The KCNH1 gene codes for the K+-selective, non-inactivating, voltage-dependent channel, referred to as Kv10.1 or Eag1.1,2 Outside the central nervous system Eag1 expression is associated with a cancerous phenotype.3,4 This was first demonstrated by the laboratory of Luis A Pardo and Walter Stühmer who showed that CHO cells upon stably expressing Eag1, but not other K+ channels, acquire the hallmark characteristic of cancer cells; and that injection of Eag1 expressing cells to immunosuppressed mice leads to the development of massive tumors in these animals.4
Naturally, the question arises as to what particular structural characteristic(s), as compared with other K+ channels, endow(s) Kv10.1 with its tumor promoting features, and which of them could be pharmacologically targeted in an effort to lessen the tumorous effect of Kv10.
The Eag family presents a unique structural characteristic, namely a cytoplasmic, Per-Arnt-Sim (PAS) domain, which interacts with the voltage-sensor (S1 to S4 segments) domain.5,6 PAS domains are present in proteins engaged in pathways that regulate cellular responses to environmental stimuli,7 as such these domains mediate protein-protein interactions and bind diverse chemical molecules.7,8 These properties suggest that PAS domain could be an interesting target to test drugs aimed to lessen the tumor promoting properties of Kv10.1.
In addition, Eag1 channels present other unique structural, and functional, features, for example:
-
1)
Eag1 channels present a long pore-loop that forms glycosylated helical structures which surround the pore opening. It has been proposed that this arrangement may hinder the binding of peptide toxins.9 This means that in the quest to find pharmacological tools against Kv10.1 studies using non-peptide compounds, as mibefradil, are instrumental.
-
2)
The pore lumen presents a rather uniform diameter along its full length. In other words Eag1 channels do not present the pore widening, or central cavity of KscA and Shaker-related channels.9,10 The central cavity is the place where internally added TEA,11 and numerous hydrophobic cations, as for example quinidine (e.g., reference 12 and references therein cited), blocks the open-pore of Shaker-related channels.11
-
3)
Eag1 channels endure a voltage-dependent gating even in the absence of a covalent linkage between S4 and S5 segments.13 This shows that communication between voltage-sensor and pore modules presents characteristics not shared with canonical Kv channels.9,13,14
The aforementioned properties indicate that care must be taken to translate drug-interaction models, initially developed on canonical Shaker-related K+ channels, to Eag1 channels.
In addition to the above mentioned features, a functional hallmark of Eag channels is the presence of both, a noticeably lag or time-shift for current surge,15 and the development of a markedly sigmoidal time course of current activation, as the holding potential (HP) becomes more negative;16,17 the latter arises from a rate limiting transition that accompanies the passage from deep closed states to sates near the open state (e.g; references 9,16,17 and references therein cited). This combined HP effect is customarily referred to as the Cole-Moore shift of Eag channels.
Recently, we studied the effect of the anti-hypertensive drug mibefradil on Kv10.1, and found that it exerts 2 major effects, depending on the initial conditions and on the time window of channel activity.18 Upon prolonged depolarizations, externally added mibefradil induces an apparent open-state inactivation, that inhibits the K+ conductance, and shifts the conductance (G) vs. voltage (V) curve to the left. On the other hand, by looking at the first tens of milliseconds of channel activity it was found that, strikingly, mibefradil inhibits the Cole-Moore shift of Kv10.1 (see Fig. 1), pointing out to the voltage-sensor domain as a site of action of mibefradil.18
Figure 1.
