attenuated in replication in RAW 264.7 mouse macrophages.32 It was also proposed that during infection of BALB/c mice, S.Typhimurium strain SR-11 grows in a mixed mode using limiting glycolytic sugars and either amino acids or tricarboxylic acid (TCA) cycle intermediates, allowing continuous formation of pyruvate from malate.30 The authors found that not enough pyruvate is generated through glycolysis to satisfy the metabolic requirements of the bacteria and for full virulence, suggesting that SR-11 grows in vivoin an environment scarce in glycolytic sugars.30
On the other hand, a group of studies highlighted the relevance of lipid metabolism for the virulence and survival of Salmonella during infection. Salmonella, as Escherichia coli, contains the complete set of β-oxidation genes for the catabolism of fatty acids (fad), and is able to grow in phosphatidyl-serine and oleic acid as unique carbon sources.33-36 Moreover, it was reported that the expression of the acyl-coenzyme A dehydrogenase gen (fadE) from the β-oxidation cycle, is induced during infection in epithelial MDCK cells,37 suggesting that the intravacuolar environment contains fatty acids available to be used as carbon and energy sources. In line with this hypothesis, limited concentrations of glucose were measured intracellularly in this model. fadBthat encodes for a key β-oxidation enzyme with several enzymatic activities is one of the most induced genes during mice infection.38 Malic enzyme39 and isocitrate liase aceBA,40 which are central enzymes of the glyoxylate cycle, are also required for normal growth and persistence of Salmonella in vivo.Yet, fadgenes are not required for full virulence in mouse.29,40 These results suggest that the accessibility of different metabolites in the SCV could evolve during the course of infection, increasing the dependence of fatty acids and acetate utilization during chronic infection.
In general, these metabolic studies are based on methods that provide an average measurement for the total bacterial population. Therefore, a new and relevant aspect of the infection process to be evaluated is the possibility that sub-populations of bacteria with different metabolic states or with diverse availability of nutrients coexist during the process of infection. In this work, we studied the expression of Salmonella genes involved in the main catabolic pathways during infection of RAW 264.7 macrophages and found the concomitant existence of sub-populations of bacteria expressing genes corresponding to different catabolic pathways for the utilization of diverse carbon sources. The proportion of these populations was modified with the presence of different metabolizable substrates, suggesting the coexistence of Salmonella with diverse metabolic states during the infection.
Results
Analysis of the expression of the β-oxidation genes in axenic cultures and during macrophage infection using fluorescence probes
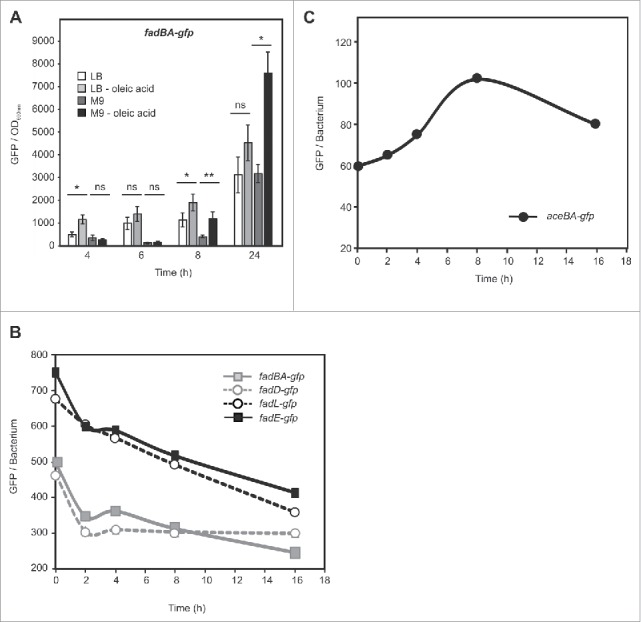
Differential gene expression profiling (DGEP) and13C-isotopologue profiling analysis (13C-IPA) suggest that glucose, glucose-6P and gluconate are the main carbon sources for intracellular S. Typhimurium,25 while other studies provide evidences that fatty acid catabolism and glyoxylate shunt are relevant metabolic pathways during certain steps of the infection process.37-41 To gain insights about the metabolic relevance of these two pathways in the course of infection, five transcriptional fusions were constructed by cloning the promoter region of different genes involved in β-oxidation cycle and the glyoxylate shunt upstream of the reporter gene gfpmut3A,42-45 and used to transform a wild-type strain of Salmonella. We first evaluated the promoter activity of the following genes: fadBA,encoding for the fatty acid oxidizing complex; fadL, encoding for long-chain fatty acid outer membrane transporter; fadD,encoding for the acyl-CoA synthase; fadE,encoding for the acyl-CoA dehydrogenase (also named as fadF,37); and aceBA,encoding for the malate synthase A and the isocitrate lyase, respectively. A Salmonella strain harboring fadBA-gfp fusion was grown in Luria-Bertani (LB) broth and minimal medium (M9) supplemented or not with oleic acid and the GFP fluorescence of bacteria was analyzed at different time points (Fig. 1A). The rich LB broth contains a low concentration of glucose and other sugars than can be metabolized by bacteria allowing a very short steady-state growth. Then the cells must switch their metabolism to use amino acids as a carbon source.46 M9 minimal medium contains salts, phosphate, sulfate, ammonium minerals and 0.2% of glucose as carbon source. In both media the presence of oleic acid led to an increase of the fadBApromoter activity (Fig. 1A), suggesting that the fadBA-gfp reporter is functional and responds to the presence of this fatty acid. The activation of the promoter was significant after 8 hours of growth, in late exponential phase, time at which the concentration of glucose in the medium decreases, thereby releasing the catabolic repression of the promoter. A Salmonella strain porting the empty pFPV25 plasmid was used as a control; the very low basal fluorescence value obtained for this construction was subtracted to those values obtained for all the reporter fusions used in this study (Fig S1A). A dose-dependent induction of the fadBA-gfp fusion was also observed when the bacteria were submitted to different amounts of oleic acid (Fig. S1B). These results were confirmed by recording the β-galactosidase activity of a Salmonella strain harboring a single chromosomal copy of fadBA-lacZ reporter fusion and grown in LB or M9 media with or without addition of oleic acid (Fig. S1C). In the same way, similar results were obtained for other β-oxidation transcriptional fusions: fadD-gfp, fadL-gfp, and fadE-gfp (Fig. S1D and data not shown). Correspondingly, the aceBA-gfp reporter presented an increased activation when the Salmonella strain harboring this fusion was grown in presence of acetate (Fig. S1E and F).
Figure 1.
Analysis of Salmonellaβ-oxidation and glyoxylate shunt pathways in axenic cultures and during macrophages infection. (A) Wild-type Salmonella (12023) carrying the fadBA-gfp fusion was grown in LB or M9 supplemented or not with oleic acid. GFP synthesis was recorded over 24 hours using a fluorimeter. The fluorescence levels shown on the graphics were calculated as the GFP values reported to the OD600. Values are means ± SD of 3 independent experiments. Unpaired t-test was used to determine whether two values were significantly different. P-values: ns, not significant; *, P < 0.05; **, P < 0.01. (B and C) Opsonized wild-type strains carrying the fadBA-gfp, fadD-gfp, fadL-gfp, fadE-gfp or aceBA-gfp fusions were phagocytized by activated RAW 264.7 cells. 0, 2, 4, 8 and 16 hours post infection, macrophages were lysed, and the mean fluorescence of bacteria extracted from them was determined by flow cytometry and plotted as a function of time. These data are representative of at least three independent experiments.
On the basis of these results, we next aimed to detect if intracellular Salmonella activates fatty acid catabolism and/or the glyoxylate shunt. For this, we performed macrophage infection assays with Salmonella strains containing the fadBA-gfp, fadD-gfp, fadL-gfp, fadE-gfp, or aceBA-gfp reporters. The bacteria were grown in M9 during 3.5 hours in order to maintain a low induction of the reporters, and used to infect activated RAW 264.7 macrophages grown in DMEM medium. Intracellular bacteria were extracted after different times of infection, and their fluorescence levels monitored by flow cytometry. Single peak of GFP histograms were recorded for the different Salmonella strains extracted from macrophages, indicating that the entire population of intracellular bacteria was responding to the vacuolar environment (data not shown). The mean GFP levels of the four strains, carrying the fad reporters, decrease significantly soon after infection (Fig. 1B). Conversely, intracellular bacteria harboring the aceBA-gfp reporter presented a rapid increase of the promoter activity until 8 hours after infection (Fig. 1C). At later time points, the GFP level decreased. These results indicate that Salmonella does not use the β-oxidation pathway during RAW 264.7 macrophages infection in DMEM medium under these conditions; however, the glyoxylate shunt is activated during the first hours of infection. Confocal microscopy analysis of RAW 264.7 macrophages infected with Salmonella strains porting the fusions fadBA-gfp, fadD-gfp, or aceBA-gfp, or the plasmids pFPV25 and pFPV25.1 as controls, confirmed the results obtained by flow cytometric analysis (Suppl. Fig 2).
Figure 2.
Cytometry analysis of Salmonella β-oxidation and glyoxylate shunt pathways during in vivo infections. (A and B) C57BL/6 mice were infected for 48 hours with wild-type strains carrying the fluorescence fusions. The relative fluorescence intensity of the injected bacteria and bacteria present in spleens was determined by flow cytometry. (A) Histogram graphs that display the relative GFP fluorescence versus the number of events shows a Gaussian distribution indicating GFP expressing bacteria. Wild-type Salmonella carrying the aceBA-gfp fusion before infection (light blue, control), extracted from spleens (blue, green, red, and orange, each color correspond to a different mouse) (n = 4). (B) C57BL/6 mice (n = 4) were infected for 48 hours with wild-type strains carrying the fadBA-gfp, fadD-gfp, or aceBA-gfp fusions. The relative fluorescence intensity of the injected bacteria and bacteria present in spleens was determined by flow cytometry. Unpaired t-test was used to determine whether two values were significantly different. P-values: *, P < 0.05; ***, P < 0.001.
