Abstract
Accurate and reliable measurements of exposure to tobacco products are essential for identifying and confirming patterns of tobacco product use and for assessing their potential biological effects in both human populations and experimental systems. Due to the introduction of new tobacco-derived products and the development of novel ways to modify and use conventional tobacco products, precise and specific assessments of exposure to tobacco are now more important than ever. Biomarkers that were developed and validated to measure exposure to cigarettes are being evaluated to assess their use for measuring exposure to these new products. Here, we review current methods for measuring exposure to new and emerging tobacco products, such as electronic cigarettes, little cigars, water pipes, and cigarillos. Rigorously validated biomarkers specific to these new products have not yet been identified. Here, we discuss the strengths and limitations of current approaches, including whether they provide reliable exposure estimates for new and emerging products. We provide specific guidance for choosing practical and economical biomarkers for different study designs and experimental conditions. Our goal is to help both new and experienced investigators measure exposure to tobacco products accurately and avoid common experimental errors. With the identification of the capacity gaps in biomarker research on new and emerging tobacco products, we hope to provide researchers, policymakers, and funding agencies with a clear action plan for conducting and promoting research on the patterns of use and health effects of these products.
Keywords: exposure, NNAL, tobacco, biomarker, cotinine
we define new and emerging tobacco and nicotine delivery products as products that have been introduced to the United States market in the past 15 years, products that have become significantly more popular in the past 15 years, or products that are being modified and used in new ways. We limit our focus to products currently used by >1% of the U.S. population, based on nationally representative survey data. The products that currently meet these criteria are electronic cigarettes (e-cigarettes), little cigars, water pipes, and cigarillos.
NEW AND EMERGING PRODUCTS
For researchers who are new to tobacco and nicotine delivery product exposure biomarkers or new to the use of biomarkers to study e-cigarettes, water pipe, cigars, little cigars, and cigarillos, this paper offers guidance on choosing biomarkers that support specific study goals and are financially and practically feasible. For physiologists, this paper describes the challenges presented by new and emerging tobacco and nicotine delivery products and potential solutions to these problems. For pulmonary physiologists, this paper offers a discussion of biomarkers in samples collected in the respiratory tract. For agencies and policymakers who fund research on tobacco and nicotine delivery products, this paper outlines an action plan for promoting research on the use and health effects of these products.
Biomarkers of Exposure
The use of tobacco products results in the uptake of nicotine and a wide range of other chemicals. These chemicals and their metabolites, measured in bodily fluids and tissues, constitute biomarkers of exposure. Biomarkers of exposure to tobacco and nicotine delivery products are limited to the chemicals taken up during product use or during exposure to product emissions. Thousands of chemicals are present in tobacco smoke, and hundreds have been identified in e-cigarette aerosols and liquids. Although some biomarkers of exposure to tobacco and nicotine delivery products are metabolites of known toxicants or carcinogens, e.g., 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL), in this paper we focus on biomarkers of exposure, not on biomarkers of potential health effects.
No Validated Biomarkers Specific for E-Cigarettes and Other New Products
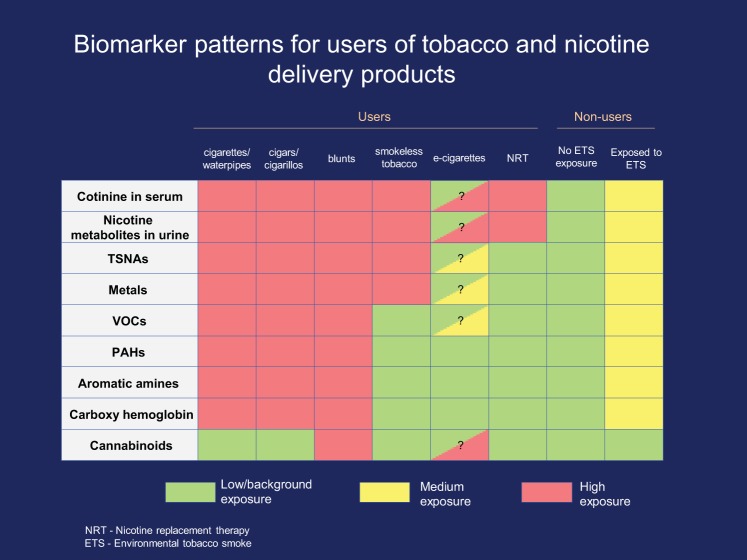
The lack of validated biomarkers for e-cigarettes is an urgent public health problem. The market for e-cigarettes has expanded rapidly since they came to market in 2007. In 2015, estimated U.S. sales totaled $3.5 billion (179, 267). To measure the health effects of e-cigarettes, researchers need biomarkers for exposure to both e-cigarettes that contain nicotine and those that do not. However, no validated biomarkers specific to nicotine-containing or nicotine-free e-cigarettes are currently available. At present, a biological specimen that tests positive for nicotine metabolites and negative for metabolites of combustion products and tobacco-specific nitrosamine (TSNA) metabolites suggests either the use of e-cigarettes with nicotine or of a nicotine replacement therapy (NRT) product, such as nicotine gum. Questionnaire data can be used to provide more accurate answers (67), but the known biomarkers of tobacco and nicotine exposure cannot. A second gap is the current lack of validated biomarkers that differentiate among the use of various combustible products (e.g., cigars, little cigars, cigarillos, water pipes, and cigarettes), as shown in Fig. 1. To support research on new products, it is essential to identify product-specific biomarkers and to develop sensitive, accurate, and affordable assays. Currently, few laboratories perform assays that can differentiate cigarette use from the use of other nicotine-containing products, and the existing assays are expensive.
Fig. 1.
Biomarker patterns for users of tobacco and nicotine.
BIOMARKERS OF EXPOSURE TO TOBACCO AND NICOTINE
Overview
There were three major categories of tobacco products on the market in 2016: combustion, heat delivery, and smokeless products (Table 1). Combustion products, which generate smoke enriched with nicotine and other chemicals (248), include cigarettes, cigars, little cigars, cigarillos, and water pipes. Many of the chemicals present in tobacco products and/or generated by combustion are taken up by the body in appreciable quantities. Therefore, exposure can be assessed by measuring these chemicals or their metabolites in biological specimens (261a) from various compartments of the body.
Table 1.
Categories of tobacco and nicotine delivery products
| Tobacco Combustion | Heat Delivery | Smokeless |
|---|---|---|
| Cigarettes | E-cigarettes Heated, tobacco-based, noncombusting cigarettes |
Lozenges Gums Smokeless tobacco Chewing tobacco Snuff Snus |
| Cigars | ||
| Cigarillos | ||
| Little cigars | ||
| Water pipes |
Heat delivery products, such as e-cigarettes, heat a solution of humectants, flavors, and tobacco extract to generate a nicotine-containing aerosol (45). Although the chemical composition of e-cigarette aerosols is simpler than that generated by combustible tobacco systems (58), they often contain a large number of flavorings and other additives. Furthermore, specific biomarkers of exposure to e-cigarettes and their constituents have not been identified.
Smokeless tobacco products—chewing tobacco, snuff and snus, and NRT products, such as gums, patches, lozenges, and sprays—do not require combustion or heating to deliver nicotine. To date, only nicotine, nicotine metabolites, and TSNAs have been used to assess exposure to smokeless tobacco products (44, 106, 115, 152, 187, 241, 243, 261). Nicotine and nicotine metabolites are also the only known exposure biomarkers for nicotine replacement products. In this paper, we discuss biomarkers of exposure to tobacco and nicotine in the three defined categories and highlight the limitations in identifying selective markers of exposure to specific tobacco products.
What Makes a Good Biomarker?
Exposure to a chemical or group of chemicals can be quantified by measuring the chemical or its metabolites in the body or excreta. A good biomarker has four key traits: 1) a clear dose-response relationship with exposure: the concentration of the biomarker chemical increases with an increase in exposure and decreases upon cessation of exposure with a known time course, 2) qualitative and quantitative identification over a wide range of concentrations so that both low and high levels of exposure are accurately estimated, 3) detection in readily collected biospecimens (e.g., saliva, urine, and blood), and 4) stability upon storage for prospective analyses.
Biomarkers of exposure, such as nicotine or nicotine metabolites (including cotinine) and TSNA metabolites (e.g., NNAL), are specific to the use of tobacco and nicotine delivery products (of all the categories discussed above). Dietary or environmental exposures contribute little to the body burden of these chemicals. Nevertheless, other less-specific biomarkers of tobacco product exposure, such as carbon monoxide (CO), volatile organic chemicals (VOCs), and polycyclic aromatic hydrocarbons (PAHs), can provide additional information relevant to a more comprehensive exposure assessment and can help relate exposure to injury. Because some chemicals or their metabolites accumulate in tissues, their measurement can provide exposure estimates that integrate the duration and extent of exposure, as well as the rate of chemical or metabolite clearance. Therefore, in selecting a biomarker to quantify exposure, it is important to consider the source, persistence, and pharmacokinetics of the biomarker, dose of exposure, as well as duration between exposure and measurement.
Biomarkers of Nicotine
Nicotine was one of the first biomarkers to be used for assessing exposure to cigarette smoke (26, 215). However, its short half-life (t1/2; ~2 h) and variable rate of metabolism led to the use of cotinine and other nicotine metabolites as biomarkers of nicotine exposure (28, 29). Cotinine is the major metabolite of nicotine, and its longer t1/2 (16–18 h) makes it a good biomarker for nicotine uptake in various biological fluids and tissues (47, 138, 159, 198, 228, 264). Nicotine and its metabolites are discussed in detail in Nicotine in Blood through Over-the-Counter Cotinine Tests.
Biomarkers of Tobacco Use Other Than Nicotine
Tobacco and nicotine delivery product chemicals originate from the tobacco production process, the product manufacturing process, chemical reactions during product storage, or combustion or pyrolysis during product use. Table 2 summarizes the major categories of these chemicals and the corresponding biomarkers commonly used to assess tobacco and nicotine delivery product exposure (102, 173). These include exhaled CO, VOCs, PAHs, and TSNAs [mainly 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) and its metabolite, NNAL]. NNAL is a biomarker for the use of combustible and smokeless tobacco products. Because e-cigarettes do not attain true combustion temperatures, they do not emit CO or as many different VOCs and PAHs in measureable quantities (97, 104, 145, 177). Thus exhaled CO and metabolites of PAHs are not associated with the use of e-cigarettes.
Table 2.
Biomarkers of tobacco and nicotine delivery product use and exposure
| Smoke Constituent | Example/Biomarker | Measured in | References |
|---|---|---|---|
| Nicotine | Cotinine | Blood Urine Saliva Respiratory fluids |
(22, 27, 28, 38) |
| Tobacco-specific nitrosamines | NNK/NNAL | Urine | (41, 49, 53, 62, 98, 118, 144) |
| Volatile organic compounds (VOCs) | 1,3-Butadiene/N-acetyl-S-(4-hydroxy-2-buten-1-yl)-l-cysteine(MHBMA-3) | Urine | (151, 223) |
| Polycyclic aromatic hydrocarbons(PAHs) | Pyrene/1-hydroxypyrene | Urine | (87, 221) |
| Metals | Cadmium | Blood Urine |
(158, 175) |
| CO | Exhaled CO carboxyhemoglobin |
Blood Breath |
(31, 63, 134, 153, 208, 218, 220) |
Biological Cut-Point Values
Biological cut-point values differentiate tobacco and nicotine delivery product users from nonusers (e.g., smokers from nonsmokers) and can be used to identify product-use patterns. Specific cut-point values that separate populations with different tobacco product exposures are established by obtaining biomarker data from a large number of users and nonusers who are classified using a separate method (e.g., self-report) to arrive statistically at an optimum cutoff value (90). Both the sample matrix (e.g., blood vs. urine) and metabolic variations among individuals influence these values. For example, the cutoff value of cotinine in urine is much higher than that of cotinine in serum, because cotinine concentration is four to six times higher in the urine than in blood (14, 274). In addition, optimum cutoffs may vary slightly, based on the subjects’ genetic backgrounds (269). For example, the optimal serum cotinine level to separate adult smokers from nonsmokers is 6 ng/ml for non-Hispanic blacks, but 5 and 1 ng/ml for non-Hispanic Whites and Mexican Americans, respectively (21). Moreover, in recent years, biological cut-point values have decreased as people tend to smoke less and are less likely to be exposed to secondhand smoke (SHS) because of indoor smoking bans (14, 123, 137, 139, 229).
It is relatively simple to separate nonsmokers from typical daily smokers using any of the aforementioned markers, as plots of such data typically result in two distinct distribution curves. However, it is not always possible to arrive at a clear distinction between the two groups. For example, biomarker values for heavy SHS exposure overlap with those from occasional smokers for some biomarkers (14, 121, 124). Hence, to distinguish SHS exposure from intermittent smoking, biomarker measurement should be supplemented with data from questionnaires. In case of mismatches, a positive biomarker value may be more reliable than self-reported data.
PLANNING FOR BIOMARKER ANALYSIS
Biomarkers of tobacco and nicotine exposure are present in trace concentration in chemically complex biological fluids. The first step in selecting a method for biomarker measurement is to determine the lowest concentration of the chemical or metabolite that must be quantified to address the primary study aims. This lower limit is defined by biological cut-point values separating active tobacco product users from nonusers and by biomarker patterns separating users of one product from users of other products (Fig. 1, and see Table 5). Biomarkers of tobacco exposure have been measured in almost every biological material, including hair and nails, although the vast majority of information has come from measurements in blood (serum or plasma) and urine. The matrices of choice are discussed in conjunction with each biomarker. The measurement of biomarkers of exposure in animals is useful and often critical for understanding human disease, because these models allow experiments and samples that are not feasible in human studies.
Table 5.
Validated biological cut-point values separating smokers and nonsmokers
LOD and LLOQ
The limit of detection (LOD) and lower limit of quantitation (LLOQ) define limits of identification and quantification, respectively. The LOD is the lowest concentration of a chemical that can be detected over the background noise in a given biological sample. LOD is technology and assay dependent: assays with elaborate sample preparation procedures that remove interfering compounds often have a lower LOD than those with simpler sample preparation. However, it is easier to detect a chemical in a specimen than to quantify it. The coefficient of variation (CV; reproducibility) at the LOD may be much larger than that at the LLOQ, and thus data may not be acceptably precise at the LOD.
The LLOQ is the concentration at and above which the assay is sufficiently reliable and reproducible for measuring the quantity of a chemical in a given specimen. For example, the acceptable LLOQ for precise cotinine measurement following SHS exposure is lower than that for precise cotinine measurement following cigarette smoking. In general, the smaller the CV, the better the assay can discriminate among experimental groups or conditions. Whereas multiple methods exist for defining LOD and LLOQ (182, 252), the guidelines typically used for tobacco studies involving biomarkers are those developed for drug clinical trials (225, 266). These guidelines also describe criteria for method development, validation, and quality control and for compound quantification (e.g., setting the LLOQ at CV < 20%). Commonly available assays and the LLOQ for each are listed (see Table 6).
Table 6.
Assay sensitivity, cost, and availability
| Biomarker | Specimen | Method | LLOQ | Cost Per Sample, $ | Availability |
|---|---|---|---|---|---|
| Exhaled CO | Breath | Portable instrument | 0.5 ppm | 200–3,000 for instrumenta | Commercial |
| Cotinine | Urine | OTC kit | NA (200 ng/mlb) | 0.50–5.00 | Commercial |
| Nicotine + metabolites | Urine | GC-MS | 2 ng/ml | 120–200 | Commercial |
| Nicotine + metabolites | Blood | GC-MS | 2 ng/ml | 180–300 | Commercial |
| Nicotine and cotinine | Urine, saliva, blood | GC-NPD | 1 and 10 ng/ml, respectively | 50–100 | Academic/govt.c |
| Nicotine | Urine, saliva, blood | GC-MS/MS | 0.2 ng/ml | 75–200 (for nic + cot) | Academic/govt.c |
| Cotinine | Urine, saliva, blood | GC-MS/MS | 2 ng/ml | Academic/govt.c | |
| Cotinine | Urine | LC-MS/MS | 0.05 ng/ml | 100–200 (for cot + 3-OH cot) | Academic/govt.c |
| 3-OH cotinine | Urine | LC-MS/MS | 0.1 ng/ml | Academic/govt.c | |
| Total nicotine equivalents | Urine | LC-MS/MS | Not applicable | 180–300 | Academic/govt.c |
| NNAL | Urine | LC-MS/MS | 0.25 pg/ml | 180–300 | Academic/govt.c |
| Menthol glucuronide | Urine, plasma | LC-MS/MS | Plasma: 4 ng/ml, urine: 500 ng/ml | 40–100 | Academic/govt.c |
| VOC metabolite panel | Urine | See Table 3 | See Table 3 | 180–300 | Academic/govt.c |
| PAH metabolite panel | Urine | See Table 4 | See Table 4 | 180–300 | Academic/govt.c |
Costs and LLOQs are constantly changing. This table was accurate at the time of publication. Please contact the laboratories when you are creating your budget and sample collection plans. The minimum sample volume for most chromatographic assays is 1 ml. NNAL and PAH metabolite assays require 3 ml. However, it is best practice to provide enough volume to allow for losses in pipetting and for repeat testing. Thus the optimal sample volumes are 2.1 ml for most tests and 6.2 ml for NNAL and PAH tests. NPD, nitrogen phosphorous detector; nic, nicotine; cot, cotinine; 3-OH, 3-hydroxycotinine.
Cost per test depends on frequency of use after instrument acquisition.
As this is a qualitative test, there is no LLOQ [not applicable (NA)]. A positive result is at or above 200 ng/ml.
Investigators must contact the laboratory in advance to ascertain availability, cost, and turnaround time for tests at academic and government laboratories.
Nicotine in Blood
Blood nicotine concentration—whether measured in whole blood, serum, or plasma—is a key determinant of the pharmacologic effects of tobacco products. The time course of nicotine in the body and resultant pharmacologic effects are highly dependent on dose, as well as the route and rate of dosing. Smoking a cigarette, for example, delivers nicotine rapidly to the pulmonary venous circulation, from which it moves quickly to the left ventricle of the heart and to the systemic arterial circulation and brain. Venous blood concentration after smoking a single cigarette ranges from 5 to 30 ng/ml, depending on how the cigarette is smoked. The mean nicotine boost in a large study of smokers was 10.9 ng/ml (195). Nicotine concentrations in arterial blood after smoking a cigarette can be quite high (up to 100 ng/ml) but usually range between 20 and 60 ng/ml (101, 119, 166, 213). For most purposes, a single blood sample within 1 or 2 min of smoking a cigarette will give an acceptable estimate of the peak nicotine concentration. However, to capture the true “peak,” multiple blood samples must be collected during and immediately after use.
Blood nicotine levels typically peak at the end of smoking a cigarette and decline rapidly over the next 20 min due to tissue distribution. The initial t1/2 of nicotine decline, during which the drug distributes into tissue, averages ~8 min. Peak venous blood levels of nicotine are similar among cigarette smokers, cigar smokers, snuff users, and chewing tobacco users, although the rate of rise of nicotine is faster among cigarette smokers (20). Pipe smokers, particularly those who have previously smoked cigarettes, may have blood and urine levels of nicotine and cotinine as high as cigarette smokers (174, 273). Cigar and pipe smokers who have previously smoked cigarettes may inhale more deeply and achieve higher blood levels of nicotine than primary cigar or pipe smokers (258). Water-pipe users attain blood nicotine levels that are, on average, lower than those seen with cigarette smoking, but their total nicotine exposure can be higher, because the duration of use is much longer (128, 235). E-cigarette use yields variable blood nicotine levels, dependent on the type of device used, the power output of the device, the nicotine content of the e-liquid, and the user’s puffing behavior (249). Cigarette-like e-cigarettes usually generate much lower blood nicotine levels, whereas tank or modifiable devices generate peak levels as high as seen with cigarette smoking (237, 263).
The elimination t1/2 of nicotine in blood is determined by a combination of clearance rate and redistribution of nicotine out-of-body tissues. Based on an average t1/2 of ~2 h, one would predict a progressive rise in nicotine blood and tissue levels over 6–8 h (3 to 4 t1/2) of regular smoking and persistence of significant levels for 6–8 h after cessation of smoking. Studies of nicotine blood levels in regular cigarette smokers confirm these predictions (32). Peak and trough levels follow each cigarette, but as the day progresses, trough levels rise, and the influence of peak levels becomes less important. Thus regular smoking is a multidosing situation, where nicotine concentrations rise during waking hours and decline during sleep, but because the nicotine t1/2 is 2 h, levels persist at significant levels for 24 h each day. Light, intermittent smoking results in less nicotine accumulation in the body over the day, and oscillations in nicotine blood levels are more prominent. Plasma nicotine t1/2 in rodents is generally shorter than in humans: 45 min in the rat and 6 to 7 min in the mouse. This means that studies in rodents require higher daily doses of nicotine to achieve blood nicotine concentrations similar to those seen in smokers (172). Perhaps the best measure of the nicotine-related pharmacologic effect is the area under the blood nicotine concentration time curve, which reflects the time-weighted exposure of body tissues to nicotine. This measure has been used to study the pharmacology of cigarettes, smokeless tobacco, and water pipes (26, 30, 128). The plasma nicotine concentration curves for various tobacco products are presented in Fig. 2.
Fig. 2.
Average blood nicotine concentration comparison. A: average blood nicotine concentrations in 10 subjects during and after cigarette smoking for 9 min (34); B: oral snuff (2.5 g) (34); C: chewing tobacco (average 7.9 g) (34); and D: nicotine gum (2, 2-mg pieces) (34). E: average plasma nicotine concentrations, corrected for baseline level, in 14 experienced e-cigarette users after 15 puffs from their usual brand of e-cigarette (237). F: average plasma nicotine concentration for hookah (water pipe) users over 24 h after 2+ sessions of hookah use between 900 and 1800 (128).
Analytical methods.
Generally, GC-based methods are most suitable for measuring blood nicotine concentrations and are reasonably economical. For the utmost sensitivity that may be required for studies of occasional tobacco users or users of products delivering low nicotine levels, GC-tandem MS (GC-MS/MS) is the method of choice (235).
Cotinine in Blood, Saliva, and Urine
Cotinine is the major proximate metabolite of nicotine and is the most widely used biomarker of nicotine exposure. On average, 75–80% of nicotine is converted to cotinine, primarily by the liver enzyme cytochrome P-450 family 2 subfamily A member 6 (CYP2A6) (126). Cotinine can be measured in whole blood, serum, plasma, saliva, and urine. Because its t1/2 (16–18 h) is longer than that of nicotine (2 h), cotinine concentrations fluctuate much less than nicotine concentrations throughout the day, making it the most practical biomarker for measuring nicotine exposure (27, 126). Cotinine concentrations in blood and saliva are highly correlated, with saliva concentrations averaging 15–20% higher than plasma (239). Urine cotinine concentrations, on average, are four to six times higher than blood or saliva levels, making urine a more sensitive matrix to detect low-dose exposure (22). Whereas the t1/2 of 16 h makes cotinine a more stable biomarker than nicotine, cotinine levels still reflect a relatively short-term exposure to tobacco, over the past 3 to 4 days.
