The discovery that the Escherichia coli focA gene product facilitates the transmembrane exchange of formate1 sparked research on proteins that turned out to be functionally situated between classical transporters and channels.2,3 The strictly microbial protein family was named formate-nitrite transporters (FNT) according to the physiologic substrates of the 2 initially characterized members. However, subsequently, the substrate spectrum was extended and comprises several weak monoacids up to the size of lactic acid. On the cellular level, FNTs regulate the metabolic substrate flow and fulfill vital functions, for instance the Plasmodium falciparum PfFNT that we and others identified as the malaria parasite's long-sought lactic acid transporter.3,4 Blockade of PfFNT by small drug-like molecules kills the parasites rendering PfFNT a novel antimalarial drug target.5,6 Their physiologic relevance as well as structural peculiarities merit a deeper look into the inner workings of the FNTs.
High resolution crystal data revealed a common homopentameric structure.2 The protomers consist of 6 transmembrane spans with both termini at the cytoplasmic side. Surprisingly, FNTs almost perfectly mimic the fold of the aquaporin water and solute channels despite unrelated amino acid sequences. The substrate path through each FNT protomer harbors 2 hydrophobic constrictions, which sandwich the imidazole sidechain of a highly-conserved histidine. The transport mechanism, however, remained unclear regarding to the question whether the substrate anion, e.g. formate, is required to be neutralized by protonation to pass the lipophilic constriction sites.
Initially, electrophysiology was used to determine substrate selectivity and pH dependent transport of FocA.2 These studies showed electrogenic, i.e. anion, transport at neutral pH and very low, i.e., channel-like, substrate affinity in the high millimolar range. Further, a sudden loss of anion transport was observed when the buffer pH was slightly shifted toward acidic conditions suggesting a gating mechanism for which different conformations of the N-terminal domain were made responsible.2 Together, FocA seemed to behave as a pH-gated anion channel.
Due to their weak acidity, e.g., pKa/formate 3.8 and pKa/acetate 4.8, the FNT substrates interconvert between the negatively charged anion and the protonated, neutral acid in a chemical equilibrium. To detect not only the electrogenic anion transport of FocA and PfFNT but additionally capture the transport of the neutral acid substrate species, we used radiolabeled substrates in our assays.3,7 Strikingly and contrary to the electrophysiology studies, we observed increasing transport rates with increasing acidity of the assay media. Apparently, neither FocA nor PfFNT exhibited closure or gating. Further, the alkalization of the media during transport indicated that protons are co-transported with the substrate and disruption of the transmembrane proton gradient revealed that transport is driven by a proton-motive force.7 Together, our data show that FNTs act as non-gated anion/proton co-transporters and the major portion of the substrate is transported as the protonated, neutral monoacid.
Where and how does the protonation occur? Initially, the central histidine was suggested to act as the proton transfer site.2 Yet, due to the lipophilic constrictions posing large energy barriers against ion passage this seems questionable. Instead, we identified a lysine at the bottom of the periplasmic vestibule to be indispensable for anion/proton co-transport as it electrostatically attracts substrate anions into the vestibule (Fig. 1). Positive amino acid residues at the opposite cytoplasmic FNT entry suggest a similar transport mechanism for the export direction. Toward the lysine, the interior of the vestibule is becoming less accessible for water and more hydrophobic. In fact, in solvation simulations, water molecules from the bulk never approached the lysine within hydrogen bond distance. When a substrate anion is, thus, drawn toward the lysine and faces an increasingly hydrophobic environment, its acidity will concomitantly drop. From titration experiments, we determined shifts in substrate acidity up to 3 pKa units, i.e., 1000fold, compared with the substrate acidity in the aqueous solvent. Determination of the corresponding dielectric constant, ε, i.e., 78 in water, yielded values in the range of 20–30 for the vestibule. Even at these conditions, the lysine is too strong a base to act as a proton donor. Hence, we conclude that the proton is derived from the bulk water and transfer occurs when the substrate reaches a certain point at its way downwards the dielectric slide.
Figure 1.