Analysis of Salmonella β-oxidation and glyoxylate shunt pathways during mouse infection
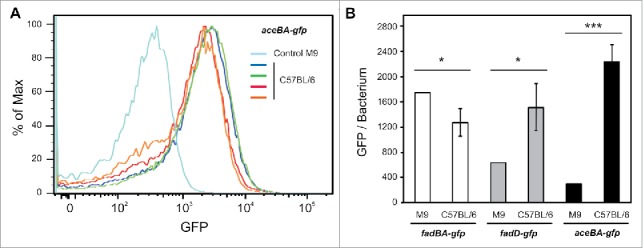
During in vivoinfection Salmonella is found in various organs and different cell types, and infection of macrophages may not necessarily reflect the complexity of environments encountered in vivo. Thus, we also analyzed the activity of the fadBA, fadD and aceBA promoters during mice infection. For this C57BL/6 mice were infected with Salmonella strains containing the reporters, and after 2 days the bacteria present in the spleens were extracted and analyzed by flow cytometry. We found that the GFP level corresponding to the fadBA promoter was slightly lower compared with bacteria of the inoculum, while the fluorescence corresponding to the aceBA promoter was noticeably increased (Fig. 2A and B and Suppl. Fig. 3). These results are similar to those obtained for these two reporters in RAW 264.7 macrophages. Interestingly, the activity of the fadD promoter also increased significantly in vivo(Fig. 2B). Taken together, these results show that, under these conditions, the β-oxidation pathway is repressed and glyoxylate shunt activated in mice and in cultured cells. However, the discrepancy observed for the activity of the fadD promoter between both models of infection, suggests that the environment of the SCV is probably richer in fatty acids in vivo, and that infection experiments performed in cultured cells do not reflect the complexity of conditions found in the context of infected animals. Nevertheless, even if fatty acids are internalized by bacteria, they are probably not catabolized by the β-oxidation pathway since the expression of fadBA genes is repressed.
Figure 3.
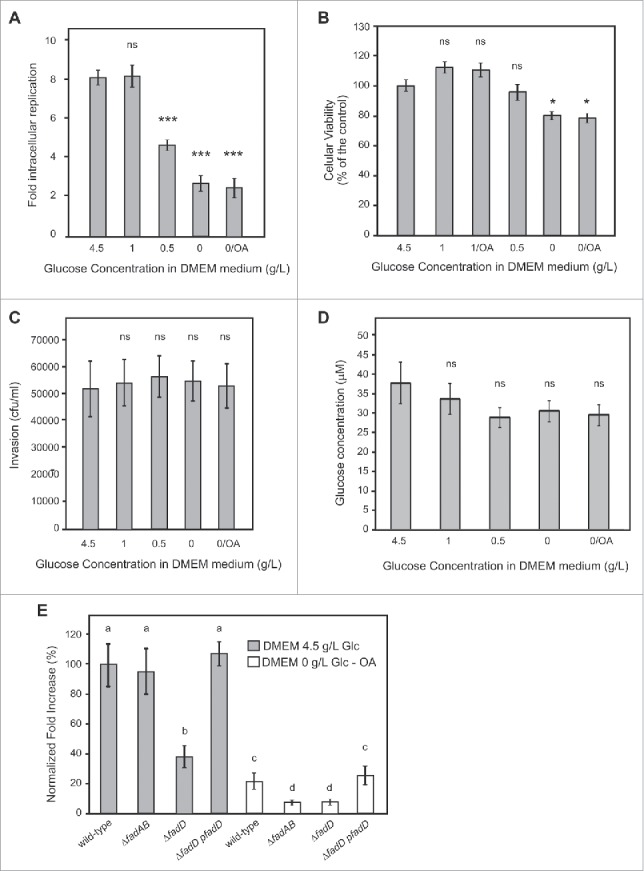
Analysis of Salmonella intracellular replication, internal glucose concentration, and macrophages viability and phagocytic capacity in presence of different concentration of glucose in the infection medium. (A-D) Activated RAW 264.7 macrophages grown in the presence of different concentrations of glucose (from 0 to 4.5 g/L) or with oleic acid were used for different assays. (A) RAW 264.7 cells were infected with wild-type strains and lysed at 2 and 16 hours post infection for enumeration of intracellular bacteria. The values shown represent the fold increase calculated as a ratio of the intracellular bacteria between 16 and 2 hours. Values are means ± SD (n = 4). (B) Cell viability was evaluated by MTT assay. (C) RAW 264.7 macrophages were infected with wild-type strains and lysed at 2 hours for enumeration of intracellular bacteria. (D) RAW 264.7 cells were grown for 24 hours, collected by centrifugation, and lysed. Glucose concentration was determined by a fluorometric assay.74 (A-D) Values are means ± SD of 3 independent experiments. An unpaired t-test was used to determine whether a value was significant different from the control. ns, not significant; *, P < 0.05; ***, P < 0.001. (E) Activated RAW 264.7 macrophages were infected with wild-type, β-oxidation mutants (ΔfadBA, ΔfadD), or a complemented (ΔfadD-pfadD) strains and lysed at 2 and 16 hours post infection for enumeration of intracellular bacteria. The values shown represent the fold increases calculated as a ratio of the intracellular bacteria between 16 and 2 hours and normalized to that of the wild-type strain in DMEM medium supplemented with 4.5 g/L of glucose. Normalized fold increase of wild type strain in DMEM media supplemented with oleic acid or mutants in both media against the wild-type (WT) strain in DMEM media supplemented 4.5 g/l of glucose were determined. Values are means ± SD of 3 independent experiments. One-way ANOVA and Tukey post-tests were used to determine whether the values were significantly different. Different letters (a, b, c, and d) indicate statistically significant differences between groups (mean ± SE). P-values: a vs. b, P < 0.05; a vs. c and a vs. d, P < 0.001; c vs. d P < 0.01.
The extracellular concentration of glucose influences Salmonella survival in RAW 264.7 macrophages
DMEM medium, commonly used to grow macrophages and other cell lines and to carry out infection experiments contains 4.5 g/L of glucose, which is a very high concentration compared with the physiological level of this sugar in the blood of vertebrates.47-49 Moreover, the host cells can be exposed to different concentration of glucose in different organs. It has also been demonstrated that S. Typhimurium preferentially associates with anti-inflammatory/M2 macrophages, which degrade and use fatty acids as a source of carbon and energy.28 To further understand the physiological state of Salmonella during infection, we analyzed its replication in RAW 264.7 macrophages previously exposed, during 24 hours, to different concentrations of glucose (from 0 to 4.5 g/L) in DMEM medium. Furthermore, with the purpose of generating a SCV environment enriched in fatty acids, we grew macrophages during 24 hours in DMEM medium in the absence of glucose and with the supplementation of oleic acid (OA) in the presence of BSA as carrier.50,51 We observed that concentrations of glucose of 0.5 g/L or below lead to a replication defect in macrophages (Fig. 3A). Fig. 3B shows that RAW 264.7 macrophages cultured in the absence of glucose during 24 hours presented a reduction of viability of 20%, whereas the intracellular replication decreased 3.2-fold independently of the presence of oleic acid. Also, the viability of macrophages was not altered in the presence of 0.5 g/L of glucose (Fig. 3B) while this condition leads to an intracellular Salmonella replication defect of 1.8-fold compared with those infecting macrophages grown with a concentration of glucose > 1 g/L (Fig. 3A). We then analyzed if the DMEM concentration of glucose had an effect on the levels of Salmonella uptake. For these experiments, we enumerated bacteria 2 hours post infection (PI), and found that the growth of macrophages with different concentrations of glucose did not affect the uptake of Salmonella by the host (Fig. 3C). Then, we measured the intracellular concentration of glucose 28 after 24 hours of incubation in DMEM supplemented with different concentrations of the sugar and in the presence or not of OA. We found that intracellular levels of glucose were mildly affected under the tested conditions (Fig. 3D). The intracellular concentration of glucose decreased less than 25% when macrophages were grown in a concentration of this carbohydrate ≤ 0.5 g/L. These results indicate that temporary reductions in the external concentration of glucose do not strikingly affect the intracellular concentration of this sugar, nor the phagocytic capacity of macrophages, but have a marked effect on the replication of bacteria into the SCV, suggesting that the availability of the carbon source inside the vacuole is limited. Finally, we infected RAW 264.7 macrophages with Salmonella wild type and with mutant strains deficient in β-oxidation (ΔfadBA) or in fatty acid transport (ΔfadD). These experiments were performed in the context of the two extreme conditions analyzed before: DMEM supplemented with 4.5 g/L of glucose (DMEM-highGlc) and DMEM without glucose and supplemented with 400 µM of OA (DMEM-nonGlc-OA). In DMEM-highGlc the intracellular replication of the ΔfadBA mutant did not statistically differ from the wild type strain whereas those of the ΔfadD mutant was 2.79-fold lower (Fig. 3E). In DMEM-nonGlc-OA, the wild type strain showed a marked deficiency in replication, compared with the same strain in DMEM-highGlc (Fig. 3A and E). The effect was even more substantial when infections were performed with mutant strains ΔfadBA and ΔfadD in DMEM-nonGlc-OA, showing a reduction in replication of 2.38- and 2.53-fold compared with the wild type strain in the same medium. An efficient trans complementation was observed when the ΔfadD strain was transformed with a FadD expressing plasmid (Fig. 3E). All together these results indicate that, in macrophages, restricted external concentrations of glucose during 24 hours lead to limited levels of glucose into the SCV, and directly affect the intracellular replication of Salmonella. Also, the β-oxidation pathway, which is dispensable in the presence of glucose gains relevance in a medium starved for glucose. Interestingly, the product of the fadD gene, the acyl-CoA synthetase, appears to be necessary for replication even in the presence of high glucose concentration in the medium, suggesting that this enzyme could have an additional role besides the β-oxidation.