Cotinine blood concentrations average ~150–250 ng/ml in daily cigarette smokers. Due to its longer t1/2 than nicotine, cotinine levels rise gradually during the day, peaking at the end of smoking, and persisting at high concentrations overnight. The daily variation in blood cotinine levels throughout the day in regular smokers is ~30%. Blood cotinine concentrations are similar in smokers and regular smokeless tobacco users (3). Urine cotinine levels are generally lower in exclusive pipe and cigar smokers compared with cigarette smokers (92, 210). To date, the limited available data indicate that plasma and saliva cotinine levels in regular e-cigarette users are similar to those of smokers. However, because most e-cigarette users also smoke cigarettes, it is difficult to disentangle the contributions of each (82, 177, 265).
The mathematical relationship between nicotine intake and steady-state cotinine blood levels, based on steady-state exposure conditions, can be expressed as follows: Dnic = CLCOT × CCOT/f, where Dnic is the intake (dose) of nicotine, CLCOT is the clearance of cotinine, CCOT is the steady-state blood concentration of cotinine, and f is the fraction of nicotine converted to cotinine (27). With the rearrangement of the equation, Dnic = (CLCOT/f) × CCOT = K × CCOT, where K is a constant that converts a given blood level of cotinine to nicotine intake. On average, K = 0.08 mg⋅24 h−1⋅ng−1⋅ml−1 (range 0.05–1.1, CV = 21.9%) (27). Thus a cotinine level of 200 ng/ml in blood corresponds, on average, to a nicotine intake of 16 mg/day. The K value is an average based on a small group of healthy volunteer smokers and is expected to vary among smokers and to be influenced by genetic and environmental factors that influence nicotine and cotinine metabolism and, therefore, to vary in smokers of different racial groups. Results from specific populations may vary.
Whereas cotinine functions well as a marker of nicotine intake, individual variation in metabolism makes it an imperfect biomarker of exposure. The pathway from nicotine to cotinine is affected by genetic variation in the liver enzyme CYP2A6; race; sex; use of certain medications, including estrogen-containing hormones (e.g., oral contraceptives); alcohol use; pregnancy; and existing liver or kidney disease (126). Certain CYP2A6 gene variants slow cotinine formation and removal, although unequally, generally resulting in higher cotinine levels for a given daily nicotine intake (286). Because African Americans and Asians have, on average, lower CYP2A6 activity, they tend to have higher cotinine levels than whites for the same daily nicotine dose. However, in rare cases with extremely low CYP2A6 activity, little cotinine is generated, so levels of this biomarker are lower than expected for a given daily nicotine dose (35). Cotinine levels are also higher in African Americans because of lower rates of conversion to cotinine-N-glucuronide by uridine diphosphate-glucuronosyltransferase 2B10 (UGT2B10) (185). The same is true for men compared with women, whose higher estrogen levels induce higher CYP2A6 activity (33). For these reasons, the most accurate biomarker of daily nicotine intake is urine total nicotine equivalents (TNEs; see Total Nicotine Metabolites in Urine).
Analytical methods.
GC (72, 130) and HPLC (105, 254) are appropriate methods for quantifying cotinine concentrations in specimens from daily tobacco users. Liquid chromatography (LC)-MS/MS is the best method for quantifying cotinine concentrations in samples from nondaily users and those exposed to SHS (39, 132).
Total Nicotine Metabolites in Urine
Assessment of daily nicotine intake in tobacco product users is important, as daily nicotine intake is related to nicotine/tobacco dependence. Urine TNE is defined as the molar sum of nicotine and all of its known metabolites in urine. It is considered the “gold standard” biomarker of daily nicotine intake. TNE levels are independent of factors that affect the rate and pattern of nicotine metabolism, such as genetics, sex, diet, and medication use. Cotinine is the proximate metabolite of nicotine and primarily a product of CYP2A6-mediated nicotine metabolism (126). Cotinine is metabolized further by CYP2A6 to trans-3′-hydroxycotinine (3-HC) and then primarily through the action of UGT enzymes (UGT2B10) to cotinine glucuronide (56, 126). Nicotine is metabolized, to a lesser extent, to its glucuronide by UGT2B10 and to nicotine N′-oxide by flavin-containing monooxygenase 3 (56, 126, 143). Given the high prevalence of polymorphisms in genes that encode the major nicotine metabolizing enzymes and the influence of factors, such as sex hormones and diet, on the rate of various nicotine metabolic pathways, a single nicotine metabolite cannot comprehensively assess daily nicotine intake. For example, African Americans usually have slower CYP2A6 activity and/or slower UGT2B10 activity and thus have higher cotinine levels for a given nicotine exposure compared with those with normal enzymatic activity (33, 286).
The combination of metabolites included in the term TNE may vary among studies. Most commonly, TNE is based on the six main metabolites: nicotine, cotinine, 3-HC, cotinine-N-glucuronide, nicotine-N-glucuronide, and 3-HC-O-glucuronide. Nornicotine, norcotinine, nicotine 1′-N-oxide, cotinine N-oxide, 4-hydroxy-4-(3-pyridyl)butanoic acid (“hydroxyacid”), and other glucuronide metabolites that are present in low abundance can be measured but are rarely included in TNE determinations. When measured at steady state, these compounds account for ~80–90% of a daily nicotine dose (29, 86, 126). Defined this way, TNE is highly correlated with daily nicotine intake, as validated by administration of labeled nicotine in steady-state conditions (23).
Analytical methods.
The method of choice is LC-MS/MS. It provides high sensitivity and specificity and can measure multiple metabolites in one analytical run. Administration of stable, isotope-labeled nicotine to human study participants has been used to correlate TNE with daily nicotine intake in multiple studies (131a, 132).
Biomarkers of Exposure in the Airways
The airways, directly exposed to the ambient atmosphere, are the body’s first point of contact with inhaled tobacco products. Theoretical models and studies of airway casts predict that most inhaled tobacco smoke deposits in the central airways (209). Smoke and other aerosol deposition is influenced by breathing patterns and the ways particles change in the airways and move as a cloud (12, 122, 164, 170, 171, 184, 199). For example, low-tar tobacco products prompt more intense smoking and variability among smokers; in the high humidity of the airways, smoke particles enlarge and coagulate, which changes their deposition pattern (209).
Exposure biomarkers have been studied less in the airways and lung than in blood and urine. Because tobacco components deposit directly into the airways, sampling from airway surfaces provides a reliable and local assessment of smoke exposure. The upper airways include the nasal cavity, pharynx, and larynx, and the lower airways include the trachea, bronchi, and bronchioles. All airway surfaces are lined with a thin film (~7 µm in depth) of airway surface liquid (ASL), which is approximately isotonic with plasma, has a pH between 7 and 7.4, and contains ~1,000 proteins, many of which are involved in innate immune defense (65, 250, 251, 279). Nicotine is inhaled into the lung and then converted to cotinine by cytochrome P-450 (25). Most nicotine metabolism by P-450 enzymes occurs in the liver (25). Although cytochrome P-450s are expressed in airway epithelia, these enzymes are not present in the ASL (146, 200), suggesting that any cotinine in the ASL is likely to be from the underlying airway epithelia or from the liver via the bloodstream. The reason to test ASL for exposure biomarkers is to estimate the local concentration in airway tissues.
The three main specimen options for ASL assessment are nasal lavage fluid (NLF), sputum, and bronchoalveolar lavage (BAL) fluid. These first two are noninvasive, and samples can be easily obtained in the field. BAL requires sedation and bronchoscopy to obtain but is considered the gold standard and perhaps the specimen most representative of the deep lung. However, all three specimen types are reliable for assessing environmental tobacco exposure. Solid-phase microextraction of volatile and semivolatile compounds using extraction fiber is a common method for sample extraction and for estimation of nicotine and cotinine in sputum (83).
Nasal lavage fluid.
NLF collection is a noninvasive way to procure specimens for tobacco product exposure assessment. Typically, subjects simply expel NLF into a specimen cup after spraying a saline solution into both nostrils. After centrifugation to remove cells and other solids, cotinine levels in NLF, measured by a competitive immunoassay, provide a specific and sensitive measurement of smoking with a cutoff of exposure of 1 ng cotinine/ml NLF (188). To normalize samples and to facilitate comparisons among subjects, the total protein concentration can be measured using standard bicinchoninic acid assays. However, whereas nasal cotinine can be a useful marker of active smoking, SHS exposure does not increase cotinine in NLF when quantified using the competitive immunoassay technology (188).
Sputum samples.
Induced sputum samples represent ASL from the large/central airways in the lung. The relatively easy sample collection method involves inhalation of hypertonic saline mist, followed by coughing to expel the sputum. Rinsing of the mouth minimizes sample contamination with salivary secretions (64). Carefully collected sputum samples are a reliable tool to identify nicotine and cotinine levels after smoking and could possibly be used to assess SHS exposure (64). Sputum samples are first treated with 0.1% DTT to break down the mucins and then filtered and centrifuged. The resulting supernatant can be used to measure nicotine and cotinine by HPLC-MS/MS. Sputum samples collected immediately following smoke exposure have been shown to have greater nicotine and cotinine levels than predicted from serum and plasma. Cotinine levels (6.5 ± 1.1 μM) were lower than nicotine (33.6 ± 5.5 μM). In contrast, nicotine and cotinine plasma levels were in the nanomolar and micromolar ranges, respectively (64, 131). Similar data have been generated in vitro using well-differentiated human bronchial epithelial cultures, followed by ASL lavage and subsequent MS analysis (64). A strong correlation was observed between different tobacco smoke dilutions and nicotine level, as well as cotinine level of ASL in vitro. In all dilutions, the nicotine level detected was in the micromolar range, although consistent with the lack of expression of cytochrome P-450 enzymes in the ASL, cotinine concentration was much lower than nicotine concentration; the ratio of nicotine follows: cotinine was ~6:1 in sputum and ~100:1 in ASL in vitro (64). Furthermore, whereas Clunes et al. (64) observed ~33 μM nicotine in sputum, they observed varying nicotine levels in vitro (from ~3,001 μM), suggesting that this technique can be used to adjust dosimetry for in vitro experiments.
BAL samples.
BAL samples are collected by squirting saline solution into the lung and aspirating the solution from lung surface fluid and cellular components. Although evaluation of BAL fluid for tobacco components and metabolites may prove a critical tool for analyzing pathogenesis of tobacco smoke-induced pulmonary diseases and evidence of exposure, most BAL fluid analysis is focused on proteomics indicating the exposure effects. The only study, to date, relating smoke-induced effects identified aluminum silicate crystals in “black macrophages” in BAL samples from cigarette smokers (167).
Breath biomarkers.
The breath is a well-validated indicator of concentrations of volatile chemicals in the respiratory tract. Benzene, 2,5-dimethylfuran, toluene, and xylenes have been measured in breath samples of smokers and nonsmokers. The use of each of these chemicals as a smoking biomarker, however, varies. The VOC, 2,5-dimethylfuran is invariably detected in smokers, regardless of use patterns, but not in nonsmokers, suggesting that it is a useful biomarker of combustible tobacco product exposure (6). Elevations in acrolein levels in smokers are matched by elevations of lipid oxidation products, including malondialdehyde and hydroxynonenal, which suggests that the acrolein may derive partially from lipid oxidation rather than cigarette smoke (11); the measurable presence of microgram quantities of acrolein in the smoke from each cigarette indicates that some of the acrolein exposure biomarkers measured in smokers also likely arise directly from smoke acrolein (78, 197). Development of similar noninvasive techniques to assess exposure to e-cigarettes and other novel tobacco products would facilitate large population studies.
Over-the-Counter Cotinine Tests
Although sometimes referred to as “nicotine” or “smoking” tests, the analyte measured by over-the-counter (OTC) test kits is cotinine. These kits require no instrumentation and are the fastest and least expensive cotinine assays available. With the exception of NicCheck I (Mossman Associate, Milford, MA), which is an older, colorimetric device, all currently available OTC cotinine test kits use some form of lateral diffusion immunoanalysis on disposable strips containing cotinine antibodies. These strips are designed to give a simple, qualitative smoker/nonsmoker response, based on a cutoff level. Most kits are meant for use with urine samples and according to the vendors, have a lower cutoff for a positive result of 200 ng/ml. As described earlier, most smokers and other active tobacco users typically have urine cotinine concentrations significantly higher than 200 ng/ml, so these strips, when positive, would indicate a likely tobacco user. These strips, however, are not sensitive enough to detect exposure to SHS and may not detect nondaily smoking.
Similar kits are available for use with saliva and have been reported to have greater sensitivity. These include the 1-Step Cotinine Rapid Saliva Test (20 ng/ml cutoff; Alere, Waltham, MA), the iScreen OFD Test (30 ng/ml cutoff; Alere), and the “Second Hand Smoke” NicoTest (10 ng/ml cutoff; USHealthTests, Albany NY). Since cotinine concentrations in saliva are much lower than in urine, saliva tests must be more sensitive to discriminate between tobacco users and nonusers. Some of these kits include supplies and devices for sample collection, whereas others do not. In general, with the assumption that these OTC kits perform accurately at indicated cutoff values, a positive result indicates an active tobacco user, whereas a negative result indicates a likely nonsmoker, infrequent smoker, or a user of a low-nicotine e-cigarette.
Although all of the kits described here use standard, lateral diffusion immunoassays, specific information about the antibodies or other test-strip components has not been published. With the exception of the NicAlert strips (Nymox Pharmaceutical, Hasbrouck Heights, NJ) described below, most of these kits, although marketed under various brand names, appear to be made by one manufacturer: Gemc Technology in Shenzhou, China. These tests are usually described by the manufacturer as providing preliminary results, requiring a more specific method, such as GC-MS, for confirmation.
Nymox Pharmaceutical’s NicAlert and TobacAlert tests are similar in concept but use multiple band (“reland,” or release ligand) regions that vary in their affinity for the analyte and use colloidal gold particles coated with cotinine conjugate for visual detection. This approach produces a more complex result pattern on the strip, which is visually evaluated by the user based on the lowest colored band. The manufacturer states that this approach provides for a semiquantitative assay. The lowest band cutoff value is 10 ng/ml, with progressively higher cutoffs assigned to higher bands, enabling the use of these strips with urine or saliva.
Cotinine test strips can provide a simple, inexpensive, and noninstrumental approach to assessing an individual’s current tobacco exposure and potentially distinguishing between regular tobacco users and nonusers. Although these devices lack the sensitivity necessary to address low-level exposures reliably, including SHS and nondaily tobacco use, and generally provide results with greater variability than standard laboratory assays, they can be helpful for certain applications. One important advantage is the ability to provide nearly immediate feedback to a subject, as is also the case with breath CO measurements.
TSNA METABOLITES IN URINE
The TSNAs include the potent lung carcinogen NNK and the oral cavity and esophageal carcinogen N′-nitrosonornicotine (NNN) and are—as indicated by their common name—regarded as completely specific to tobacco. Consequently, these compounds and their metabolites are among the most important biomarkers for monitoring tobacco exposure and evaluating tobacco and nicotine delivery products (109, 110, 118). Urine is the preferred biospecimen, and the primary biomarker—considered as specific as nicotine or cotinine for tobacco exposure—is NNAL, a metabolite of NNK and itself a carcinogen. A key benefit of NNAL assays is the compound’s estimated terminal t1/2 of 10–18 days (112), which is longer than other tobacco biomarkers. The main disadvantage is that the urinary concentration of NNAL is many times lower than that of cotinine, so the assay is more technically challenging and expensive to perform. Measurements of NNAL typically require extensive sample prep, with analysis by LC-MS/MS, and fewer laboratories can reliably measure NNAL than cotinine or nicotine.
Figure 3 shows the formation of the nitrosamines NNN and NNK from nicotine. Whereas nicotine is converted to cotinine in the body, the formation of NNK and NNN from nicotine occurs mainly within tobacco itself, partly during plant development but predominantly by nitrosation of nicotine during tobacco leaf processing and curing (88, 89). (NNN and NNK are also formed from nornicotine and pseudo-oxy-nicotine during curing.) Additional TSNAs can be formed from related tobacco alkaloids, but NNN and NNK are believed to be the primary carcinogenic forms in humans. There is strong evidence that the TSNAs are carcinogenic in both experimental animals and humans and that NNAL is not only a biomarker of tobacco exposure but also an indicator of cancer risk (117, 244).
Fig. 3.
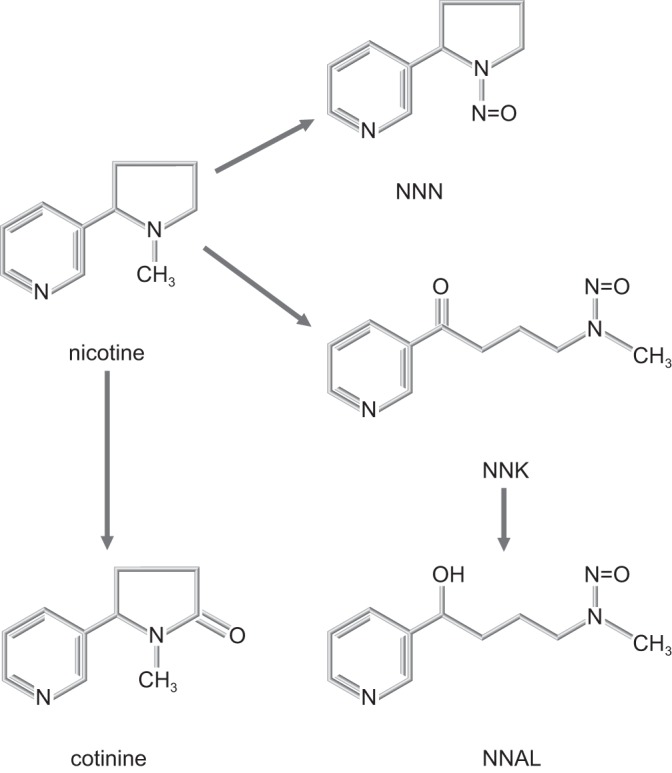
Tobacco-specific nitrosamine formation from nicotine, a process that occurs mainly during the curing and processing of tobacco [modified from Kotandeniya et al. (150)].
NNAL, the NNK metabolite, is the most stable and abundant TSNA metabolite in urine samples, occurring both free and as a glucuronide. NNN is also present in urine, but its concentration is lower than that of NNAL, making it more difficult to detect and quantify. Measurements made after hydrolyzing the glucuronides of NNAL provide “total” NNAL values, which are the values most commonly used. Whereas NNAL is usually measured in urine, where its concentration is highest, it has also been measured in blood and in toenail clippings (a potentially longer term storage site) (242).
TSNAs are released into the air when tobacco is burned, and nonsmokers are also exposed (46, 114). There is evidence that nicotine can continue to form NNK in the environment (224, 231). Nonsmokers exposed to SHS may have a higher NNAL-to-cotinine ratio in their urine (96). NNAL concentrations in urine of both smokers and nonsmokers have been reported in several large studies, including a multiethnic cohort study (193) and in all U.S. National Health and Nutrition Examination (NHANES) surveys since 2007 (37a, 277, 280). The total NNAL geometric mean for nontobacco users in the 2011–2012 NHANES survey of the U.S. population was 1.19 pg/mg creatinine (95% confidence interval 1.09, 1.29), whereas it was 216 pg/mg creatinine (95% confidence interval 182, 257) among cigarette smokers (277). Although creatinine concentrations vary with age, sex, and muscle mass, they average 0.5–1.5 mg/dl in healthy adult humans and 0.1–0.5 mg/dl in healthy adult mice. Mean values were lower in pipe and cigar smokers and much higher in oral tobacco users, although the sample sizes for the latter two groups were smaller. In a multinational study of 631 smokers and nonsmokers, the cut point for total NNAL between smokers and nonsmokers was 47.3 pg/ml (96). It is likely that occasional nonsmokers, with heavy SHS exposure, may exceed this cut point, but most nonsmokers fall well below this limit (96).
Since TSNAs are found in the tobacco leaf and also form during combustion, they are also delivered via other tobacco products, such as chewing tobacco, cigars, and pipes. Relatively high TSNA exposure has been reported among some smokeless tobacco users (115) and may contribute to the increased oral cancer risk associated with the use of smokeless tobacco products. Purified nicotine, such as that found in nicotine patches, gums, and lozenges, should contain no TSNA. However, NNN has been found in the urine of users of some oral NRT products, possibly through endogenous formation by nitrosation of nornicotine (241). Additionally, studies have shown that some e-cigarettes and e-cigarette liquids provide low-level nitrosamine exposure through their aerosols (99, 147). However, urinary NNAL concentrations decrease in smokers who switch to e-cigarettes (97, 203), and urinary NNAL concentrations in sole users of e-cigarettes are 1–10% of the concentrations seen in cigarette smokers (113, 226, 268) (e.g., 1.47 pg/mg creatinine vs. 53.4 pg/mg) (226).
Concentrations of TSNA in tobacco can be reduced by selecting specific types of tobacco and by modifying the curing and manufacturing process. Lower TSNA deliveries have been reported for Swedish Snus (a smokeless tobacco product that uses tobacco with relatively low TSNA content), the Omni “reduced carcinogen” cigarette, and a medicinal nicotine patch (107). The authors noted, however, that the patch delivered significantly lower TSNA than any of the other recreational products studied, and only trace levels of NNK have been detected in a typical nicotine patch (243). The specificity of NNAL to tobacco and its role as a human carcinogen make it a fundamental marker for any evaluation of a new tobacco or nicotine delivery product.
VOC METABOLITES IN URINE
VOCs are a diverse group of chemicals that are abundant in tobacco product emissions and in the atmosphere, even where no one is smoking (73, 74, 78, 94, 260). Many VOCs are formed by incomplete combustion of organic materials, and tobacco is not the only source of exposure. VOCs are also present in foods and beverages. In addition to exogenous sources, VOCs, such as acrolein, are generated by endogenous processes, such as inflammation and lipid peroxidation. Acrolein is a product of the reactions catalyzed by myeloperoxidase (10), and it is also generated as a result of lipid peroxidation reactions (259). Given that smoking increases both oxidative stress and inflammation, the measured levels of acrolein exposure biomarkers likely reflect a combination of inhaled acrolein from tobacco smoke (197) and endogenous inflammatory responses and lipid peroxidation. Hence, measurements of VOC metabolites in the urine provide a somewhat nonspecific estimate of exposure to tobacco products. Nevertheless, the levels of many VOCs and VOC metabolites are elevated in smokers’ urine compared with nonsmokers (48, 77, 168, 238). Concentrations of VOCs, such as acrolein and crotonaldehyde, are up to two orders of magnitude higher in cigarette smoke than in ambient air (78). Therefore, many VOC metabolites are found at background levels in all urine samples, and cigarette smoking increases these exposure biomarkers above that background.
