ABSTRACT
Opioids are among the most powerful analgesics for managing pain, yet their repeated use can lead to the development of severe adverse effects. In a recent study, we identified the microglial pannexin-1 channel (Panx1) as a critical substrate for opioid withdrawal. Here, we investigated whether microglial Panx1 contributes to opioid-induced hyperalgesia (OIH) and opioid analgesic tolerance using mice with a tamoxifen-inducible deletion of microglial Panx1. We determined that escalating doses of morphine resulted in thermal pain hypersensitivity in both Panx1-expressing and microglial Panx1-deficient mice. In microglial Panx1-deficient mice, we also found that acute morphine antinociception remained intact, and repeated morphine treatment at a constant dose resulted in a progressive decline in morphine antinociception and a reduction in morphine potency. This reduction in morphine antinociceptive potency was indistinguishable from that observed in Panx1-expressing mice. Notably, morphine tolerant animals displayed increased spinal microglial reactivity, but no change of microglial Panx1 expression. Collectively, our findings indicate microglial Panx1 differentially contributes to opioid withdrawal, but not the development of opioid-induced hyperalgesia or tolerance.
KEYWORDS: microglia, opioid analgesic tolerance, opioid-induced hyperalgesia, opioid withdrawal, pannexin-1
Introduction
The dramatic rise in opioid prescriptions and off-label use has resulted in a concomitant increase in opioid-related hospitalizations and deaths.1,2 Yet, opioids remain an indispensible class of analgesics for managing moderate to severe pain, and account for approximately 20% of medications used to treat noncancer chronic pain in the United States.3 An over-reliance on opioids, and the potential for severe adverse opioid effects or abuse, have fueled growing concerns about the safety of long-term opioid use.
In a recent study, we interogated the underlying mechanisms of opioid physical dependence, a phenomenon characterized by a severe and debilitating withdrawal syndrome upon terminating opioid use. We identified the microglial pannexin-1 (Panx1) channel as an unexpected and novel therapeutic target in opioid withdrawal.4 Panx1 are expressed on neurons and glia within the central nervous system, and when activated allow the passage of molecules up to 1 kDa.5,6 Flow cytometric analysis revealed that escalating doses of morphine selectively increases Panx1 expression in spinal microglia, and blockade or genetic deletion of microglial Panx1 alleviates the autonomic and somatic symptoms of opioid withdrawal.
The cessation or reduction of opioid use can lead to a withdrawal syndrome in opioid dependent individuals.7 However, long-term and repeated opioid exposure can also result in other adverse effects, such as opioid-induced hyperalgesia (OIH) and analgesic tolerance. OIH manifests as a paradoxical increase in pain sensitivity,8 while analgesic tolerance is characterized by a progressive reduction in pain relieving effects.9 In an attempt to reestablish adequate pain control, opioid dose is often increased; this dose escalation can further exacerbate OIH and increase the risk of withdrawal symptoms upon discontinuation of opioids.
Opioid withdrawal, hyperalgesia, and analgesic tolerance have been linked to increased microglial reactivity within the dorsal horn of the spinal cord,4,10-15 a key site of opioid analgesia. There is, however, increasing evidence that divergent cellular mechanisms mediate these adverse effects12,13,16 – in other words, opioid tolerance, OIH, and withdrawal may have distinct mechanisms that can be differentially targeted. In the present addendum, we examined whether genetic deletion of microglial Panx1 affects the development of morphine analgesic tolerance and OIH.
