The free cytosolic Ca2+ concentration and voltage across the plasma membrane are major determinants of cell function. Both are regulated by Ca2+-permeable, non-selective cation channels, which are varied and numerous. Several superfamilies of protein are involved, one of which is the Transient Receptor Potential (TRP) family of membrane proteins encoded by 28 genes in mammals.1 TRP proteins form channels by assembling as tetramers (Fig. 1A) and heteromultimerisation (mixing together of different TRPs in a tetramer) significantly increases the number of possible TRP channels—exactly how many distinct TRP channels exist, however, remains unclear. One of the subfamilies of mammalian TRP is formed by the Canonical TRPCs, so called because they are closest in amino acid sequence to the archetypal D. melanogaster TRP.2 There are 7 TRPCs, all of which exist in humans except TRPC2.
Figure 1.
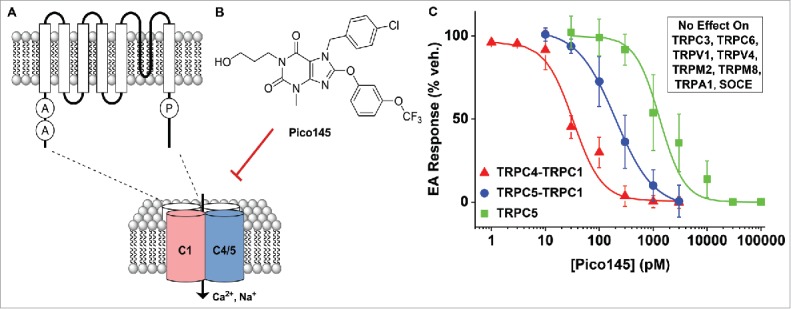
Picomolar small-molecule inhibition of TRPC1/4/5 channels and its lack of effect on other TRPs and store-operated calcium entry channels (SOCE).
TRPCs are striking for their widespread expression and tendency to form heteromers.2,3 One of the heteromerising clusters consists of TRPC1, TRPC4 and TRPC5. TRPC4 and TRPC5 are each able to form homomeric channels that are biophysically similar to each other. In contrast, TRPC1 does not form homomeric channels, or does so poorly, yet it readily forms heteromers with TRPC4 and TRPC5, generating ion channels with distinct biophysical characteristics.4 Importantly, TRPC1 is widely expressed across many cell types, suggesting that TRPC4 and TRPC5 commonly exist in heteromers with TRPC1. However, the tools with which to determine the contributions of homomers, heteromers or combinations thereof have been lacking.
TRPC1/4/5 channels seem not to have a single physiologic activator. Instead they are promiscuous— multiple modulators exist. Not all are endogenous physiologic substances; rather, some are exogenous chemicals from plants, consistent with a general idea that TRP channels help mammals integrate with their external environment. Modulators of the TRPC1/4/5 channels include receptor agonists, hydrogen peroxide, mild acidification, toxic metal ions, oxidised phospholipids, galangin and ω-3 fatty acids.3 The most powerful, potent and selective activator is (−)-Englerin A, generated by the East African plant Phyllanthus engleri.5,6 The physiologic significance of the (−)-Englerin A-induced TRPC1/4/5 activation is unknown. In mice, (−)-Englerin A is toxic.6
Despite limitations in tools for studying TRPC1/4/5 channels, there is encouraging evidence for roles in pathophysiology, for example in epilepsy, anxiety, pain, adverse cardiac and vascular remodelling, rheumatoid arthritis and cancer.6 A physiologic necessity or survival advantage of TRPC1/4/5 channels has remained unclear but suggested pro-pathophysiological roles have led to interest in the channels as targets in therapeutic drug discovery efforts. Whether the target should be TRPC1, TRPC4, TRPC5—or some combination of these—has nevertheless often been unclear.
The motivation to discover and develop potent, selective small-molecule inhibitors of TRPC1/4/5 channels has therefore been 2-fold: first to facilitate basic research efforts aimed at better understanding the roles of TRPC1/4/5 channels across species, tissues and (patho)physiologic processes, and second to enable proof-of-concept drug discovery studies and foundations for intellectual property.