Several VOCs in tobacco smoke, including acrolein, benzene, and 1,3-butadiene, are high-priority chemicals on the U.S. Food and Drug Administration’s list of harmful and potentially harmful tobacco product constituents (261b). Acrolein can cause cardiovascular and lung damage. Benzene is a human carcinogen (International Agency for Research on Cancer, Class 1A) known to cause leukemia. 1, 3-Butadiene is also a human carcinogen. Acrolein forms during heating of glycerol (glycerin) or glycerol-derived fats (e.g., triglycerides), making it of particular interest for e-cigarettes, which commonly use glycerol (“vegetable glycerin”) in their e-liquids (240). Benzene exposure from hookah use may be higher than from cigarette smoking, possibly due to the burning charcoal generally placed on top of the moist fruit–tobacco mixture (128).
A number of harmful VOCs can be measured directly in human blood, urine, and breath (13, 43, 100). Furthermore, many toxic VOCs are metabolized to forms, such as mercapturic acids, that are useful biomarkers of exposure (8). Some mercapturic acid biomarkers that are useful in tobacco studies are listed in Table 3. The acrylonitrile metabolites 2-cyanoethylmercapturic acid and/or N-acetyl-S-(2-cyanoethyl)-l-cysteine are highly selective biomarkers of smoke exposure that effectively assess toxic acrylonitrile exposure and serve as a surrogate measure for smoke exposure (128, 135, 180, 219, 222, 223). Nevertheless, it is important to remember that several VOCs, such as acrolein and crotonaldehyde, and their metabolites are highly reactive and readily form covalent adducts with cell constituents, such as proteins, DNA, lipids, and carbohydrates, and therefore, are retained in tissues for extended periods. Consequently, the absence of urinary metabolites of VOCs, especially at low levels of exposure, cannot be taken to indicate absence of exposure. Conversely, because VOCs, such as acrolein, can also be generated by inflammation (10) and oxidative stress (259), the presence of tissue acrolein–protein adducts in smokers may not be entirely attributable to exposure from tobacco smoke.
Table 3.
Volatile organic compounds and their biomarkers
| VOC | Biomarker | Abbreviation |
|---|---|---|
| Acrolein | 3-Hydroxypropylmercapturic acid | 3-HPMA |
| Acrylamide | 2-Carbamoylethylmercapturic acid (acrylamide mercapturic acid) | AAMA |
| Acrylonitrile | 2-Cyanoethylmercapturic acid | CNEMA, CYMA |
| Benzene | Phenylmercapturic acid | PMA |
| 1,3-Butadiene | N-Acetyl-S-(4-hydroxy-2-buten-1-yl)-l-cysteine | MHBMA-3 |
| Crotonaldehyde | 3-Hydroxy-1-methyl-l-propylmercapturic acid | HMPMA |
| N,N-Dimethylformamide | N-Acetyl-S-(N-methylcarbamoyl)-l-cysteine | AMCC |
| Ethylbenzene | Phenylglyoxylic acid | PGA |
| Ethylene, ethylene oxide | 2-Hydroxyethylmercapturic acid | HEMA |
| Methylating agents | Methylmercapturic acid | MMA |
| Propylene, propylene oxide | 2-Hydroxypropylmercapturic acid | 2-HPMA |
| Styrene | N-Acetyl-S-(1-phenyl-2-hydroxyethyl)-l-cysteine + N-acetyl-S-(2-phenyl-2-hydroxyethyl)-l-cysteine + mandelic acid | PHEMA, MA |
| Xylene | N-Acetyl-S-(2,4-dimethylphenyl)-l-cysteine, methylhippuric acids | DPMA, 2MHA, 2MPHA, 4MPHA |
Analytical Methods
Characterization and quantitation of VOC mercapturates usually require chromatographic separation—both GC and LC have been used—followed by MS analyses. GC-MS is especially useful for the detection and quantitation of low molecular weight VOCs and VOC metabolites, such as short-chain carboxylic acids, some mercapturic acid conjugates, phenols, and alcohols. These analytes are usually detected after extraction and derivatization (79, 142, 148, 155, 214, 219, 256, 262). The development of ultrahigh performance LC and highly sensitive MS detectors with ultrahigh scan speeds can eliminate the need for sample derivatization, enhance sensitivity, and decrease assay time (<10 min). These advances have led to the development of a new generation of multimetabolite, high-throughput assays, such as the one used by the Centers for Disease Control and Prevention for detection of 28 VOC metabolites (8).
With advances in MS technology and especially the advent of high-accuracy and high-resolution MS time-of-flight and Orbitrap mass analyzers, the monitoring and identification of tens or even hundreds of compounds in one chromatographic run are becoming a possibility. These new methods have enabled the discovery of new mercapturates (140, 270, 271). New assays are required to characterize and quantify signature metabolites of emerging tobacco products, such as e-cigarettes, cigarillos, and hookahs, as well as the flavoring reagents used in these products.
PAH METABOLITES IN URINE
PAH formation results from the incomplete combustion of organic compounds, including tobacco, during smoking. The lower molecular weight PAHs, comprising two or three aromatic rings, occur mostly in the gas phase of tobacco smoke and appear noncarcinogenic, except naphthalene. However, a number of the higher molecular weight PAHs and their alkyl derivatives, which occur mainly in the particulate matter of tobacco smoke, are strong carcinogens and are considered to be major factors in the development of lung cancer (13), due to their conversion to reactive metabolites that form DNA adducts. Exposure to both mainstream and sidestream tobacco smoke is associated with increased risk of lung cancer, cardiovascular disease, and chronic obstructive pulmonary disease. Because tobacco is often dried and cured using fire and smoke, smokeless tobacco products also contain PAHs. The quantitative pattern of PAH exposure can differ for different tobacco products, such as cigarettes vs. water pipes, as discussed later. Yet, PAHs are not specific to tobacco, and exposures also come from air pollution, food, and the workplace. Adducts of PAHs with DNA or proteins have been measured as carcinogen exposure biomarkers. Yet these PAH adduct biomarkers give only nonspecific information about the source of carcinogens and are difficult to measure, often yielding low or negative numbers (111).
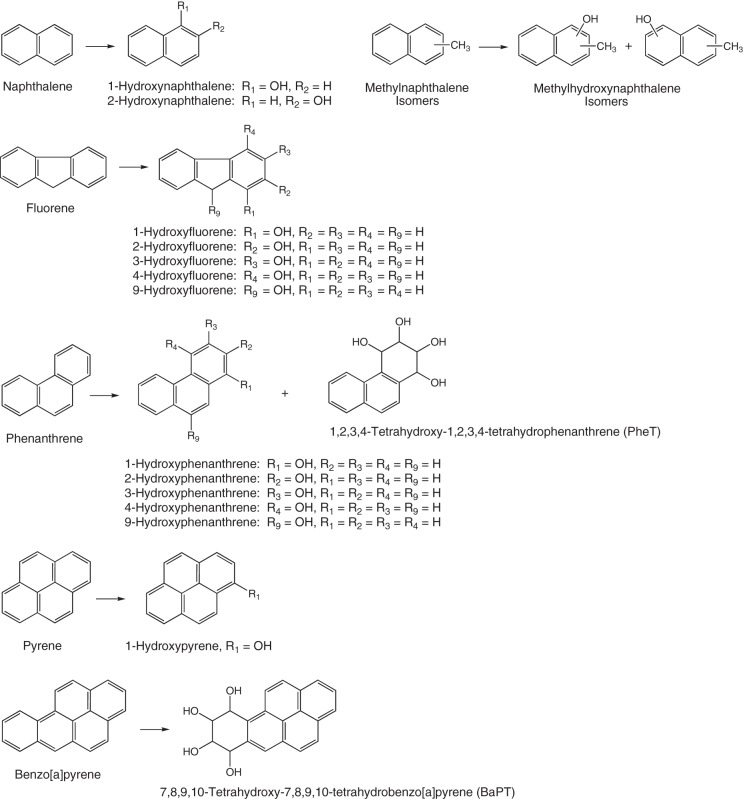
PAH biomarkers of tobacco smoke exposure that are commonly measured include 1-hydroxypyrene (1-HOP); 1-, 2-, and 3-fluorenols; 1- and 2-naphthols; monohydroxyphenanthrenes; and 1,2,3,4-tetrahydroxy-1,2,3,4-tetrahydrophenanthrene (Fig. 4) (284, 285). Tetrahydroxy-7,8,9,10-tetrahydrobenzo[a]pyrene (284) and hydroxylated metabolites of methylnaphthalenes (MeNs) (160) have also been reported in smokers, but the potential toxicity and health effects of MeNs are not well studied (160). PAH metabolites are found in biofluids, mainly in the conjugated (glucuronide and sulfate) forms, and deconjugation is typically performed before quantitative analysis. Table 4 shows levels of various PAH metabolites measured in U.S. smokers and nonsmokers.
Fig. 4.
Main PAH metabolites measured as biomarkers in urine of tobacco smokers.
Table 4.
PAH metabolites in urine from smokers and nonsmokers (pg/ml)
| Smokers | Nonsmokers | |||
|---|---|---|---|---|
| Biomarker | Least-Squares Geometric Means | 95%Confidence Interval | Least-Squares Geometric Means | 95%Confidence Interval |
| 1-Hydroxypyrene (1-HOP) | 104 | 91–119 | 40 | 35–46 |
| 1-Hydroxynaphthalene (1-NAP) | 6,293 | 5,570–7,111 | 1,523 | 1,367–1,697 |
| 2- Hydroxynaphthalene (2-NAP) | 8,597 | 7,325–10,090 | 1,682 | 1,466–1,931 |
| 2-Hydroxyfluorene (2-FLUO) | 990 | 871–1,125 | 236 | 211–265 |
| 3-Hydroxyfluorene (3-FLUO) | 592 | 516–679 | 90 | 81–100 |
| 9-Hydroxyfluorene (9-FLUO) | 342 | 310–377 | 200 | 174–231 |
| 1-Hydroxyphenanthrene (1-PHEN) | 193 | 170–219 | 132 | 117–150 |
| 2-Hydroxyphenanthrene (2-PHEN) | 88 | 75–103 | 48 | 38–57 |
| 3-Hydroxyphenanthrene (3-PHEN) | 194 | 167–224 | 91 | 81–102 |
| 4-Hydroxyphenanthrene (4-PHEN) | 53 | 40–70 | 39 | 28–54 |
| 9-Hydroxyphenanthrene (9-PHEN) | 88 | 77–102 | 26 | 23–30 |
1-HOP has been most commonly used as a PAH biomarker of tobacco smoke exposure. It is a four-member aromatic ring compound found predominantly in the particulate phase and is thought to be the best surrogate biomarker for the potent carcinogen, benzo[a]pyrene, a PAH, present in extremely low levels and whose metabolite biomarkers are difficult to measure in urine. Several studies have shown increased concentrations of 1-HOP in smokers; after smoking cessation, levels of urinary 1-HOP were reduced by 50% (50). 1,2,3,4-Tetrahydroxy-1,2,3,4-tetrahydrophenanthrene is also increased in smokers’ urine compared with nonsmokers (282). In one study, levels of urinary MeNs were elevated 37-fold in smokers, on average (160), but larger cohort studies are needed to confirm this finding.
2-Naphthol and hydroxylated fluorenes appear more specifically related to smoking exposure and nicotine intake than 1-HOP, having correlation coefficients of 0.66 and 0.71, respectively, with urine nicotine equivalents, and are the best PAH measures for daily smoke exposure (236). Naphthalene metabolites in smokers are present in the highest concentration, in the >10-ng/ml range and may be 5–10 times higher than levels in nonsmokers. Levels of fluorenols are lower but were found to have the highest probability of predicting smokers from nonsmokers in both the United States and Poland (236). However, fluorene is not known to be carcinogenic and is not metabolized to diol epoxides, which are often highly potent proximate carcinogens derived from higher molecular weight PAHs. Fluorene’s predictive value for smoke exposure does not, therefore, predict cancer risk, unless it is determined that these lower molecular weight PAHs are produced in the same ratio during smoking, as the higher molecular weight carcinogenic PAHs.
Because PAH metabolites are not specific for tobacco smoke exposure, their main use is in distinguishing the use of combusted vs. noncombusted tobacco products and in characterizing different types of combusted tobacco products. For example, pyrene metabolite levels are higher and naphthalene and fluorene metabolite levels are lower in water-pipe users than in cigarette smokers, presumably reflecting the contribution of the burning charcoal in the water pipe (128). Similarly, different PAH profiles were observed between cigarette smokers in the United States and in China, presumably reflecting differences in both tobacco blends and environmental exposures (24).
There are several limitations on the use of PAH metabolite measurement to assess tobacco smoke exposure. First, the environmental sources of PAH exposure may vary across regions and countries, resulting in regional and national differences between nonsmokers’ and smokers’ test values. Additionally, the ratio of lower to higher molecular weight PAHs varies with region and type of tobacco-filler composition (236). Second, there are no clear-cut points for PAH metabolites that can differentiate between smokers and nonsmokers and are generalizable across regions. For example, optimal cut points were found to be at least two times higher for Polish than for U.S. samples, because Polish subjects were exposed to higher background levels of PAHs (236). Regional, normal ranges are therefore necessary to establish baseline background exposure. Third, since PAH metabolites have short t1/2 (4–10 h), metabolite levels in a spot urine sample reflect the time interval between the subject’s last smoking episode and urine collection (236). Fourth, concentrations of urinary PAH biomarkers are generally low in picograms/milliliters.
Because hydroxylated PAH metabolites are excreted mainly as conjugates, PAH exposure is generally quantified after de-conjugation with β-glucuronidase. Because of the complexity of the urine matrix and the low concentrations of PAH metabolites present, extractive clean-up procedures are generally necessary. Mass spectrometric methods with stable isotope-labeled internal standards provide the greatest sensitivity, precision, and specificity and are most commonly used. An exception is 1-HOP, for which HPLC with fluorescence detection has provided satisfactory results (52, 59).
Currently, the most commonly used instrumental methods are GC-MS (51, 103, 116, 212) and LC-MS/MS (129, 186, 205, 281). Derivatization is necessary for GC-MS methods, and generally, trimethylsilyl derivatives have been used (51, 103, 116, 212). High specificity and sensitivity in GC-MS analysis have been achieved by using an accurate mass/high-resolution mass spectrometer (212). Derivatization has been used in LC-MS/MS analysis to increase the sensitivity and specificity (129). Specific urinary biomarkers of tobacco exposure, such as TNEs and NNAL, can be correlated with the nonspecific PAH metabolite biomarkers and used as a measure of tobacco exposure.
METALS
Tobacco plants readily absorb metal ions and compounds from the soil. The amount of metals absorbed is influenced by the concentrations of metals in the soil and in soil amendments, such as phosphate fertilizers, animal waste, or sewage sludge (2, 15, 183). Cadmium is a toxic and carcinogenic metal that is absorbed from the soil by tobacco plants and is, therefore, present at high concentrations in mainstream tobacco smoke (192, 261a). Cadmium levels are higher in the blood and urine of current smokers than in former smokers or nonsmokers (1, 108, 176, 196, 207). During combustion, cadmium is reduced to the neutral form and is transported through the cigarette rod in the gas phase before condensation (16, 191). Pulmonary elimination of cadmium after inhaling tobacco smoke is slow. The biological t1/2 of cadmium is 14–23 yr (91, 189, 247). Its slow elimination from the lungs after inhalation exposure from combustible tobacco products is consistent with exposure to cadmium in the poorly soluble, neutral form. It is measured in blood and urine using inductively coupled plasma MS (108, 178, 207, 230). Toxicokinetic studies suggest that cadmium concentration in blood reflects recent exposure (1, 136), whereas its concentration in urine reflects chronic exposure (1, 211). However, cadmium is a nonspecific biomarker of tobacco exposure, because cadmium in human specimens may also reflect dietary and occupational exposures. Cadmium levels increase with age, and the average urinary cadmium concentration for nonsmokers in the 1988–2004 NHANES was 0.21 ng/ml (253). For smokers, it was 0.40 ng/ml, and values above 5 ng/ml were associated with the occupational history of working with metal.
With few exceptions, exposure to toxic metals from noncombustible-inhaled tobacco products, such as e-cigarettes, is likely to be lower than for combustible products. In e-cigarettes, the aerosolization temperature is lower than combustion temperature. These temperatures are not high enough to cause evaporation of metals from the heating element. However, any metal in the e-liquid may be sputtered and entrained in the heated aerosol as the heating element boils the liquid from the wick. Moreover, the tobacco extract used as a source of nicotine in e-juices is not the complete tobacco matrix. Temperatures between 200 and 300°C are sufficient to aerosolize propylene glycol or glycerol—the most common e-cigarette solvents. However, the temperatures of the heating element are sufficient to cause thermal decomposition of some extract and solvent constituents, which may, in turn, contribute to e-cigarette users’ exposure (Fig. 5) (81). E-cigarettes components—including exposed wires, wire coatings, solder joints, electrical connectors, heating element material, and vitreous fiber wick material—constitute the second major source of inorganic toxicants to which e-cigarette users may be exposed. Figure 5 shows an example of an e-cigarette’s nichrome heating element wrapped around a vitreous fiber wick. Thermal decomposition of some substances and possibly heat-induced breakage of the wick fibers are apparent in the vicinity of the heating element after use.
Fig. 5.
Light microscope image of the nichrome heating element coiled around the vitreous fiber wick of an e-cigarette before (left) and after (right) use. Note the evidence of thermally decomposed organic substances and fragmented vitreous fibers in the vicinity of the heating element.
Figure 6 shows corrosion on a brass electrical connector from an e-cigarette. The corrosion is reflected in the copper- and zinc-containing particulate in e-liquid that was trapped with polytetrafluoroethylene filters (Fig. 7). This source might account for the elevated levels of copper and zinc in the aerosol produced by some e-cigarettes (278). Similarly, a tin solder joint could undergo corrosion (Fig. 8), leading potentially to elevated levels of tin in some e-cigarette liquids (278).
Fig. 6.

Zinc and copper corrosion has apparently occurred on the surface of this brass electrical connector from an e-cigarette. [Image obtained using scanning electron microscopy–energy dispersive spectroscopy (EDS).] The orange-colored fibers are fragments of the vitreous wick fibers composed of silicate.
Fig. 7.
Piles of fine and nano-sized particles obtained by filtration of the liquid from an e-cigarette before (left) and after (right) use. The violet and magenta colors represent copper and zinc in the particulate. The orange and yellow–green particles are calcium silicate and silica particles.
Fig. 8.

A tin solder connection on a battery of an e-cigarette appears to have undergone some corrosion.
EXHALED CO
CO is formed by incomplete combustion of organic materials and is prevalent in the environment at low concentrations due to its presence in motor vehicle exhaust. However, high CO levels are generated during tobacco combustion, making exhaled CO a useful and validated marker for identifying individuals who have recently used a combustible tobacco product. Exhaled CO can be measured easily and reliably by asking study participants to blow into a portable CO monitor after holding their breath for 15 s. The concentration of exhaled CO [measured in parts per million (ppm)] correlates well (r > 0.95) with the concentration of carboxyhemoglobin in blood (percent hemoglobin saturation), and so measurement of exhaled CO has become a standard method for assessing recent smoking (25a, 272).
Although CO is produced in the human body (largely via heme-oxygenase-1) and therefore, can affect both blood carboxyhemoglobin and exhaled CO (227), the contribution of biologically generated CO to exhaled CO is small (in the range of 1–5 ppm) relative to the increase in exhaled CO directly attributable to active smoking (typically in the range of 7–60 ppm). For example, Cheng et al. (57) reported on health correlates of exhaled CO in the Framingham community sample. In that group, although approximately one-third included smokers, 78% of those with an exhaled CO > 5 ppm were self-reported tobacco smokers, whereas only 10% of them were never smokers (of whom some could have been misreporting their smoking). Studies that have confirmed abstinence from smoking using cotinine have found that <3% of recent ex-smokers have an exhaled CO > 4 ppm. Based on these data, some investigators have recommended that the cutoff for exhaled CO should be lowered to 4 ppm to confirm smoking abstinence (70), although values of 6 ppm continue to be used (see below).
Cut Points
Smoking cessation.
The t1/2 of exhaled CO varies from 2 h in someone actively exercising (e.g., jogging) to 8 h in someone sleeping, with the average of 4 h (25a). The initial CO cut point for verification of self-reported abstinence in a smoking cessation study was 8–10 ppm, and CO < 10 ppm has been widely used in smoking cessation clinical trials (typically to verify self-reported smoking abstinence for the past 7 days). However, ambient indoor CO levels have decreased as smoking prevalence and SHS exposure have fallen over the past two decades. Many studies now suggest that CO < 6 ppm (169) may be an optimal cut point to verify self-reported smoking cessation for at least 1 wk (Table 5), and this value is now being used in clinical trials (17, 216).
Because it is readily measured, exhaled CO is frequently used in tobacco research to confirm smoking status. Both 8 h “overnight abstinence” and 12 h abstinence are common in studies that do not require zero blood nicotine concentrations at baseline. If zero blood nicotine is necessary, then 16–24 h abstinence may be necessary, as the average nicotine t1/2 can reach 4 h in slow metabolizers. Without real-time blood nicotine measurement, however, it is difficult to decide which exhaled CO cut point to use to verify abstinence compliance. For example, many compliant volunteers will likely have exhaled CO > 6 or > 10 ppm after overnight abstinence, partly due to the longer t1/2 of CO during sleep. If an afternoon baseline CO measurement (with normal smoking) is available, then a verification cut point at least 50, 60, or 70% lower for overnight (8 h) and 12 and 24 h abstinence is reasonable. If no baseline smoking CO measure is available, then absolute cut points of <16, <12, and <10 ppm are reasonable to verify overnight (8 h) and 12 and 24 h of smoking abstinence. Although exhaled CO does not offer “perfect” validation of short periods of smoking abstinence, these guidelines may be practical for laboratory studies. If saliva or urinary nicotine is also measured, then researchers can exclude subjects with unacceptably high nicotine concentrations.
Exhaled CO As An Estimate of Smoke Inhalation
A measurement of exhaled CO is generally regarded as a valid and reliable estimate of recent smoke inhalation. However, the measure increases by 1–8 ppm with every cigarette smoked and then immediately starts falling, with an average t1/2 of 4 h. Thus the time of day and time since last smoke are highly relevant, with afternoon or evening measurement preferable to early morning. Moreover, in a repeated-measures study, it is preferable to repeat CO measurements at the same time of day and at the time of measurement to record how many cigarettes a subject has smoked that day and the time since the last smoke. With regular smoking, afternoon CO measurements correlate well with intake of nicotine and other tobacco toxicants (141). As research participants may be exposed to a range of tobacco/nicotine products, as well as nontobacco sources of inhaled CO (e.g., smoked marijuana) (181, 206), it may be advisable to exclude recent users of products, other than the product of interest, to enable greater confidence in the source of measured CO (and nicotine).