Results
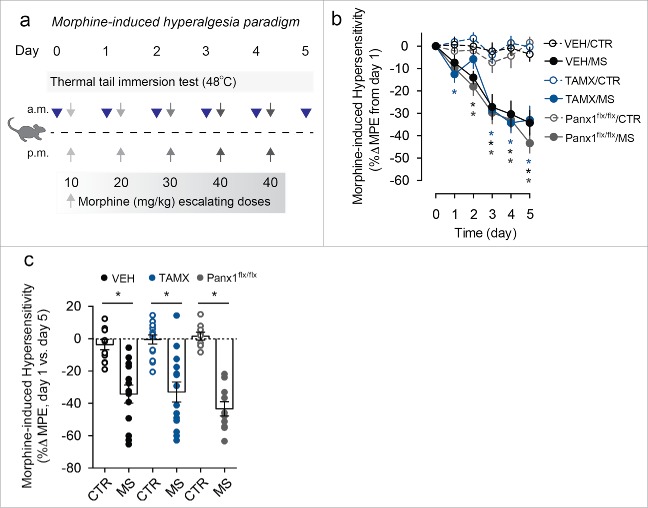
To assess the role of microglial Panx1 in OIH and analgesic tolerance, we used mice with a tamoxifen-inducible deletion of Panx1 from CX3CR1 expressing cells (microglial cells within the central nervous system; Cx3cr1-CreERT2::Panx1flx/flx). First, we investigated the contribution of microglial Panx1 in OIH using a behavioral paradigm where thermal tail withdrawal latencies were assessed daily before each morning injection of morphine (Fig. 1a). In both Panx1-expressing mice (vehicle treated Cx3cr1-CreERT2::Panx1flx/flx) and Panx1-deficient mice (tamoxifen treated Cx3cr1-CreERT2::Panx1flx/flx), there was a progressive reduction in tail withdrawal latencies over the course of 5 d of morphine treatment, indicating the development of pain hypersensitivity that was not observed with saline injections (Fig. 1b,c). To control for the effects of tamoxifen, we treated mice lacking Cre recombinase (Panx1flx/flx) with tamoxifen, and found that these Panx1-expressing mice also developed thermal pain hypersensitivity with repeated morphine treatment (Fig. 1b,c). These findings indicate that microglial Panx1 is not required for the development of morphine-induced hyperalgesia.
Figure 1.
Mice lacking microglial Panx1 develop opioid-induced hyperalgesia. (a) Schematic depicting morphine dosing and behavioral testing paradigm for opioid-induced hyperalgesia (OIH) experiments. (b) Assessment of daily response to a nociceptive thermal stimulus throughout the 5 d OIH paradigm. Behavior was assessed using the thermal tail immersion test at baseline before morning injections of morphine sulfate (MS) or saline (CTR) in vehicle (VEH) and tamoxifen (TAMX) treated Cx3cr1-CreERT2::Panx1flx/flx mice, and tamoxifen treated Panx1flx/flx mice. (VEH/CTR n = 11, VEH/MS n = 13, TAMX/CTR n = 14, TAMX/MS n = 14, Panx1flx/flx/CTR n = 9, Panx1flx/flx/MS n = 10). Two-way repeated-measures ANOVA (significant effect of time (F5,325 = 41.70), treatment (F5,65 = 16.47), and interaction (F25,325 = 7.21)) and Sidak post-hoc test. (c) Quantification of percent change in baseline nociceptive response before administration of saline (CTR) or morphine (MS) on day 0 compared with responses on day 5 following repeated CTR or MS treatment. VEH/CTR n = 11, VEH/MS n = 13, TAMX/CTR n = 14, TAMX/MS n = 14, Panx1flx/flx/CTR n = 9, Panx1flx/flx/MS n = 10. One-way ANOVA (F5,65 = 18.01) and Sidak post-hoc test. In each panel, error bars represent s.e.m; each circle represents data from an individual animal. *P<0.05.
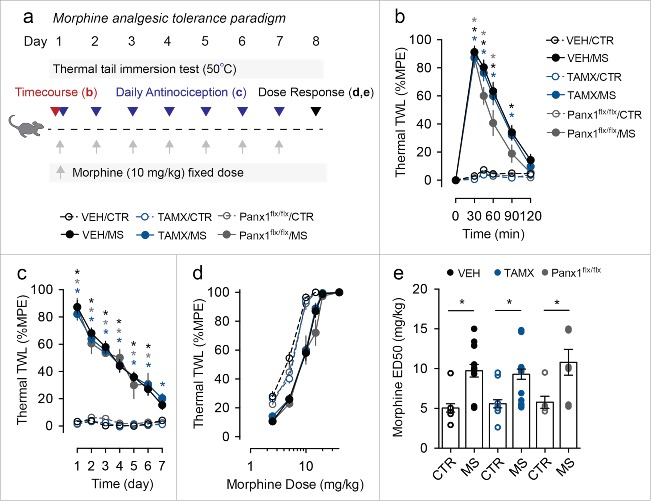
We next evaluated the acute antinociceptive response of Panx1-expressing and microglial Panx1-deficient mice to a single injection of morphine (10 mg per kg) (Fig. 2a). Morphine antinociception was measured at various time points after the injection of morphine. We found that both the duration and peak antinociceptive response to a single dose of morphine were indistinguishable between Panx1-expressing and deficient mice (Fig. 2b). Thus, acute morphine antinociception is intact in microglial Panx1-deficient mice.