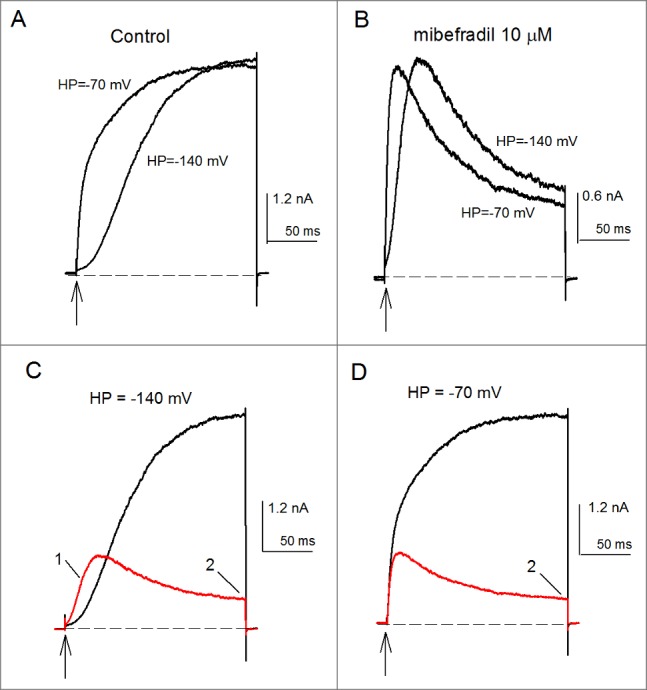
Mibefradil inhibition of the Cole-Moore shift and K+-conductance of Kv10.1 channels. (A) Control IK evoked by a 200-ms pulse to +30mV applied from the HP of either -70 or -140 mV, as indicated. The rate of IK activation depends on the HP from which channels are activated. IK activated from -140 mV is markedly slower than IK evoked from the HP of -70mV. (B) As in A, but in the presence of 10 µM mibefradil. (C) Superposed IK in control (black trace) vs. 10 µM mibefradil conditions (red trace). The currents were activated as in A from the HP of -140 mV. Notice the clear mibefradil inhibition of the Cole-Moore shift. As a consequence of this, the initial IK of mibefradil-modified channels surpasses control IK (labeled 1). Also note the marked mibefradil inhibition of GK at pulse end (labeled 2). (D) As in C but currents were activated from the relatively depolarized HP of -70 mV. Note that, as expected, in this case mibefradil only inhibits GK at pulse end. The vertical arrows indicate the time of delivery of the constant +30 mV activation pulse. The dashed line is the zero current level.
The exhaustive Competition-plot of Cornish-Bowden,19 performed between quinidine and mibefradil, along with other observations, excluded open-pore block as the mechanism underlying mibefradil-induced inactivation. Hence, we concluded that both the mibefradil inhibition of the Cole-Moore effect and its induced inactivation were the result of the drug binding within the voltage-sensor domain.
Regarding the above, and supporting our proposal of the voltage-sensor domain as the site of action of mibferdil, it has been reported that (a) amino-acid deletions of the PAS domain inhibit the Cole-Moore effect, shift the G vs. V curve to the left, and induce an open-state inactivation,6 similar to the effects of mibefradil on Kv10.1; on the other hand, regarding the prototypical Shaker B channel it was reported that, (b) progressive elimination of functional S4 segments, performed by neutralizations of arginine residues, shifts the G vs. V curve to the left, and inhibits the Cole-Moore shift. The authors hypothesized that the introduced mutations left S4 segments in a pre-activated like state.20 Similar to our hypothesis regarding the gating modification of Kv10.1, namely that mibefradil binds to the voltage-sensor domain inducing an apparent inactivation and accelerating the rate limiting transition that accompanies the passage from deep closed states to states near the open state.17,18 It remains to be determined where on the voltage-sensor domain mibefradil binds, thus exerting the aforementioned effects, and how this relates to the particular way of communication between voltage-sensor and pore domains of Kv10.1.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors thank Professors Walter Stühmer and Luis A Pardo for kindly providing the HEK cells expressing Kv10.1 channels.
Funding
Funding is provided by DGAPA-UNAM IN224616-RN224616 and CONACYT 153504 grants.
References
- [1].Warmke JW, Ganetzky B. A family of potassium channel genes related to eag in Drosophila and mammals. Proc Natl Acad Sci USA 1994; 91:3438-42; PMID:8159766; https://doi.org/ 10.1073/pnas.91.8.3438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Occhiodoro T, Bernheim L, Liu JH, Bijlenga P, Sinnreich M, Bader RC, Fischer-Lougheed J. Cloning of a human ether‐à‐go‐go potassium channel expressed in myoblasts at the onset of fusion. FEBS Letters 1998; 434:177-82; PMID:9738473; https://doi.org/ 10.1016/S0014-5793(98)00973-9 [DOI] [PubMed] [Google Scholar]
- [3].Pardo LA, Stühmer W. The role of K+ channels in cancer. Nat Rev Cancer 2014; 14:39-48; PMID:24336491; https://doi.org/ 10.1038/nrc3635 [DOI] [PubMed] [Google Scholar]
- [4].Pardo LA, del Camino D, Sánchez A, Alves F, Bruggemann A, Beckh S, Stühmer W. Oncogenic potential of EAG channels. EMBO J 1999; 18:5540-47; PMID:10523298; https://doi.org/ 10.1093/emboj/18.20.5540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Morais Cabral JH, Lee A, Cohen SL, Chait BT, Min L, Mackinnon R. Crysral structure and functional analysis of the HERG Potassium Channel N terminus: A eukaryotic PAS domain. Cell 1998; 95:649-55; PMID:9845367; https://doi.org/ 10.1016/S0092-8674(00)81635-9 [DOI] [PubMed] [Google Scholar]