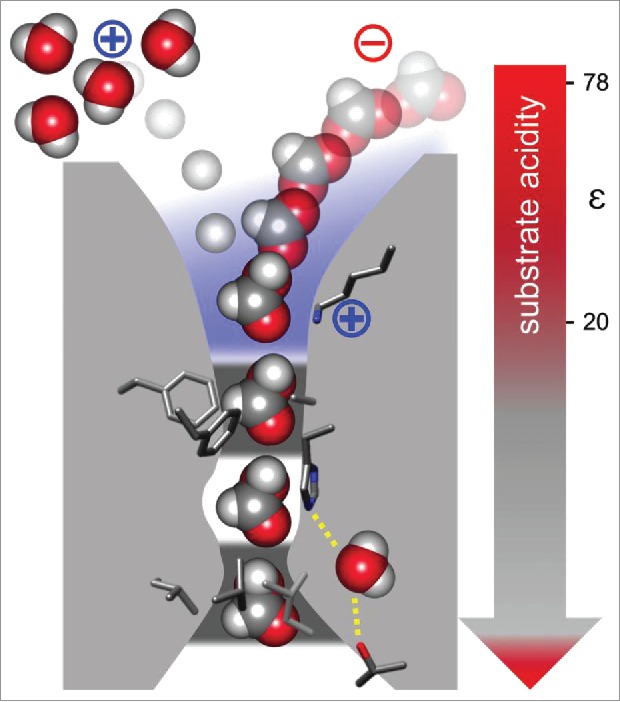
Transport mechanism of formate-nitrite transporters: The substrate anion, e.g., formate, is electrostatically attracted by a conserved lysine (blue shading) and slides into a hydrophobic vestibule. Substrate acidity decreases concomitantly with the lowering dielectricity, ε, facilitating proton transfer from the bulk water. The protonated, neutral substrate is largely preferred over the anion to pass the 2 lipophilic constriction sites (dark gray).
This mechanism is complementary to that of ammonium transporters, e.g., E. coli AmtB, which are thought to abstract a proton from the ammonium cation, NH4+, for neutralization and subsequent passage of neutral ammonia, NH3, via a hydrophobic transport path.8 Weak acid and base neutralizing transporters, thus, make use of the same mechanistic principle.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was funded by the Deutsche Forschungsgemeinschaft grant no. Be2253/6-3.
References
- [1].Suppmann B, Sawers G. Isolation and characterization of hypophosphite-resistant mutants of Escherichia coli: identification of the FocA protein, encoded by the pfl operon, as a putative formate transporter. Mol Microbiol 1994; 11:965-82; PMID:8022272; https://doi.org/ 10.1111/j.1365-2958.1994.tb00375.x [DOI] [PubMed] [Google Scholar]
- [2].Lü W, Du J, Schwarzer NJ, Wacker T, Andrade SL, Einsle O. The formate/nitrite transporter family of anion channels. Biol Chem 2013; 394:715-27; 23380538; PMID:23380538; https://doi.org/ 10.1515/hsz-2012-0339 [DOI] [PubMed] [Google Scholar]
- [3].Wu B, Rambow J, Bock S, Holm-Bertelsen J, Wiechert M, Soares AB, Spielmann T, Beitz E. Identity of a Plasmodium lactate/H+ symporter structurally unrelated to human transporters. Nat Commun 2015; 6:6284; 25669138; PMID:25669138; https://doi.org/ 10.1038/ncomms7284 [DOI] [PubMed] [Google Scholar]
- [4].Marchetti RV, Lehane AM, Shafik SH, Winterberg M, Martin RE, Kirk K. A lactate and formate transporter in the intraerythrocytic malaria parasite, Plasmodium falciparum. Nat Commun 2015; 6:6721; 25823844; PMID:25823844; https://doi.org/ 10.1038/ncomms7721 [DOI] [PubMed] [Google Scholar]
- [5].Golldack A, Henke B, Bergmann B, Wiechert M, Erler H, Blancke Soares A, Spielmann T, Beitz E. Substrate-analogous inhibitors exert antimalarial action by targeting the Plasmodium lactate transporter PfFNT at nanomolar scale. PLoS Pathog 2017; 13:e1006172; 28178358; PMID:28178358; https://doi.org/ 10.1371/journal.ppat.1006172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Hapuarachchi SV, Cobbold SA, Shafik SH, Dennis AS, McConville MJ, Martin RE, Kirk K, Lehane AM. The malaria parasite's lactate transporter PfFNT is the target of antiplasmodial compounds identified in whole cell phenotypic screens. PLoS Pathog 2017; 13:e1006180; 28178359; PMID:28178359; https://doi.org/ 10.1371/journal.ppat.1006180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Wiechert M, Beitz E. Mechanism of formate-nitrite transporters by dielectric shift of substrate acidity. EMBO J 2017; 36:949-58; 28250043; PMID:28250043; https://doi.org/ 10.15252/embj.201695776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Khademi S, O'Connell J 3rd, Remis J, Robles-Colmenares Y, Miercke LJ, Stroud RM. Mechanism of ammonia transport by Amt/MEP/Rh: structure of AmtB at 1.35 Å. Science 2004; 305:1587-94; 15361618; PMID:15361618; https://doi.org/ 10.1126/science.1101952 [DOI] [PubMed] [Google Scholar]