Figure 2.
Genetic deletion of microglial Panx1 does not impact acute response to morphine or the development of morphine analgesic tolerance. (a) Schematic depicting morphine dosing and behavioral testing paradigm for morphine analgesic tolerance experiments. (b) Time course of antinociceptive response to an acute injection of morphine sulfate (MS) or saline (CTR) in vehicle (VEH) and tamoxifen (TAMX) treated Cx3cr1-CreERT2::Panx1flx/flx mice, and tamoxifen treated Panx1flx/flx mice. (VEH/CTR n = 11, VEH/MS n = 13, TAMX/CTR n = 14, TAMX/MS n = 18, Panx1flx/flx/CTR n = 6, Panx1flx/flx/MS n = 7). Two-way repeated-measures ANOVA (significant effect of time (F5,315 = 236.9), treatment (F5,63 = 56.45), and interaction (F25,315 = 52.49)) and Sidak post-hoc test. (c) Assessment of daily morphine antinociception throughout the 7 d tolerance paradigm. Behavior was assessed using the thermal tail immersion test 30 min after injection of morphine (MS) or saline (CTR) and was normalized to daily baseline in Cx3cr1-CreERT2:Panx1flx/flx mice treated with vehicle (VEH/CTR n = 12, VEH/MS n = 17) or tamoxifen (TAMX/CTR n = 15, TAMX/MS n = 21), or Panx1flx/flx mice that received tamoxifen (Panx1flx/flx/CTR n = 6, Panx1flx/flx/MS n = 7). Two-way repeated-measures ANOVA (significant effect of time (F6,432 = 73.80), treatment (F5,72 = 109.5), and interaction (F30,432 = 16.67)) and Sidak post-hoc test. (d-e) Morphine dose-response curve, (d), and median effective dose (ED50), (e), in mice that received 7 d of morphine (MS) or saline (CTR). VEH/CTR n = 10, VEH/MS n = 14, TAMX/CTR n = 13, TAMX/MS n = 18, Panx1flx/flx/CTR n = 6, Panx1flx/flx/MS n = 7. One-way ANOVA (F5,62 = 9.15) and Sidak post-hoc test. In each panel, error bars represent s.e.m; each circle represents data from an individual animal. *P<0.05.
To test whether genetic deletion of microglial Panx1 affects the development of morphine analgesic tolerance, mice were treated with a fixed dose of morphine (10 mg per kg, i.p.) over 7 consecutive days (Fig. 2a). Morphine antinociception was assessed 30 min after each daily injection of morphine. In Panx1-expressing mice, we observed a progressive decline in thermal tail flick latency with repeated morphine treatment, and after 7 days, morphine had no effect on thermal withdrawal thresholds compared with control animals (Fig. 2c). Repeated morphine treatment also produced a rightward shift in the dose response curve (Fig. 2d), and a significantly higher median effective dose (ED50) in morphine-treated mice compared with saline-treated controls (Fig. 2e). Similar to Panx1-expressing mice, littermate microglial Panx1-deficient mice displayed a progressive decline in thermal tail flick latency with repeated morphine, and a rightward shift in morphine dose response concomitant with increased morphine ED50 (Fig. 2c-e). These findings suggest that genetic deletion of microglial Panx1 does not impact the development of morphine analgesic tolerance in mice.