CONFOUNDING EXPOSURES FOR NONSPECIFIC BIOMARKERS
Metabolites of VOCs, PAHs, exhaled CO, and metal are not specific to the use of and exposure to tobacco products. Although the smoking of tobacco products causes characteristic increases in the concentrations of these biomarkers, diet and occupational and recreational exposure to smoke, vehicle exhaust, and welding fumes can also cause increases in these biomarkers (7, 18, 135, 162, 202, 217, 275, 276). With the measurement of nonspecific biomarkers of exposure to tobacco and nicotine delivery products, it is very helpful to collect questionnaire data on recreational, environmental, and occupational exposure to smoke, exhaust, dust, and metal fumes.
The use of cannabis (marijuana) is an important potential, confounding exposure for VOCs, PAHs, CO, and metals. Cannabis can be smoked, heated, and aerosolized; used as a concentrate in an e-cigarette; or consumed orally. The prevalence of cannabis use is higher among smokers than among nonsmokers (204, 206, 245). The compounds in cannabis smoke are similar to those in tobacco smoke, except for differences in the concentrations of nicotine, TSNAs, and cannabinoids (181). The smoking of cannabis may cause increases in nonspecific biomarkers of combustion aerosol exposure, including CO and metabolites of VOCs and PAHs. The aerosolizing of cannabis and the use of cannabis extracts in e-cigarettes may also cause increases in metabolites of VOCs and PAHs. The transfer of metals during the use of cannabis products has not been studied sufficiently. The screening of subjects in tobacco and nicotine delivery studies for biomarkers of exposure to cannabis can improve the interpretation of results from these nonspecific biomarker tests, reduce the prevalence of anomalous findings, and improve data quality.
There are two federal cut-point concentrations for cannabis metabolites: one for the initial immunoassay test (50 ng/ml) and a second for confirmation of a positive initial test, usually by GC- or LC-MS (15 ng/ml) (75a). These concentrations do not correlate with intoxication. The initial 50-ng/ml test may be performed using an OTC test or by submitting a specimen to an accredited testing laboratory. The OTC tests are usually less expensive and offer low levels of false-positive and -negative results (71).
Tetrahydrocannabinol (THC), the primary psychoactive compound in cannabis, is lipophilic, so the t1/2 for elimination of the primary metabolite of THC (delta-9-THC-9-carboxylic acid) can be long. People who smoke cannabis more than once a day, every day, can test positive at the initial federal cutoff (50 ng/ml) for over 24 days after cessation (165). In infrequent users, a single use will not be detectable after 3–4 days (54). Likewise, even very heavy secondhand exposure to cannabis smoke is unlikely to result in a positive drug test after 12 h (66).
When a study participant smokes both tobacco and cannabis, analysis of cotinine and NNAL will yield data that can quantify tobacco use. Where spectrophotometric analysis of THC metabolites is available, it is also possible to quantify cannabis use. However, in dual users, it is not possible to apportion the sources of CO, VOCs, and PAHs. In individuals who use e-cigarettes with nicotine and cannabis regularly, the expected pattern would be cotinine above 30 ng/ml in urine and THC metabolite levels between 20 and 60 ng/ml.
POTENTIAL BIOMARKERS OF E-CIGARETTE USE
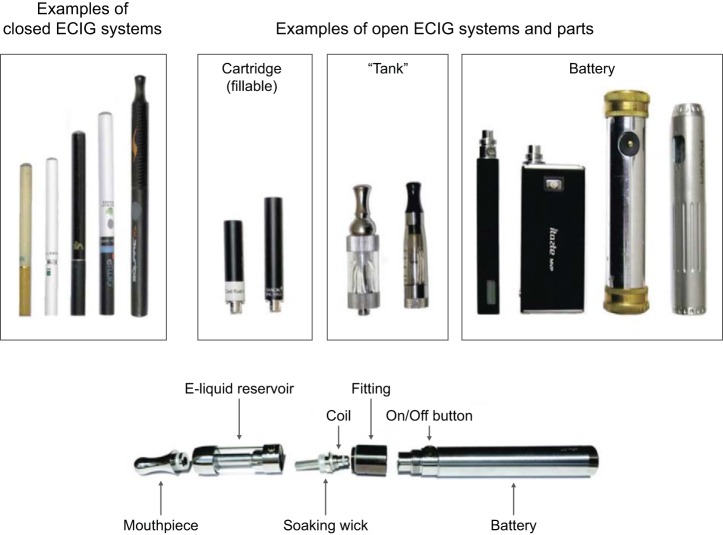
E-cigarettes are electrically powered devices that heat and aerosolize a flavored liquid to produce an inhalable aerosol without combustion (42, 45). Most e-cigarettes contain nicotine. First-generation e-cigarettes (cig-alikes) are frequently disposable and resemble combustible cigarettes but generally deliver less nicotine (42). Second-generation e-cigarettes (e.g., “tank-style,” “vape pens,” “e-Gos”) are refillable, have easily assembled components, are usually cigar sized, and are more likely rechargeable than disposable (163). The nicotine delivery is more similar or in some cases, higher than a combustible cigarette (80, 85, 237). Third-generation devices (e.g., “mods,” “rebuildables,” or “advanced personal vaporizers”) come in a large array of customizable formats that generally include stronger batteries, variable voltage, low amperage coils, and refillable tanks for e-liquid (Fig. 9) (75, 249). These combinations affect toxicant emissions in the e-cigarette aerosol (93, 95, 149, 283).
Fig. 9.
Example of e-cigarette (ECIG) system and parts.
E-cigarette solution, known as e-liquid, can be a mixture of propylene glycol and glycerol (typically, 75:25) or glycerol alone, water, tobacco–nicotine extract, and flavorings. Flavorings can include menthol, sugars, esters, and pyrazines (161). More than 7,760 flavored e-liquids are now available (255, 287). E-liquid can also be contaminated with alkaloids other than nicotine, carbonyls, VOCs, PAHs, TSNAs, and metals (58, 127).
E-liquid is a starting point for the composition of e-cigarette aerosol, but the aerosol is the source of human exposure and thus, potential biomarkers. The physical composition of the aerosol can be altered by many factors: the temperature of the metal coil, rate of e-liquid flow through the heated coil, chemical composition of the coil, the coil connection to the power source, the wicking material transporting e-liquid, and the hot aerosol contacts.
It is challenging to identify exposure biomarkers specific to e-cigarette use because many e-liquid components are also found in common foods and personal care products. For example, the flavorings used in e-liquids are also used in many foods. Similarly, propylene glycol and glycerol are in many baked goods, beverages, sauces, soaps, lotions, and toothpaste. The body metabolizes propylene glycol to d- and l-lactic acid. Whereas l-lactate is a normal, endogenous metabolic product, production of d-lactate is characteristic of propylene glycol exposure, absent other causes of d-lactate acidosis (61). The extent to which plasma d-lactate is increased following frequent e-cigarette use is unknown and worthy of further study. Glycerol is an endogenous constituent in the synthesis and catabolism of triglycerides. Nicotine and its metabolites are present in the body fluids of consumers of all tobacco products, including nicotine-containing e-cigarettes.
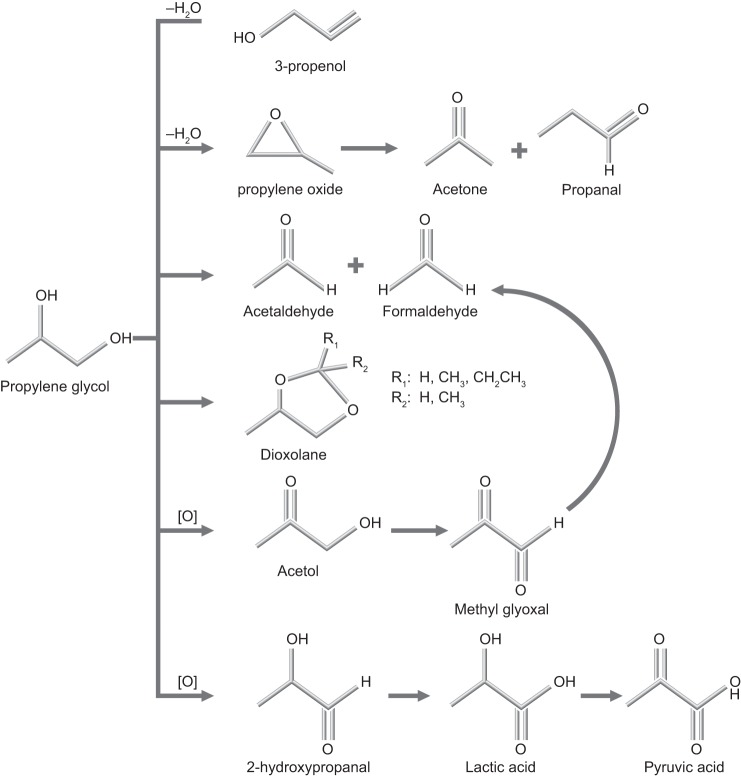
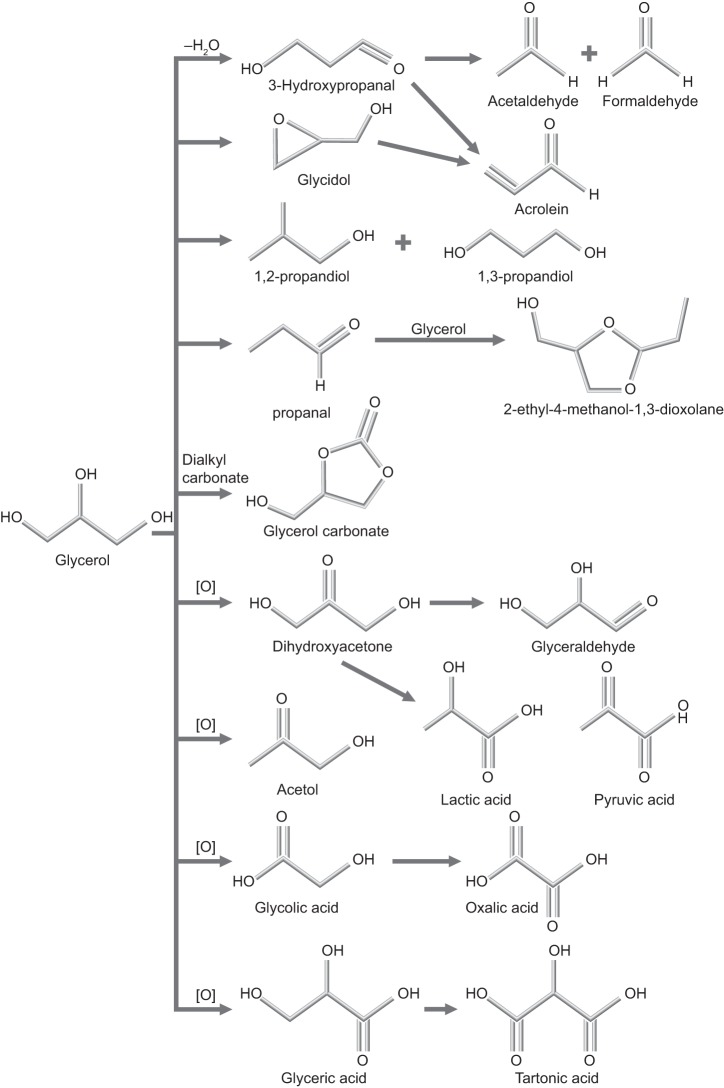
During e-cigarette use, propylene glycol and glycerol can react by dehydration and oxidation pathways to yield methyl glyoxal (201, 232, 246); glyceraldehyde (60); propylene oxide (154); glycidol (190); dioxolanes (69, 76, 125); and oxalic, lactic, and pyruvic acids (156, 257), as shown in Figs. 10 and 11. In addition, the heating of e-liquids creates low molecular weight carbonyls (formaldehyde, acrolein, acetaldehyde) and reactive oxygen species (19, 99, 120, 233). However, all of these chemicals are also found in cigarette smoke (248). As discussed earlier, at this time, the best way to identify use of nicotine-containing e-cigarettes is to confirm the presence of cotinine and the absence of other biomarkers for the use of combustible tobacco products. It is also necessary to exclude the use of NRT.
Fig. 10.
Degradation pathways and products of propylene glycol.
Fig. 11.
Degradation pathways and products of glycerol.
In comparison with cigarette smoke, e-cigarette aerosols contain undetectable or low concentrations of TSNAs (84, 147). The levels of TSNAs, such as NNAL, are expected to be lower in e-cigarette users than in smokers or oral tobacco users. Likewise, levels of PAH metabolites and some VOC metabolites should be lower in e-cigarette users than in smokers. Some less-than-specific constituents of e-cigarettes and/or their metabolites—including nicotine and menthol (as menthol glucuronide)—may still be suitable as biomarkers of e-cigarette use in well-characterized subjects whose prior exposure to non-e-cigarette nicotine sources is known and can be controlled and baseline concentrations established.
Flavor Biomarkers
Flavored noncigarette tobacco products are widely available, and they may have particular appeal to youth (9, 68). Among e-cigarette users who responded to an online survey, fruit flavors are most preferred, and the most prevalent reason for e-cigarette is the belief that they might be less harmful than cigarettes (36). These results suggest that at least some users are attracted to the various e-cigarette flavorings available and have an unsubstantiated perception of the relative safety of these products. Health effects of these flavorant additives are not well understood. E-cigarette flavor compounds include eucalyptol, camphor, menthol, methyl salicylate, pulegone, ethyl salicylate, cinnamaldehyde, eugenol, diphenyl ether, and coumarin (161). Except for the menthol biomarker, menthol glucuronide, biomarkers for potentially toxic flavorants—including cinnamaldehyde and cherry flavorants—have not been well studied. Because flavors are often created from a mixture of synthetic compounds, their exact compositions can vary, even among flavors with the same name. For example, different strawberry-flavored products, including cigarettes, e-cigarette liquid, snus, and hookah tobacco, were found to contain different flavoring compounds. These compounds included esters and aldehydes; the terpenes linalool; α-terpineol; nerolidol and limonene; as well as the lactones γ-decalactone, γ-dodecalactone, and γ-undecalactone (194). Several studies using in vitro assays indicate that these flavorants may cause oxidative and inflammatory responses in lung cells (human) and tissues (rodents) (157). Together, these studies indicate that flavored tobacco product users are exposed to potentially harmful chemicals; however, we do not currently have the data to link these inhaled exposures to health effects. Although we now have considerable data on e-liquid constituents and aerosols, the derivation of useful and specific exposure biomarkers from these data remains a challenge because of the ubiquity of propylene glycol, glycerol, and flavorants in consumer products.
TOBACCO EXPOSURE BIOMARKER RESEARCH AND CAPACITY GAPS
Expanding Access to Biomarkers of Exposure
Biomarkers of exposure to tobacco and nicotine delivery products are critical to establish links between tobacco product use and subsequent health effects. These biomarkers allow us to quantify exposure, ascertain dose-response relationships, and in some cases, identify sources of exposure to tobacco product toxicants. Accurate and sensitive biomarkers are essential for characterizing exposure patterns and adverse health effects of new and emerging tobacco and nicotine delivery products.
Few laboratories can currently perform the most sophisticated and sensitive assays of all of the biomarkers of exposure to tobacco and nicotine delivery products. Although assays for metabolites of nicotine, NNK, VOCs, and PAHs are necessary to confirm nicotine-containing e-cigarette use and to differentiate between active product use and SHS exposure, this suite of assays is costly and time consuming, especially for large studies and sample sets. Limited laboratory capacity impedes research at a time when the market for tobacco and nicotine delivery products is undergoing rapid change, and information is needed to inform policymaking and regulations. In addition to expanding access to existing assays, new biomarker assays are needed to assess population harm attributable to new and emerging tobacco and nicotine delivery products.
The development of biomarker assays proceeds through distinct stages. First is the identification of new candidate biomarkers. Next is assay development: the painstaking process of finding the optimal way to quantify a biomarker. This effort may include synthesis of the molecule of interest as an internal standard, experimentation with different types of chromatographic techniques, development of methods for preliminary sample purification and/or derivatization, and development of well-characterized control and test specimens to demonstrate specificity and reproducibility.
Once identified, the biomarker must be validated. The process of validation should address the following questions: how well does the candidate biomarker correlate with tobacco and nicotine delivery product use and exposure? If the biomarker does correlate well, is it the best biomarker for exposure to a specific product type or group of toxins, or are there better candidates? Can the assay be improved? Does the assay generate reliable and valid results in samples from different populations? Can cut-point values be determined to distinguish among populations with different use and exposure patterns? As a scientific consensus develops around a particular detection method, the details are disseminated to other research laboratories, which then test the same samples using the same or similar assays and compare findings—so-called interlaboratory testing (37). Concurrence of findings suggests that the assay is valid and reproducible; discordant findings require additional research and investigation. Moreover, interlaboratory validation assures that unavoidable, minor differences in equipment and methods across laboratories do not affect the quality of the results (37).
Currently, we are in the discovery and identification stage for biomarkers of e-cigarettes, little cigars, cigarillos, and water pipes. Validated biomarkers of use and exposure to these tobacco products are urgently needed. Cut-point values distinguishing between active cigarette use and SHS exposure have been identified for serum cotinine (21) and for NNAL (96) (see Table 5). The high-sensitivity LC-MS/MS assay for urinary cotinine is in interlaboratory testing, and the high-sensitivity LC-MS/MS assay for blood cotinine has completed interlaboratory testing and been published (37). The candidate biomarker, NNAL, appears to be the optimal biomarker for TSNA exposure, and many laboratories are using the assay validated for distinguishing between active smoking and SHS exposure to test samples from populations with different product use and exposure patterns. Several laboratories are in the early stages of validating biomarkers of VOC and PAH exposure (debating the best biomarkers, refining methods, and comparing results from different sample sets).
The measurement of nicotine exposure in smokers can be costly (Table 6). Laboratories with limited throughput may be able to analyze these samples at a lower cost but often have longer turnaround times, slowing study progress. Studies with large sample sets routinely face long turnaround times. In addition, researchers often need to measure several biomarkers to create a detailed profile reflecting exposure to new, emerging, and traditional tobacco products. For example, to ascertain whether a study participant uses nicotine-containing e-cigarettes or nicotine-replacement products, but does not use combustible tobacco products, researchers must measure cotinine, NNAL, and/or VOC or PAH metabolites in a single sample. This multivariate analysis can be cost prohibitive but is necessary for an accurate exposure and outcome profile.
The equipment needed for these biomarker analyses is highly sensitive and expensive. Additionally, it must often be housed in a controlled environment and connected to uninterrupted and consistent electrical power. High-quality GC-MS/MS, LC-MS/MS, and ultrahigh performance LC-MS instruments cost hundreds of thousands of dollars and need expensive technical support to install, calibrate, and maintain. Newer instruments offer greater sensitivity and throughput than older systems, but few laboratories have the resources required to acquire and maintain a critical core of instrumentation and staff.
The expansion of access to tobacco and nicotine delivery system biomarker tests requires additional work across the entire continuum of biomarker development. For example, research is needed to investigate potential biomarkers of e-cigarette use, including flavors and the pyrolysis and metabolism products of propylene glycol and glycerol (all typical e-liquid constituents). Laboratories now validating assays for biomarkers, such as VOC and PAH metabolites, need access to more specimens to identify specific biomarkers for combustible products. Interlaboratory NNAL validation testing is also a high priority to distinguish between light and infrequent smoking and SHS exposure—an important distinction as product use patterns change.
Expanded biomarker testing will also expand exposure data sets that improve our ability to link biomarker measurements with the use of specific tobacco products and subsequent health effects, which is critical to inform public health policymaking. To further this effort, we recommend five strategies to assess better exposure to new and emerging tobacco and nicotine delivery products.
Leverage advanced biomarker research.
Advanced approaches will yield significant improvements in test sensitivity, selectivity, and throughput—three crucial quality metrics. Advanced instrumentation and innovative sample preparation can improve sensitivity and selectivity. Increased automation in sample prep and data evaluation can drive down costs while increasing throughput, making large population studies feasible to characterize population harm.
Build capacity for established biomarker methods.
We must build capacity for established biomarker methods by disseminating information about validated assays to more laboratories, including commercial and hospital/clinical laboratories. This strategy requires additional training and regular interlaboratory testing. With the increase in the number of facilities able to perform biomarker assays, we can increase access and enhance collaboration among researchers toward the common goal of assay improvement.
Educate researchers and policymakers.
We must educate researchers and policymakers about the importance of the individual and quantitative exposure data provided by biomonitoring. The generation of such data requires additional resources but is crucial to attribute health effects accurately in populations that are using new tobacco and nicotine delivery products and potentially using more than one product or subject to other recreational or occupational exposures . Increased biomonitoring will help researchers to characterize better exposure and subsequent health effects caused by new and emerging tobacco and nicotine delivery products.
Establish biospecimen repositories and data archives.
Establishment of biospecimen repositories and data archives is necessary to support assay development and validation. Large numbers of well-characterized samples are needed to establish biomarker cut-point values and to support optimization of assay protocols. The proposed biospecimen repositories could include samples from well-characterized small studies and from large existing studies, such as NHANES and the Population Assessment of Tobacco and Health study.
Develop and validate OTC kits.
OTC kits need to be developed and validated so that that they can detect more reliably cotinine at the current biological cut-point values, distinguishing smokers from nonsmokers based on both urine and saliva specimens. Such tests might be useful in clinical settings to screen patients for tobacco exposure or to assess response to smoking cessation therapy. Furthermore, they could be administered in research settings to assess rapidly eligibility for experimental studies by determining smoking status. As noted in Over-the-Counter Cotinine Tests, there are commercial products that claim levels of sensitivity that meet these needs, but they are not yet validated by use and may need further development to be effective. Affordable, effective OTC kits will vastly increase the pace of research on new and emerging tobacco products.
Biomarkers of exposure to tobacco and nicotine delivery products are critical tools for identifying and quantifying the health effects of tobacco products. The absence of unique biomarkers for new and emerging tobacco products, especially e-cigarettes, is an urgent problem. The implementation of these five strategies will dramatically improve the characterization of harmful and addictive exposures related to tobacco and nicotine delivery products, as well as characterize the resulting health effects of these products on individuals and populations.
DISCLAIMERS
The findings and conclusions in this report are solely those of the authors and do not represent the official position of the Centers for Disease Control and Prevention, the U.S. Food and Drug Administration, and the U.S. National Institutes of Health (including NCI, NIDA, NIEHS, and NHLBI).
GRANTS
This work was supported and provided, in part, by grants from the National Cancer Institute (NCI) of the U.S. National Institutes of Health (NIH) and the Center for Tobacco Products (CTP) of the U.S. Food and Drug Administration (FDA): P50CA180890 (to S. F. Schick, P. Jacob 3rd, G. St. Helen, and N. L. Benowitz); National Institute on Drug Abuse (NIDA) of the NIH and the CTP of the FDA: P50DA036105 (to N. A. Saliba, A. El Hellani, and J. Foulds), P50DA036151 (to P. Jatlow), and P50DA036107 (to J. Foulds); National Heart Lung and Blood Institute (NHLBI) of the NIH and the CTP of the FDA: P50HL120100 (to A. Ghosh, J. C. Gomez, J. R. Martin, R. Tarran, and C. M. Doerschuk) and P50HL120163 (to S. Srivastava, P. K Lorkiewicz, and A. Bhatnagar); NIDA: P30DA012393 (to P. Jacob 3rd, G. St. Helen, and N. L. Benowitz) and R21DA038775 (to J. Foulds); National Institute of Environmental Health Sciences (NIEHS): P30ES013508 and P42ES023720 (both to C. Mesaros and I. A. Blair); NCI: CA81301 (to S. S. Hecht) and U54CA189222; and NHLBI: R01HL120746 (to S. Srivastava and P. K. Lorkiewicz).
