ABSTRACT
We recently reported key physiologic roles for Ca2+-activated transient receptor potential melastatin 4 (TRPM4) channels in detrusor smooth muscle (DSM). However, the Ca2+-signaling mechanisms governing TRPM4 channel activity in human DSM cells are unexplored. As the TRPM4 channels are activated by Ca2+, inositol 1,4,5-trisphosphate receptor (IP3R)-mediated Ca2+ release from the sarcoplasmic reticulum represents a potential Ca2+ source for TRPM4 channel activation. We used clinically-characterized human DSM tissues to investigate the molecular and functional interactions of the IP3Rs and TRPM4 channels. With in situ proximity ligation assay (PLA) and perforated patch-clamp electrophysiology, we tested the hypothesis that TRPM4 channels are tightly associated with the IP3Rs and are activated by IP3R-mediated Ca2+ release in human DSM. With in situ PLA, we demonstrated co-localization of the TRPM4 channels and IP3Rs in human DSM cells. As the TRPM4 channels and IP3Rs must be located within close apposition to functionally interact, these findings support the concept of a potential Ca2+-mediated TRPM4-IP3R regulatory mechanism. To investigate IP3R regulation of TRPM4 channel activity, we sought to determine the consequences of IP3R pharmacological inhibition on TRPM4 channel-mediated transient inward cation currents (TICCs). In freshly-isolated human DSM cells, blocking the IP3Rs with the selective IP3R inhibitor xestospongin-C significantly decreased TICCs. The data suggest that IP3Rs have a key role in mediating the Ca2+-dependent activation of TRPM4 channels in human DSM. The study provides novel insight into the molecular and cellular mechanisms regulating TRPM4 channels by revealing that TRPM4 channels and IP3Rs are spatially and functionally coupled in human DSM.
KEYWORDS: TRPM4 channel, patch-clamp, proximity ligation assay, xestospongin-C
Introduction
The functions of the urinary bladder—the principle organ responsible for the storage and periodic release of urine—are mediated by the activity of detrusor smooth muscle (DSM). In DSM cells, it is well-established that Ca2+ influx through L-type voltage-gated Ca2+ channels underlies the depolarization phase of spontaneous action potentials while the large-conductance voltage- and Ca2+-activated K+ (BK) channels, in addition to other voltage-gated KV channels, work in opposition to L-type voltage-gated Ca2+ channel activity to promote membrane hyperpolarization.1-4 Albeit these mechanisms have been known for many years in DSM, recent studies from our group have identified an important role for an additional Ca2+-sensitive cationic conductance regulating DSM function in rodents and humans.5-8 Indeed, Ca2+-activated monovalent cation channels known as the transient receptor potential (TRP) melastatin 4 (TRPM4) channels are functionally expressed in rat, guinea pig, and human DSM.5-8
Of the 28 members that constitute the TRP channel superfamily, TRPM4 channels, in addition to TRPM5, are the only TRP channel members selective for monovalent cations (K+ and Na+) over Ca2+.9,10 TRPM4 channel functional roles in excitable cells and tissues, including rodent6-8 and human DSM,5-8 have begun to emerge in recent years following studies using the novel and selective TRMP4 channel inhibitor, 9-phenanthrol.6-8,11-13 We recently provided the first detailed study demonstrating the expression and key functional roles for TRPM4 channels in human DSM excitability and contractility using the selective TRPM4 channel inhibitor 9-phenanthrol.5 Our novel study was the central topic of an editorial focus in the American Journal of Physiology – Cell Physiology,14 which highlighted the attractiveness of the TRPM4 channels as potential novel targets for the pharmacological control of the urinary bladder. Under pathophysiological conditions, DSM dysfunction is associated with some forms of overactive bladder.15 The TRPM4 channels, in addition to the cellular mechanisms regulating their activity, represent an exciting and novel therapeutic approach to the treatment of various bladder disorders and the collateral effects these conditions inflict on patient quality of life. Nonetheless, significant work still remains to ascertain the cellular mechanisms regulating TRPM4 channel activity in human DSM function to further validate their potential therapeutic role for bladder disorders.
It has been suggested that Ca2+ release from the sarcoplasmic reticulum (SR) inositol 1,4,5-trisphosphate (IP3) receptors (IP3Rs) may constitute a primary Ca2+ source for TRPM4 channel activation.16 The concept of IP3R-mediated Ca2+ release serving as critically important Ca2+ source for TRPM4 channel activation, as opposed to Ca2+ influx through L-type voltage-gated Ca2+ channels, was supported by the observation that removal of extracellular Ca2+ had no acute effects on TRPM4 channel activity.16 Whether this TRPM4-IP3R regulatory mechanism exists in human DSM, however, is completely unknown.