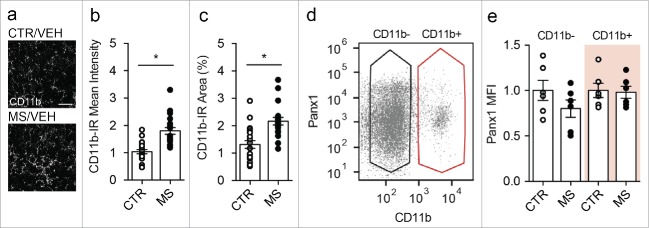
We recently reported that treatment with escalating doses of morphine increased microglial reactivity (as indicated by increased CD11b immunoreactivity) within the spinal dorsal horn, and preferentially upregulated Panx1 expression in CD11b-positive cells (microglia) isolated from the spinal cord of morphine dependent animals. Here, we investigated whether repeated morphine (10 mg per kg) treatment leading to the development of antinociceptive tolerance might affect CD11b and microglial Panx1 expression. We found that morphine tolerant animals showed a significant increase in mean intensity and percent area of CD11b positive staining in the spinal dorsal horn compared with saline treated control animals (Fig. 3a-c). Using a flow cytometry approach, we assessed Panx1 expression in CD11b positive (microglia) and CD11b negative (neurons and astrocytes) cells acutely isolated from the spinal cord of adult mice treated with 7 d of morphine (10 mg per kg) or saline. We found that Panx1 expression in both CD11b positive and CD11b negative populations was comparable in morphine and saline control (Fig. 3d-e), indicating that 7 d of repeated morphine treatment increased spinal microglial reactivity without affecting Panx1 expression.
Figure 3.
Morphine tolerant mice exhibit increased microglial reactivity, but no change in microglial Panx1 expression (a-e) Mice received daily morphine sulfate (MS, 10 mg per kg) or saline (CTR) for 7 d. (a) Representative images of CD11b immunoreactivity (IR) in spinal dorsal horn sections taken from CTR and MS mice. Scale bar = 25 μm. (b-c) Quantification of (b) mean intensity of CD11b-immunoreactivity (CD11b-IR) or (c) percent area of CD11b-IR in CTR (n = 21) and MS (n = 19) treated mice. N values indicate the number of sections analyzed. Two-tailed t-test (mean intensity, t = 5.38 df = 38; percent area, t = 4.28 df = 38). (d) Dot plot of flow cytometric analysis representing gating parameters used for CD11b negative (CD11b-, black) and CD11b positive (CD11b+, red) populations used to calculate mean fluorescent intensity (MFI) of Panx1 expression. (e) Quantification of Panx1 mean fluorescent intensity (MFI) in CD11b- or CD11b+ spinal cord cell populations obtained from saline (CTR) or morphine (MS) treated mice. MFI values from MS cells are normalized to respective CD11b- or CD11b+ control cells. N = 6 experimental replicates from 4 CTR and 4 MS animals. One-way ANOVA (F3,20 = 1.17, p = 0.35). In each panel, error bars represent s.e.m; each circle represents data from an individual experiment. *P<0.05.
Discussion
We recently discovered that activation of Panx1 on microglia is critically involved in opioid withdrawal.4 Here, we show that microglial Panx1 is not required for the development of opioid analgesic tolerance or OIH. Thus, microglial Panx1 may be a mechanistic point of divergence that underlies opioid withdrawal, but not tolerance or hyperalgesia.