DISCLOSURES
N. L. Benowitz is a consultant to pharmaceutical companies that market smoking-cessation medications and has been a paid expert witness in litigation against tobacco companies. J. Foulds does paid consulting for pharmaceutical companies making smoking cessation medicines (e.g., Pfizer) and has received a research grant from Pfizer, but there is no conflict between that work and the content of this biomarkers paper.
AUTHOR CONTRIBUTIONS
R.S.P. conceived and designed research; J.T.B. and R.S.P. performed experiments; J.T.B. and R.S.P. analyzed data; J.T.B. and R.S.P. interpreted results of experiments. S.F.S., B.C.B., N.A.S., A.E.H., R.S.P., C.M., G.S.H., and C.M.D. prepared figures; S.F.S., B.C.B., P. Jacob, N.A.S., J.T.B., A.E.H., P. Jatlow, R.S.P., L.W., J.F., A.G., S.S.H., J.C.G., J.R.M., C.M., S.S., G.S.H., R.T., P.K.L., I.A.B., H.L.K., C.M.D., and N.L.B. drafted manuscript; S.F.S., B.C.B., P. Jacob, L.W., S.S.H., R.T., H.L.K., N.L.B., and A.B. edited and revised manuscript; S.F.S. and A.B. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Norma Minkoff (Center for Evaluation and Coordination of Training and Research in Tobacco Regulatory Science, Westat) for organizing the collaborative writing process and creating the bibliography and Margaret Peng for assistance in reviewing material on PAHs, and Mary Halstead for providing light and scanning electron microscope images used in this study.
REFERENCES
- 1. Adams SV, Newcomb PA. Cadmium blood and urine concentrations as measures of exposure: NHANES 1999-2010. J Expo Sci Environ Epidemiol 24: 163–170, 2014. doi: 10.1038/jes.2013.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adamu CA, Bell PF, Mulchi C, Chaney R. Residual metal concentrations in soils and leaf accumulations in tobacco a decade following farmland application of municipal sludge. Environ Pollut 56: 113–126, 1989. doi: 10.1016/0269-7491(89)90170-X. [DOI] [PubMed] [Google Scholar]
- 3.Agaku IT, King BA. Validation of self-reported smokeless tobacco use by measurement of serum cotinine concentration among US adults. Am J Epidemiol 180: 749–754, 2014. doi: 10.1093/aje/kwu182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alonso M, Castellanos M, Sanchez JM. Evaluation of potential breath biomarkers for active smoking: assessment of smoking habits. Anal Bioanal Chem 396: 2987–2995, 2010. doi: 10.1007/s00216-010-3524-z. [DOI] [PubMed] [Google Scholar]
- 7.Alshaarawy O, Elbaz HA, Andrew ME. The association of urinary polycyclic aromatic hydrocarbon biomarkers and cardiovascular disease in the US population. Environ Int 89-90: 174–178, 2016. doi: 10.1016/j.envint.2016.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alwis KU, Blount BC, Britt AS, Patel D, Ashley DL. Simultaneous analysis of 28 urinary VOC metabolites using ultra high performance liquid chromatography coupled with electrospray ionization tandem mass spectrometry (UPLC-ESI/MSMS). Anal Chim Acta 750: 152–160, 2012. doi: 10.1016/j.aca.2012.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ambrose BK, Day HR, Rostron B, Conway KP, Borek N, Hyland A, Villanti AC. Flavored tobacco product use among US youth aged 12–17 years, 2013–2014. JAMA 314: 1871–1873, 2015. doi: 10.1001/jama.2015.13802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anderson MM, Hazen SL, Hsu FF, Heinecke JW. Human neutrophils employ the myeloperoxidase-hydrogen peroxide-chloride system to convert hydroxy-amino acids into glycolaldehyde, 2-hydroxypropanal, and acrolein. A mechanism for the generation of highly reactive alpha-hydroxy and alpha,beta-unsaturated aldehydes by phagocytes at sites of inflammation. J Clin Invest 99: 424–432, 1997. doi: 10.1172/JCI119176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andreoli R, Manini P, Corradi M, Mutti A, Niessen WM. Determination of patterns of biologically relevant aldehydes in exhaled breath condensate of healthy subjects by liquid chromatography/atmospheric chemical ionization tandem mass spectrometry. Rapid Commun Mass Spectrom 17: 637–645, 2003. doi: 10.1002/rcm.960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Asgharian B, Price OT, Yurteri CU, Dickens C, McAughey J. Component-specific, cigarette particle deposition modeling in the human respiratory tract. Inhal Toxicol 26: 36–47, 2014. doi: 10.3109/08958378.2013.851305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ashley DL, Bonin MA, Cardinali FL, McCraw JM, Wooten JV. Measurement of volatile organic compounds in human blood. Environ Health Perspect 104, Suppl 5: 871–877, 1996. doi: 10.1289/ehp.96104s5871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Avila-Tang E, Al-Delaimy WK, Ashley DL, Benowitz N, Bernert JT, Kim S, Samet JM, Hecht SS. Assessing secondhand smoke using biological markers. Tob Control 22: 164–171, 2013. doi: 10.1136/tobaccocontrol-2011-050298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bache CA, Lisk DJ, Doss GJ, Hoffmann D, Adams JD. Cadmium and nickel in mainstream particulates of cigarettes containing tobacco grown on a low-cadmium soil-sludge mixture. J Toxicol Environ Health 16: 547–552, 1985. doi: 10.1080/15287398509530762. [DOI] [PubMed] [Google Scholar]
- 16.Bache CA, Lisk DJ, Shane BS, Hoffmann D, Adams JD. Effectiveness of cigarette filter tips for reducing cadmium in relation to other mainstream smoke constituents. Drug Chem Toxicol 10: 189–193, 1987. doi: 10.3109/01480548709042981. [DOI] [PubMed] [Google Scholar]
- 17.Baker TB, Piper ME, Stein JH, Smith SS, Bolt DM, Fraser DL, Fiore MC. Effects of Nicotine patch vs varenicline vs combination nicotine replacement therapy on smoking cessation at 26 weeks: a randomized clinical trial. JAMA 315: 371–379, 2016. doi: 10.1001/jama.2015.19284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bartolomé M, Ramos JJ, Cutanda F, Huetos O, Esteban M, Ruiz-Moraga M, Calvo E, Pérez-Gómez B, González O, Castaño A; BIOAMBIENT.ES . Urinary polycyclic aromatic hydrocarbon metabolites levels in a representative sample of the Spanish adult population: The BIOAMBIENT.ES project. Chemosphere 135: 436–446, 2015. doi: 10.1016/j.chemosphere.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 19.Bekki K, Uchiyama S, Ohta K, Inaba Y, Nakagome H, Kunugita N. Carbonyl compounds generated from electronic cigarettes. Int J Environ Res Public Health 11: 11192–11200, 2014. doi: 10.3390/ijerph111111192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benowitz NL. Drug therapy. Pharmacologic aspects of cigarette smoking and nicotine addiction. N Engl J Med 319: 1318–1330, 1988. doi: 10.1056/NEJM198811173192005. [DOI] [PubMed] [Google Scholar]
- 21.Benowitz NL, Bernert JT, Caraballo RS, Holiday DB, Wang J. Optimal serum cotinine levels for distinguishing cigarette smokers and nonsmokers within different racial/ethnic groups in the United States between 1999 and 2004. Am J Epidemiol 169: 236–248, 2009. doi: 10.1093/aje/kwn301. [DOI] [PubMed] [Google Scholar]
- 22.Benowitz NL, Dains KM, Dempsey D, Herrera B, Yu L, Jacob P 3rd. Urine nicotine metabolite concentrations in relation to plasma cotinine during low-level nicotine exposure. Nicotine Tob Res 11: 954–960, 2009. doi: 10.1093/ntr/ntp092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Benowitz NL, Dains KM, Dempsey D, Yu L, Jacob P 3rd. Estimation of nicotine dose after low-level exposure using plasma and urine nicotine metabolites. Cancer Epidemiol Biomarkers Prev 19: 1160–1166, 2010. doi: 10.1158/1055-9965.EPI-09-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Benowitz NL, Gan Q, Goniewicz ML, Lu W, Xu J, Li X, Jacob P 3rd, Glantz S. Different profiles of carcinogen exposure in Chinese compared with US cigarette smokers. Tob Control 24: e258–e263, 2015. doi: 10.1136/tobaccocontrol-2014-051945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benowitz NL, Hukkanen J, Jacob P 3rd. Nicotine chemistry, metabolism, kinetics and biomarkers. Handb Exp Pharmacol 192: 29–60, 2009. doi: 10.1007/978-3-540-69248-5_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25a.Benowitz NL, Jacob P 3rd, Ahijevych K, Jarvis MJ, Hall S, LeHouezec J, Hansson A, Lichtenstein E, Henningfield J, Tsoh J, Hurt RD, Velicer W; SRNT Subcommittee on Biochemical Verification . Biochemical verification of tobacco use and cessation. Nicotine Tob Res 4: 149–159, 2002. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- 26.Benowitz NL, Jacob P 3rd. Daily intake of nicotine during cigarette smoking. Clin Pharmacol Ther 35: 499–504, 1984. doi: 10.1038/clpt.1984.67. [DOI] [PubMed] [Google Scholar]
- 27.Benowitz NL, Jacob P 3rd. Metabolism of nicotine to cotinine studied by a dual stable isotope method. Clin Pharmacol Ther 56: 483–493, 1994. doi: 10.1038/clpt.1994.169. [DOI] [PubMed] [Google Scholar]
- 28.Benowitz NL, Jacob P 3rd. Nicotine and cotinine elimination pharmacokinetics in smokers and nonsmokers. Clin Pharmacol Ther 53: 316–323, 1993. doi: 10.1038/clpt.1993.27. [DOI] [PubMed] [Google Scholar]
- 29.Benowitz NL, Jacob P 3rd, Fong I, Gupta S. Nicotine metabolic profile in man: comparison of cigarette smoking and transdermal nicotine. J Pharmacol Exp Ther 268: 296–303, 1994. [PubMed] [Google Scholar]
- 30.Benowitz NL, Jacob P 3rd, Yu L. Daily use of smokeless tobacco: systemic effects. Ann Intern Med 111: 112–116, 1989. doi: 10.7326/0003-4819-111-2-112. [DOI] [PubMed] [Google Scholar]
- 31.Benowitz NL, Jacob P 3rd, Yu L, Talcott R, Hall S, Jones RT. Reduced tar, nicotine, and carbon monoxide exposure while smoking ultralow- but not low-yield cigarettes. JAMA 256: 241–246, 1986. doi: 10.1001/jama.1986.03380020103032. [DOI] [PubMed] [Google Scholar]
- 32.Benowitz NL, Kuyt F, Jacob P 3rd. Circadian blood nicotine concentrations during cigarette smoking. Clin Pharmacol Ther 32: 758–764, 1982. doi: 10.1038/clpt.1982.233. [DOI] [PubMed] [Google Scholar]
- 33.Benowitz NL, Lessov-Schlaggar CN, Swan GE, Jacob P 3rd. Female sex and oral contraceptive use accelerate nicotine metabolism. Clin Pharmacol Ther 79: 480–488, 2006. doi: 10.1016/j.clpt.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 34.Benowitz NL, Porchet H, Sheiner L, Jacob P 3rd. Nicotine absorption and cardiovascular effects with smokeless tobacco use: comparison with cigarettes and nicotine gum. Clin Pharmacol Ther 44: 23–28, 1988. doi: 10.1038/clpt.1988.107. [DOI] [PubMed] [Google Scholar]
- 35.Benowitz NL, St Helen G, Dempsey DA, Jacob P 3rd, Tyndale RF. Disposition kinetics and metabolism of nicotine and cotinine in African American smokers: impact of CYP2A6 genetic variation and enzymatic activity. Pharmacogenet Genomics 26: 340–350, 2016. doi: 10.1097/FPC.0000000000000222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berg CJ. Preferred flavors and reasons for e-cigarette use and discontinued use among never, current, and former smokers. Int J Public Health 61: 225–236, 2016. doi: 10.1007/s00038-015-0764-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bernert JT, Jacob P 3rd, Holiday DB, Benowitz NL, Sosnoff CS, Doig MV, Feyerabend C, Aldous KM, Sharifi M, Kellogg MD, Langman LJ. Interlaboratory comparability of serum cotinine measurements at smoker and nonsmoker concentration levels: a round-robin study. Nicotine Tob Res 11: 1458–1466, 2009. doi: 10.1093/ntr/ntp161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37a.Bernert JT, Pirkle JL, Xia Y, Jain RB, Ashley DL, Sampson EJ. Urine concentrations of a tobacco-specific nitrosamine carcinogen in the U.S. population from secondhand smoke exposure. Cancer Epidemiol Biomarkers Prev 19: 2969–2977, 2010. doi: 10.1158/1055-9965.EPI-10-0711. [DOI] [PubMed] [Google Scholar]
- 38.Bernert JT Jr, McGuffey JE, Morrison MA, Pirkle JL. Comparison of serum and salivary cotinine measurements by a sensitive high-performance liquid chromatography-tandem mass spectrometry method as an indicator of exposure to tobacco smoke among smokers and nonsmokers. J Anal Toxicol 24: 333–339, 2000. doi: 10.1093/jat/24.5.333. [DOI] [PubMed] [Google Scholar]
- 39.Bernert JT Jr, Turner WE, Pirkle JL, Sosnoff CS, Akins JR, Waldrep MK, Ann Q, Covey TR, Whitfield WE, Gunter EW, Miller BB, Patterson DG Jr, Needham LL, Hannon WH, Sampson EJ. Development and validation of sensitive method for determination of serum cotinine in smokers and nonsmokers by liquid chromatography/atmospheric pressure ionization tandem mass spectrometry. Clin Chem 43: 2281–2291, 1997. [PubMed] [Google Scholar]
- 41.Bhat SH, Gelhaus SL, Mesaros C, Vachani A, Blair IA. A new liquid chromatography/mass spectrometry method for 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) in urine. Rapid Commun Mass Spectrom 25: 115–121, 2011. doi: 10.1002/rcm.4824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bhatnagar A, Whitsel LP, Ribisl KM, Bullen C, Chaloupka F, Piano MR, Robertson RM, McAuley T, Goff D, Benowitz N; American Heart Association Advocacy Coordinating Committee, Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology, and Council on Quality of Care and Outcomes Research . Electronic cigarettes: a policy statement from the American Heart Association. Circulation 130: 1418–1436, 2014. doi: 10.1161/CIR.0000000000000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Blount BC, Kobelski RJ, McElprang DO, Ashley DL, Morrow JC, Chambers DM, Cardinali FL. Quantification of 31 volatile organic compounds in whole blood using solid-phase microextraction and gas chromatography-mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 832: 292–301, 2006. doi: 10.1016/j.jchromb.2006.01.019. [DOI] [PubMed] [Google Scholar]
- 44.Boffetta P, Hecht S, Gray N, Gupta P, Straif K. Smokeless tobacco and cancer. Lancet Oncol 9: 667–675, 2008. doi: 10.1016/S1470-2045(08)70173-6. [DOI] [PubMed] [Google Scholar]
- 45.Breland A, Soule E, Lopez A, Ramoa C, El-Hellani A, Eissenberg T. Electronic cigarettes: what are they and what do they do? Ann NY Acad Sci, 1394: 5–30, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brunnemann KD, Cox JE, Hoffmann D. Analysis of tobacco-specific N-nitrosamines in indoor air. Carcinogenesis 13: 2415–2418, 1992. doi: 10.1093/carcin/13.12.2415. [DOI] [PubMed] [Google Scholar]
- 47.Byrd GD, Davis RA, Ogden MW. A rapid LC-MS-MS method for the determination of nicotine and cotinine in serum and saliva samples from smokers: validation and comparison with a radioimmunoassay method. J Chromatogr Sci 43: 133–140, 2005. doi: 10.1093/chromsci/43.3.133. [DOI] [PubMed] [Google Scholar]
- 48.Calafat AM, Barr DB, Pirkle JL, Ashley DL. Reference range concentrations of N-acetyl-S-(2-hydroxyethyl)-L-cysteine, a common metabolite of several volatile organic compounds, in the urine of adults in the United States. J Expo Anal Environ Epidemiol 9: 336–342, 1999. doi: 10.1038/sj.jea.7500032. [DOI] [PubMed] [Google Scholar]
- 49.Carmella SG, Akerkar S, Hecht SS. Metabolites of the tobacco-specific nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in smokers’ urine. Cancer Res 53: 721–724, 1993. [PubMed] [Google Scholar]
- 50.Carmella SG, Chen M, Han S, Briggs A, Jensen J, Hatsukami DK, Hecht SS. Effects of smoking cessation on eight urinary tobacco carcinogen and toxicant biomarkers. Chem Res Toxicol 22: 734–741, 2009. doi: 10.1021/tx800479s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Carmella SG, Chen M, Yagi H, Jerina DM, Hecht SS. Analysis of phenanthrols in human urine by gas chromatography-mass spectrometry: potential use in carcinogen metabolite phenotyping. Cancer Epidemiol Biomarkers Prev 13: 2167–2174, 2004. [PubMed] [Google Scholar]
- 52.Carmella SG, Le KA, Hecht SS. Improved method for determination of 1-hydroxypyrene in human urine. Cancer Epidemiol Biomarkers Prev 13: 1261–1264, 2004. [PubMed] [Google Scholar]
- 53.Carmella SG, Ming X, Olvera N, Brookmeyer C, Yoder A, Hecht SS. High throughput liquid and gas chromatography-tandem mass spectrometry assays for tobacco-specific nitrosamine and polycyclic aromatic hydrocarbon metabolites associated with lung cancer in smokers. Chem Res Toxicol 26: 1209–1217, 2013. doi: 10.1021/tx400121n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cary P. The Marijuana Detection Window: Determining the Length of Time Cannabinoids Will Remain Detectable in Urine Following Smoking: A Critical Review of Relevant Research and Cannabinoid Detection Guidance for Drug Courts. In: Drug Court Review. Alexandria, VA: National Drug Court Institute, 2005, vol. V, p. 23–58. [Google Scholar]
- 56.Chen G, Blevins-Primeau AS, Dellinger RW, Muscat JE, Lazarus P. Glucuronidation of nicotine and cotinine by UGT2B10: loss of function by the UGT2B10 Codon 67 (Asp>Tyr) polymorphism. Cancer Res 67: 9024–9029, 2007. doi: 10.1158/0008-5472.CAN-07-2245. [DOI] [PubMed] [Google Scholar]
- 57.Cheng S, Lyass A, Massaro JM, O’Connor GT, Keaney JF Jr, Vasan RS. Exhaled carbon monoxide and risk of metabolic syndrome and cardiovascular disease in the community. Circulation 122: 1470–1477, 2010. doi: 10.1161/CIRCULATIONAHA.110.941013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cheng T. Chemical evaluation of electronic cigarettes. Tob Control 23, Suppl 2: ii11–ii17, 2014. doi: 10.1136/tobaccocontrol-2013-051482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chetiyanukornkul T, Toriba A, Kizu R, Makino T, Nakazawa H, Hayakaw K. Determination of 1-hydroxypyrene in human urine by high-performance liquid chromatography with fluorescence detection using a deuterated internal standard. J Chromatogr A 961: 107–112, 2002. doi: 10.1016/S0021-9673(02)00363-1. [DOI] [PubMed] [Google Scholar]
- 60.Cho HJ, Chang CC, Fan W. Base free, one-pot synthesis of lactic acid from glycerol using a bifunctional Pt/Sn-MFI catalyst. Green Chem 16: 3428–3433, 2014. doi: 10.1039/c4gc00723a. [DOI] [Google Scholar]
- 61.Christopher MM, Eckfeldt JH, Eaton JW. Propylene glycol ingestion causes d-lactic acidosis. Lab Invest 62: 114–118, 1990. [PubMed] [Google Scholar]
- 62.Church TR, Anderson KE, Le C, Zhang Y, Kampa DM, Benoit AR, Yoder AR, Carmella SG, Hecht SS. Temporal stability of urinary and plasma biomarkers of tobacco smoke exposure among cigarette smokers. Biomarkers 15: 345–352, 2010. doi: 10.3109/13547501003753881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Clemente Jiménez ML, Pérez-Trullén A, Rubio Aranda E, Marrón Tundidor R, Herrero Labarga I. [Correlation between carbon-monoxide levels in exhaled air and nicotine-dependence measurements systems (DSM-IV, Fagerström test and ARU-SMQ-9) in adolescent smokers]. Med Clin (Barc) 121: 89–94, 2003. [PubMed] [Google Scholar]
- 64.Clunes LA, Bridges A, Alexis N, Tarran R. In vivo versus in vitro airway surface liquid nicotine levels following cigarette smoke exposure. J Anal Toxicol 32: 201–207, 2008. doi: 10.1093/jat/32.3.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Clunes LA, Davies CM, Coakley RD, Aleksandrov AA, Henderson AG, Zeman KL, Worthington EN, Gentzsch M, Kreda SM, Cholon D, Bennett WD, Riordan JR, Boucher RC, Tarran R. Cigarette smoke exposure induces CFTR internalization and insolubility, leading to airway surface liquid dehydration. FASEB J 26: 533–545, 2012. doi: 10.1096/fj.11-192377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cone EJ, Bigelow GE, Herrmann ES, Mitchell JM, LoDico C, Flegel R, Vandrey R. Non-smoker exposure to secondhand cannabis smoke. I. Urine screening and confirmation results. J Anal Toxicol 39: 1–12, 2015. doi: 10.1093/jat/bku116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Connor Gorber S, Schofield-Hurwitz S, Hardt J, Levasseur G, Tremblay M. The accuracy of self-reported smoking: a systematic review of the relationship between self-reported and cotinine-assessed smoking status. Nicotine Tob Res 11: 12–24, 2009. doi: 10.1093/ntr/ntn010. [DOI] [PubMed] [Google Scholar]
- 68.Corey CG, Ambrose BK, Apelberg BJ, King BA. Flavored tobacco product use among middle and high school students—United States, 2014. MMWR Morb Mortal Wkly Rep 64: 1066–1070, 2015. doi: 10.15585/mmwr.mm6438a2. [DOI] [PubMed] [Google Scholar]
- 69.Courtney TD, Nikolakis V, Mpourmpakis G, Chen JG, Vlachos DG. Liquid-phase dehydration of propylene glycol using solid-acid catalysts. Appl Catal A Gen 449: 59–68, 2012. doi: 10.1016/j.apcata.2012.09.034. [DOI] [Google Scholar]