Therefore, the current study sought to investigate the molecular and functional interaction of the TRPM4 channels and IP3Rs in human DSM. This was achieved via a combined experimental approach using in situ proximity ligation assays (PLA) and perforated patch-clamp electrophysiology in combination with pharmacological tools. This study reveals the novel mechanism whereby SR IP3Rs regulate the excitability of human DSM through molecular and functional interactions with TRPM4 channels.
Materials and methods
Human DSM tissue acquisition
Human DSM tissues were acquired from donor patients undergoing routine open bladder surgeries as described previously.5,17,18 All procedures attendant upon the collection of human DSM tissues have been reviewed and approved by the Institutional Review Board of the Medical University of South Carolina (protocol number Pro00045232). 6 Caucasian patients (3 men and 3 women; average age 64.5 ± 11.2 years) without preoperative histories of overactive bladder and American Urological Association symptom scores <8 were used for this study. Following surgeries, human DSM tissues were stored on ice in a container containing HEPES-buffered dissection solution (see Solutions and Drugs) for immediate transport to the laboratory for DSM cell isolation.
DSM cell isolation
Human DSM single cells were enzymatically isolated using a combination of papain and collagenase as described previously.5,17,18 Freshly-isolated DSM cells were used for in situ PLA or electrophysiological experiments within 12 h following isolation.
In situ proximity ligation assay (PLA)
In situ PLA was performed on freshly-isolated DSM cells using the Duolink® in situ orange starter kit goat/rabbit (Cat. No: DUO92106, Sigma-Aldrich, St. Louis, MO) according to the manufacturer's instructions. DSM cells underwent fixation in 2% paraformaldehyde (10 min, 37°C) followed by 2 15-min washes in phosphate buffered saline. DSM cells were blocked in Duolink blocking solution (30 min, 37°C). Subsequently, DSM cells were incubated with the anti-rabbit IP3R and goat anti-TRPM4 channel antibodies (Santa Cruz Biotechnology, Dallas, TX; catalog numbers SC-27540 TRPM4; SC-28614 IP3R), respectively, in Duolink antibody diluent solution overnight at 4°C, followed by 2 × 5 min washes in Duolink 1x wash buffer A solution. The rabbit anti-Kv7.5 antibody was also obtained from Santa Cruz (catalog number SC-50416). DSM cells were then incubated with the Duolink PLA probe anti-rabbit MINUS and anti-goat PLUS oligonucleotide-conjugated secondary antibodies (1 h, 37°C), followed by 2 × 5 min washes in Duolink 1x wash buffer A solution. DSM cells then underwent incubation in ligase-ligation solution (30 min, 37°C), which hybridizes the 2 PLA MINUS and PLUS probes when in close proximity (< 40 nm). Following ligation, DSM cells were washed 2 × 2-min in Duolink 1x wash buffer A solution. Finally, DSM cells were incubated in amplification-polymerase solution (100 min, 37°C) containing nucleotides and fluorescently-labeled oligonucleotides. The oligonucleotide arm of one PLA probe is a primer for rolling-circle amplification. The fluorescently-labeled oligonucleotides hybridize to the rolling-circle amplification product thus permitting detection. Following amplification, DSM cells were washed 2 × 10 min in 1x wash buffer B and then a 1 min wash in 0.01x wash buffer B. Slides were then mounted using minimal volume of Duolink in situ mounting medium with 4',6-diamidino-2-phenylindole (DAPI). PLA signals (Duolink In Situ Detection Reagents Orange (λexcitation/emission = 554/576 nm)) were detected using laser scanning LSM 700 confocal microscope (Carl Zeiss, Germany) with a 63x oil immersion objective.
Patch-clamp electrophysiology
The amphotericin B-perforated whole cell patch-clamp technique was used in all of the electrophysiological recordings as described previously.5,7,8,19 At the holding potential of −70 mV (corrected for junction potential), the total open channel probability (NPo) of TRPM4 channel-mediated transient inward cation currents (TICCs) before and after the addition of xestospongin-C was analyzed. A stable recording period of at least 5-10 min before and after the addition of xestospongin-C was used for analysis with pCLAMP version 10.2 software (Molecular Devices, Union City, CA).