Several microglial mechanisms have been implicated in the adverse actions of opioid analgesics (for review see11,17,18). For example, the P2X4R-BDNF-KCC2 signaling pathway is a core microglial mechanism causally involved in OIH.12,16 Activation of P2X4 receptors (P2X4R) triggers the release of BDNF from microglia, which signals to downregulate the K+-Cl− cotransporter KCC2 resulting in disinhibition of spinal lamina I nociceptive neurons.12 Blocking key nodes of the P2X4R-BDNF-KCC2 signaling ensemble effectively alleviates OIH,16 but has no bearing on morphine analgesic tolerance or withdrawal.12 P2X7 receptors (P2X7R), on the other hand, are important for opioid tolerance and withdrawal. In opioid tolerance, microglial P2X7R activation causes phosphorylation of p38 mitogen-activated protein kinases (MAPK) and upregulation of the proinflammatory cytokine IL-18, which potentiates spinal NMDAR function and opposes opioid analgesia.15 In opioid withdrawal, activation of P2X7R opens Panx1 channels to drive release of ATP, a key spinal substrate for the autonomic and somatic signs of opioid withdrawal.4 Our conclusion that microglial Panx1 is a mechanistic point of divergence supports growing evidence that opioid withdrawal, tolerance, and hyperalgesia do not necessarily reflect a single underlying mechanism, but rather these adverse effects may be a consequence of both overlapping and distinct signaling pathways involving multiple cell types.12,13,16
Although Panx1 channels on microglia may not be required for development of morphine tolerance or hyperalgesia, microglia nonetheless remain promising therapeutic targets for improving opioid pain therapy. In rodents, broad-spectrum inhibitors of glial function, such as propentofylline, ibudilast, or minocycline, have been shown to reduce tolerance, OIH, and withdrawal.14,19-21 Furthermore, intrathecal administration of a neutralizing antibody against the fractalkine receptor (CX3CR1), which in the central nervous system is localized on microglia, significantly attenuates hyperalgesia and tolerance,10 whereas depletion of spinal microglia suppresses hyperalgesia and withdrawal.4,12
The dissociation between increased CD11b staining and no change in Panx1 expression in response to morphine treatment provides a potential cellular explanation for the lack of Panx1 involvement in the development of morphine antinociceptive tolerance. It is important to note that in contrast to the tolerance paradigm where morphine was administered at a constant dose over 7 days, establishment of OIH and physical dependence involved escalating doses of morphine over 5 days, which increased both microglial reactivity and Panx1 expression within the dorsal horn.4 Based on our finding that genetic deletion of microglial Panx1 does not impact OIH, we surmise that the increase in spinal microglial Panx1 expression and function may be preferentially unmasked and expressed as autonomic and somatic physical symptoms during opioid withdrawal, but are not required for the development of OIH.
In addition to the classical definition of OIH as pain hypersensitivity that develops with repeated opioid use, OIH has also been described as a symptom of opioid withdrawal.22 Our data suggest that the cellular adaptations that underlie physical withdrawal symptoms and opioid-induced pain hypersensitivity develop differently. For instance, synaptic facilitation underlying OIH has been shown to represent a presynaptic mechanism within the dorsal horn,13 whereas we demonstrate that opioid withdrawal manifests as postsynaptic facilitation.4 Furthermore, OIH has been shown to depend on microglial-BDNF dependent disinhibition of dorsal horn synapses, a pathway that is not critical for withdrawal.12 Therefore, our findings together with other developments in the field, point to divergent mechanisms underlying OIH and withdrawal.
In conclusion, we have identified microglial Panx1 as a cellular point of divergence that critically underlies opioid withdrawal, but not opioid tolerance or hyperalgesia. Since these side effects are mechanistically separable, it may be possible to differentially target opioid withdrawal, tolerance and OIH.
Methods
Animals
Adult male and female mice aged 8–14 weeks were used for all experiments. Mice were housed under a 12-h/12-h light/dark cycle with ad libitum access to food and water. Mice were randomly allocated to different test groups, and experimenters were blind to drug treatment and genetic profile of mice. All experiments were approved by the University of Calgary Animal Care Committees and are in accordance with the guidelines of the Canadian Council on Animal Care.
Morphine dosing paradigms and nociceptive behavioral models
Morphine sulfate (PCCA) prepared in sterile saline solution was administered via intraperitoneal (i.p.) injection into male and female Cx3cr1-CreERT2::Panx1flx/flx or Panx1flx/flx mice (weight ranging 18–32 g). Thermal nociceptive thresholds were assessed using the tail immersion test. Briefly, the distal portion of the tail was submerged in a 50 °C (tolerance) or 48 °C (hyperalgesia) water bath, and time latency to vigorous tail flicking was recorded and averaged over 3 consecutive responses. A maximum cutoff time was set at 10 s for 50 °C and 20 s for 48 °C to prevent tissue damage. For the hyperalgesia paradigm, mice were treated with escalating doses of morphine twice daily for 5 d (Day 1: 10 mg per kg; Day 2: 20 mg per kg; Day 3: 30 mg per kg; Day 4 and 5: 40 mg per kg). Nociceptive measurements were taken before all morning morphine injections throughout the testing paradigm. All values were adjusted to baseline on day 0 and maximum cut-off time of 20 s (%MPE). On day 1 of the tolerance paradigm (in a subset of mice), a time course of morphine-induced antinociception was performed at 30, 45, 60, 90 and 120 min after the first injection of morphine (10 mg per kg). Mice then received 7 consecutive days of fixed dose morphine treatment (10 mg per kg). Nociceptive measurements were taken before and 30 min after morphine injections, and values were adjusted to daily baseline and maximum cut-off time (% MPE). On day 8 (following 7 d of morphine or saline treatment) a subset of mice were subjected to a morphine dose response. During the dose response, mice were injected with escalating doses of morphine (2.5 mg per kg to 40 mg per kg), until tail flick latencies reached the maximal cutoff time of 10 s. ED50 was calculated from the dose response curve using GraphPad Prism 6 software.