The extracellular concentration of glucose modifies the metabolic state of Salmonella in RAW 264.7 macrophages
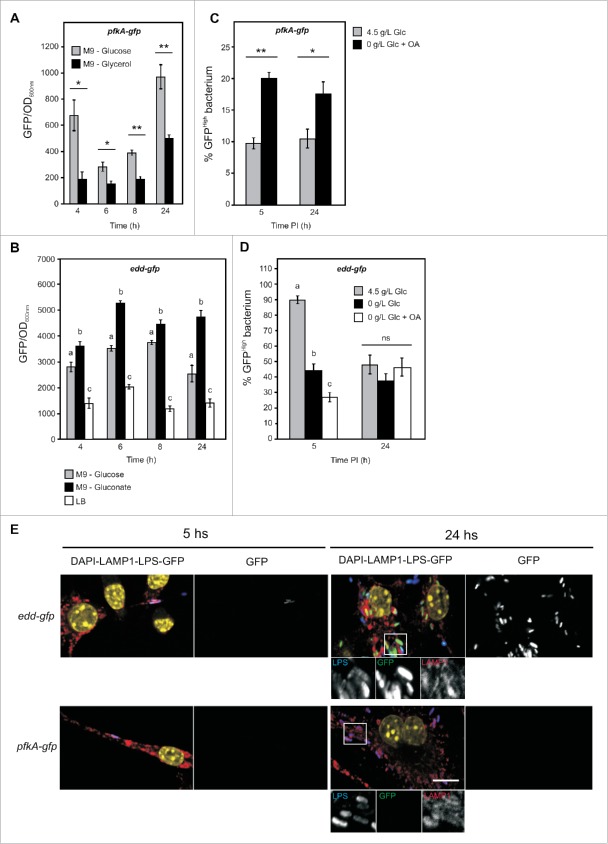
Next we analyzed, by flow cytometry, if changes in the external concentration of glucose induce any modification in the expression profile of Salmonella genes involved in fatty acid catabolism and/or glyoxylate shunt during infections of macrophages. In order to not induce the probe before the infection, we grew a Salmonella strain harboring the fadBA-gfp reporter during 3.5 hours in M9 (Fig. 1A, and Suppl. Fig. 4) and infected activated RAW 264.7 macrophages grown in different DMEM media. We observed a clear reduction of the mean GFP levels during the infections, regardless of the presence or the absence of glucose in the external medium (Fig. 1B and Fig. 4A). At 5 hours post infection, most of the Salmonella extracted from macrophages grown in DMEM-nonGlc-OA showed GFP fluorescence levels (blue curve) similar to those found for the bacteria extracted from macrophages grown in DMEM-highGlc (black curve) (Fig. 4B). This indicates that the majority of bacteria within RAW 264.7 macrophages exhibit a very low or no expression of fadBA. However, in DMEM-nonGlc-OA, we also observed a second population of bacteria with a higher GFP fluorescence mean. This population, hereafter referred to as GFPhigh bacteria, corresponds to Salmonella in which expression of fadBA is induced. Although, this GFPhigh population did not exceed 12% of the total Salmonella population, it was significantly more prevalent in macrophages grown in DMEM-nonGlc-OA that in those grown in DMEM-highGlc (6% approximately), at different times of infection (Fig. 4C). Similar results were observed for bacteria harboring the reporter fadD-gfp (Fig. 4D and Suppl. Fig. 5), indicating that a subpopulation of intracellular bacteria was responding to the limiting concentration of glucose and the presence of fatty acids. Remarkably, a different GFP expression profile was observed for the aceBA promoter (Fig. 4E). In this case, the percentage of GFPhigh population was prevalent in DMEM-highGlc, resulting in values of 30.9 and 70.9% at 5 and 16 hours, respectively, compared with 9.2 and 16.4% obtained in DMEM-nonGlc-OA. Interestingly, at 24 hours post infection, the recovered GFPhigh population represented 5.2% of the total bacteria in DMEM-highGlc, and 14.5% in DMEM-nonGlc-OA. These results indicate that even in the presence of high concentrations of glucose, Salmonella induces and maintains the expression of the glyoxylate shunt until 16 hours post infection in RAW 264.7 macrophages. At all time points evaluated in DMEM-nonGlc-OA medium, aceBA-gfp was induced in a low percentage of bacteria (below 16.4%), suggesting that this metabolic pathway is less representative under this condition.
Figure 4.

Salmonella present sub-populations expressing genes corresponding to different source of carbon utilization pathways during macrophage infection. (A) Wild-type strain, carrying the fadBA-gfp fusion, was cultured in M9 during 3.5 hours, bacteria were then opsonized and phagocytized by activated RAW 264.7 cells. 0, 5, 16 and 24 hours post infection, macrophages were lysed, and the mean fluorescence of bacteria extracted from them was determined by flow cytometry and plotted as a function of time. These data are representative of at least three independent experiments. (B) At 5 hours post infection, the relative GFP fluorescence intensity of bacteria extracted from macrophages, cultured in DMEM-highGlc (black) or DMEM-nonGlc-OA (blue), was determined by flow cytometry. Between 5000 and 10.000 bacteria were analyzed for each sample. A small population of Salmonella extracted from infected macrophages is highly fluorescent (GFPhigh). (C-E) Wild-type strains, carrying the fadBA-gfp (C), fadD-gfp (D), or aceB-gfp (E) fusions, were cultured in M9 during 3.5 h, opsonized and phagocytized by activated RAW 264.7 cells, cultured in DMEM-highGlc or DMEM-nonGlc-OA. 5, 16, and 24 hours post infection, macrophages were lysed, and the fraction of GFP high bacteria extracted from macrophages was determined by flow cytometry. An unpaired t-test was used to determine whether the values were significantly different. P-values: *, P<0.05; **, P<0.01; ***, P<0.001. (F) Wild-type strain, carrying the fadBA-gfp fusion, was cultured in M9 during 16 hours, bacteria were then opsonized and phagocytized by activated RAW 264.7 cells, cultured in different media. 5 and 24 hours post infection, macrophages were lysed, and the mean fluorescence of bacteria extracted from macrophages was determined by flow cytometry. One-way ANOVA was used to determine whether the values were significantly different. Different letters (a, b, c, and d) indicate statistically significant differences between groups (mean ± SE, P<0.05). ns, not significant.
Figure 5.
Salmonella prioritizes the Entner-Doudoroff pathway during infections in macrophages. Wild-type Salmonella carrying the pfkA-gfp (A) or edd-gfp (B) reporters were grown in LB or M9 medium supplemented with glucose, glycerol, or gluconate. Bacterial growth was resumed and GFP synthesis was recorded over 24 h using a fluorimeter. The fluorescence levels shown on the graphics were calculated as the GFP values reported to the OD600. Values are means ± SD of 3 independent experiments. (A) An unpaired t-test was used to determine whether the values were significantly different. P-values: *, P<0.05; **, P<0.01. (B) One-way ANOVA was used to determine whether the values were significantly different. Different letters (a, b and c) indicate statistically significant differences between groups (mean ± SE, P<0.05). Opsonized wild-type strain carrying the pfkA-gfp (C) or edd-gfp (D) fusions were phagocytized by activated RAW 264.7 cells, cultured in different DMEM media. 5 and 24 hours post infection, macrophages were lysed, and fraction of GFPhigh bacteria extracted from them was determined by flow cytometry. (C) An unpaired t-test was used to determine whether the values were significantly different. P-values: *, P<0.05; **, P<0.01. (D) One-way ANOVA was used to determine whether the values were significantly different. Different letters (a, b and c) indicate statistically significant differences between groups (mean ± SE, P<0.05). ns, not significant. (E) Opsonized wild-type strain carrying the pfkA-gfp or edd-gfp fusions were phagocytosed by RAW 264.7 cells grown on coverslips in DMEM medium supplemented with 4.5 g/L of glucose. 5 or 24 hours post infection macrophages were fixed, immunostained for LPS and LAMP1, and imaged by confocal microscopy for GFP (green), LPS (blue), LAMP1 (red), and nuclei (yellow). The voltage gain of the GFP photomultiplier was increased until Salmonella expressing GFP under the control of a reference constitutive promoter (rpsM-gfp, shown in Fig S2 presented few saturated pixels and was kept unchanged to image macrophages infected with other Salmonella carrying other reporters. Magnified insets showing grayscale images for LPS (left), GFP (middle) and LAMP1 (right) are presented (scale bar, 10 µm). Glc is abbreviation for glucose.
In order to evaluate if the initial metabolic state of Salmonella influences its intracellular physiology or behavior, we grew a Salmonella strain harboring the fadBA-gfp reporter cassette during 16 hours in M9 medium and infected RAW 264.7 macrophages grown in four different DMEM media: 0.5 g/L of glucose and supplemented with OA, 1 g/L of glucose and supplemented or not with OA, and 4.5 g/L of glucose. Under these conditions the fadBA-gfp reporter was already induced at the moment of the infection (Fig. 1A and Suppl. Fig. 4). Fig. 4F shows that more than 40% of the bacteria maintain the induction of fadBA promoter until 5 hours post infection compared with the 5.2% of GFPhigh population observed for Salmonella which have not induced the pathway previous to the infection (Fig. 4C). In these experiments the only difference consists in the inoculum of the pathogen. In addition, for the fadBA-gfp fusion, at 5 h post infection, the percentage of GFPhigh populations were slightly higher when infection medium had lower glucose concentration or was supplemented with OA. At 24 hours post infection, the levels of bacteria expressing high GFP fluorescence were markedly reduced in the four media evaluated, presenting a minimum of 5.3% in DMEM-highGlc, and a maximum of 26.2% in DMEM 0.5 g/L of glucose and supplemented with OA (Fig. 4F). These results suggest that various populations of bacteria, with different metabolic state, coexist into the macrophages during the infection. Also, the initial metabolic states of the pathogen in the inoculum and the medium utilized for the infection can influence the proportion of bacteria expressing genes corresponding to a particular pathway.
Entner-Doudoroff is the main metabolic pathway utilized by Salmonella during infections in RAW 264.7 macrophages
Since a small fraction of bacteria activates the β-oxidation cycle during infection of macrophages, we decided to study the prevalence of different catabolic pathways for glucose. For this we generated two new reporters to analyze the level of transcription of the 6-phosphofructokinase from glycolysis (pfkA-gfp) and of the phosphogluconate dehydratase from the Entner-Doudoroff (ED) pathway (edd-gfp). A Salmonella strain carrying the pfkA-gfp fusion was grown in medium M9 supplemented with glucose or glycerol as unique source of carbon, and tested for GFP fluorescence levels. A clear induction of the pfkA promoter was evidenced in the presence of glucose (Fig. 5A). A similar analysis was performed for a strain with the edd-gfp fusion, which was grown in medium M9 supplemented with glucose or gluconate or in LB medium. As compared with bacteria grown, in LB in which the concentration of glucose and other metabolizable sugars is very limited, the edd-gfp reporter was strongly induced in M9 in the presence of glucose and even more in the presence of gluconate (Fig. 5B). These data indicate that both reporters are able to sense the availability of different carbon sources. A dose-dependent induction of the pfkA-gfp and edd-gfp fusions was also observed (Suppl. Fig. 6).
Figure 6.