Solutions and drugs
The Ca2+-free dissection solution had the following composition (in mM): 80 monosodium glutamate, 55 NaCl, 6 KCl, 2 MgCl2, 10 HEPES, and 10 glucose, adjusted to pH 7.3 with NaOH. The extracellular (bath) solution used in the patch-clamp experiments in the gap-free mode (both voltage- and current-clamp) contained (in mM): 134 NaCl, 6 KCl, 1 MgCl2, 2 CaCl2, 10 glucose, and 10 HEPES, pH adjusted to 7.4 with NaOH. The patch-clamp pipette solution for these experiments contained (in mM): 110 potassium aspartate, 30 KCl, 10 NaCl, 1 MgCl2, 10 HEPES, and 0.05 EGTA, pH adjusted to 7.2 with NaOH and supplemented with freshly dissolved (every 1–2 h) 200 μg/ml amphotericin-B in dimethyl sulfoxide. Bovine serum albumin was obtained from Thermo Fisher Scientific (Fair Lawn, NJ). All other compounds were obtained from Sigma-Aldrich.
Data analysis and statistics
The TICCs were analyzed as open channel probability (NPo) as described previously.5 Data are summarized as means ± SEM for n (the number of DSM cells) isolated from N (the number of patients). Data were compared using a paired Student's t-test. A P value <0.05 was considered statistically significant.
Results
TRPM4 channels are colocalized with the IP3Rs in human DSM cells
In order for the discrete Ca2+ release events from SR IP3Rs to regulate TRPM4 channel activity in human DSM, the IP3Rs and TRPM4 channels must be located within close apposition. To determine TRPM4 channel colocalization with IP3Rs, we conducted Duolink in situ PLA on freshly-isolated human DSM cells. To elucidate TRPM4/IP3R colocalization, human DSM cells were incubated with the anti-TRPM4 goat and anti-IP3R rabbit antibodies, respectively. As shown in Fig. 1A, co-incubation of TRPM4 and IP3R antibodies positively confirmed that TRPM4 channels and IP3Rs are located in close proximity (< 40 nm) to the cell membrane as indicated by the bright red fluorescence. Fluorescent detection was not observed when the anti-TRPM4 goat antibody was omitted (Fig. 1B). As a negative control, we used a rabbit antibody specific for the voltage-gated KV7.5 channel subtype, which is expressed in human DSM,20,21 but not expected to interact with TRPM4 channels. Incubation of human DSM cells with anti-TRPM4 and anti-KV7.5 rabbit antibodies, respectively, resulted in no observable fluorescent signal (Fig. 1). Results were confirmed in at least 3 human DSM cells.
Figure 1.
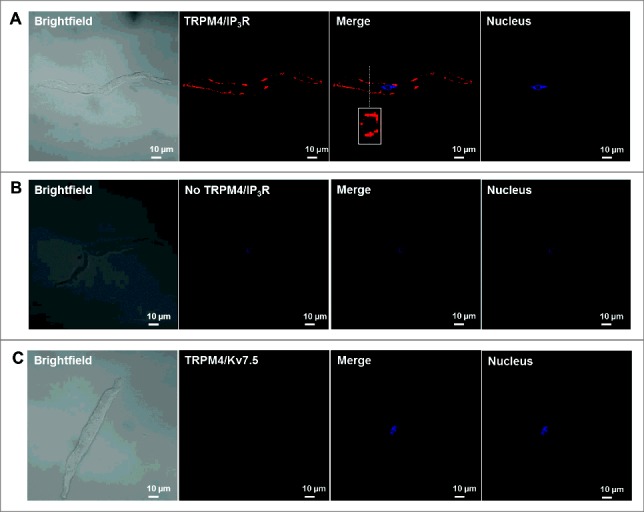
TRPM4 channels are colocalized with the IP3R of the sarcoplasmic reticulum in freshly-isolated human DSM cells. (A) Representative PLA image of a human DSM cell stained with the anti-TRPM4 goat polyclonal antibody and anti-IP3R rabbit polyclonal antibody. As demonstrated in the merge panel in (A), the orthogonal cross-section image of the human DSM cell is shown in the rectangular box and represents the cross-sectional plane as indicated by the white dashed line. (B) Representative image of a human DSM cells stained with only the anti-IP3R rabbit polyclonal antibody (negative control, no signal detected). (C) Representative image of a human DSM cell stained with the anti-TRPM4 goat polyclonal antibody and the anti-KV7.5 rabbit polyclonal antibody (negative control, no signal detected). As shown in (A), positive co-localization of anti-TRPM4 and anti-IP3R antibodies is exemplified by red fluorescent staining. Results were confirmed in at least 3 human DSM cells isolated from 3 different patients.