Generation of Cx3cr1-CreERT2::Panx1flx/flx mice
Mice with microglial-specific deletion of Panx1 were generated using a Cre-loxP system as described previously.4 Briefly, Panx1flx/flx homozygote mice with loxP sequences flanking exon 2 of the Panx1 gene were crossed with C57BL6/J mice expressing Cre-ERT2 fusion protein under the Cx3cr1 promoter (Jax mice: B6.129P2(Cg)-Cx3cr1tm2.1(cre/ERT)Litt/WganJ, stock number 021160). To induce Cre recombination, mice were injected i.p. with tamoxifen (1 mg, Sigma) over 5 d in the tolerance paradigm and 2 d in the hyperalgesia paradigm. Comparable levels of recombination were observed in mice receiving 5 d versus 2 d of tamoxifen. Control mice were littermates that received vehicle injections (sunflower oil with 10% ethanol), while tamoxifen-related effects were controlled for using Panx1flx/flx (no Cre expression) littermate mice that received tamoxifen injections. All experiments were conducted at least 21 d after the first tamoxifen or vehicle injection to control for the effects of peripheral Cx3cr1-expressing cells.
Immunohistochemistry
Mice were treated for 7 d with saline or morphine (10 mg per kg). On day 7, mice were anesthetized with isoflurane and perfused transcardially with PBS. Following dissection, the spinal lumbar segment was post-fixed in 4% PFA, then cryoprotected in 30% sucrose for at least 24 hr. Spinal cords were sliced at 30 μm into free-floating sections, then incubated overnight at 4°C in rat antibody to CD11b (1:1000, Abcam, ab8878). Sections were then washed and incubated at 4°C with fluorochrome-conjugated secondary antibodies (1:2000 donkey anti-rat IgG Alexa Fluor 647, Abcam, ab150155). Images were taken with a Nikon A1R multiphoton microscope and image quantification was performed using Image J (NIH).
Flow cytometry
Mice were treated for 7 d with saline or morphine (10 mg per kg). On day 7, mice were anaesthetized with isoflurane and perfused transcardially with PBS. The lumbar segment of the spinal cord was isolated and submerged in HBSS. Following blunt dissection, spinal cord contents were filtered through a 70 μm cell strainer into DMEM containing 10 mM HEPES and 2% FBS. Isotonic Percoll (density 1,23 g/mL, GE Biosciences) was mixed into the cell suspension, followed by a 1,08 g/mL Percoll underlay. Samples were spun at 3000 r.p.m. for 30 min at 20 °C, after which myelin debris was discarded and the interface between Percoll gradients was collected and transferred to fresh medium. Samples were centrifuged again at 1350 r.p.m. for 10 min at 4 °C, and the pellet was reconstituted in PBS containing 1% BSA. Cells were fixed with 2% PFA for 10 min, washed, then permeabilized in 0.1% Triton-X for 10 min. Cells were then stained with fluorophore-conjugated CD11b/c-PE (1:500 eBioscience, 12–0110–82) and rabbit antibody to Panx1 (1:400 Pierce, PA5–34475, and 1:400 Life Technologies) pre-incubated with fluorophore-conjugated anti-rabbit secondary antibody (1:500 Cell Signaling Technology, 4414S) for 1 hr at 20 °C. Cell fluorescence was measured by an Attune Acoustic Focusing Cytometer (Applied Biosystems). Viable cell population was gated using forward and side scatter plots. CD11b- and Panx1-positive staining were gated using BL2 and RL1 intensities, in single stained compared with unstained cells.