- [6].Terlau H, Heinemann S, Stühmer W, Pongs O, Ludwig J. Amino terminal-dependent gating of the potassium channel rat eag is compensated by a mutation in the S4-segment. J. Physiol 1997; 502:537-43; PMID:9279806; https://doi.org/ 10.1111/j.1469-7793.1997.537bj.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].McIntosh BE, Hogenesch JB, Bradfield CA. Mammalian Per-Arnt-Sim proteins in environmental adaptation. Ann Rev Physiol 2010; 72:625-45; https://doi.org/ 10.1146/annurev-physiol-021909-135922 [DOI] [PubMed] [Google Scholar]
- [8].Henry JT, Crosson S. Ligand-binding PAS domains in genomic, cellular, and structural context. Ann Rev Microbiol 2011; 65:261-86; https://doi.org/ 10.1146/annurev-micro-121809-151631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Whicher JR, MacKinnon R. Structure of the voltage-gated K+ channel Eag1 reveals an alternative voltage sensing mechanism. Science 2016; 353:664-9; PMID:27516594; https://doi.org/ 10.1126/science.aaf8070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Doyle DA, Cabral JM, Pfuetzner RA, Kuo A, Gulbis JM, Cohen SL, Chait BT, MacKinnon R. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science 1998; 280(5360):69-77; PMID:9525859; https://doi.org/ 10.1126/science.280.5360.69 [DOI] [PubMed] [Google Scholar]
- [11].Armstrong CM. Interaction of tetraethylammonium ion derivatives with the potassium channels of giant axons. J Gen Physiol 1971; 58:413-37; PMID:5112659; https://doi.org/ 10.1085/jgp.58.4.413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Gomez-Lagunas F. Quinidine interaction with Shab K+ channels: pore block and irreversible collapse of the K+ conductance. J Physiol 2010; 588:2691-706; PMID:20547671; https://doi.org/ 10.1113/jphysiol.2010.193128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Lörinczi É, Gómez-Posada JC, de la Peña P, Tomczak AP, Fernández-Trujillo J, Leipscher U, Stühmer W, Barros F, Pardo LA. Voltage-dependent gating of KCNH potassium channels lacking a covalent link between voltage-sensing and pore domains. Nat Commun 2015; 6:6672; PMID:25818916; https://doi.org/ 10.1038/ncomms7672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Tomczak A, Fernández-Trillo J, Bharill S, Papp F, Panyi G, Stuhmer W, Isacoff EY, Pardo AL. A new mechanism of voltage-dependent gating exposed by Kv10.1 channels interrupted between voltage sensor and pore. J Gen Physiol 2017; 30:577-93; https://doi.org/ 10.1085/jgp.201611742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Cole KS, Moore JW. Potasium ion current in the squid giant axon: dynamic characteristics. Biophys J 1960; 1:1-14; PMID:13694549; https://doi.org/ 10.1016/S0006-3495(60)86871-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Bauer CK, Schwarz JR. Physiology of EAG K+ channels. J Membrane Biol 2001; 182:1-15; https://doi.org/ 10.1007/s00232-001-0031-3 [DOI] [PubMed] [Google Scholar]
- [17].Silverman WR, Roux B, Papazian DM. Structural basis of two-stage voltage-dependent activation in K+ channels. Proc Natl Acad Sci USA 2003; 100:2935-40; PMID:12606713; https://doi.org/ 10.1073/pnas.0636603100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Gómez-Lagunas F, Carrillo E, Pardo LA, Stühmer W. Gating modulation of the tumor related Kv10.1 channel by Mibefradil. J Cell Physiol 2017; 232(8):2019-32; PMID:27255432; https://doi.org/ 10.1002/jcp.25448 [DOI] [PubMed] [Google Scholar]
- [19].Chevillard C, Cárdenas ML, Cornish-Bowden A. The competition plot: A simple test of whether two reactions occur at the same active site. Biochem J 1993; 289:599-604; PMID:8424801; https://doi.org/ 10.1042/bj2890599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Gagnon DG, Bezanilla F. A single charged voltage sensor is capable of gating the Shaker K+ channel. J Gen Physiol 2009; 133:467-83; PMID:19398775; https://doi.org/ 10.1085/jgp.200810082 [DOI] [PMC free article] [PubMed] [Google Scholar]