IP3R pharmacological inhibition with xestospongin-C decreases transient inward cation current activity in human DSM cells
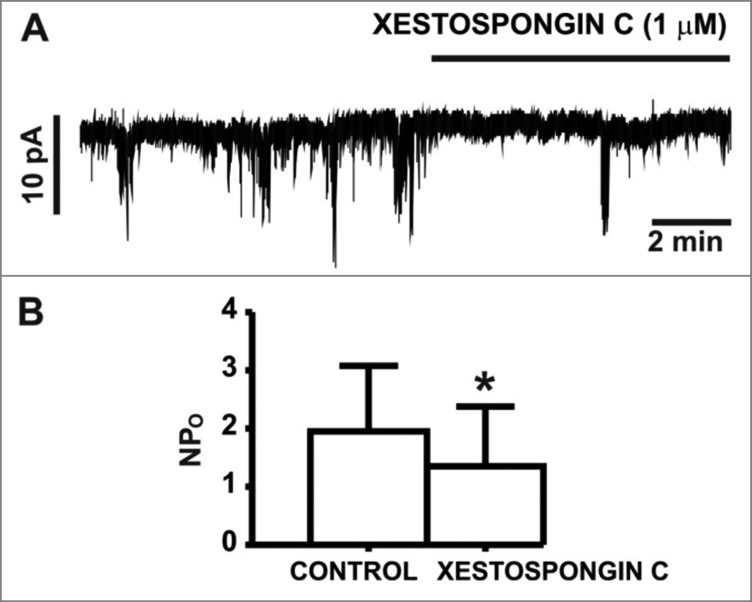
In non-DSM cells, TRPM4 channels have been shown to be regulated by Ca2+ release events originating from the SR IP3Rs.22 Therefore, SR IP3R Ca2+ release activity may constitute a fundamentally important cellular mechanism regulating TRPM4 channel activity in human DSM. To ascertain whether SR IP3Rs potentially regulate TRPM4 channel activity in human DSM, we examined the physiologic consequences of IP3R pharmacological inhibition on TRPM4 channel-mediated TICCs. At the holding potential of -70 mV, where BK channel activity is negligible,8 pharmacological inhibition of the IP3Rs with the selective inhibitor xestospongin-C (1 µM) significantly reduced TICC activity (NPo) from 2.0 ± 1.1 in control conditions to 1.3 ± 1.0 in the presence of 1 µM xestospongin-C (n = 6, N = 4; P<0.05; Fig. 2). These results support the concept that Ca2+ release events from SR IP3Rs are involved in the regulation of TRPM4 channel-mediated TICCs in human DSM cells (Fig. 3).
Figure 2.
IP3R inhibition with xestospongin-(C) decreased TICC activity in human DSM cells. (A) An original recording showing the inhibitory effect of xestospongin-C (1 µM) on TICCs in a human DSM single cell recorded at a holding potential of -70 mV. (B) Summary data illustrating the inhibitory effects of xestospongin-C (1 µM) on TICCs, analyzed as single channel open probability (NPo) (n = 6, N = 4; *P<0.05).
Figure 3.
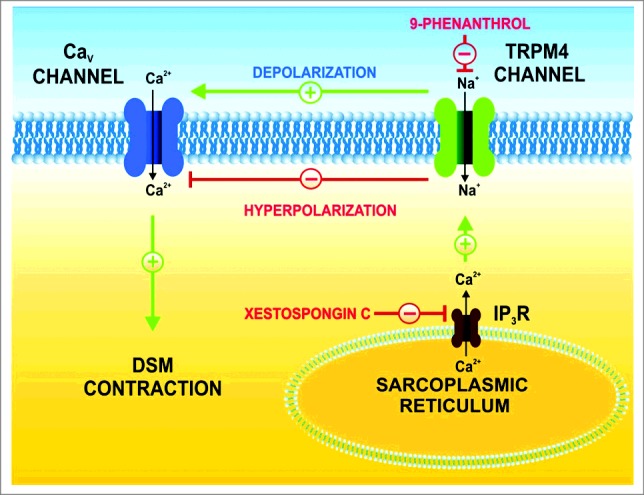
Illustration of the IP3R regulatory role of TRPM4 channels in human DSM function. TRPM4 channels conduct monovalent cations (primarily Na+ under resting conditions) and control human DSM membrane potential. TRPM4 channels are controlled by IP3R-mediated Ca2+ release from the sarcoplasmic reticulum as inhibition of the IP3Rs with xestospongin-C reduces TRPM4 channel activity. Pharmacological blockade of TRPM4 channels with 9-phenanthrol hyperpolarizes the DSM cell membrane, inhibits the voltage-gated Ca2+ channels, reduces intracellular Ca2+ levels and promotes DSM relaxation.5
Discussion
This study sought to determine whether IP3Rs of the SR regulate TRPM4 channel activity in human DSM function. This was achieved by elucidating IP3R-TRPM4 channel spatial and functional interactions in human DSM by using in situ PLA and perforated patch-clamp electrophysiology in combination with the IP3R inhibitor xestospongin-C. In situ PLA confirmed co-localization between IP3Rs and TRPM4 channels in human DSM isolated cells. Further, IP3R pharmacological inhibition with xestospongin-C led to the attenuation of TICCs. This study established the novel finding that TRPM4 channels and SR IP3Rs are physically and functionally coupled in the regulation of human DSM excitability.