Statistics
All data are presented as mean ± s.e.m., and each data point represents an individual animal or experiment. Tests of statistical difference were performed with GraphPad Prism 6 software using unpaired 2-tailed t-test, ordinary one-way ANOVA or 2-way repeated-measures ANOVA with post hoc Sidak. For all experiments, statistical significance was defined at p < 0.05.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by grants from the Natural Sciences and Engineering Research Council of Canada (RGPIN418299), the Canadian Institutes of Health Research (MOP133523), Vi Riddell Pediatric Pain Program, Rita Allen Foundation and American Pain Society, and the Canadian Foundation for Innovation to TT. N.E.B. is supported by a CIHR Doctoral Research Award. H.L-P. is a recipient of an Alberta Innovates Health Solutions Graduate Scholarship.
References
- [1].Rudd RA, Aleshire N, Zibbell JE, Gladden RM. Increases in drug and opioid overdose deaths–United States, 2000–2014. MMWR Morb Mortal Wkly Rep. 2016;64:1378-82. doi: 10.15585/mmwr.mm6450a3. PMID:26720857 [DOI] [PubMed] [Google Scholar]
- [2].Owens PL, Barrett ML, Weiss AJ, Washington RE, Kronick R. Hospital inpatient utilization related to opioid overuse among adults, 1993–2012: Statistical brief #177 In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville: (MD: ): Agency for Healthcare Research and Quality (US); 2006. Retrieved from: https://www.ncbi.nlm.nih.gov/books/NBK246983/ [PubMed] [Google Scholar]
- [3].Daubresse M, Chang HY, Yu Y, Viswanathan S, Shah ND, Stafford RS, Kruszewski SP, Alexander GC. Ambulatory diagnosis and treatment of nonmalignant pain in the United States, 2000–2010. Med Care. 2013;51:870-8. doi: 10.1097/MLR.0b013e3182a95d86. PMID:24025657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Burma NE, Bonin RP, Leduc-Pessah H, Baimel C, Cairncross ZF, Mousseau M, Shankara JV, Stemkowski PL, Baimoukhametova D, Bains JS, et al.. Blocking microglial pannexin-1 channels alleviates morphine withdrawal in rodents. Nat Med. 2017;23:355-60. doi: 10.1038/nm.4281. PMID:28134928 [DOI] [PubMed] [Google Scholar]
- [5].Penuela S, Gehi R, Laird DW. The biochemistry and function of pannexin channels. Biochim Biophys Acta. 2013;1828:15-22. doi: 10.1016/j.bbamem.2012.01.017. PMID:22305965 [DOI] [PubMed] [Google Scholar]
- [6].Boyce AKJ, Epp AL, Nagarajan A, Swayne LA. Transcriptional and post-translational regulation of pannexins. Biochim Biophys Acta. 2017; [Epub ahead of print]; doi: 10.1016/j.bbamem.2017.03.004. PMID:28279657. [DOI] [PubMed] [Google Scholar]
- [7].World Health Organization Clinical guidelines for withdrawal management and treatment of drug dependence in closed settings. WHO Press, Geneva, Switzerland. [PubMed] [Google Scholar]
- [8].Carullo V, Fitz-James I, Delphin E. Opioid-induced hyperalgesia: A diagnostic dilemma. J Pain Palliat Care Pharmacother. 2015;29:378-84. doi: 10.3109/15360288.2015.1082006. PMID:26523869 [DOI] [PubMed] [Google Scholar]
- [9].Dumas EO, Pollack GM. Opioid tolerance development: A pharmacokinetic/pharmacodynamic perspective. AAPS J. 2008;10:537-51. doi: 10.1208/s12248-008-9056-1. PMID:18989788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Johnston IN, Milligan ED, Wieseler-Frank J, Frank MG, Zapata V, Campisi J, Langer S, Martin D, Green P, Fleshner M, et al.. A role for proinflammatory cytokines and fractalkine in analgesia, tolerance, and subsequent pain facilitation induced by chronic intrathecal morphine. J Neurosci. 2004;24:7353-65. doi: 10.1523/JNEUROSCI.1850-04.2004. PMID:15317861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Watkins LR, Hutchinson MR, Johnston IN, Maier SF. Glia: Novel counter-regulators of opioid analgesia. Trends Neurosci. 2005;28:661-9. doi: 10.1016/j.tins.2005.10.001. PMID:16246435 [DOI] [PubMed] [Google Scholar]