- 70.Cropsey KL, Trent LR, Clark CB, Stevens EN, Lahti AC, Hendricks PS. How low should you go? Determining the optimal cutoff for exhaled carbon monoxide to confirm smoking abstinence when using cotinine as reference. Nicotine Tob Res 16: 1348–1355, 2014. doi: 10.1093/ntr/ntu085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Crouch DJ, Hersch RK, Cook RF, Frank JF, Walsh JM. A field evaluation of five on-site drug-testing devices. J Anal Toxicol 26: 493–499, 2002. doi: 10.1093/jat/26.7.493. [DOI] [PubMed] [Google Scholar]
- 72.Curvall M, Kazemi-Vala E, Enzell CR. Simultaneous determination of nicotine and cotinine in plasma using capillary column gas chromatography with nitrogen-sensitive detection. J Chromatogr A 232: 283–293, 1982. doi: 10.1016/S0378-4347(00)84168-7. [DOI] [PubMed] [Google Scholar]
- 73.Daisey JM, Mahanama KR, Hodgson AT. Toxic volatile organic compounds in simulated environmental tobacco smoke: emission factors for exposure assessment. J Expo Anal Environ Epidemiol 8: 313–334, 1998. [PubMed] [Google Scholar]
- 74.Darrall KG, Figgins JA, Brown RD, Phillips GF. Determination of benzene and associated volatile compounds in mainstream cigarette smoke. Analyst (Lond) 123: 1095–1101, 1998. doi: 10.1039/a708664d. [DOI] [PubMed] [Google Scholar]
- 75.Dawkins L, Kimber C, Puwanesarasa Y, Soar K. First- versus second-generation electronic cigarettes: predictors of choice and effects on urge to smoke and withdrawal symptoms. Addiction 110: 669–677, 2015. doi: 10.1111/add.12807. [DOI] [PubMed] [Google Scholar]
- 75a.Department of Health and Human Services, Substance Abuse and Mental Health Services Administration Mandatory guidelines for federal workplace drug testing programs. Fed Regist 73: 71858–71907, 2008. [Google Scholar]
- 76.Dhale AD, Myrant LK, Chopade SP, Jackson JE, Miller DJ. Propylene glycol and ethylene glycol recovery from aqueous solution via reactive distillation. Chem Eng Sci 59: 2881–2890, 2004. doi: 10.1016/j.ces.2004.02.018. [DOI] [Google Scholar]
- 77.Ding YS, Blount BC, Valentin-Blasini L, Applewhite HS, Xia Y, Watson CH, Ashley DL. Simultaneous determination of six mercapturic acid metabolites of volatile organic compounds in human urine. Chem Res Toxicol 22: 1018–1025, 2009. doi: 10.1021/tx800468w. [DOI] [PubMed] [Google Scholar]
- 78.Dong JZ, Moldoveanu SC. Gas chromatography-mass spectrometry of carbonyl compounds in cigarette mainstream smoke after derivatization with 2,4-dinitrophenylhydrazine. J Chromatogr A 1027: 25–35, 2004. doi: 10.1016/j.chroma.2003.08.104. [DOI] [PubMed] [Google Scholar]
- 79.Einig T, Dunemann L, Dehnen W. Sensitive gas chromatographic method for determination of mercapturic acids in human urine. J Chromatogr B Biomed Appl 687: 379–385, 1996. doi: 10.1016/S0378-4347(96)00259-9. [DOI] [PubMed] [Google Scholar]
- 80.El-Hellani A Sr, Salman R, El-Hage R, Talih S, Malek N, Baalbaki R, Karaoghlanian N, Nakkash R, Shihadeh A, Saliba NA. Nicotine and carbonyl emissions from popular electronic cigarette products: correlation to liquid composition and design characteristics. Nicotine Tob Res. First published October 25, 2016; doi: 10.1093/ntr/ntw280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.England L, Lisko J, Pappas R. Important considerations for providers regarding the use of electronic cigarettes. Int J Respir Pulm Med 2: 035e, 2015. [Google Scholar]
- 82.Etter JF. A longitudinal study of cotinine in long-term daily users of e-cigarettes. Drug Alcohol Depend 160: 218–221, 2016. doi: 10.1016/j.drugalcdep.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 83.Fan X, Lam M, Mathers D, Mabury S, Witter A, Klinger D. Quantitative determination of nicotine and cotinine in urine and sputum using a combined SPME-GC/MS method. J Chem Educ 79: 1257, 2002. doi: 10.1021/ed079p1257. [DOI] [Google Scholar]
- 84.Farsalinos KE, Gillman G, Poulas K, Voudris V. Tobacco-specific nitrosamines in electronic cigarettes: comparison between liquid and aerosol levels. Int J Environ Res Public Health 12: 9046–9053, 2015. doi: 10.3390/ijerph120809046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Farsalinos KE, Spyrou A, Tsimopoulou K, Stefopoulos C, Romagna G, Voudris V. Nicotine absorption from electronic cigarette use: comparison between first and new-generation devices. Sci Rep 4: 4133, 2014. doi: 10.1038/srep04133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Feng S, Kapur S, Sarkar M, Muhammad R, Mendes P, Newland K, Roethig HJ. Respiratory retention of nicotine and urinary excretion of nicotine and its five major metabolites in adult male smokers. Toxicol Lett 173: 101–106, 2007. doi: 10.1016/j.toxlet.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 87.Feng S, Roethig HJ, Liang Q, Kinser R, Jin Y, Scherer G, Urban M, Engl J, Riedel K. Evaluation of urinary 1-hydroxypyrene, S-phenylmercapturic acid, trans,trans-muconic acid, 3-methyladenine, 3-ethyladenine, 8-hydroxy-2′-deoxyguanosine and thioethers as biomarkers of exposure to cigarette smoke. Biomarkers 11: 28–52, 2006. doi: 10.1080/13547500500399730. [DOI] [PubMed] [Google Scholar]
- 88.Fischer S, Spiegelhalder B, Preussmann R. Preformed tobacco-specific nitrosamines in tobacco--role of nitrate and influence of tobacco type. Carcinogenesis 10: 1511–1517, 1989. doi: 10.1093/carcin/10.8.1511. [DOI] [PubMed] [Google Scholar]
- 89.Fisher MT, Bennett CB, Hayes A, Kargalioglu Y, Knox BL, Xu D, Muhammad-Kah R, Gaworski CL. Sources of and technical approaches for the abatement of tobacco specific nitrosamine formation in moist smokeless tobacco products. Food Chem Toxicol 50: 942–948, 2012. doi: 10.1016/j.fct.2011.11.035. [DOI] [PubMed] [Google Scholar]
- 90.Florescu A, Ferrence R, Einarson T, Selby P, Soldin O, Koren G. Methods for quantification of exposure to cigarette smoking and environmental tobacco smoke: focus on developmental toxicology. Ther Drug Monit 31: 14–30, 2009. doi: 10.1097/FTD.0b013e3181957a3b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Friberg L, Elinder C, Kjellstrom T, Nordberg G. Cadmium and Health: A Toxicological and Epidemiological Appraisal: Effects and Response. Boca Raton, FL: CRC, 1985, vol II. [Google Scholar]
- 92.Funck-Brentano C, Raphaël M, Lafontaine M, Arnould JP, Verstuyft C, Lebot M, Costagliola D, Roussel R. Effects of type of smoking (pipe, cigars or cigarettes) on biological indices of tobacco exposure and toxicity. Lung Cancer 54: 11–18, 2006. doi: 10.1016/j.lungcan.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 93.Geiss O, Bianchi I, Barrero-Moreno J. Correlation of volatile carbonyl yields emitted by e-cigarettes with the temperature of the heating coil and the perceived sensorial quality of the generated vapours. Int J Hyg Environ Health 219: 268–277, 2016. doi: 10.1016/j.ijheh.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 94.Ghilarducci DP, Tjeerdema RS. Fate and effects of acrolein. Rev Environ Contam Toxicol 144: 95–146, 1995. [DOI] [PubMed] [Google Scholar]
- 95.Gillman IG, Kistler KA, Stewart EW, Paolantonio AR. Effect of variable power levels on the yield of total aerosol mass and formation of aldehydes in e-cigarette aerosols. Regul Toxicol Pharmacol 75: 58–65, 2016. doi: 10.1016/j.yrtph.2015.12.019. [DOI] [PubMed] [Google Scholar]
- 96.Goniewicz ML, Eisner MD, Lazcano-Ponce E, Zielinska-Danch W, Koszowski B, Sobczak A, Havel C, Jacob P, Benowitz NL. Comparison of urine cotinine and the tobacco-specific nitrosamine metabolite 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) and their ratio to discriminate active from passive smoking. Nicotine Tob Res 13: 202–208, 2011. doi: 10.1093/ntr/ntq237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Goniewicz ML, Gawron M, Smith DM, Peng M, Jacob P 3rd, Benowitz NL. Exposure to nicotine and selected toxicants in cigarette smokers who switched to electronic cigarettes: a longitudinal within-subjects observational study. Nicotine Tob Res 19: 160–167, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Goniewicz ML, Havel CM, Peng MW, Jacob P 3rd, Dempsey D, Yu L, Zielinska-Danch W, Koszowski B, Czogala J, Sobczak A, Benowitz NL. Elimination kinetics of the tobacco-specific biomarker and lung carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol. Cancer Epidemiol Biomarkers Prev 18: 3421–3425, 2009. doi: 10.1158/1055-9965.EPI-09-0874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Goniewicz ML, Knysak J, Gawron M, Kosmider L, Sobczak A, Kurek J, Prokopowicz A, Jablonska-Czapla M, Rosik-Dulewska C, Havel C, Jacob P 3rd, Benowitz N. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob Control 23: 133–139, 2014. doi: 10.1136/tobaccocontrol-2012-050859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gordon SM, Wallace LA, Brinkman MC, Callahan PJ, Kenny DV. Volatile organic compounds as breath biomarkers for active and passive smoking. Environ Health Perspect 110: 689–698, 2002. doi: 10.1289/ehp.02110689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gourlay SG, Benowitz NL. Arteriovenous differences in plasma concentration of nicotine and catecholamines and related cardiovascular effects after smoking, nicotine nasal spray, and intravenous nicotine. Clin Pharmacol Ther 62: 453–463, 1997. doi: 10.1016/S0009-9236(97)90124-7. [DOI] [PubMed] [Google Scholar]
- 102.Gregg EO, Fisher AL, Lowe F, McEwan M, Massey ED. An approach to the validation of biomarkers of harm for use in a tobacco context. Regul Toxicol Pharmacol 44: 262–267, 2006. doi: 10.1016/j.yrtph.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 103.Gupta MK, Jain R, Singh P, Ch R, Mudiam MK. Determination of urinary PAH metabolites using DLLME hyphenated to injector port silylation and GC-MS-MS. J Anal Toxicol 39: 365–373, 2015. doi: 10.1093/jat/bkv023. [DOI] [PubMed] [Google Scholar]
- 104.Han S, Chen H, Zhang X, Liu T, Fu Y. Levels of selected groups of compounds in refill solutions for electronic cigarettes. Nicotine Tob Res 18: 708–714, 2016. doi: 10.1093/ntr/ntv189. [DOI] [PubMed] [Google Scholar]
- 105.Hariharan M, VanNoord T, Greden JF. A high-performance liquid-chromatographic method for routine simultaneous determination of nicotine and cotinine in plasma. Clin Chem 34: 724–729, 1988. [PubMed] [Google Scholar]
- 106.Hatsukami DK, Ebbert JO, Feuer RM, Stepanov I, Hecht SS. Changing smokeless tobacco products new tobacco-delivery systems. Am J Prev Med 33, Suppl: S368–S378, 2007. doi: 10.1016/j.amepre.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 107.Hatsukami DK, Lemmonds C, Zhang Y, Murphy SE, Le C, Carmella SG, Hecht SS. Evaluation of carcinogen exposure in people who used “reduced exposure” tobacco products. J Natl Cancer Inst 96: 844–852, 2004. doi: 10.1093/jnci/djh163. [DOI] [PubMed] [Google Scholar]
- 108.Hecht EM, Arheart K, Lee DJ, Hennekens CH, Hlaing WM. A cross-sectional survey of cadmium biomarkers and cigarette smoking. Biomarkers 21: 429–435, 2016. doi: 10.3109/1354750X.2016.1153717. [DOI] [PubMed] [Google Scholar]
- 109.Hecht SS. Biochemistry, biology, and carcinogenicity of tobacco-specific N-nitrosamines. Chem Res Toxicol 11: 559–603, 1998. doi: 10.1021/tx980005y. [DOI] [PubMed] [Google Scholar]
- 110.Hecht SS. It is time to regulate carcinogenic tobacco-specific nitrosamines in cigarette tobacco. Cancer Prev Res (Phila) 7: 639–647, 2014. doi: 10.1158/1940-6207.CAPR-14-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hecht SS. Tobacco carcinogens, their biomarkers and tobacco-induced cancer. Nat Rev Cancer 3: 733–744, 2003. doi: 10.1038/nrc1190. [DOI] [PubMed] [Google Scholar]
- 112.Hecht SS, Carmella SG, Chen M, Dor Koch JF, Miller AT, Murphy SE, Jensen JA, Zimmerman CL, Hatsukami DK. Quantitation of urinary metabolites of a tobacco-specific lung carcinogen after smoking cessation. Cancer Res 59: 590–596, 1999. [PubMed] [Google Scholar]
- 113.Hecht SS, Carmella SG, Kotandeniya D, Pillsbury ME, Chen M, Ransom BW, Vogel RI, Thompson E, Murphy SE, Hatsukami DK. Evaluation of toxicant and carcinogen metabolites in the urine of e-cigarette users versus cigarette smokers. Nicotine Tob Res 17: 704–709, 2015. doi: 10.1093/ntr/ntu218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hecht SS, Carmella SG, Murphy SE, Akerkar S, Brunnemann KD, Hoffmann D. A tobacco-specific lung carcinogen in the urine of men exposed to cigarette smoke. N Engl J Med 329: 1543–1546, 1993. doi: 10.1056/NEJM199311183292105. [DOI] [PubMed] [Google Scholar]
- 115.Hecht SS, Carmella SG, Murphy SE, Riley WT, Le C, Luo X, Mooney M, Hatsukami DK. Similar exposure to a tobacco-specific carcinogen in smokeless tobacco users and cigarette smokers. Cancer Epidemiol Biomarkers Prev 16: 1567–1572, 2007. doi: 10.1158/1055-9965.EPI-07-0227. [DOI] [PubMed] [Google Scholar]
- 116.Hecht SS, Chen M, Yagi H, Jerina DM, Carmella SG. r-1,t-2,3,c-4-Tetrahydroxy-1,2,3,4-tetrahydrophenanthrene in human urine: a potential biomarker for assessing polycyclic aromatic hydrocarbon metabolic activation. Cancer Epidemiol Biomarkers Prev 12: 1501–1508, 2003. [PubMed] [Google Scholar]
- 117.Hecht SS, Murphy SE, Stepanov I, Nelson HH, Yuan JM. Tobacco smoke biomarkers and cancer risk among male smokers in the Shanghai cohort study. Cancer Lett 334: 34–38, 2013. doi: 10.1016/j.canlet.2012.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hecht SS, Stepanov I, Carmella SG. Exposure and metabolic activation biomarkers of carcinogenic tobacco-specific nitrosamines. Acc Chem Res 49: 106–114, 2016. doi: 10.1021/acs.accounts.5b00472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Henningfield JE, Stapleton JM, Benowitz NL, Grayson RF, London ED. Higher levels of nicotine in arterial than in venous blood after cigarette smoking. Drug Alcohol Depend 33: 23–29, 1993. doi: 10.1016/0376-8716(93)90030-T. [DOI] [PubMed] [Google Scholar]
- 120.Herrington JS, Myers C. Electronic cigarette solutions and resultant aerosol profiles. J Chromatogr A 1418: 192–199, 2015. doi: 10.1016/j.chroma.2015.09.034. [DOI] [PubMed] [Google Scholar]
- 121.Ho MK, Faseru B, Choi WS, Nollen NL, Mayo MS, Thomas JL, Okuyemi KS, Ahluwalia JS, Benowitz NL, Tyndale RF. Utility and relationships of biomarkers of smoking in African-American light smokers. Cancer Epidemiol Biomarkers Prev 18: 3426–3434, 2009. doi: 10.1158/1055-9965.EPI-09-0956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hofmann W, Morawska L, Bergmann R. Environmental tobacco smoke deposition in the human respiratory tract: differences between experimental and theoretical approaches. J Aerosol Med 14: 317–326, 2001. doi: 10.1089/089426801316970277. [DOI] [PubMed] [Google Scholar]
- 123.Homa DM, Neff LJ, King BA, Caraballo RS, Bunnell RE, Babb SD, Garrett BE, Sosnoff CS, Wang L; Centers for Disease Control and Prevention (CDC) . Vital signs: disparities in nonsmokers’ exposure to secondhand smoke—United States, 1999-2012. MMWR Morb Mortal Wkly Rep 64: 103–108, 2015. [PMC free article] [PubMed] [Google Scholar]
- 124.Hsieh SJ, Ware LB, Eisner MD, Yu L, Jacob P 3rd, Havel C, Goniewicz ML, Matthay MA, Benowitz NL, Calfee CS. Biomarkers increase detection of active smoking and secondhand smoke exposure in critically ill patients. Crit Care Med 39: 40–45, 2011. doi: 10.1097/CCM.0b013e3181fa4196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Huang L, Zhu Y, Zheng H, Ding G, Li Y. Direct conversion of glycerol into 1, 3-propanediol over Cu-H4SiW12O40/SiO2 in vapor phase. Catal Lett 131: 312–320, 2009. doi: 10.1007/s10562-009-9914-1. [DOI] [Google Scholar]
- 126.Hukkanen J, Jacob P 3rd, Benowitz NL. Metabolism and disposition kinetics of nicotine. Pharmacol Rev 57: 79–115, 2005. doi: 10.1124/pr.57.1.3. [DOI] [PubMed] [Google Scholar]
- 127.Hutzler C, Paschke M, Kruschinski S, Henkler F, Hahn J, Luch A. Chemical hazards present in liquids and vapors of electronic cigarettes. Arch Toxicol 88: 1295–1308, 2014. doi: 10.1007/s00204-014-1294-7. [DOI] [PubMed] [Google Scholar]
- 128.Jacob P 3rd, Abu Raddaha AH, Dempsey D, Havel C, Peng M, Yu L, Benowitz NL. Comparison of nicotine and carcinogen exposure with water pipe and cigarette smoking. Cancer Epidemiol Biomarkers Prev 22: 765–772, 2013. doi: 10.1158/1055-9965.EPI-12-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Jacob P 3rd, Wilson M, Benowitz NL. Determination of phenolic metabolites of polycyclic aromatic hydrocarbons in human urine as their pentafluorobenzyl ether derivatives using liquid chromatography-tandem mass spectrometry. Anal Chem 79: 587–598, 2007. doi: 10.1021/ac060920l. [DOI] [PubMed] [Google Scholar]
- 130.Jacob P 3rd, Wilson M, Benowitz NL. Improved gas chromatographic method for the determination of nicotine and cotinine in biologic fluids. J Chromatogr A 222: 61–70, 1981. doi: 10.1016/S0378-4347(00)81033-6. [DOI] [PubMed] [Google Scholar]
- 131.Jacob P 3rd, Wu S, Yu L, Benowitz NL. Simultaneous determination of mecamylamine, nicotine, and cotinine in plasma by gas chromatography-mass spectrometry. J Pharm Biomed Anal 23: 653–661, 2000. doi: 10.1016/S0731-7085(00)00343-5. [DOI] [PubMed] [Google Scholar]
- 131a.Jacob P 3rd, Yu L, Benowitz N.. Determination of nicotine, its major metabolites, and deuterium-labeled isotopomers in human urine using LC-MS/MS (Abstract). Drug Metab Rev 34: 160, 2002. [Google Scholar]
- 132.Jacob P 3rd, Yu L, Duan M, Ramos L, Yturralde O, Benowitz NL. Determination of the nicotine metabolites cotinine and trans-3′-hydroxycotinine in biologic fluids of smokers and non-smokers using liquid chromatography-tandem mass spectrometry: biomarkers for tobacco smoke exposure and for phenotyping cytochrome P450 2A6 activity. J Chromatogr B Analyt Technol Biomed Life Sci 879: 267–276, 2011. doi: 10.1016/j.jchromb.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Jaffe JH, Kanzler M, Friedman L, Stunkard AJ, Verebey K. Carbon monoxide and thiocyanate levels in low tar/nicotine smokers. Addict Behav 6: 337–343, 1981. doi: 10.1016/0306-4603(81)90049-6. [DOI] [Google Scholar]
- 135.Jain RB. Distributions of selected urinary metabolites of volatile organic compounds by age, gender, race/ethnicity, and smoking status in a representative sample of U.S. adults. Environ Toxicol Pharmacol 40: 471–479, 2015. doi: 10.1016/j.etap.2015.07.018. [DOI] [PubMed] [Google Scholar]
- 136.Järup L, Elinder CG, Spång G. Cumulative blood-cadmium and tubular proteinuria: a dose-response relationship. Int Arch Occup Environ Health 60: 223–229, 1988. doi: 10.1007/BF00378700. [DOI] [PubMed] [Google Scholar]
- 137.Jarvis MJ, Fidler J, Mindell J, Feyerabend C, West R. Assessing smoking status in children, adolescents and adults: cotinine cut-points revisited. Addiction 103: 1553–1561, 2008. doi: 10.1111/j.1360-0443.2008.02297.x. [DOI] [PubMed] [Google Scholar]
- 138.Jarvis MJ, Russell MA, Benowitz NL, Feyerabend C. Elimination of cotinine from body fluids: implications for noninvasive measurement of tobacco smoke exposure. Am J Public Health 78: 696–698, 1988. doi: 10.2105/AJPH.78.6.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Jarvis MJ, Tunstall-Pedoe H, Feyerabend C, Vesey C, Saloojee Y. Comparison of tests used to distinguish smokers from nonsmokers. Am J Public Health 77: 1435–1438, 1987. doi: 10.2105/AJPH.77.11.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Jian W, Yao M, Zhang D, Zhu M. Rapid detection and characterization of in vitro and urinary N-acetyl-L-cysteine conjugates using quadrupole-linear ion trap mass spectrometry and polarity switching. Chem Res Toxicol 22: 1246–1255, 2009. doi: 10.1021/tx900035j. [DOI] [PubMed] [Google Scholar]
- 141.Joseph AM, Hecht SS, Murphy SE, Carmella SG, Le CT, Zhang Y, Han S, Hatsukami DK. Relationships between cigarette consumption and biomarkers of tobacco toxin exposure. Cancer Epidemiol Biomarkers Prev 14: 2963–2968, 2005. doi: 10.1158/1055-9965.EPI-04-0768. [DOI] [PubMed] [Google Scholar]
- 142.Kage S, Kudo K, Ikeda H, Ikeda N. Simultaneous determination of formate and acetate in whole blood and urine from humans using gas chromatography-mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 805: 113–117, 2004. doi: 10.1016/j.jchromb.2004.02.029. [DOI] [PubMed] [Google Scholar]