Several studies from our laboratory have revealed key roles for the TRPM4 channels in regulating the excitability and contractility of DSM in rodents.6-8 More recently, we further explored the translational implications of our findings in rodents by investigating the physiologic roles for the TRPM4 channels in clinically-characterized human DSM.5 As summarized in a recent editorial article in the American Journal of Physiology Cell Physiology,14 our group revealed critical roles for the TRPM4 channels in regulating DSM function. TRPM4 channel expression is abundant in human DSM and species-related differences in the functional roles of the TRPM4 channels have been reported between human and rodents.5 This previous report by our group provided compelling evidence at the cellular level in human DSM demonstrating the TRPM4 channel inhibitor 9-phenanthrol had a substantially greater inhibitory effect on DSM cell excitability in human versus rodent DSM cells.5
Utilizing the selective TRPM4 channel inhibitor 9-phenanthrol, we reported that the pharmacological inhibition of the TRPM4 channels hyperpolarized the membrane potential in human DSM cells and caused inhibition of spontaneous phasic, pharmacologically-induced, and nerve-evoked contractions in human DSM isolated strips.5 Among the mechanisms underlying the inhibitory effects of 9-phenanthrol on human DSM excitability and contractility includes the attenuation of TICCs.5-8 TICCs are caused by Na+ influx following Ca2+-dependent TRPM4 channel activation which, under physiologic conditions, functions to enhance DSM cell excitability.5,23,24 As TRPM4 channels are activated by Ca2+, it was critically important to determine the specific Ca2+-dependent molecular and cellular mechanisms involved in the regulation of TRPM4 channel activity. Albeit well-known, there are 2 key sources for the elevation of intracellular Ca2+ concentrations, including: 1) Ca2+ entry through L-type voltage-gated Ca2+ channels at the cell membrane, and 2) Ca2+ release events through SR ryanodine receptors or IP3Rs.4,24 In particular, emerging evidence from non-DSM cell types has shown that members of the TRP channel family, including TRPM4, can be activated by intracellular Ca2+ released from SR IP3Rs. IP3R-mediated activation of adjacent TRPM4 channels, as has been reported in non-DSM cell types,16,25 would lead to depolarization of the cell membrane potential, activation of L-type voltage-gated Ca2+ channels, an increase in global intracellular Ca2+ concentrations, and thus the activation of DSM contractions. In fact, in cerebral artery myocytes, sustained TRPM4 channel activity was shown to be dependent on Ca2+ released from the SR IP3Rs.26
Given the critically important role for the TRPM4 channels in human DSM excitability and contractility, we sought to examine whether IP3Rs and TRPM4 channels functionally interact to regulate human DSM function. Our results demonstrated that xestospongin-C, a selective IP3R inhibitor, significantly decreased TICC activity in human DSM cells (Fig. 2). These results are consistent with IP3R-dependent regulation of TRPM4 channels in human DSM by a similar mechanism as was suggested for cerebral artery myocytes. Ca2+ released from the SR IP3Rs are localized and transient events as has been demonstrated in murine colonic myocytes and rabbit portal vein myocytes.12,27 Hence, localized Ca2+ release events for the SR IP3Rs must be in close apposition to the plasmalemmal TRPM4 channels in order for IP3R-TRPM4 channel functional coupling. Thus, to ascertain the potential co-localization for the TRPM4 channels and IP3Rs, we used the Duolink in situ PLA technique in combination with TRPM4 channel and IP3R-specific antibodies. As illustrated by Fig. 1, in situ PLA experiments demonstrated the co-localization of IP3Rs and TRPM4 channels in freshly-isolated human DSM cells within the vicinity of the cell membrane. These results are in contrast to findings from arterial myocytes, where IP3R co-localization was confirmed with TRPC3 channels, while no co-localization with either the TRPM4 or TRPC6 channels was found.28 The existence of IP3R co-localization with TRPM4 channels in DSM, but not in arterial myocytes, may suggest TRPM4 channel-IP3R spatial interactions follow, to an extent, a certain level of tissue-specific expression.