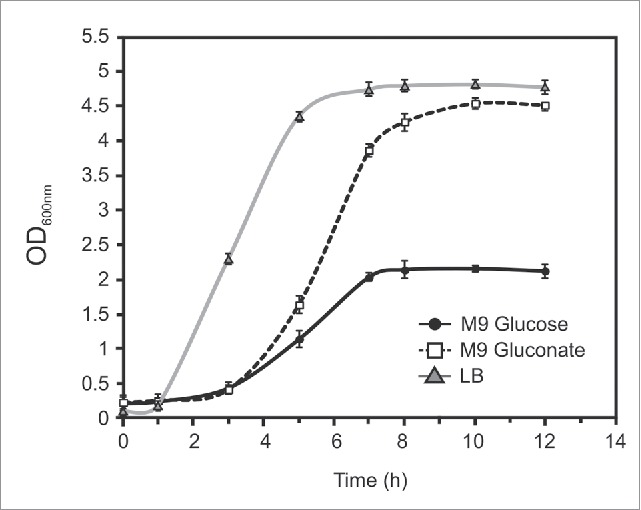
Growth curves of Salmonellain LB and M9 with glucose or gluconate. Wild-type Salmonella was grown during 12 hours in LB or M9 medium supplemented with glucose, or gluconate. OD600 was recorded. These data are representative of at least three independent experiments.
Then, both strains were used to infect RAW 264.7 macrophages cultured in DMEM-highGlc or DMEM-nonGlc-OA. We found that, like for other reporters, Salmonella recovered from macrophages presents a heterogeneous population. Interestingly, the pfkA-gfpreporter presented a higher percentage of GFPhigh population in macrophages cultured in DMEM-nonGlc-OA (Fig. 5C), both at 5 and 24 hours post infection. However, this GFPhigh population was not higher than 20% of the total Salmonella population. In the case of edd-gfp,the percentage of GFPhigh population reached 89.8%, at 5 hours post infection in DMEM-highGlc, and decreased to 44.3 and 26.9% in DMEM-nonGlc and DMEM-nonGlc-OA media, respectively (Fig. 5D). At 24 hours post infection we found between 40 and 50% of GFPhigh bacteria in the three different media. Altogether these results indicate that the Entner-Doudoroff pathway is prevalently used by intracellular Salmonella regardless the presence of glucose or fatty acids. They also show that glycolysis is less relevant even if the expression of 6-phosphofructokinase is increased when the concentration of glucose is limited. Confocal microscopy analysis of RAW 264.7 macrophages cultured in DMEM-highGlc, and infected with Salmonella strains porting the fusions pfkA-gfp, or edd-gfp, confirmed the results obtained by flow cytometric analysis (Fig. 5E).
Finally, in order to test the source of carbon that favors the development of Salmonella in axenic cultures, we grew at 37°C the wild type strain of S.Typhimurium in three different media: LB, M9 supplemented with 0.2% w/v of glucose, or 0.2% w/v of gluconate. Fig. 6 shows that the highest growth rate of Salmonella occurs in LB broth, reaching a final DO at 600 nm of approximately 4.8 at stationary phase. In M9 medium with gluconate the growth curve reached a similar density but presented a longer lag phase. Finally, for Salmonella grown in M9 supplemented with glucose, an important decrease of the growth rate and of the final OD of the cultures was observed. These results suggest that this strain of Salmonella can metabolize gluconate more efficiently than glucose as a source of carbon.
Discussion
Salmonella and other intracellular pathogens must adapt their metabolism to available nutrients and to physical conditions. Several studies have revealed that resistance to antimicrobial peptides, nitric oxide and oxidative killing are important to bacterial survival within macrophages and to virulence.3,52 Microarray studies have shown that several hundreds of S. Typhimurium genes are differentially regulated in response to the nutritional-limited phagosomal environment 27; however, the micro-environment of the SCV has remained largely unknown. Recent studies53-58 provided us with a vision of the in vivometabolism of pathogens within the host cell compartments. Though, gene expression analysis of intracellular bacteria is usually measured at the whole population level, which may mask potential cell-to-cell heterogeneity.59,60 Furthermore, a number of data exist from the comparison of the bacteria growing in LB broth, or a rich media, with those obtained from the intracellular environment, this may also affect the relative conclusions obtained from the analysis.61
In this work we used fluorescence reporter fusions to analyze the relevance of different metabolic pathways used by Salmonella during infections in RAW 264.7 macrophages. We modified the availability of glucose and fatty acids in the medium of infection in order to challenge the adaptation capability of bacteria into the SCV. First we identified that the high concentrations of glucose contained in the host cells growth media, commonly used to evaluate expression and actions of bacterial protein effectors, can mask slight metabolic tuning of the pathogen during infection. This is the case for variations of the β-oxidation genes expression in a minority fraction of the bacteria in culture media supplemented with fatty acid. In E. coliand Salmonella ssp, the expression of genes involved in fatty acid catabolism is negatively controlled by the transcriptional regulator FadR.62,63 High glucose concentrations induce carbon catabolite repression, a regulatory mechanism by which the expression of genes required for the utilization of secondary carbon sources is prevented by the CRP-cAMP complex.64,65 However, during infections in vertebrates the bacteria could face variations in glucose concentrations, and the presence of other sources of carbon. In infected mice, the promoter of the fadBA,a central gene involved in β-oxidation, showed a very low activity suggesting that this pathway is not activated. However, fadDencoding for the acyl-CoA synthetase, a key enzyme for the internalization and activation of fatty acid together with the transporter FadL, is highly expressed. These results indicate that fatty acids could be internalized but not processed by the β-oxidation cycle, during infections in vivo. We have also found that a ΔfadD mutant has a defect in replication inside macrophages, even in the presence of elevated concentration of glucose, suggesting that this enzyme has an additional role besides fatty acid catabolism. In line with these observations, Lucas et al.66 have shown that FadD is a positive regulator of the hilA expression, which is a central player for the host invasion process. The authors suggested that fatty acid and/or their derivatives, activated by FadD, may act as intracellular signals to regulate hilA expression and to increase expression of the SPI-1 Salmonella invasion genes.66,67 In addition, it was found that in E. coli and Sinorhizobium meliloti, FadD activates exogenous long-chain fatty acids (LCFA), but also plays a major role in the activation of endogenous fatty acids released from membrane.68 Recently, it has also been demonstrated that long chain unsaturated free fatty acids (LCUFAs), present in the bacterial growth medium, exert a repressive action on the PhoP/PhoQ system activity that is independent of the fatty acid β-oxidative pathway. This inhibition could be due to a FadD-mediated change in the phospholipid composition of the bacterial membranes that would perturb the catalytic activity of membrane-anchored PhoQ protein.69
The glyoxylate shunt (ICL) is necessary for persistence of Salmonella in vivo, but not for the acute phase of infection.40 However, we unexpectedly found that aceBA genes are highly expressed during infection in mice and also until 16 hours post infection in RAW 264.7 macrophages, even in the presence of elevated concentration of glucose. Then, at 24 hours post infection we observed a dramatic decrease in the percentage of bacteria expressing these genes. Also, in media lacking glucose and supplemented with oleic acid, a low population of Salmonella expressing aceBAwas detected, suggesting that the enzymes involved in the glyoxylate cycle could have less relevance in the long-term infection in macrophages. The glyoxylate cycle is commonly associated to functions in anaplerosis and gluconeogenesis, like the assimilation of C2 compounds and catabolism of fatty acids. However, in E. coli, the glyoxylate shunt can act together with the PEP carboxykinase, in the presence of glucose, in order to complete oxidation of carbohydrates to CO2, and also as a redox-cofactor balancing. Specifically these enzymes could add potential metabolic flexibility to redox metabolism by effectively decoupling catabolic carbon flow from NADPH formation that would occur in parallel with tricarboxylic acid cycle.70 Therefore in the case of Salmonella, the central enzymes of the glyoxylate cycle could be required in different stages of the infection process to optimize its metabolic and energetic status.
We also found that variations of glucose concentration in the culture media during a period of 24 hours did not decrease dramatically the macrophages viability nor cytoplasmic levels of glucose. Yet, an external concentration of glucose <0.5 g/L led to a significant decrease of Salmonella replication within the host, while the levels of bacteria up-take were not affected. These results suggest that the amount of glucose available into the SCV become limiting even if the cytoplasmic concentration of glucose is steady. A ΔfadBA mutant, defective in fatty acid catabolism, did not show reduction in replication into the RAW 264.7 cells, cultured in media with high glucose concentration. However, a ΔfadD mutant, defective in fatty acid activation and transport, showed a clear defect in replication. These results also suggest that FadD could have additional roles apart from the activation of fatty acids that are shuffled into the β-oxidation cycle. Though, both ΔfadBA and ΔfadD mutants showed an important drop for replication in macrophages cultured in low glucose concentration. Consequently, it is possible that in the presence of an excess of glucose, the β-oxidation pathway does not play a significant role in the metabolism of Salmonella during infection, while when the concentration of glucose is limited the catabolism of fatty acids gains relevance for survival and replication of the pathogen. Considering that the availability of fatty acids may vary in different organs during infection in vivo,3 S. Typhimurium may perceive these SCV environment variations and adapt the metabolic gene expression accordingly.
Then, by using variations in the media where macrophages were cultured, we detected heterogeneity of the intracellular bacterial gene expression. The percentages of bacteria expressing fadBA and fadD genes increased when we changed the medium of infection from DMEM-highGlc to DMEM-nonGlc-OA. In both media a minority fraction of bacteria activated the expression of these β-oxidation genes. Nevertheless, in DMEM-nonGlc-OA, ΔfadBA and ΔfadD mutants were defective in replication inside macrophages, suggesting that these small populations could have relevance for survival under conditions with limiting glucose concentrations. Accordingly to this, microarrays data also have showed that the expression of β-oxidation genes of S. Typhimurium in J774 macrophages was up- or down- regulated as compared with bacteria grown in RPMI medium with glucose or in LB medium, respectively.25,71 Consequently, in differential metabolic gene expression analysis it is extremely relevant to consider the origins of the RNAs comparator, since the results inferred may therefore differ substantially.27,72 Further studies also showed that Salmonella upregulates expression of fadE and fadB genes during infection in epithelial MDCK cells37 and mice.38 These data suggest that the intravacuolar environment contains fatty acids available to be used as carbon and energy sources.