- 143.Kaivosaari S, Toivonen P, Hesse LM, Koskinen M, Court MH, Finel M. Nicotine glucuronidation and the human UDP-glucuronosyltransferase UGT2B10. Mol Pharmacol 72: 761–768, 2007. doi: 10.1124/mol.107.037093. [DOI] [PubMed] [Google Scholar]
- 144.Kavvadias D, Scherer G, Cheung F, Errington G, Shepperd J, McEwan M. Determination of tobacco-specific N-nitrosamines in urine of smokers and non-smokers. Biomarkers 14: 547–553, 2009. doi: 10.3109/13547500903242883. [DOI] [PubMed] [Google Scholar]
- 145.Kavvalakis MP, Stivaktakis PD, Tzatzarakis MN, Kouretas D, Liesivuori J, Alegakis AK, Vynias D, Tsatsakis AM. Multicomponent analysis of replacement liquids of electronic cigarettes using chromatographic techniques. J Anal Toxicol 39: 262–269, 2015. doi: 10.1093/jat/bkv002. [DOI] [PubMed] [Google Scholar]
- 146.Kesimer M, Kirkham S, Pickles RJ, Henderson AG, Alexis NE, Demaria G, Knight D, Thornton DJ, Sheehan JK. Tracheobronchial air-liquid interface cell culture: a model for innate mucosal defense of the upper airways? Am J Physiol Lung Cell Mol Physiol 296: L92–L100, 2009. doi: 10.1152/ajplung.90388.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kim HJ, Shin HS. Determination of tobacco-specific nitrosamines in replacement liquids of electronic cigarettes by liquid chromatography-tandem mass spectrometry. J Chromatogr A 1291: 48–55, 2013. doi: 10.1016/j.chroma.2013.03.035. [DOI] [PubMed] [Google Scholar]
- 148.Kim S, Vermeulen R, Waidyanatha S, Johnson BA, Lan Q, Rothman N, Smith MT, Zhang L, Li G, Shen M, Yin S, Rappaport SM. Using urinary biomarkers to elucidate dose-related patterns of human benzene metabolism. Carcinogenesis 27: 772–781, 2006. doi: 10.1093/carcin/bgi297. [DOI] [PubMed] [Google Scholar]
- 149.Kosmider L, Sobczak A, Fik M, Knysak J, Zaciera M, Kurek J, Goniewicz ML. Carbonyl compounds in electronic cigarette vapors: effects of nicotine solvent and battery output voltage. Nicotine Tob Res 16: 1319–1326, 2014. doi: 10.1093/ntr/ntu078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kotandeniya D, Carmella SG, Ming X, Murphy SE, Hecht SS. Combined analysis of the tobacco metabolites cotinine and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol in human urine. Anal Chem 87: 1514–1517, 2015. doi: 10.1021/ac504047j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kotapati S, Matter BA, Grant AL, Tretyakova NY. Quantitative analysis of trihydroxybutyl mercapturic acid, a urinary metabolite of 1,3-butadiene, in humans. Chem Res Toxicol 24: 1516–1526, 2011. doi: 10.1021/tx2001306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kotlyar M, Hertsgaard LA, Lindgren BR, Jensen JA, Carmella SG, Stepanov I, Murphy SE, Hecht SS, Hatsukami DK. Effect of oral snus and medicinal nicotine in smokers on toxicant exposure and withdrawal symptoms: a feasibility study. Cancer Epidemiol Biomarkers Prev 20: 91–100, 2011. doi: 10.1158/1055-9965.EPI-10-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kozlowski LT, Sweeney CT, Pillitteri JL. Blocking cigarette filter vents with lips more than doubles carbon monoxide intake from ultra-low tar cigarettes. Exp Clin Psychopharmacol 4: 404–408, 1996. doi: 10.1037/1064-1297.4.4.404. [DOI] [Google Scholar]
- 154.Laino T, Tuma C, Moor P, Martin E, Stolz S, Curioni A. Mechanisms of propylene glycol and triacetin pyrolysis. J Phys Chem A 116: 4602–4609, 2012. doi: 10.1021/jp300997d. [DOI] [PubMed] [Google Scholar]
- 155.Lamarre SG, MacMillan L, Morrow GP, Randell E, Pongnopparat T, Brosnan ME, Brosnan JT. An isotope-dilution, GC-MS assay for formate and its application to human and animal metabolism. Amino Acids 46: 1885–1891, 2014. doi: 10.1007/s00726-014-1738-7. [DOI] [PubMed] [Google Scholar]
- 156.Len C, Luque R. Continuous flow transformations of glycerol to valuable products: an overview. Sustain Chem Process 2: 1–10, 2014. doi: 10.1186/2043-7129-2-1. [DOI] [Google Scholar]
- 157.Lerner CA, Sundar IK, Yao H, Gerloff J, Ossip DJ, McIntosh S, Robinson R, Rahman I. Vapors produced by electronic cigarettes and e-juices with flavorings induce toxicity, oxidative stress, and inflammatory response in lung epithelial cells and in mouse lung. PLoS One 10: e0116732, 2015. doi: 10.1371/journal.pone.0116732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Leung TF, Chan IH, Liu TC, Lam CW, Wong GW. Relationship between passive smoking exposure and urinary heavy metals and lung functions in preschool children. Pediatr Pulmonol 48: 1089–1097, 2013. doi: 10.1002/ppul.22801. [DOI] [PubMed] [Google Scholar]
- 159.Lewis SJ, Cherry NM, McL Niven R, Barber PV, Wilde K, Povey AC. Cotinine levels and self-reported smoking status in patients attending a bronchoscopy clinic. Biomarkers 8: 218–228, 2003. doi: 10.1080/1354750031000120125. [DOI] [PubMed] [Google Scholar]
- 160.Li Z, Romanoff LC, Trinidad DA, Pittman EN, Hilton D, Hubbard K, Carmichael H, Parker J, Calafat AM, Sjödin A. Quantification of 21 metabolites of methylnaphthalenes and polycyclic aromatic hydrocarbons in human urine. Anal Bioanal Chem 406: 3119–3129, 2014. doi: 10.1007/s00216-014-7676-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Lisko JG, Tran H, Stanfill SB, Blount BC, Watson CH. Chemical composition and evaluation of nicotine, tobacco alkaloids, pH, and selected flavors in e-cigarette cartridges and refill solutions. Nicotine Tob Res 17: 1270–1278, 2015. doi: 10.1093/ntr/ntu279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Liu B, Jia C. Effects of profession on urinary PAH metabolite levels in the US population. Int Arch Occup Environ Health 89: 123–135, 2016. doi: 10.1007/s00420-015-1057-7. [DOI] [PubMed] [Google Scholar]
- 163.Lopez AA, Eissenberg T. Science and the evolving electronic cigarette. Prev Med 80: 101–106, 2015. doi: 10.1016/j.ypmed.2015.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Lourenço RV, Klimek MF, Borowski CJ. Deposition and clearance of 2 micron particles in the tracheobronchial tree of normal subjects—smokers and nonsmokers. J Clin Invest 50: 1411–1420, 1971. doi: 10.1172/JCI106624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Lowe RH, Abraham TT, Darwin WD, Herning R, Cadet JL, Huestis MA. Extended urinary Delta9-tetrahydrocannabinol excretion in chronic cannabis users precludes use as a biomarker of new drug exposure. Drug Alcohol Depend 105: 24–32, 2009. doi: 10.1016/j.drugalcdep.2009.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Lunell E, Molander L, Ekberg K, Wahren J. Site of nicotine absorption from a vapour inhaler—comparison with cigarette smoking. Eur J Clin Pharmacol 55: 737–741, 2000. doi: 10.1007/s002280050007. [DOI] [PubMed] [Google Scholar]
- 167.Lynn WS, Kylstra JA, Sahu SC, Tainer J, Shelburne J, Pratt PC, Gutknecht WF, Shaw R, Ingram P. Investigations of black bronchoalveolar human lavage fluid. Chest 72: 483–488, 1977. doi: 10.1378/chest.72.4.483. [DOI] [PubMed] [Google Scholar]
- 168.Marco E, Grimalt JO. A rapid method for the chromatographic analysis of volatile organic compounds in exhaled breath of tobacco cigarette and electronic cigarette smokers. J Chromatogr A 1410: 51–59, 2015. doi: 10.1016/j.chroma.2015.07.094. [DOI] [PubMed] [Google Scholar]
- 169.Marrone GF, Shakleya DM, Scheidweiler KB, Singleton EG, Huestis MA, Heishman SJ. Relative performance of common biochemical indicators in detecting cigarette smoking. Addiction 106: 1325–1334, 2011. doi: 10.1111/j.1360-0443.2011.03441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Martonen TB. Deposition patterns of cigarette smoke in human airways. Am Ind Hyg Assoc J 53: 6–18, 1992. doi: 10.1080/15298669291359249. [DOI] [PubMed] [Google Scholar]
- 171.Martonen TB, Musante CJ. Importance of cloud motion on cigarette smoke deposition in lung airways. Inhal Toxicol 12, Suppl 4: 261–280, 2000. doi: 10.1080/08958370050165120. [DOI] [PubMed] [Google Scholar]
- 172.Matta SG, Balfour DJ, Benowitz NL, Boyd RT, Buccafusco JJ, Caggiula AR, Craig CR, Collins AC, Damaj MI, Donny EC, Gardiner PS, Grady SR, Heberlein U, Leonard SS, Levin ED, Lukas RJ, Markou A, Marks MJ, McCallum SE, Parameswaran N, Perkins KA, Picciotto MR, Quik M, Rose JE, Rothenfluh A, Schafer WR, Stolerman IP, Tyndale RF, Wehner JM, Zirger JM. Guidelines on nicotine dose selection for in vivo research. Psychopharmacology (Berl) 190: 269–319, 2007. doi: 10.1007/s00213-006-0441-0. [DOI] [PubMed] [Google Scholar]
- 173.Mattes W, Yang X, Orr MS, Richter P, Mendrick DL. Biomarkers of tobacco smoke exposure. Adv Clin Chem 67: 1–45, 2014. doi: 10.1016/bs.acc.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 174.McCusker K, McNabb E, Bone R. Plasma nicotine levels in pipe smokers. JAMA 248: 577–578, 1982. doi: 10.1001/jama.1982.03330050059032. [DOI] [PubMed] [Google Scholar]
- 175.McElroy JA, Shafer MM, Trentham-Dietz A, Hampton JM, Newcomb PA. Urinary cadmium levels and tobacco smoke exposure in women age 20–69 years in the United States. J Toxicol Environ Health A 70: 1779–1782, 2007. doi: 10.1080/15287390600754953. [DOI] [PubMed] [Google Scholar]
- 176.McKelvey W, Gwynn RC, Jeffery N, Kass D, Thorpe LE, Garg RK, Palmer CD, Parsons PJ. A biomonitoring study of lead, cadmium, and mercury in the blood of New York City adults. Environ Health Perspect 115: 1435–1441, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.McRobbie H, Phillips A, Goniewicz ML, Smith KM, Knight-West O, Przulj D, Hajek P. Effects of switching to electronic cigarettes with and without concurrent smoking on exposure to nicotine, carbon monoxide, and acrolein. Cancer Prev Res (Phila) 8: 873–878, 2015. doi: 10.1158/1940-6207.CAPR-15-0058. [DOI] [PubMed] [Google Scholar]
- 178.McShane WJ, Pappas RS, Wilson-McElprang V, Paschal D. A rugged and transferable method for determining blood cadmium, mercury, and lead with inductively coupled plasma-mass spectrometry. Spectrochim Acta Part B At Spectrosc 63: 638–644, 2008. doi: 10.1016/j.sab.2008.03.016. [DOI] [Google Scholar]
- 179.Mincer JR. The US vaporizer market is booming (Online). Business Insider http://www.businessinsider.com/r-in-rise-of-us-vape-shops-owners-eye-new-marijuana-market-2015-7 [29 July 2016].
- 180.Minet E, Cheung F, Errington G, Sterz K, Scherer G. Urinary excretion of the acrylonitrile metabolite 2-cyanoethylmercapturic acid is correlated with a variety of biomarkers of tobacco smoke exposure and consumption. Biomarkers 16: 89–96, 2011. doi: 10.3109/1354750X.2010.533287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Moir D, Rickert WS, Levasseur G, Larose Y, Maertens R, White P, Desjardins S. A comparison of mainstream and sidestream marijuana and tobacco cigarette smoke produced under two machine smoking conditions. Chem Res Toxicol 21: 494–502, 2008. doi: 10.1021/tx700275p. [DOI] [PubMed] [Google Scholar]
- 182.Moretti M, Sisti D, Rocchi MB, Delprete E. CLSI EP17-A protocol: a useful tool for better understanding the low end performance of total prostate-specific antigen assays. Clin Chim Acta 412: 1143–1145, 2011. doi: 10.1016/j.cca.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 183.Mulchi C, Bell P, Adamu C, Chaney R. Long term availability of metals in sludge amended acid soils. J Plant Nutr 10: 1149–1161, 1987. doi: 10.1080/01904168709363643. [DOI] [Google Scholar]
- 184.Muller WJ, Hess GD, Scherer PW. A model of cigarette smoke particle deposition. Am Ind Hyg Assoc J 51: 245–256, 1990. doi: 10.1080/15298669091369600. [DOI] [PubMed] [Google Scholar]
- 185.Murphy SE, Park SS, Thompson EF, Wilkens LR, Patel Y, Stram DO, Le Marchand L. Nicotine N-glucuronidation relative to N-oxidation and C-oxidation and UGT2B10 genotype in five ethnic/racial groups. Carcinogenesis 35: 2526–2533, 2014. doi: 10.1093/carcin/bgu191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Onyemauwa F, Rappaport SM, Sobus JR, Gajdosová D, Wu R, Waidyanatha S. Using liquid chromatography-tandem mass spectrometry to quantify monohydroxylated metabolites of polycyclic aromatic hydrocarbons in urine. J Chromatogr B Analyt Technol Biomed Life Sci 877: 1117–1125, 2009. doi: 10.1016/j.jchromb.2009.02.067. [DOI] [PubMed] [Google Scholar]
- 187.Osterdahl BG. The migration of tobacco-specific nitrosamines into the saliva of chewers of nicotine-containing chewing gum. Food Chem Toxicol 28: 619–622, 1990. doi: 10.1016/0278-6915(90)90169-N. [DOI] [PubMed] [Google Scholar]
- 188.Ozdener MH, Yee KK, McDermott R, Cowart BJ, Vainius AA, Dalton P, Rawson NE. Assessment of smoking status based on cotinine levels in nasal lavage fluid. Tob Induc Dis 5: 11, 2009. doi: 10.1186/1617-9625-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Pääkkö P, Kokkonen P, Anttila S, Kalliomäki PL. Cadmium and chromium as markers of smoking in human lung tissue. Environ Res 49: 197–207, 1989. doi: 10.1016/S0013-9351(89)80065-9. [DOI] [PubMed] [Google Scholar]
- 190.Pagliaro M, Ciriminna R, Kimura H, Rossi M, Della Pina C. From glycerol to value-added products. Angew Chem Int Ed Engl 46: 4434–4440, 2007. doi: 10.1002/anie.200604694. [DOI] [PubMed] [Google Scholar]
- 191.Pappas RS, Fresquez MR, Watson CH. Cigarette smoke cadmium breakthrough from traditional filters: implications for exposure. J Anal Toxicol 39: 45–51, 2015. doi: 10.1093/jat/bku115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Pappas RS, Polzin GM, Zhang L, Watson CH, Paschal DC, Ashley DL. Cadmium, lead, and thallium in mainstream tobacco smoke particulate. Food Chem Toxicol 44: 714–723, 2006. doi: 10.1016/j.fct.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 193.Park SL, Carmella SG, Ming X, Vielguth E, Stram DO, Le Marchand L, Hecht SS. Variation in levels of the lung carcinogen NNAL and its glucuronides in the urine of cigarette smokers from five ethnic groups with differing risks for lung cancer. Cancer Epidemiol Biomarkers Prev 24: 561–569, 2015. doi: 10.1158/1055-9965.EPI-14-1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Paschke M, Hutzler C, Henkler F, Luch A. Toward the stereochemical identification of prohibited characterizing flavors in tobacco products: the case of strawberry flavor. Arch Toxicol 89: 1241–1255, 2015. doi: 10.1007/s00204-015-1558-x. [DOI] [PubMed] [Google Scholar]
- 195.Patterson F, Benowitz N, Shields P, Kaufmann V, Jepson C, Wileyto P, Kucharski S, Lerman C. Individual differences in nicotine intake per cigarette. Cancer Epidemiol Biomarkers Prev 12: 468–471, 2003. [PubMed] [Google Scholar]
- 196.Paukovits WR, Moser MH, Rutter R, Paukovits JB. Inhibition of hematopoietic stem cell proliferation by hemoregulatory peptide pyroGlu-Glu-Asp-Cys-Lys (pEEDCK) provides protection against short-term neutropenia and long-term damage. Ann N Y Acad Sci 628: 92–104, 1991. doi: 10.1111/j.1749-6632.1991.tb17227.x. [DOI] [PubMed] [Google Scholar]
- 197.Pazo DY, Moliere F, Sampson MM, Reese CM, Agnew-Heard KA, Walters MJ, Holman MR, Blount BC, Watson CH, Chambers DM. Mainstream smoke levels of volatile organic compounds in 50 U.S. domestic cigarette brands smoked with the ISO and Canadian intense protocols. Nicotine Tob Res 18: 1886–1894, 2016. doi: 10.1093/ntr/ntw118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Pérez-Stable EJ, Benowitz NL, Marín G. Is serum cotinine a better measure of cigarette smoking than self-report? Prev Med 24: 171–179, 1995. doi: 10.1006/pmed.1995.1031. [DOI] [PubMed] [Google Scholar]
- 199.Phalen R, Oldham M, Schum G. The deposition of concentrated cigarette smoke in airway models. Ann Occup Hyg 46, Suppl 1: 343–345, 2002. https://www.researchgate.net/publication/31468849_The_Deposition_of_Concentrated_Cigarette_Smoke_in_Airway_Models [Google Scholar]
- 200.Pickett G, Seagrave J, Boggs S, Polzin G, Richter P, Tesfaigzi Y. Effects of 10 cigarette smoke condensates on primary human airway epithelial cells by comparative gene and cytokine expression studies. Toxicol Sci 114: 79–89, 2010. doi: 10.1093/toxsci/kfp298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Pinxt HH, Kuster BF, Marin GB. Promoter effects in the Pt-catalysed oxidation of propylene glycol. Appl Catal A Gen 191: 45–54, 2000. doi: 10.1016/S0926-860X(99)00304-X. [DOI] [Google Scholar]
- 202.Pluym N, Gilch G, Scherer G, Scherer M. Analysis of 18 urinary mercapturic acids by two high-throughput multiplex-LC-MS/MS methods. Anal Bioanal Chem 407: 5463–5476, 2015. doi: 10.1007/s00216-015-8719-x. [DOI] [PubMed] [Google Scholar]
- 203.Pulvers K, Emami AS, Nollen NL, Romero DR, Strong DR, Benowitz NL, Ahluwalia JS. Tobacco Consumption and Toxicant Exposure of Cigarette Smokers Using Electronic Cigarettes. Nicotine Tob Res. First published December 21, 2016; doi: 10.1093/ntr/ntw333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Ramo DE, Prochaska JJ. Prevalence and co-use of marijuana among young adult cigarette smokers: an anonymous online national survey. Addict Sci Clin Pract 7: 5, 2012. doi: 10.1186/1940-0640-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Ramsauer B, Sterz K, Hagedorn HW, Engl J, Scherer G, McEwan M, Errington G, Shepperd J, Cheung F. A liquid chromatography/tandem mass spectrometry (LC-MS/MS) method for the determination of phenolic polycyclic aromatic hydrocarbons (OH-PAH) in urine of non-smokers and smokers. Anal Bioanal Chem 399: 877–889, 2011. doi: 10.1007/s00216-010-4355-7. [DOI] [PubMed] [Google Scholar]
- 206.Richter KP, Kaur H, Resnicow K, Nazir N, Mosier MC, Ahluwalia JS. Cigarette smoking among marijuana users in the United States. Subst Abus 25: 35–43, 2004. doi: 10.1300/J465v25n02_06. [DOI] [PubMed] [Google Scholar]
- 207.Richter PA, Bishop EE, Wang J, Swahn MH. Tobacco smoke exposure and levels of urinary metals in the U.S. youth and adult population: the National Health and Nutrition Examination Survey (NHANES) 1999-2004. Int J Environ Res Public Health 6: 1930–1946, 2009. doi: 10.3390/ijerph6071930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Rickert WS, Robinson JC. Estimating the hazards of less hazardous cigarettes. II. Study of cigarette yields of nicotine, carbon monoxide, and hydrogen cyanide in relation to levels of cotinine, carboxyhemoglobin, and thiocyanate in smokers. J Toxicol Environ Health 7: 391–403, 1981. doi: 10.1080/15287398109529990. [DOI] [PubMed] [Google Scholar]
- 209.Robinson RJ, Yu CP. Deposition of cigarette smoke particles in the human respiratory tract. Aerosol Sci Technol 34: 202–215, 2001. doi: 10.1080/027868201300034844. [DOI] [Google Scholar]
- 210.Rodriguez J, Jiang R, Johnson WC, MacKenzie BA, Smith LJ, Barr RG. The association of pipe and cigar use with cotinine levels, lung function, and airflow obstruction: a cross-sectional study. Ann Intern Med 152: 201–210, 2010. doi: 10.7326/0003-4819-152-4-201002160-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Roels HA, Hoet P, Lison D. Usefulness of biomarkers of exposure to inorganic mercury, lead, or cadmium in controlling occupational and environmental risks of nephrotoxicity. Ren Fail 21: 251–262, 1999. doi: 10.3109/08860229909085087. [DOI] [PubMed] [Google Scholar]
- 212.Romanoff LC, Li Z, Young KJ, Blakely NC 3rd, Patterson DG Jr, Sandau CD. Automated solid-phase extraction method for measuring urinary polycyclic aromatic hydrocarbon metabolites in human biomonitoring using isotope-dilution gas chromatography high-resolution mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 835: 47–54, 2006. doi: 10.1016/j.jchromb.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 213.Rose JE, Behm FM, Westman EC, Coleman RE. Arterial nicotine kinetics during cigarette smoking and intravenous nicotine administration: implications for addiction. Drug Alcohol Depend 56: 99–107, 1999. doi: 10.1016/S0376-8716(99)00025-3. [DOI] [PubMed] [Google Scholar]
- 214.Ruppert T, Scherer G, Tricker AR, Adlkofer F. trans,trans-muconic acid as a biomarker of non-occupational environmental exposure to benzene. Int Arch Occup Environ Health 69: 247–251, 1997. doi: 10.1007/s004200050143. [DOI] [PubMed] [Google Scholar]