While our current data provide strong evidence to support the spatial and functional coupling of the IP3Rs and TRPM4 channels in human DSM (Figs. 1-2), we cannot exclude potential involvement of alternative signaling pathways. IP3 and Ca2+ are the 2 central modulators of SR IP3R activity.29 Indeed, IP3R activity has been shown to be regulated by IP3 generated downstream from the activation of Gq/11-coupled muscarinic receptor pathways involving phospholipase C hydrolysis of phosphatidylinositol bisphosphate (PIP2).29 Further, more recent studies have shown Ca2+-permeable TRPC6 channels are spatially and functionally coupled with the SR IP3Rs in cerebral arterial myocytes.30 Thus, TRPC6 activation of the IP3Rs leads to Ca2+ release events from the SR IP3Rs, which then activate adjacent TRPM4 channels at the cell membrane.30 Also, in the vasculature, protein kinase C was shown to modulate vascular myogenic tone in a TRPM4 channel-dependent manner.31,32 Consistently, in a separate study on HEK-293 cells expressing recombinant TRPM4 channels, the sensitivity for TRPM4 channels to Ca2+-dependent activation is enhanced by PKC phosphorylation.33 Whether these potential TRPM4 channel regulatory mechanisms exist in human DSM is yet to be explored. Our data confirm IP3R-TRPM4 channel functional coupling, and this work represents a foundational study in the human urinary bladder and provides a solid platform for subsequent mechanistic studies in human DSM (Fig. 3).
The key functional role of the TRPM4 channels in promoting DSM excitability has provided an intriguing opportunity for potential therapeutic exploitation in the context of overactive bladder syndrome. Specifically, pharmacological modulators of the TRPM4 channels or mechanisms controlling their activity may represent a promising novel approach for treatment of bladder dysfunction. Given the critical role of the TRPM4 channels in human DSM function and dysfunction, further efforts to ascertain TRPM4 channel regulatory mechanisms are critical for confirming and validating their potential therapeutic utility. The current study has provided novel mechanistic insight into TRPM4 channel regulation by SR IP3Rs in human DSM. As current treatments of overactive bladder lack desired efficacy, research efforts to identify novel therapeutic modalities are urgently needed and examining TRPM4 channel physiologic roles and mechanisms of regulation is of scientific and clinical relevance.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We would like to thank Drs. Kiril L. Hristov and Shankar P. Parajuli for their help with the patch-clamp experiments. We thank the Medical University of South Carolina (MUSC) Urology staff surgeons: Drs. Thomas Keane, Harry Clarke, Stephen Savage, Ross Rames, Sandip Prasad, and Jonathan Picard, as well as the MUSC Urology Residents: Ryan Levey, Austin Younger, Mark Currin, Nima Baradaran, Olugbemisola McCoy, Alyssa Greiman, Sarah Starosta, Bryce Wyatt and Tracy Tipton for their help with human tissue collection. We also thank Drs. Whitney Kellett and Damiano Angoli for the critical evaluation of the manuscript.
Funding
This study was supported by a grant from the National Institutes of Health R01 DK106964 to Georgi V. Petkov. Aaron Provence was supported by an NIH pre-doctoral fellowship F31 DK104528 under Dr. Petkov's mentorship.