- [12].Ferrini F, Trang T, Mattioli T-AM, Laffray S, Del'Guidice T, Lorenzo L-E, Castonguay A, Doyon N, Zhang W, Godin AG, et al.. Morphine hyperalgesia gated through microglia-mediated disruption of neuronal Cl− homeostasis. Nat Neurosci. 2013;16:183-92. doi: 10.1038/nn.3295. PMID:23292683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Corder G, Tawfik VL, Wang D, Sypek EI, Low SA, Dickinson JR, Sotoudeh C, Clark JD, Barres BA, Bohlen CJ, et al.. Loss of μ opioid receptor signaling in nociceptors, but not microglia, abrogates morphine tolerance without disrupting analgesia. Nat Med. 2017;23:164-73. doi: 10.1038/nm.4262. PMID:28092666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Raghavendra V, Tanga FY, DeLeo JA. Attenuation of morphine tolerance, withdrawal-induced hyperalgesia, and associated spinal inflammatory immune responses by propentofylline in rats. Neuropsychopharmacology. 2004;29:327-34. doi: 10.1038/sj.npp.1300315. PMID:14532913 [DOI] [PubMed] [Google Scholar]
- [15].Chen ML, Cao H, Chu YX, Cheng LZ, Liang LL, Zhang YQ, Zhao ZQ. Role of P2X7 receptor-mediated IL-18/IL-18R signaling in morphine tolerance: Multiple glial-neuronal dialogues in the rat spinal cord. J Pain. 2012;13:945-58; doi: 10.1016/j.jpain.2012.06.007. PMID:22968128 [DOI] [PubMed] [Google Scholar]
- [16].Ferrini F, Lorenzo LE, Godin AG, Quang ML, De Koninck Y. Enhancing KCC2 function counteracts morphine-induced hyperalgesia. Sci Rep. 2017;7:3870. doi: 10.1038/s41598-017-04209-3. PMID:28634406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hutchinson MR, Shavit Y, Grace PM, Rice KC, Maier SF, Watkins LR. Exploring the Neuroimmunopharmacology of opioids: An integrative review of mechanisms of central immune signaling and their implications for opioid analgesia. Pharmacol Rev. 2011;63:772-810. doi: 10.1124/pr.110.004135. PMID:21752874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Trang T, Al-Hasani R, Salvemini D, Salter MW, Gutstein H, Cahill CM. Pain and Poppies: The good, the bad, and the ugly of opioid analgesics. J Neurosci. 2015;35:13879-88. doi: 10.1523/JNEUROSCI.2711-15.2015. PMID:26468188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ledeboer A, Hutchinson MR, Watkins LR, Johnson KW. Ibudilast (AV-411). A new class therapeutic candidate for neuropathic pain and opioid withdrawal syndromes. Expert Opin Investig Drugs. 2007;16:935-50. doi: 10.1517/13543784.16.7.935. PMID:17594181 [DOI] [PubMed] [Google Scholar]
- [20].Hutchinson MR, Lewis SS, Coats BD, Skyba DA, Crysdale NY, Berkelhammer DL, Brzeski A, Northcutt A, Vietz CM, Judd CM, et al.. Reduction of opioid withdrawal and potentiation of acute opioid analgesia by systemic AV411 (ibudilast). Brain Behav Immun. 2009;23:240-50. doi: 10.1016/j.bbi.2008.09.012. PMID:18938237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Zhang Y, Li H, Li Y, Sun X, Zhu M, Hanley G, Lesage G, Yin D. Essential role of toll-like receptor 2 in morphine-induced microglia activation in mice. Neurosci Lett. 2011;489:43-7. doi: 10.1016/j.neulet.2010.11.063. PMID:21130144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Lee M, Silverman SM, Hansen H, Patel VB, Manchikanti L. A comprehensive review of opioid-induced hyperalgesia. Pain Physician. 2011;14:145-61. PMID:21412369 [PubMed] [Google Scholar]