- 215.Russell MA, Wilson C, Patel UA, Feyerabend C, Cole PV. Plasma nicotine levels after smoking cigarettes with high, medium, and low nicotine yields. BMJ 2: 414–416, 1975. doi: 10.1136/bmj.2.5968.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Schane RE, Prochaska JJ, Glantz SA. Counseling nondaily smokers about secondhand smoke as a cessation message: a pilot randomized trial. Nicotine Tob Res 15: 334–342, 2013. doi: 10.1093/ntr/nts126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Schantz MM, Benner BA Jr, Heckert NA, Sander LC, Sharpless KE, Vander Pol SS, Vasquez Y, Villegas M, Wise SA, Alwis KU, Blount BC, Calafat AM, Li Z, Silva MJ, Ye X, Gaudreau É, Patterson DG Jr, Sjödin A. Development of urine standard reference materials for metabolites of organic chemicals including polycyclic aromatic hydrocarbons, phthalates, phenols, parabens, and volatile organic compounds. Anal Bioanal Chem 407: 2945–2954, 2015. doi: 10.1007/s00216-014-8441-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Scherer G. Carboxyhemoglobin and thiocyanate as biomarkers of exposure to carbon monoxide and hydrogen cyanide in tobacco smoke. Exp Toxicol Pathol 58: 101–124, 2006. doi: 10.1016/j.etp.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 219.Scherer G, Engl J, Urban M, Gilch G, Janket D, Riedel K. Relationship between machine-derived smoke yields and biomarkers in cigarette smokers in Germany. Regul Toxicol Pharmacol 47: 171–183, 2007. doi: 10.1016/j.yrtph.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 220.Scherer G, Richter E. Biomonitoring exposure to environmental tobacco smoke (ETS): a critical reappraisal. Hum Exp Toxicol 16: 449–459, 1997. doi: 10.1177/096032719701600806. [DOI] [PubMed] [Google Scholar]
- 221.Scherer G, Urban M, Hagedorn HW, Feng S, Kinser RD, Sarkar M, Liang Q, Roethig HJ. Determination of two mercapturic acids related to crotonaldehyde in human urine: influence of smoking. Hum Exp Toxicol 26: 37–47, 2007. doi: 10.1177/0960327107073829. [DOI] [PubMed] [Google Scholar]
- 222.Scherer G, Urban M, Hagedorn HW, Serafin R, Feng S, Kapur S, Muhammad R, Jin Y, Sarkar M, Roethig HJ. Determination of methyl-, 2-hydroxyethyl- and 2-cyanoethylmercapturic acids as biomarkers of exposure to alkylating agents in cigarette smoke. J Chromatogr B Analyt Technol Biomed Life Sci 878: 2520–2528, 2010. doi: 10.1016/j.jchromb.2010.02.023. [DOI] [PubMed] [Google Scholar]
- 223.Schettgen T, Musiol A, Alt A, Ochsmann E, Kraus T. A method for the quantification of biomarkers of exposure to acrylonitrile and 1,3-butadiene in human urine by column-switching liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem 393: 969–981, 2009. doi: 10.1007/s00216-008-2510-1. [DOI] [PubMed] [Google Scholar]
- 224.Schick SF, Glantz S. Concentrations of the carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in sidestream cigarette smoke increase after release into indoor air: results from unpublished tobacco industry research. Cancer Epidemiol Biomarkers Prev 16: 1547–1553, 2007. doi: 10.1158/1055-9965.EPI-07-0210. [DOI] [PubMed] [Google Scholar]
- 225.Shah VP, Midha KK, Findlay JW, Hill HM, Hulse JD, McGilveray IJ, McKay G, Miller KJ, Patnaik RN, Powell ML, Tonelli A, Viswanathan CT, Yacobi A. Bioanalytical method validation--a revisit with a decade of progress. Pharm Res 17: 1551–1557, 2000. doi: 10.1023/A:1007669411738. [DOI] [PubMed] [Google Scholar]
- 226.Shahab L, Goniewicz ML, Blount BC, Brown J, McNeill A, Alwis KU, Feng J, Wang L, West R. Nicotine, carcinogen, and toxin exposure in long-term e-cigarette and nicotine replacement therapy users: a cross-sectional study. Ann Intern Med 166: 390–400, 2017. doi: 10.7326/M16-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Shaoqing Y, Ruxin Z, Yingjian C, Jianqiu C, Yanshen W, Genhong L. A meta-analysis of the association of exhaled carbon monoxide on asthma and allergic rhinitis. Clin Rev Allergy Immunol 41: 67–75, 2011. doi: 10.1007/s12016-009-8195-1. [DOI] [PubMed] [Google Scholar]
- 228.Shin HS, Kim JG, Shin YJ, Jee SH. Sensitive and simple method for the determination of nicotine and cotinine in human urine, plasma and saliva by gas chromatography-mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 769: 177–183, 2002. doi: 10.1016/S1570-0232(02)00007-7. [DOI] [PubMed] [Google Scholar]
- 229.Sims M, Mindell JS, Jarvis MJ, Feyerabend C, Wardle H, Gilmore A. Did smokefree legislation in England reduce exposure to secondhand smoke among nonsmoking adults? Cotinine analysis from the Health Survey for England. Environ Health Perspect 120: 425–430, 2012. doi: 10.1289/ehp.1103680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Skoczyńska A, Smolik R. The effect of combined exposure to lead and cadmium on serum lipids and lipid peroxides level in rats. Int J Occup Med Environ Health 7: 263–271, 1994. [PubMed] [Google Scholar]
- 231.Sleiman M, Gundel LA, Pankow JF, Jacob P 3rd, Singer BC, Destaillats H. Formation of carcinogens indoors by surface-mediated reactions of nicotine with nitrous acid, leading to potential thirdhand smoke hazards. Proc Natl Acad Sci USA 107: 6576–6581, 2010. doi: 10.1073/pnas.0912820107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Sleiman M, Logue JM, Montesinos VN, Russell ML, Litter MI, Gundel LA, Destaillats H. Emissions from electronic cigarettes: key parameters affecting the release of harmful chemicals. Environ Sci Technol 50: 9644–9651, 2016. doi: 10.1021/acs.est.6b01741. [DOI] [PubMed] [Google Scholar]
- 233.Soussy S, El-Hellani A, Baalbaki R, Salman R, Shihadeh A, Saliba NA. Detection of 5-hydroxymethylfurfural and furfural in the aerosol of electronic cigarettes. Tob Control 25, Suppl 2: ii88–ii93, 2016. doi: 10.1136/tobaccocontrol-2016-053220. [DOI] [PubMed] [Google Scholar]
- 235.St Helen G, Benowitz NL, Dains KM, Havel C, Peng M, Jacob P 3rd. Nicotine and carcinogen exposure after water pipe smoking in hookah bars. Cancer Epidemiol Biomarkers Prev 23: 1055–1066, 2014. doi: 10.1158/1055-9965.EPI-13-0939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.St Helen G, Goniewicz ML, Dempsey D, Wilson M, Jacob P 3rd, Benowitz NL. Exposure and kinetics of polycyclic aromatic hydrocarbons (PAHs) in cigarette smokers. Chem Res Toxicol 25: 952–964, 2012. doi: 10.1021/tx300043k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.St Helen G, Havel C, Dempsey DA, Jacob P 3rd, Benowitz NL. Nicotine delivery, retention and pharmacokinetics from various electronic cigarettes. Addiction 111: 535–544, 2016. doi: 10.1111/add.13183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.St Helen G, Jacob P 3rd, Peng M, Dempsey DA, Hammond SK, Benowitz NL. Intake of toxic and carcinogenic volatile organic compounds from secondhand smoke in motor vehicles. Cancer Epidemiol Biomarkers Prev 23: 2774–2782, 2014. doi: 10.1158/1055-9965.EPI-14-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.St Helen G, Novalen M, Heitjan DF, Dempsey D, Jacob P 3rd, Aziziyeh A, Wing VC, George TP, Tyndale RF, Benowitz NL. Reproducibility of the nicotine metabolite ratio in cigarette smokers. Cancer Epidemiol Biomarkers Prev 21: 1105–1114, 2012. doi: 10.1158/1055-9965.EPI-12-0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Stein YS, Antal MJ Jr, Jones M Jr. A study of the gas-phase pyrolysis of glycerol. J Anal Appl Pyrolysis 4: 283–296, 1983. doi: 10.1016/0165-2370(83)80003-5. [DOI] [Google Scholar]
- 241.Stepanov I, Carmella SG, Briggs A, Hertsgaard L, Lindgren B, Hatsukami D, Hecht SS. Presence of the carcinogen N′-nitrosonornicotine in the urine of some users of oral nicotine replacement therapy products. Cancer Res 69: 8236–8240, 2009. doi: 10.1158/0008-5472.CAN-09-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Stepanov I, Hecht SS, Lindgren B, Jacob P 3rd, Wilson M, Benowitz NL. Relationship of human toenail nicotine, cotinine, and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol to levels of these biomarkers in plasma and urine. Cancer Epidemiol Biomarkers Prev 16: 1382–1386, 2007. doi: 10.1158/1055-9965.EPI-07-0145. [DOI] [PubMed] [Google Scholar]
- 243.Stepanov I, Jensen J, Hatsukami D, Hecht SS. Tobacco-specific nitrosamines in new tobacco products. Nicotine Tob Res 8: 309–313, 2006. doi: 10.1080/14622200500490151. [DOI] [PubMed] [Google Scholar]
- 244.Stepanov I, Sebero E, Wang R, Gao YT, Hecht SS, Yuan JM. Tobacco-specific N-nitrosamine exposures and cancer risk in the Shanghai Cohort Study: remarkable coherence with rat tumor sites. Int J Cancer 134: 2278–2283, 2014. doi: 10.1002/ijc.28575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Substance Abuse and Mental Health Services Administration. Population Data/NSDUH: Results from the 2011 and 2012 National Survey on Drug Use and Health: Summary of National Findings and Detailed Tables. http://www.samhsa.gov/data/population-data-nsduh/reports?tab=38 [12 Sept 2014].
- 246.Sugiyama S, Tanaka H, Bando T, Nakagawa K, Sotowa K-I, Katou Y, Mori T, Yasukawa T, Ninomiya W. Liquid-phase oxidation of propylene glycol using heavy-metal-free Pd/C under pressurized oxygen. Catal Today 203: 116–121, 2013. doi: 10.1016/j.cattod.2012.02.064. [DOI] [Google Scholar]
- 247.Suwazono Y, Kido T, Nakagawa H, Nishijo M, Honda R, Kobayashi E, Dochi M, Nogawa K. Biological half-life of cadmium in the urine of inhabitants after cessation of cadmium exposure. Biomarkers 14: 77–81, 2009. doi: 10.1080/13547500902730698. [DOI] [PubMed] [Google Scholar]
- 248.Talhout R, Schulz T, Florek E, van Benthem J, Wester P, Opperhuizen A. Hazardous compounds in tobacco smoke. Int J Environ Res Public Health 8: 613–628, 2011. doi: 10.3390/ijerph8020613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Talih S, Balhas Z, Eissenberg T, Salman R, Karaoghlanian N, El Hellani A, Baalbaki R, Saliba N, Shihadeh A. Effects of user puff topography, device voltage, and liquid nicotine concentration on electronic cigarette nicotine yield: measurements and model predictions. Nicotine Tob Res 17: 150–157, 2015. doi: 10.1093/ntr/ntu174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Tarran R. Regulation of airway surface liquid volume and mucus transport by active ion transport. Proc Am Thorac Soc 1: 42–46, 2004. doi: 10.1513/pats.2306014. [DOI] [PubMed] [Google Scholar]
- 251.Tarran R, Trout L, Donaldson SH, Boucher RC. Soluble mediators, not cilia, determine airway surface liquid volume in normal and cystic fibrosis superficial airway epithelia. J Gen Physiol 127: 591–604, 2006. doi: 10.1085/jgp.200509468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Taylor JK. Quality Assurance of Chemical Measurements. Boca Raton, FL: CRC, 1987. [Google Scholar]
- 253.Tellez-Plaza M, Navas-Acien A, Caldwell KL, Menke A, Muntner P, Guallar E. Reduction in cadmium exposure in the United States population, 1988-2008: the contribution of declining smoking rates. Environ Health Perspect 120: 204–209, 2012. doi: 10.1289/ehp.1104020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Thuan NT, Migueres ML, Roche D, Roussel G, Mahuzier G, Chretien J, Ekindjian OG. Elimination of caffeine interference in HPLC determination of urinary nicotine and cotinine. Clin Chem 35: 1456–1459, 1989. [PubMed] [Google Scholar]
- 255.Tierney PA, Karpinski CD, Brown JE, Luo W, Pankow JF. Flavour chemicals in electronic cigarette fluids. Tob Control 25, e1: e10–e15, 2016. doi: 10.1136/tobaccocontrol-2014-052175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Tomcik K, Ibarra RA, Sadhukhan S, Han Y, Tochtrop GP, Zhang GF. Isotopomer enrichment assay for very short chain fatty acids and its metabolic applications. Anal Biochem 410: 110–117, 2011. doi: 10.1016/j.ab.2010.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Tsujino T, Ohigashi S, Sugiyama S, Kawashiro K, Hayashi H. Oxidation of propylene glycol and lactic acid to pyruvic acid in aqueous phase catalyzed by lead-modified palladium-on-carbon and related systems. J Mol Catal 71: 25–35, 1992. doi: 10.1016/0304-5102(92)80005-2. [DOI] [Google Scholar]
- 258.Turner JA, Sillett RW, McNicol MW. Effect of cigar smoking on carboxyhaemoglobin and plasma nicotine concentrations in primary pipe and cigar smokers and ex-cigarette smokers. BMJ 2: 1387–1389, 1977. doi: 10.1136/bmj.2.6099.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Uchida K. Current status of acrolein as a lipid peroxidation product. Trends Cardiovasc Med 9: 109–113, 1999. doi: 10.1016/S1050-1738(99)00016-X. [DOI] [PubMed] [Google Scholar]
- 260.Uchiyama S, Tomizawa T, Inaba Y, Kunugita N. Simultaneous determination of volatile organic compounds and carbonyls in mainstream cigarette smoke using a sorbent cartridge followed by two-step elution. J Chromatogr A 1314: 31–37, 2013. doi: 10.1016/j.chroma.2013.09.019. [DOI] [PubMed] [Google Scholar]
- 261.Underner M, Perriot J. [Smokeless tobacco]. Rev Mal Respir 28: 978–994, 2011. doi: 10.1016/j.rmr.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 261a.U.S. Department of Health and Human Services. How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2010. [Google Scholar]
- 261b.U.S. Food and Drug Administration Harmful and potentially harmful constituents in tobacco products and tobacco smoke: established list. Fed Regist 77: 20034–20037, 2012. [Google Scholar]
- 262.van Sittert NJ, Megens HJ, Watson WP, Boogaard PJ. Biomarkers of exposure to 1,3-butadiene as a basis for cancer risk assessment. Toxicol Sci 56: 189–202, 2000. doi: 10.1093/toxsci/56.1.189. [DOI] [PubMed] [Google Scholar]
- 263.Vansickel AR, Weaver MF, Eissenberg T. Clinical laboratory assessment of the abuse liability of an electronic cigarette. Addiction 107: 1493–1500, 2012. doi: 10.1111/j.1360-0443.2012.03791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Vartiainen E, Seppälä T, Lillsunde P, Puska P. Validation of self reported smoking by serum cotinine measurement in a community-based study. J Epidemiol Community Health 56: 167–170, 2002. doi: 10.1136/jech.56.3.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Vélez de Mendizábal N, Jones DR, Jahn A, Bies RR, Brown JW. Nicotine and cotinine exposure from electronic cigarettes: a population approach. Clin Pharmacokinet 54: 615–626, 2015. doi: 10.1007/s40262-014-0221-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Viswanathan CT, Bansal S, Booth B, DeStefano AJ, Rose MJ, Sailstad J, Shah VP, Skelly JP, Swann PG, Weiner R. Quantitative bioanalytical methods validation and implementation: best practices for chromatographic and ligand binding assays. Pharm Res 24: 1962–1973, 2007. doi: 10.1007/s11095-007-9291-7. [DOI] [PubMed] [Google Scholar]
- 267.Vonder Haar M. Nielsen: e-cig sales growth continues to decline. Segment is down, despite strong showing from Vuse. CSP Magazine. http://www.cspdailynews.com/category-news/tobacco/articles/nielsen-e-cig-sales-growth-continues-decline [3 Feb 2016].
- 268.Wagener TL, Floyd EL, Stepanov I, Driskill LM, Frank SG, Meier E, Leavens EL, Tackett AP, Molina N, Queimado L. Have combustible cigarettes met their match? The nicotine delivery profiles and harmful constituent exposures of second-generation and third-generation electronic cigarette users. Tob Control 26: e23–e28, 2017. doi: 10.1136/tobaccocontrol-2016-053041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Wagenknecht LE, Cutter GR, Haley NJ, Sidney S, Manolio TA, Hughes GH, Jacobs DR. Racial differences in serum cotinine levels among smokers in the Coronary Artery Risk Development in (Young) Adults study. Am J Public Health 80: 1053–1056, 1990. doi: 10.2105/AJPH.80.9.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Wagner S, Scholz K, Donegan M, Burton L, Wingate J, Völkel W. Metabonomics and biomarker discovery: LC-MS metabolic profiling and constant neutral loss scanning combined with multivariate data analysis for mercapturic acid analysis. Anal Chem 78: 1296–1305, 2006. doi: 10.1021/ac051705s. [DOI] [PubMed] [Google Scholar]
- 271.Wagner S, Scholz K, Sieber M, Kellert M, Voelkel W. Tools in metabonomics: an integrated validation approach for LC-MS metabolic profiling of mercapturic acids in human urine. Anal Chem 79: 2918–2926, 2007. doi: 10.1021/ac062153w. [DOI] [PubMed] [Google Scholar]
- 272.Wald NJ, Idle M, Boreham J, Bailey A. Carbon monoxide in breath in relation to smoking and carboxyhaemoglobin levels. Thorax 36: 366–369, 1981. doi: 10.1136/thx.36.5.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Wald NJ, Idle M, Boreham J, Bailey A, Van Vunakis H. Serum cotinine levels in pipe smokers: evidence against nicotine as cause of coronary heart disease. Lancet 2: 775–777, 1981. doi: 10.1016/S0140-6736(81)90187-2. [DOI] [PubMed] [Google Scholar]
- 274.Wall MA, Johnson J, Jacob P, Benowitz NL. Cotinine in the serum, saliva, and urine of nonsmokers, passive smokers, and active smokers. Am J Public Health 78: 699–701, 1988. doi: 10.2105/AJPH.78.6.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Watzek N, Scherbl D, Feld J, Berger F, Doroshyenko O, Fuhr U, Tomalik-Scharte D, Baum M, Eisenbrand G, Richling E. Profiling of mercapturic acids of acrolein and acrylamide in human urine after consumption of potato crisps. Mol Nutr Food Res 56: 1825–1837, 2012. doi: 10.1002/mnfr.201200323. [DOI] [PubMed] [Google Scholar]
- 276.Wei B, Alwis KU, Li Z, Wang L, Valentin-Blasini L, Sosnoff CS, Xia Y, Conway KP, Blount BC. Urinary concentrations of PAH and VOC metabolites in marijuana users. Environ Int 88: 1–8, 2016. doi: 10.1016/j.envint.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Wei B, Blount BC, Xia B, Wang L. Assessing exposure to tobacco-specific carcinogen NNK using its urinary metabolite NNAL measured in US population: 2011-2012. J Expo Sci Environ Epidemiol 26: 249–256, 2016. doi: 10.1038/jes.2014.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Williams M, To A, Bozhilov K, Talbot P. Strategies to reduce tin and other metals in electronic cigarette aerosol. PLoS One 10: e0138933, 2015. doi: 10.1371/journal.pone.0138933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Worthington EN, Tarran R. Methods for ASL measurements and mucus transport rates in cell cultures. Methods Mol Biol 742: 77–92, 2011. doi: 10.1007/978-1-61779-120-8_5. [DOI] [PubMed] [Google Scholar]
- 280.Xia Y, Bernert JT, Jain RB, Ashley DL, Pirkle JL. Tobacco-specific nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) in smokers in the United States: NHANES 2007-2008. Biomarkers 16: 112–119, 2011. doi: 10.3109/1354750X.2010.533288. [DOI] [PubMed] [Google Scholar]
- 281.Xu X, Zhang J, Zhang L, Liu W, Weisel CP. Selective detection of monohydroxy metabolites of polycyclic aromatic hydrocarbons in urine using liquid chromatography/triple quadrupole tandem mass spectrometry. Rapid Commun Mass Spectrom 18: 2299–2308, 2004. doi: 10.1002/rcm.1625. [DOI] [PubMed] [Google Scholar]
- 282.Yuan JM, Butler LM, Stepanov I, Hecht SS. Urinary tobacco smoke-constituent biomarkers for assessing risk of lung cancer. Cancer Res 74: 401–411, 2014. doi: 10.1158/0008-5472.CAN-13-3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Zhao T, Shu S, Guo Q, Zhu Y. Effects of design parameters and puff topography on heating coil temperature and mainstream aerosols in electronic cigarettes. Atmos Environ 134: 61–69, 2016. doi: 10.1016/j.atmosenv.2016.03.027. [DOI] [Google Scholar]
- 284.Zhong Y, Carmella SG, Hochalter JB, Balbo S, Hecht SS. Analysis of r-7,t-8,9,c-10-tetrahydroxy-7,8,9,10-tetrahydrobenzo[a]pyrene in human urine: a biomarker for directly assessing carcinogenic polycyclic aromatic hydrocarbon exposure plus metabolic activation. Chem Res Toxicol 24: 73–80, 2011. doi: 10.1021/tx100287n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Zhong Y, Carmella SG, Upadhyaya P, Hochalter JB, Rauch D, Oliver A, Jensen J, Hatsukami D, Wang J, Zimmerman C, Hecht SS. Immediate consequences of cigarette smoking: rapid formation of polycyclic aromatic hydrocarbon diol epoxides. Chem Res Toxicol 24: 246–252, 2011. doi: 10.1021/tx100345x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Zhu AZ, Renner CC, Hatsukami DK, Swan GE, Lerman C, Benowitz NL, Tyndale RF. The ability of plasma cotinine to predict nicotine and carcinogen exposure is altered by differences in CYP2A6: the influence of genetics, race, and sex. Cancer Epidemiol Biomarkers Prev 22: 708–718, 2013. doi: 10.1158/1055-9965.EPI-12-1234-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Zhu SH, Sun JY, Bonnevie E, Cummins SE, Gamst A, Yin L, Lee M. Four hundred and sixty brands of e-cigarettes and counting: implications for product regulation. Tob Control 23, Suppl 3: iii3–iii9, 2014. doi: 10.1136/tobaccocontrol-2014-051670. [DOI] [PMC free article] [PubMed] [Google Scholar]