References
- [1].Hashitani H, Brading AF. Ionic basis for the regulation of spontaneous excitation in detrusor smooth muscle cells of the guinea-pig urinary bladder. Br J Pharmacol 2003; 140:159-69; PMID:12967945; https://doi.org/ 10.1038/sj.bjp.0705320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Petkov GV. Role of potassium ion channels in detrusor smooth muscle function and dysfunction. Nat Rev Urol 2012; 9:30-40; PMID:22158596; https://doi.org/ 10.1038/nrurol.2011.194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Hashitani H, Brading AF. Electrical properties of detrusor smooth muscles from the pig and human urinary bladder. Br J Pharmacol 2003; 140:146-58; PMID:12967944; https://doi.org/ 10.1038/sj.bjp.0705319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Petkov GV. Central role of the BK channel in urinary bladder smooth muscle physiology and pathophysiology. Am J Physiol Regul Integr Comp Physiol 2014; 307:R571-84; PMID:24990859; https://doi.org/ 10.1152/ajpregu.00142.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Hristov KL, Smith AC, Parajuli SP, Malysz J, Rovner ES, Petkov GV. Novel regulatory mechanism in human urinary bladder: Central role of transient receptor potential melastatin 4 channels in detrusor smooth muscle function. Am J Physiol Cell Physiol 2016; 310(7):C600-11; PMID:26791488; https://doi.org/ 10.1152/ajpcell.00270.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Parajuli SP, Hristov KL, Sullivan MN, Xin W, Smith AC, Earley S, Malysz J, Petkov GV. Control of urinary bladder smooth muscle excitability by the TRPM4 channel modulator 9-phenanthrol. Channels (Austin) 2013; 7:537-40; PMID:24037125; https://doi.org/ 10.4161/chan.26289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Smith AC, Hristov KL, Cheng Q, Xin W, Parajuli SP, Earley S, Malysz J, Petkov GV. Novel role for the transient potential receptor melastatin 4 channel in guinea pig detrusor smooth muscle physiology. Am J Physiol Cell Physiol 2013; 304:C467-77; PMID:23302778; https://doi.org/ 10.1152/ajpcell.00169.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Smith AC, Parajuli SP, Hristov KL, Cheng Q, Soder RP, Afeli SA, Earley S, Xin W, Malysz J, Petkov GV. TRPM4 channel: A new player in urinary bladder smooth muscle function in rats. Am J Physiol Renal Physiol 2013; 304:F918-29; PMID:23283997; https://doi.org/ 10.1152/ajprenal.00417.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Launay P, Fleig A, Perraud AL, Scharenberg AM, Penner R, Kinet JP. TRPM4 is a Ca2+-activated nonselective cation channel mediating cell membrane depolarization. Cell 2002; 109:397-407; PMID:12015988; https://doi.org/ 10.1016/S0092-8674(02)00719-5 [DOI] [PubMed] [Google Scholar]
- [10].Earley S, Brayden JE. Transient receptor potential channels in the vasculature. Physiol Rev 2015; 95:645-90; PMID:25834234; https://doi.org/ 10.1152/physrev.00026.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Earley S. TRPM4 channels in smooth muscle function. Pflugers Arch 2013; 465:1223-31; PMID:23443854; https://doi.org/ 10.1007/s00424-013-1250-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Guinamard R, Hof T, Del Negro CA. The TRPM4 channel inhibitor 9-phenanthrol. Br J Pharmacol 2014; 171:1600-13; PMID:24433510; https://doi.org/ 10.1111/bph.12582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Gonzales AL, Garcia ZI, Amberg GC, Earley S. Pharmacological inhibition of TRPM4 hyperpolarizes vascular smooth muscle. Am J Physiol Cell Physiol 2010; 299:C1195-202; PMID:20826763; https://doi.org/ 10.1152/ajpcell.00269.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hamilton KL. New life in overactive bladder. focus on “novel regulatory mechanism in human urinary bladder: Central role of transient receptor potential melastatin 4 channels in detrusor smooth muscle function." Am J Physiol Cell Physiol 2016; 310:C597-9; PMID:26888821; https://doi.org/ 10.1152/ajpcell.00039.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Andersson KE, Wein AJ. Pharmacology of the lower urinary tract: Basis for current and future treatments of urinary incontinence. Pharmacol Rev 2004; 56:581-631; PMID:15602011; https://doi.org/ 10.1124/pr.56.4.4 [DOI] [PubMed] [Google Scholar]