We also obtained different gene expression profiles for fadBA-gfp fusion when the Salmonella strain used to infect macrophages was in exponential or in stationary phase of growth in M9 media. In macrophages infected with an inoculum of Salmonella grown in M9 during more than 8 hours, the fadBA-gfp fusion has already a significant level of promoter activity before the infection (Fig. 1A) and approximately half of the population conserves an elevated expression level of fadBA genes early during infection (Fig. 4F). Then, after 24 hours of infection in a medium with elevated concentration of glucose, the percentage of bacteria with high level of fadBA-gfp expression decreases significantly. However, the reduction of the GFPhigh population is moderated when glucose concentration in the medium decreases and/or the media is supplemented with oleic acid. Consequently, variations in the carbon and energy sources availability before and during the infection have inference in the metabolic pathway activated for the bacteria. Consequently, it is critical to consider the initial metabolic state of the bacteria, since the conclusion obtained could be influenced or biased by the origin of the inoculum.
The pfkA and edd genes involved in two different pathways of glucose catabolism, glycolysis and Entner-Doudoroff, respectively, were tested in order to identify which pathway is prioritized by the bacteria for survival and replication inside macrophage. In both media, high and low glucose and supplemented with fatty acids, the fraction of bacteria expressing the 6-phosphogluconate dehydratase was more important than those expressing the phosphofructokinase. This result together with the expression profile observed for aceBA suggest that the metabolism of Salmonella could alternate between glycolysis and ED pathways and between Krebb cycle and glyoxylate shunt in order to optimize the intracellular concentrations of NADH and NADPH and the redox balance of bacteria during the different stages of infection. This could allow the bacteria to fine-tune their fitness and to adapt to different environmental changes.
Finally, we determined that Salmonella grows more efficiently in M9 minimal media using gluconate than glucose as carbon source. Other studies also proposed that Salmonella can use the ED pathway to survive in different host cells, like Caco-2 cells,26 J774-A.1 macrophages,27,71 and HeLa cells.71, 72
Recent studies have shown that low-pH conditions can regulate the expression of virulence and metabolism Salmonella genes.73,74 Consequently, we evaluated ED and β–oxidation pathways in anexic cultures at pH 5.2 and found that the expression of edd gene is not altered by the lower pH conditions (Suppl. Fig. 7). On the other hand, at late time points of growth (more than 8 hours), fadBA expression level showed a decrease at pH 5.2. This effect was more significant in the absence of oleic acid. These results indicate that the expression of certain Salmonella genes, involved in the catabolism of carbon sources, can also be regulated by the pH.
In conclusion, we found that major populations of bacteria survive using glucose and/or gluconate as substrates, while a minority uses fatty acid. The gfpfusion method is a unique tool for the measurement of gene expression in individual bacteria in response to complex environments such as the phagosome, and presents multiple advantages: its specificity to a dedicated pathway and its sensitivity. Moreover, this reporter system can be used in different conditions and can be monitored using various methods (fluorimeter, flow cytometry, quantitative microscopy). Yet, we should keep in mind that the use of plasmids might sometime lead to artifacts arising from their high copy number, their loss in the absence of antibiotic during long-term infection experiments, or an abnormal regulation of promoters. Yet, in this study all the gfp transcriptional reports were evaluated first in axenic culture and the fadBA promoter profile was also validated by a chromosomal single copy construction using a lacZreporter. A specific LPS Salmonella antibody was used to detect bacteria, and constitutive gfp construction was used as control for plasmid maintenance in the flow cytometry assays. Though, in future analysis it will be important to consider the nutritional variables involved in different models of infection, infection conditions, and the initial metabolic state of the bacteria. Also, if the metabolism of intracellular and cultured in media bacteria is compared, we must evaluate if the ‘comparator bacteria’ derive from a rich media with poorly defined nutrient and carbon substrate compositions or a well-defined medium, since the deducing details about central carbon and other key metabolisms depend on these data. Our results suggest that Salmonella could use diverse strategies to adapt its metabolism to the variable conditions found in the environment of the SCV in different stages of the infection. Also, every population could have a particular relevance to facilitate the proliferation or to establish a chronic infection or latency in different cell types or organs encountered by the pathogen in the host organism.
Experimental procedures
Chemicals, strains, plasmids and DNA manipulation
The Salmonella Typhimurium strains used in this work were wild-type 12023 (NTCC) and its isogenic derivatives. The Salmonella and E. coli strains, and plasmids used in this study are listed in Table 1. Ampicillin was added at 100 µg ml−1.
Table 1.
Bacterial strains and plasmids.
| Strain | Relevant genotype and/or information | Source or reference |
|---|---|---|
| 12023 | Wild-type | Laboratory stock |
| PB7956 | 12023 - ΔfadBA | García-Véscovi |
| PB8099 | 12023 - ΔfadD | 68 |
| ΔfadD - pfadD | 12023 - ΔfadD - pfadD | This study |
| Plasmids | ||
| pFPV25 | GFP reporter fusion vector (ApR) | 42 |
| pMAN-3 | pFPV25 derivative carrying the fadAB::gfpmut3a promoter (ApR) | This study |
| pMAN-4 | pFPV25 derivative carrying the fadL::gfpmut3a promoter (ApR) | This study |
| pMAN-5 | pFPV25 derivative carrying the fadD::gfpmut3a promoter (ApR) | This study |
| pMAN-6 | pFPV25 derivative carrying the fadE::gfpmut3a promoter (ApR) | This study |
| pMAN-11 | pFPV25 derivative carrying the aceBA::gfpmut3a promoter (ApR) | This study |
| pMAN-12 | pFPV25 derivative carrying the pfkA::gfpmut3a promoter (ApR) | This study |
| pMAN-13 | pFPV25 derivative carrying the edd::gfpmut3a promoter (ApR) | This study |
| p28LL | pET28a(+) carrying gene fadD, as a His-tag fusión (KmR) | This study |
Culture conditions
E. coli and Salmonella strains harbouring the indicated plasmids were grown at 37°C in Luria Bertani medium (Difco, San Jose, CA), or in minimal medium (M9, glucose 0.2%, MgSO4 1 mM, CaCl2 200 mM, thiamine 1 µg ml−1, casamino acids 1 mg ml−1). When indicated, glucose in M9 was replaced by gluconate 0.2%, glycerol 0.5%, acetate 0.5%, or oleic acid (Sigma) 0.2% and brij 0.4%.
Fluorescence analysis with microplate reader
Fluorescence from bacteria grown in LB or M9 medium was analyzed with a Synergy 2 Multi-Mode Microplate Reader (BioTek). 2 ml of bacteria were grown at 37°C with aeration and 200 µl were transferred to the 96-well plate at each time point. For each sample fluorescence detected at 482 and 515 nm excitation and emission wavelengths, respectively, was related to the absorbance at 600 nm to make it proportional to bacterial cell concentration. For dose-dependent experiments, wild-type strains carrying the analyzed fusion were grown in M9 minimal medium or LB to an OD600 of 0.4. Then, different concentrations of oleic acid, glucose, acetate or gluconate were added and GFP levels were recorded over 9 hours. GFP synthesis was recorded over using a fluorimeter.
Eukaryotic cells and culture conditions
RAW 264.7 were grown routinely in DMEM (GibcoBRL, Gaithersburg, MD) supplemented with 10% FCS (Life Technologies, Rockville, MD), 2 mM nonessential amino acids, and glutamine (GibcoBRL) at 37°C in 5% CO2. In experiments in which the concentration of glucose was changed, RAW-264.7 cells were first grown in classical DMEM during 24 hours. Then the medium was changed for DMEM without glucose, supplemented with 0, 0.5, 1 or 4.5 g/L of glucose, or 400 µM of oleic acid complexed with BSA (1:4 molar ratio),50 for the rest of the experiment.
Gene cloning and plasmid construction
The cloning vector used was pFPV25, carrying promotorless gfpmut3agene (Valdivia and Falkow, 1997). The inserts carrying 300–400 bp upstream of fadBA, fadD, fadL, fade, aceBA, pfkA, and eddstart codons were PCR-amplified from S. typhimurium 12023 by using the synthetic primers listed in Table 2. PCR products were digested using XbaIand BamHIor KpnI, cloned into pFPV25 vector to generate the plasmids pMAN3 to pMAN13. For trans complementation experiments, the cloning vector pET28a(+) (Novagen) was used to express the gene fadD. The insert corresponding to the coding sequence of fadD of approximately 1700 pb was PCR-amplified from S. typhimurium 12023 by using the synthetic primers listed in Table 2. PCR product was digested using NdeIand BamHI, cloned into the pET28a(+) vector to generate the plasmid p28LL. The inserts were verified by DNA sequencing.
Table 2.
Oligonucleotides used in this study.
| Oligonucleotide | Sequence (5′ to 3′) | Restriction site |
|---|---|---|
| fadBAfw | CCCGGATCCCTCGCCAGAATGAATAAGTAACG | BamHI |
| fadBArev | CCCTCTAGAGTCAGTCTCCTGAATCCAC | XbaI |
| fadDfw | CCCGGATCCCAGGTCGTCATGCCTCCAC | BamHI |
| fadDrev | CCCTCTAGACACAACCCCAGTTAATAAAC | XbaI |
| fadLfw | CCCGGATCCGGCATTCGCCTCTCCAGTC | BamHI |
| fadLrev | CCCTCTAGAAACCTCATTGATTATTTTTATAC | XbaI |
| fadEfw | CCCGGATCCAAAAATTAGCCAGCGTTTCC | BamHI |
| fadErev | CCCTCTAGAAACGAAAAGCTCCCTTGC | XbaI |
| aceBAfw | CAGATGGATCCATATGATCTGCGTCACATG | BamHI |
| aceBArev | TGTGGTCTAGACATGCAGCTCCTCGTTGTTG | XbaI |
| pfkAup | GACTGGTACCATTCAGATTCATTTGG | KpnI |
| pfkAdn | TTCTTTCTAGAGACTACCTCTGAACTTTGG | XbaI |
| eddfw | GCGCTGGATCCCCGCCGTGACGAAGTGG | BamHI |
| eddrev | CCCCCTCTAGACATAGAGGCTCCTGAAA | XbaI |
| fadDup(P) | AGAGGTCATATGTTGAAGAAGGTTTGGCTTAAC | NdeI |
| fadDdn(P)1 | AGCTGGGATCCCGCTCAGGCTTTATTGTC | BamHI |
MTT assay
The viability of the RAW 246.7 cells was evaluated by the MTT assay, based on the protocol described by.73,75 Briefly, RAW 246.7 macrophages were cultured for 24 hours in DMEM medium in 24 wells-plates. Then the medium was substituted for DMEM with different concentrations of glucose or with oleic acid, for 24 hours. At the end of the incubation time, cells were incubated for 4 hours with 0.5 mg/ml of MTT, dissolved in serum free DMEM medium. Washing with PBS was followed by the addition of DMSO (200 µl), gentle shaking for 10 min so that complete dissolution was achieved. Absorbance was recorded at 540 nm using a Synergy 2 Multi-Mode Microplate Reader (BioTek).