Unfortunately, potent and selective pharmacological agents for TRPC1/4/5 channels have been hard to come by.7 (−)-Englerin A was a significant breakthrough, demonstrating the possibility for very effective, potent and selective modulation. But (−)-Englerin A is an activator of the channels and it discriminates poorly between different combination of TRPC1/4/5. Despite these limitations, (−)-Englerin A is an important tool for TRPC1/4/5 channel studies because it enables robust and clear activation of the channels in overexpression and endogenous systems; previously it was often difficult to clearly distinguish TRPC1/4/5 signals from other signals or to perform efficient compound screening. Another limitation was lack of clarity over whether a functional signal resulting from overexpression of TRPC1, TRPC4 and/or TRPC5 was mediated by a homomer or a heteromer. We provided clarity by generating and characterizing functional concatemers of TRPC1 with TRPC4 or TRPC5, balancing expression of TRPC1 with its partner.4,8 An observation made with concatemers was that heteromeric channels are less sensitive to known inhibitors of the channels such as ML204,4 suggesting that such agents are not necessarily good for determining the relevance of TRPC4 in cell, tissue and whole animal studies.
Our recent publication demonstrates the characterization of a high-quality, potent and selective inhibitor of TRPC1/4/5 channels, which we named “Pico145” (Fig. 1B).4 Pico145 can be readily synthesized, in only 3 steps, from commercially available starting materials, facilitating its wide-spread use by investigators. Its potency ranges from 9 to 1300 pM depending on the TRPC1/4/5 subtype and activation mechanism while a range of other investigated channel types were unaffected (Fig. 1C). The findings suggest identification of an important chemical probe of TRPC1/4/5 channels, with potency orders of magnitude better than previously reported agents, impressive specificity, and graded subtype selectivity within the TRPC1/4/5 channel family (Fig. 1C). Pico145 should greatly facilitate future studies of the TRPC1/4/5 channels.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- [1].Damann N, Voets T, Nilius B. TRPs in our senses. Curr Biol 2008; 18:R880-9; PMID:18812089; http://dx.doi.org/ 10.1016/j.cub.2008.07.063 [DOI] [PubMed] [Google Scholar]
- [2].Abramowitz J, Birnbaumer L. Physiology and pathophysiology of canonical transient receptor potential channels. FASEB J 2009; 23:297-328; http://dx.doi.org/ 10.1096/fj.08-119495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Beech DJ. Characteristics of transient receptor potential canonical calcium-permeable channels and their relevance to vascular physiology and disease. Circulation J 2013; 77:570-9; http://dx.doi.org/ 10.1253/circj.CJ-13-0154 [DOI] [PubMed] [Google Scholar]
- [4].Rubaiy HN, Ludlow MJ, Henrot M, Gaunt HJ, Miteva K, Cheung SY, Tanahashi Y, Hamzah N, Musialowski KE, Blythe NM, et al.. Picomolar, selective and subtype specific small-molecule inhibition of TRPC1/4/5 channels. J Biol Chem 2017. http://dx.doi.org/ 10.1074/jbc.M116.773556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Akbulut Y, Gaunt HJ, Muraki K, Ludlow MJ, Amer MS, Bruns A, Vasudev NS, Radtke L, Willot M, Hahn S, et al.. (−)-Englerin A is a potent and selective activator of TRPC4 and TRPC5 calcium channels. Angewandte Chemie 2015; 54:3787-91; PMID:25707820; http://dx.doi.org/ 10.1002/anie.201411511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Gaunt HJ, Vasudev NS, Beech DJ. Transient receptor potential canonical 4 and 5 proteins as targets in cancer therapeutics. Eur Biophys J 2016; 45:611-20; PMID:27289383; http://dx.doi.org/ 10.1007/s00249-016-1142-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Bon RS, Beech DJ. In pursuit of small molecule chemistry for calcium-permeable non-selective TRPC channels— mirage or pot of gold? Br J Pharmacol 2013; 170:459-74; PMID:23763262; http://dx.doi.org/ 10.1111/bph.12274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ludlow MJ, Gaunt HJ, Rubaiy HN, Musialowski KE, Blythe NM, Vasudev NS, Muraki K, Beech DJ. (−)-Englerin A-evoked Cytotoxicity is mediated by Na+ Influx and Counteracted by Na+/K+-ATPase. J Biol Chem 2017; 292:723-31; PMID:27875305; http://dx.doi.org/ 10.1074/jbc.M116.755678 [DOI] [PMC free article] [PubMed] [Google Scholar]