- [16].Gonzales AL, Earley S. Endogenous cytosolic Ca2+ buffering is necessary for TRPM4 activity in cerebral artery smooth muscle cells. Cell Calcium 2012; 51:82-93; PMID:22153976; https://doi.org/ 10.1016/j.ceca.2011.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hristov KL, Afeli SA, Parajuli SP, Cheng Q, Rovner ES, Petkov GV. Neurogenic detrusor overactivity is associated with decreased expression and function of the large conductance voltage- and Ca2+-activated K+ channels. PLoS One 2013; 8:e68052; PMID:23861849; https://doi.org/ 10.1371/journal.pone.0068052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hristov KL, Chen M, Kellett WF, Rovner ES, Petkov GV. Large-conductance voltage- and Ca2+-activated K+ channels regulate human detrusor smooth muscle function. Am J Physiol Cell Physiol 2011; 301:C903-12; PMID:21697543; https://doi.org/ 10.1152/ajpcell.00495.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Hristov KL, Chen M, Afeli SA, Cheng Q, Rovner ES, Petkov GV. Expression and function of KV2-containing channels in human urinary bladder smooth muscle. Am J Physiol Cell Physiol 2012; 302:C1599-608; PMID:22422395; https://doi.org/ 10.1152/ajpcell.00447.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Provence A, Hristov KL, Parajuli SP, Rovner ES, Petkov GV. Voltage-gated KCNQ channels in human detrusor smooth muscle contractility: A novel target for the pharmacological treatment of overactive bladder. J Urol 2015; 193:e188; https://doi.org/ 10.1016/j.juro.2015.02.912 [DOI] [Google Scholar]
- [21].Provence A, Angoli D, Rovner ES, Petkov GV. KV7 channel pharmacological modulation in human detrusor: A promising two-way street for the potential treatment of overactive and underactive bladder. J Urol 2017; 197:e1353; https://doi.org/ 10.1016/j.juro.2017.02.3162 [DOI] [Google Scholar]
- [22].Gonzales AL, Earley S. Regulation of cerebral artery smooth muscle membrane potential by Ca2+-activated cation channels. Microcirculation 2013; 20:337-47; PMID:23116477; https://doi.org/ 10.1111/micc.12023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Fliegert R, Glassmeier G, Schmid F, Cornils K, Genisyuerek S, Harneit A, Schwarz JR, Guse AH. Modulation of Ca2+ entry and plasma membrane potential by human TRPM4b. FEBS J 2007; 274:704-13; PMID:17288552; https://doi.org/ 10.1111/j.1742-4658.2006.05614.x [DOI] [PubMed] [Google Scholar]
- [24].Amberg GC, Navedo MF. Calcium dynamics in vascular smooth muscle. Microcirculation 2013; 20:281-9; PMID:23384444; https://doi.org/ 10.1111/micc.12046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Xi Q, Adebiyi A, Zhao G, Chapman KE, Waters CM, Hassid A, Jaggar JH. IP3 constricts cerebral arteries via IP3 receptor-mediated TRPC3 channel activation and independently of sarcoplasmic reticulum Ca2+ release. Circ Res 2008; 102:1118-26; PMID:18388325; https://doi.org/ 10.1161/CIRCRESAHA.108.173948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Gonzales AL, Amberg GC, Earley S. Ca2+ release from the sarcoplasmic reticulum is required for sustained TRPM4 activity in cerebral artery smooth muscle cells. Am J Physiol Cell Physiol 2010; 299:C279-88; PMID:20427713; https://doi.org/ 10.1152/ajpcell.00550.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Gordienko DV, Bolton TB. Crosstalk between ryanodine receptors and IP3 receptors as a factor shaping spontaneous Ca2+-release events in rabbit portal vein myocytes. J Physiol 2002; 542:743-62; PMID:12154176; https://doi.org/ 10.1113/jphysiol.2001.015966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Adebiyi A, Zhao G, Narayanan D, Thomas-Gatewood CM, Bannister JP, Jaggar JH. Isoform-selective physical coupling of TRPC3 channels to IP3 receptors in smooth muscle cells regulates arterial contractility. Circ Res 2010; 106:1603-12; PMID:20378853; https://doi.org/ 10.1161/CIRCRESAHA.110.216804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Hill-Eubanks DC, Werner ME, Heppner TJ, Nelson MT. Calcium signaling in smooth muscle. Cold Spring Harb Perspect Biol 2011; 3:a004549; PMID:21709182; https://doi.org/ 10.1101/cshperspect.a004549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Gonzales AL, Yang Y, Sullivan MN, Sanders L, Dabertrand F, Hill-Eubanks DC, Nelson MT, Earley S. A PLCgamma1-dependent, force-sensitive signaling network in the myogenic constriction of cerebral arteries. Sci Signal 2014; 7:ra49; PMID:24866019; https://doi.org/ 10.1126/scisignal.2004732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Earley S, Straub SV, Brayden JE. Protein kinase C regulates vascular myogenic tone through activation of TRPM4. Am J Physiol Heart Circ Physiol 2007; 292:H2613-22; PMID:17293488; https://doi.org/ 10.1152/ajpheart.01286.2006 [DOI] [PubMed] [Google Scholar]
- [32].Earley S, Waldron BJ, Brayden JE. Critical role for transient receptor potential channel TRPM4 in myogenic constriction of cerebral arteries. Circ Res 2004; 95:922-9; PMID:15472118; https://doi.org/ 10.1161/01.RES.0000147311.54833.03 [DOI] [PubMed] [Google Scholar]
- [33].Nilius B, Prenen J, Tang J, Wang C, Owsianik G, Janssens A, Voets T, Zhu MX. Regulation of the Ca2+ sensitivity of the nonselective cation channel TRPM4. J Biol Chem 2005; 280:6423-33; PMID:15590641; https://doi.org/ 10.1074/jbc.M411089200 [DOI] [PubMed] [Google Scholar]