Glucose measurements
RAW 264.7 macrophages were seeded at a density of 2.5 × 106 cells per well in 6-well tissue culture plates containing DMEM (GibcoBRL) with 10% fetal calf serum (FCS) (HyClone) and cultured during 24 hours, then medium was changed as indicated for DMEM with different glucose concentration or 400 µM oleic acid for another 24 hours. Cells were washed twice with cold PBS, and 100 mM Tris-HCl buffer, pH 7.6, was added onto the cells. Macrophages were then scraped, passed through a 26 gauge needle 10 times to disrupt cells, and then clarified by centrifugation.28, 76 Glucose in the supernatant was specifically converted to 6-phosphogluconate in a coupled enzymatic reaction (hexokinase, glucose 6-phosphate dehydrogenase), with formation of NADPH, which was determined fluorometrically at 340 nm in a Synergy 2 Multi-Mode Microplate Reader (BioTek).77
Bacterial infection of macrophages
RAW 264.7 macrophages were seeded at a density of 4×105 cells per well in 6-well tissue culture plates in DMEM-based growing medium with different glucose concentration or 400 µM oleic acid. Macrophages were supplemented with IFN-γ (10 U ml−1, ImmunoTools) 24 hours before use. Bacteria were cultured during 3.5, 8 or 16 hours at 37°C in M9 minimal medium with shaking, and opsonized for 30 min in DMEM containing FCS and 10% normal mouse serum (Perbio). Bacteria were added to the monolayers at a m.o.i. ≈70:1, centrifuged at 500 g for 5 min at 4°C, and incubated for 30 min at 37°C in 5% CO2. The macrophages were washed three times with PBS, and incubated with growing medium supplemented with 100 µg ml−1 gentamicin for 90 min, after that the gentamicin concentration was decreased to 10 µg ml−1 for the remainder of the experiment. For the replication assays, bacteria were recovered and enumerated after plating a dilution series onto LB agar and LB agar with the appropriate antibiotics. Normalized fold increase were determined for the ratio between the mutants or the differential condition and wild-type strains within the output (bacteria recovered from the cells after 16 h post infection) divided by their ratios within the input (bacteria recovered from the cells after 2 h post infection).
Mouse infections
Eight to 10 week-old C57BL/6 mice were inoculated intraperitonealy with bacterial strains for a total of 105 bacteria per mouse. The spleens were harvested 48 hours after inoculation and homogenized.
Flow cytometry analysis of bacteria extracted from macrophages or spleen
Infected macrophages were washed twice with PBS, lysed with 0.1% Triton X-100 in PBS and immediately fixed in two volumes of 3% paraformaldehyde for 1 h. Large debris and nuclei were removed by centrifugation for 5 min at 200 g and bacteria were pelleted at 20 000 g for 10 min. Spleens were removed from infected mice and homogenized on a 70 µm sieve in 4 ml 0.1% Tx-100. Debris were removed by centrifugation for 5 min at 200 g and bacteria were pelleted at 20 000 g for 10 min. Pellets were resuspended in 0.5 ml 3% paraformaldehyde and fixed for 1 h and then bacteria were pelleted again. Bacteria extracted from macrophages or spleen were resuspended in 0.5 ml of 10 mM NH4Cl in PBS and immunolabelled with a mouse anti-Salmonella 1E6 (Abcam) and a donkey anti-mouse PE (Abcam), both diluted 1:1000. For flow cytometric analysis, bacteria were gated in FL2 and analyzed for the expression of GFP in FL1. Data were acquired with a FACScalibur (BD Biosciences) equipped with blue argon laser (488 nm) and analyzed with the FlowJo software (Tree Star Inc., Ashland, OR) on 2000 to 10000 events finally identified as S. Typhimurium particles. Statistical analyses were performed using Prism (GraphPad, San Diego, CA, USA).
Immunofluorescence staining and confocal microscopy
Cells grown on coverslips were fixed with 3% paraformaldehyde (pH 7.4) in PBS at room temperature for 10 min. Fixed cells were washed three times in PBS and permeabilized with 0.1% saponin in PBS. Primary and secondary antibodies were diluted in PBS containing 0.1% saponin and 5% horse serum. Coverslips were incubated with primary antibodies for 30–60 min at room temperature, washed in PBS containing 0.1% saponin, and then incubated with appropriate secondary antibodies. Salmonella and SCVs were labeled using anti LPS and anti lysosomal LAMP1 antibodies, respectively. Slides were mounted in Prolong Gold (Invitrogen) and nucleus were visualized by DAPI staining. Infected cells were observed and imaged with a Zeiss LSM 710 confocal microscope (Carl Zeiss, Jena, Germany).
Antibodies and reagents
The mouse anti-Salmonella 1E6 was obtained from Abcam. The mouse monoclonal antibody against LAMP1, H4A3 [developed by J. T. August and J. E. K. Hildreth (Johns Hopkins University School of Medicine, Baltimore, MD), obtained from the Developmental Studies Hybridoma Bank (Iowa City, IA), developed under the auspices of the National Institute of Child Health and Human Development, and maintained by the University of Iowa (Iowa City, IA)] was used at a dilution of 10−3.
Ethic statement
Animal experimentation was conducted in strict accordance with good animal practice as defined by the French animal welfare bodies (Law 87–848 dated 19 October 1987 modified by Decree 2001–464 and Decree 2001–131 relative to European Convention, EEC Directive 86/609). All animal work was approved by the Direction Départementale des Services Vétérinaires des Bouches du Rhône (authorization number 13.118 to S.M.).
Supplementary Material
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We kindly thank Mara Ojeda for flow cytometry assistance, and Dolores Campos for cell biology assistance, and Lucas Daurelio for generous help in statistical analysis. We thank Eleonora García-Véscovi and Gastón Viarengo for their kind gifts of Salmonella mutant strains.
Funding
This work was supported by grants ANPCyT to LD (P. BID PICT 2010-1901 and P. BID PICT 2014-1454), and Gob. Santa Fe to LD (SF 2010-122-11). LD was recipient of fellowship Programa AVE-UNR.
References
- [1].Pang T, Bhutta ZA, Finlay BB, Altwegg M. Typhoid fever and other salmonellosis a continuing challenge. Trends Microbiol 1995; 3(7):253-5; PMID:7551636; http://dx.doi.org/ 10.1016/S0966-842X(00)88937-4 [DOI] [PubMed] [Google Scholar]
- [2].Boyle EC, Bishop JL, Grassl GA, Finlay BB. Salmonella: From pathogenesis to therapeutics. J Bacteriol 2007; 189(5):1489-95; PMID:17189373; http://dx.doi.org/ 10.1128/JB.01730-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Haraga A, Ohlson MB, Miller SI. Salmonellae interplay with host cells. Nat Rev Microbiol 2008; 6(1):53-66; PMID:18026123; http://dx.doi.org/ 10.1038/nrmicro1788 [DOI] [PubMed] [Google Scholar]
- [4].Cirillo DM, Valdivia RH, Monack DM, Falkow S. Macrophage-dependent induction of the Salmonellapathogenicity island 2 type III secretion system and its role in intracellular survival. Mol Microbiol 1998; 30(1):175-88; PMID:9786194; http://dx.doi.org/ 10.1046/j.1365-2958.1998.01048.x [DOI] [PubMed] [Google Scholar]
- [5].Hensel M, Shea JE, R S, Mundy R, Nikolaus T, Banks G. Genes encoding putative effector proteins of the type III secretion system of. Mol Microbiol 1998; 30:163-74; PMID:9786193; http://dx.doi.org/ 10.1046/j.1365-2958.1998.01047.x [DOI] [PubMed] [Google Scholar]
- [6].Ochman H, Soncini FC, Solomon F, Grosiman EA. Identification of a pathogenicity island required for Salmonella enteropathogenicity. Proc Natl Acad Sci U S A 1996; 93(15):7800-4; PMID:8755556; http://dx.doi.org/ 10.1073/pnas.93.15.7800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Shea JE, Beuzon CR, Gleeson C, Mundy R, Holden DW. Influence of the Salmonella typhimurium pathogenicity island 2 type III secretion system on bacterial growth in the mouse. Infect Immun 1999; 67(1):213-9; PMID:9864218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Shea JE, Hensel M, Gleeson C, Holden DW. Identification of a virulence locus encoding a second type III secretion system in Salmonella typhimurium. Proc Natl Acad Sci U S A 1996; 93(6):2593-7; PMID:8637919; http://dx.doi.org/ 10.1073/pnas.93.6.2593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Waterman SR, Holden DW. Functions and effectors of the Salmonella pathogenicity island 2 type III secretion system. Cell Microbiol 2003; 5(8):501-11; PMID:12864810; http://dx.doi.org/ 10.1046/j.1462-5822.2003.00294.x [DOI] [PubMed] [Google Scholar]
- [10].Lossi NS, Rolhion N, Magee AI, Boyle C, Holden DW. The Salmonella SPI-2 effector SseJ exhibits eukaryotic activator-dependent phospholipase A and glycerophospholipid: Cholesterol acyltransferase activity. Microbiology 2008; 154(9):2680-8; PMID:18757801; http://dx.doi.org/ 10.1099/mic.0.2008/019075-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Nawabi P, Catron DM, Haldar K. Esterification of cholesterol by a type III secretion effector during intracellular Salmonella infection. Mol Microbiol 2008; 68(1):173-85; PMID:18333886; http://dx.doi.org/ 10.1111/j.1365-2958.2008.06142.x [DOI] [PubMed] [Google Scholar]
- [12].Mazurkiewicz P, Thomas J, Thompson JA, Liu M, Arbibe L, Sansonetti P, Holden DW. SpvC is a Salmonella effector with phosphothreonine lyase activity on host mitogen-activated protein kinases. Mol Microbiol 2008; 67(6):1371-83; PMID:18284579; http://dx.doi.org/ 10.1111/j.1365-2958.2008.06134.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Poh J, Odendall C, Spanos A, Boyle C, Liu M, Freemont P, Holden DW. SteC is a Salmonella kinase required for SPI-2-dependent F-actin remodelling. Cell Microbiol 2008; 10(1):20-30; PMID:17645553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Quezada CM, Hicks SW, Galán JE, Stebbins CE. A family of Salmonella virulence factors functions as a distinct class of autoregulated E3 ubiquitin ligases. Proc Natl Acad Sci U S A 2009; 106(12):4864-9; PMID:19273841; http://dx.doi.org/ 10.1073/pnas.0811058106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Pegues D, Hantman M, Behlau I, Miller SI. PhoP / PhoQ transcriptional repression of Salmonella typhimurium invasion protein secretion genes: evidence for a role in protein secretion. Mol Microbiol 1995; 17(1):169-81; PMID:7476203; http://dx.doi.org/ 10.1111/j.1365-2958.1995.mmi_17010169.x [DOI] [PubMed] [Google Scholar]
- [16].Ohl ME, Miller SI. Salmonella: A Model for Bacterial Pathogenesis. Annu Rev Med 2001; 52:259-74; PMID:11160778; http://dx.doi.org/ 10.1146/annurev.med.52.1.259 [DOI] [PubMed] [Google Scholar]
- [17].Richter-dahlfors BA, Buchan AMJ, Finlay BB. Murine Salmonellosis Studied by Confocal Microscopy: Salmonella typhimurium Resides Intracellularly Inside Macrophages and Exerts a Cytotoxic Effect on. J Exp Med 1997; 186(4):569-80; PMID:9254655; http://dx.doi.org/ 10.1084/jem.186.4.569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Fields PI, Swanson R V, Haidaris CG, Heffron F. Mutants of Salmonella typhimurium that cannot survive within the macrophage are avirulent. Proc Natl Acad Sci U S A 1986; 83(14):5189-93; PMID:3523484; http://dx.doi.org/ 10.1073/pnas.83.14.5189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].O'Callaghan D, Maskell D, Liew FY, Easmon CS, Dougan G. Characterization of aromatic- and purine-dependent Salmonella typhimurium: attention, persistence, and ability to induce protective immunity in BALB/c mice. Infect Immun 1988; 56(2):419-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ray K, Marteyn B, Sansonetti PJ, Tang CM. Life on the inside: the intracellular lifestyle of cytosolic bacteria. Nat Rev Microbiol 2009; 7(5):333-40; PMID:19369949; http://dx.doi.org/ 10.1038/nrmicro2112 [DOI] [PubMed] [Google Scholar]
- [21].Smith H. Host factors that influence the behaviour of bacterial pathogens in vivo. Int J Med Microbiol 2000; 290(3):207-13; PMID:10959722; http://dx.doi.org/ 10.1016/S1438-4221(00)80117-4 [DOI] [PubMed] [Google Scholar]
- [22].Munoz-Elias EJ, McKinney JD. Carbon metabolism of intracellular bacteria. Cell Microbiol 2006; 8(1):10-22; PMID:16367862; http://dx.doi.org/ 10.1111/j.1462-5822.2005.00648.x [DOI] [PubMed] [Google Scholar]
- [23].Brown SA, Palmer KL, Whiteley M. Revisiting the host as a growth medium. Nat Rev Microbiol 2008; 6(9):657-66; PMID:18679171; http://dx.doi.org/ 10.1038/nrmicro1955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Macfarlane GT, Macfarlane S. Human colonic microbiota: ecology, physiology and metabolic potential of intestinal bacteria. Scand J Gastroenterol Suppl 1997; 222:3-9; PMID:9145437; http://dx.doi.org/ 10.1080/00365521.1997.11720708 [DOI] [PubMed] [Google Scholar]
- [25].Eisenreich W, Dandekar T, Heesemann J, Goebel W. Carbon metabolism of intracellular bacterial pathogens and possible links to virulence. Nat Rev Microbiol 2010; 8(6):401-12; PMID:20453875; http://dx.doi.org/ 10.1038/nrmicro2351 [DOI] [PubMed] [Google Scholar]
- [26].Götz A, Eylert E, Eisenreich W, Goebel W. Carbon metabolism of enterobacterial human pathogens growing in epithelial colorectal adenocarcinoma (Caco-2) cells. PLoS One 2010; 5(5):e10586; http://dx.doi.org/ 10.1371/journal.pone.0010586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Eriksson S, Lucchini S, Thompson A, Rhen M, Hinton JC. Unravelling the biology of macrophage infection by gene expression profiling of intracellular Salmonella enterica. Mol Microbiol 2003; 47(1):103-18; PMID:12492857; http://dx.doi.org/ 10.1046/j.1365-2958.2003.03313.x [DOI] [PubMed] [Google Scholar]
- [28].Eisele NA, Ruby T, Jacobson A, Manzanillo PS, Cox JS, Lam L, Mukundan L, Chawla A, Monack DM. Salmonella require the fatty acid regulator PPARd for the establishment of a metabolic environment essential for long-term persistence. Cell Host Microbe 2013; 14(2):171-82; PMID:23954156; http://dx.doi.org/ 10.1016/j.chom.2013.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Yimga MT, Leatham MP, Allen JH, Laux DC, Conway T, Cohen PS. Role of gluconeogenesis and the tricarboxylic acid cycle in the virulence of Salmonella enterica serovar Typhimurium in BALB/c mice. Infect Immun 2006; 74(2):1130-40; PMID:16428761; http://dx.doi.org/ 10.1128/IAI.74.2.1130-1140.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Mercado-Lubo R, Leatham MP, Conway T, Cohen PS. Salmonella enterica serovar typhimurium mutants unable to convert malate to pyruvate and oxaloacetate are avirulent and immunogenic in BALB/c mice. Infect Immun 2009; 77(4):1397-405; PMID:19168732; http://dx.doi.org/ 10.1128/IAI.01335-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Lundberg BE, Wolf RE Jr., Dinauer MC, Xu Y, Fang FC. Glucose 6-phosphate dehydrogenase is required for Salmonella typhimurium virulence and resistance to reactive oxygen and nitrogen intermediates. Infect Immun 1999; 67(1):436-8; PMID:9864251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Bowden SD, Rowley G, Hinton JCD, Thompson A. Glucose and glycolysis are required for the successful infection of macrophages and mice by Salmonella enterica serovar Typhimurium. Infect Immun 2009; 77(7):3117-26; PMID:19380470; http://dx.doi.org/ 10.1128/IAI.00093-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Clark DP, Cronan JE. Two-Carbon Compounds and Fatty Acids as Carbon Sources. (Neidhardt F. C., Ingraham J. L., Lin E. C. C., Low K. B., Magasanik B., Reznikoff W. S., Riley M., Schaechter M., and Umbarger H. E. (ed.) RCIII, ed.). Washington, D.C: ASM Press; 1996. [Google Scholar]
- [34].Cronan CO, Rock JE. Biosynthesis of Membrane Lipids. (Neidhardt F. C., Ingraham J. L., Lin E. C. C., Low K. B., Magasanik B., Reznikoff W. S., Riley M., Schaechter M., and Umbarger H. E. (ed.) RCIII, ed.). Washington, D.C: ASM Press; 1996. [Google Scholar]
- [35].Zhang YM, Rock CO. Transcriptional regulation in bacterial membrane lipid synthesis. J Lipid Res 2009; 50 Suppl:S115-9; PMID:18941141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Iram SH, Cronan JE. The beta-oxidation systems of Escherichia coli and Salmonella enterica are not functionally equivalent. J Bacteriol 2006; 188(2):599-608; PMID:16385050; http://dx.doi.org/ 10.1128/JB.188.2.599-608.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Spector MP, DiRusso CC, Pallen MJ, Garcia del Portillo F, Dougan G, Finlay BB. The medium-/long-chain fatty acyl-CoA dehydrogenase (fadF) gene of Salmonella typhimurium is a phase 1 starvation-stress response (SSR) locus. Microbiology 1999; 145 (Pt 1):15-31; PMID:10206693; http://dx.doi.org/ 10.1099/13500872-145-1-15 [DOI] [PubMed] [Google Scholar]
- [38].Mahan MJ, Tobias JW, Slauch JM, Hanna PC, Collier RJ, Mekalanos JJ. Antibiotic-based selection for bacterial genes that are specifically induced during infection of a host. Proc Natl Acad Sci U S A 1995; 92(January):669-73; PMID:7846034; http://dx.doi.org/ 10.1073/pnas.92.3.669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Valentine PJ, Devore BP, Heffron F. Identification of three highly attenuated Salmonella typhimurium mutants that are more immunogenic and protective in mice than a prototypical aroA mutant. Infect Immun 1998; 66(7):3378-83; PMID:9632608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Fang FC, Libby SJ, Castor ME, Fung AM. Isocitrate lyase (AceA) is required for Salmonella persistence but not for acute lethal infection in mice. Infect Immun 2005; 73(4):2547-9; PMID:15784602; http://dx.doi.org/ 10.1128/IAI.73.4.2547-2549.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Becker D, Selbach M, Rollenhagen C, Ballmaier M, Meyer TF, Mann M, Bumann D. Robust Salmonella metabolism limits possibilities for new antimicrobials. Nature 2006; 440(7082):303-7; PMID:16541065; http://dx.doi.org/ 10.1038/nature04616 [DOI] [PubMed] [Google Scholar]
- [42].Valdivia RH, Falkow S. Bacterial genetics by flow cytometry: rapid isolation of Salmonella typhimurium acid-inducible promoters by differential fluorescence induction. Mol Microbiol 1996; 22(2):367-78; PMID:8930920; http://dx.doi.org/ 10.1046/j.1365-2958.1996.00120.x [DOI] [PubMed] [Google Scholar]
- [43].Aussel L, Zhao W, Hebrard M, Guilhon AA, Viala JP, Henri S, Chasson L, Gorvel JP, Barras F, Méresse S. Salmonella detoxifying enzymes are sufficient to cope with the host oxidative burst. Mol Microbiol 2011; 80(3):628-40; PMID:21362067; http://dx.doi.org/ 10.1111/j.1365-2958.2011.07611.x [DOI] [PubMed] [Google Scholar]
- [44].Viala JP, Meresse S, Pocachard B, Guilhon AA, Aussel L, Barras F. Sensing and adaptation to low pH mediated by inducible amino acid decarboxylases in Salmonella. PLoS One 2011; 6(7):e22397; PMID:21799843; http://dx.doi.org/ 10.1371/journal.pone.0022397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].De la Cruz MA, Zhao W, Farenc C, Gimenez G, Raoult D, Cambillau C, Gorvel JP, Méresse S. A Toxin-Antitoxin Module of Salmonella Promotes Virulence in Mice. PLoS Pathog 2013; 9(12):1-13; http://dx.doi.org/ 10.1371/journal.ppat.1003827 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [46].Sezonov G, Joseleau-Petit D, D'Ari R. Escherichia coli physiology in Luria-Bertani broth. J Bacteriol 2007; 189(23):8746-9; PMID:17905994; http://dx.doi.org/ 10.1128/JB.01368-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Borch-Johnsen K, Group DS, Diabetes E, Study E. Will new diagnostic criteria for diabetes mellitus change phenotype of patients with diabetes? Reanalysis of European epidemiological data. DECODE Study Group on behalf of the European Diabetes Epidemiology Study Group. BMJ 1998; 317(7155):371-5; http://dx.doi.org/ 10.1136/bmj.317.7155.371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Liu F, Adi D, Xie X, Li XM, Fu ZY, Shan CF, Huang Y, Chen BD, Gai MT, Gao XM, et al.. Prevalence of Isolated Diastolic Hypertension and Associated Risk Factors among Different Ethnicity Groups in Xinjiang, China. PLoS One 2015; 10(12):e0145325; PMID:26694755; http://dx.doi.org/ 10.1371/journal.pone.0145325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Nankervis SA, Mitchell JM, Charchar FJ, McGlynn MA, Lewandowski PA. Consumption of a low glycaemic index diet in late life extends lifespan of Balb/c mice with differential effects on DNA damage. Longev Heal 2013; 2(1):4; http://dx.doi.org/ 10.1186/2046-2395-2-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Wiesenfeld PW, Babu US, O'Donnell MW. Effect of long-chain fatty acids in the culture medium on fatty acid composition of WEHI-3 and J774A.1 cells. Comp Biochem Physiol - B Biochem Mol Biol 2001; 128(1):123-34; PMID:11163311; http://dx.doi.org/ 10.1016/S1096-4959(00)00305-5 [DOI] [PubMed] [Google Scholar]
- [51].Lee W, VanderVen BC, Fahey RJ, Russell DG. Intracellular Mycobacterium tuberculosis exploits host-derived fatty acids to limit metabolic stress. J Biol Chem 2013; 288(10):6788-800; PMID:23306194; http://dx.doi.org/ 10.1074/jbc.M112.445056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Diacovich L, Gorvel JP. Bacterial manipulation of innate immunity to promote infection. Nat Rev Microbiol 2010; 8(2):117-28; PMID:20075926; http://dx.doi.org/ 10.1038/nrmicro2295 [DOI] [PubMed] [Google Scholar]
- [53].Saenz HL, Dehio C. Signature-tagged mutagenesis: Technical advances in a negative selection method for virulence gene identification. Curr Opin Microbiol 2005; 8(5):612-9; PMID:16126452; http://dx.doi.org/ 10.1016/j.mib.2005.08.013 [DOI] [PubMed] [Google Scholar]
- [54].Jansen A, Yu J. Differential gene expression of pathogens inside infected hosts. Curr Opin Microbiol 2006; 9(2):138-42; PMID:16459132; http://dx.doi.org/ 10.1016/j.mib.2006.01.003 [DOI] [PubMed] [Google Scholar]
- [55].Sauer U. Metabolic networks in motion: 13C-based flux analysis. Mol Syst Biol 2006; 2:62; PMID:17102807; http://dx.doi.org/ 10.1038/msb4100109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Eylert E, Schär J, Mertins S, Stoll R, Bacher A, Goebel W, Eisenreich W. Carbon metabolism of Listeria monocytogenes growing inside macrophages. Mol Microbiol 2008; 69(4):1008-17; PMID:18627458; http://dx.doi.org/ 10.1111/j.1365-2958.2008.06337.x [DOI] [PubMed] [Google Scholar]
- [57].Munger J, Bennett BD, Parikh A, Feng XJ, McArdle J, Rabitz HA, Shenk T, Rabinowitz JD. Systems-level metabolic flux profiling identifies fatty acid synthesis as a target for antiviral therapy. October 2010; 26(10):1179-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Breitling R, Vitkup D, Barrett MP. New surveyor tools for charting microbial metabolic maps. Nat RevMicrobiol 2008; 6(1740-1534 (Electronic)):156-61 [DOI] [PubMed] [Google Scholar]
- [59].Helaine S, Holden DW. Heterogeneity of intracellular replication of bacterial pathogens. Curr Opin Microbiol 2013; 16(2):184-91; PMID:23485258; http://dx.doi.org/ 10.1016/j.mib.2012.12.004 [DOI] [PubMed] [Google Scholar]
- [60].Alberdi L, Méresse S. Single-cell analysis: Understanding infected cell heterogeneity. Virulence 2016; 1-2; PMID:27786599; http://dx.doi.org/ 10.1080/21505594.2016.1253659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Garcia-del Portillo F, Nunez-Hernandez C, Eisman B, Ramos-Vivas J. Growth control in the Salmonella-containing vacuole. Curr Opin Microbiol 2008; 11(1):46-52; PMID:18282735; http://dx.doi.org/ 10.1016/j.mib.2008.01.001 [DOI] [PubMed] [Google Scholar]
- [62].Iram SH, Cronan JE. Unexpected functional diversity among FadR fatty acid transcriptional regulatory proteins. J Biol Chem 2005; 280(37):32148-56; PMID:16027119; http://dx.doi.org/ 10.1074/jbc.M504054200 [DOI] [PubMed] [Google Scholar]
- [63].Feng Y, Cronan JE. Crosstalk of Escherichia coli FadR with Global Regulators in Expression of Fatty Acid Transport Genes. PLoS One 2012; 7(9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Stülke J, Hillen W. Carbon catabolite repression in bacteria. Curr Opin Microbiol 1999; 2(2):195-201; PMID:10322165; http://dx.doi.org/ 10.1016/S1369-5274(99)80034-4 [DOI] [PubMed] [Google Scholar]
- [65].Nanchen A, Schicker A, Revelles O, Sauer U. Cyclic AMP-dependent catabolite repression is the dominant control mechanism of metabolic fluxes under glucose limitation in Escherichia coli. J Bacteriol 2008; 190(7):2323-30; PMID:18223071; http://dx.doi.org/ 10.1128/JB.01353-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Lucas RL, Lostroh CP, DiRusso CC, Spector MP, Wanner BL, Lee CA. Multiple factors independently regulate hilA and invasion gene expression in Salmonella enterica serovar typhimurium. J Bacteriol 2000; 182(7):1872-82; PMID:10714991; http://dx.doi.org/ 10.1128/JB.182.7.1872-1882.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Baxter MA, Jones BD. The fimYZ genes regulate Salmonella enterica Serovar Typhimurium invasion in addition to type 1 fimbrial expression and bacterial motility. Infect Immun 2005; 73(3):1377-85; PMID:15731035; http://dx.doi.org/ 10.1128/IAI.73.3.1377-1385.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Pech-Canul A, Nogales J, Miranda-Molina A, Álvarez L, Geiger O, Soto MJ, López-Lara IM. FadD is required for utilization of endogenous fatty acids released from membrane lipids. J Bacteriol 2011; 193(22):6295-304; PMID:21926226; http://dx.doi.org/ 10.1128/JB.05450-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Viarengo G, Sciara MI, Salazar MO, Kieffer PM, Furlán RLE, Véscovi EG. Unsaturated long chain free fatty acids are input signals of the Salmonella enterica PhoP/PhoQ regulatory system. J Biol Chem 2013; 288(31):22346-58; PMID:23782700; http://dx.doi.org/ 10.1074/jbc.M113.472829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Fischer E, Sauer U. A Novel Metabolic Cycle Catalyzes Glucose Oxidation and Anaplerosis in Hungry Escherichia coli. J Biol Chem 2003; 278(47):46446-51; PMID:12963713; http://dx.doi.org/ 10.1074/jbc.M307968200 [DOI] [PubMed] [Google Scholar]
- [71].Hautefort I, Thompson A, Eriksson-Ygberg S, Parker ML, Lucchini S, Danino V, Bongaerts RJ, Ahmad N, Rhen M, Hinton JC. During infection of epithelial cells Salmonella enterica serovar Typhimurium undergoes a time-dependent transcriptional adaptation that results in simultaneous expression of three type 3 secretion systems. Cell Microbiol 2008; 10(4):958-84; PMID:18031307; http://dx.doi.org/ 10.1111/j.1462-5822.2007.01099.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Lucchini S, Hinton JCD, Yu J. Transcriptional Adaptation of of Shigella flexneri during Infection of Macrophages and Epithelial Cells: Insights into the Strategies of a Cytosolic Bacterial Pathogen. Infect Immun 2005; 73(1):88-102; PMID:15618144; http://dx.doi.org/ 10.1128/IAI.73.1.88-102.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Chakraborty S, Mizusaki H, Kenney LJ. A FRET-based DNA biosensor tracks OmpR-dependent acidification of Salmonella during macrophage infection. PLoS Biol 2015; 13(4):e1002116; PMID:25875623; http://dx.doi.org/ 10.1371/journal.pbio.1002116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Wang KC, Hsu YH, Huang YN, Chen TH, Lin JH, Hsuan SL, Chien MS, Lee WC, Yeh KS. A low-pH medium in vitro or the environment within a macrophage decreases the transcriptional levels of fimA, fimZ and lrp in Salmonella enterica serovar Typhimurium. J Biosci 2013; 38(3):499-507; PMID:23938383; http://dx.doi.org/ 10.1007/s12038-013-9347-2 [DOI] [PubMed] [Google Scholar]
- [75].Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods 1983; 65(1-2):55-63; PMID:6606682; http://dx.doi.org/ 10.1016/0022-1759(83)90303-4 [DOI] [PubMed] [Google Scholar]
- [76].Bondar RJL, Mead DC. Evaluation of glucose 6 phosphate dehydrogenase from Leuconostoc mesenteroides in the hexokinase method for determining glucose in serum. Clin Chem 1974; 20(5):586-90; PMID:4363766 [PubMed] [Google Scholar]
- [77].Aguirre A, Peiru S, Eberhardt F, Vetcher L, Cabrera R, Menzella HG. Enzymatic hydrolysis of steryl glucosides, major contaminants of vegetable oil-derived biodiesel. Appl Microbiol Biotechnol 2014; 98(9):4033-40; PMID:24265025; http://dx.doi.org/ 10.1007/s00253-013-5345-4 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.