Abstract
Design
We evaluated the subclinical shedding of 6 different herpesviruses in antiretroviral drug-treated HIV(+) men who have sex with men (MSM), and determined how this is associated with markers of inflammation and immune activation.
Methods
We obtained blood, semen, throat washing, urine, and stool from 15 antiretroviral-treated HIV-1-infected MSM with CD4+ T cell reconstitution, and 12 age-matched HIV(−) MSM from the Multicenter AIDS Cohort Study at 4 timepoints over 24 weeks to measure DNA levels of cytomegalovirus (CMV), Epstein-Barr virus (EBV), herpes simplex virus 1 and 2, human herpesvirus 6 (HHV6), and HHV8. T cell activation and plasma levels of soluble markers of inflammation and activation were also measured at the corresponding timepoints.
Results
HIV(+) participants had a trend for higher total herpesvirus shedding rate. HIV(+) participants also had a significantly higher rate of shedding EBV and CMV compared to the HIV(−) group. Herpesvirus shedding was mostly seen in throat washings. In the HIV(+) group, herpesvirus shedding rate inversely correlated with plasma levels of interferon gamma-induced protein 10 (IP-10) and soluble CD163 (sCD163). CMV DNA levels negatively correlated with levels of T cell activation. There was a trend for a positive correlation between EBV shedding rate and plasma soluble CD14. HHV6 shedding rate negatively correlated with plasma levels of interleukin-6, sCD163, and IP-10. Correlations were not observed among HIV(−) individuals.
Conclusions
Among treated HIV-infected MSM, there are higher subclinical shedding rates of some herpesviruses that occurs in different body compartments and negatively correlates with levels of inflammation and immune activation.
Keywords: HIV, herpesvirus shedding, inflammation, immune activation, ART
INTRODUCTION
Despite the increase in life expectancy of HIV-1-infected patients on effective antiretroviral therapy (ART), chronic HIV infection has been associated with increased risk for non-AIDS-associated chronic diseases such as cardiovascular disease, dementia, osteoporosis, and HIV-associated malignancies.[1–5] This increased risk has been linked to elevated levels of systemic inflammation.[6–8] It is hypothesized that elevated levels of T cell immune activation in chronic, treated HIV could be due to: a) preferential depletion of CD4+ T-cells in the gut mucosa early in infection leading to the translocation of microbial products;[9, 10] b) reactivation of other viruses such as cytomegalovirus (CMV) and Epstein Barr virus (EBV);[11–15] and c) T cell dysregulation.[16, 17] Any one or a combination of these mechanisms could lead to the elevated levels of T-cell activation and increased systemic inflammation.
A significant proportion of humans, depending on geographic locale, age, socioeconomic status, person-to-person close contact, and HIV-1 infection, have latent and life-long herpesvirus infections. Herpesviruses infections are common in men who have sex with men (MSM), with CMV and EBV having a >90–95% seroprevalence in HIV-1-infected and seronegative MSM in the Multicenter AIDS Cohort Study (MACS).[18] Indeed, CMV-specific memory T cells are found in large numbers in the blood of both HIV(+) and HIV(−) elderly individuals, presumably due to repeated T cell expansion in response to persistent CMV reactivation and antigen turnover from latent reservoirs,[19, 20] resulting in an exhausted and senescent T cell phenotype.[21]
In a study by Hunt et al., HIV-infected individuals with incomplete T cell recovery on ART who received the CMV drug valganciclovir, had a significant decrease in CD8+ T cell activation compared to those who received placebo,[22] suggesting that CMV reactivation in blood or other anatomic compartments could be driving T cell activation. In addition, CMV replication in semen of virally suppressed HIV-infected individuals has been associated with seminal shedding of HIV as well as higher levels of proviral HIV DNA in blood.[12, 23] A similar association has been seen between higher levels of EBV DNA and HIV DNA in blood,[24] and concomitant seminal shedding of EBV and CMV with HIV-1 levels in blood and semen.[25] Apart from semen and blood, herpesviruses have also been detected in the oral cavity of treated HIV-infected children.[26] Furthermore, given the altered enteric virome in pathogenic simian immunodeficiency virus infection[27] and in AIDS,[28] persistent herpesvirus shedding could also be present in stool of treated individuals. As such, evaluation of these different compartments for herpesvirus shedding over time will be important in determining whether asymptomatic reactivation could propel chronic, persistent immune activation and elevated levels of systemic inflammation. In this study, we therefore explored the presence of subclinical reactivation of 6 different herpesviruses in 5 body compartments among 15 treated HIV(+) MSM compared with 12 age-matched, CMV antibody (Ab)(+), HIV(−) controls at 4 different time points over 24 weeks, and evaluated whether herpesvirus reactivation correlated with levels of immune activation and inflammation.
METHODS
Participants and Samples
Participants for this study were recruited from the Pittsburgh clinical site in the MACS, an ongoing prospective cohort study of HIV infection, comprised of MSM from Pittsburgh, PA.[29] This study was approved by the University of Pittsburgh (IRB# PRO12030691). Written informed consent was obtained from all study participants.
We enrolled HIV-1-infected, CMV IgG Ab(+) individuals who were virally suppressed on ART for at least 48 weeks and had CD4+ T cell counts ≥500 cells/mm3 [HIV(+)]. We also enrolled age-matched (±2y) CMV IgG Ab(+) HIV-1-seronegative controls [HIV(−)]. Participants receiving acyclovir, valacyclovir, famciclovir, ganciclovir, or valganciclovir were excluded, and use of these medications was asked at every study visit. Blood (plasma and whole blood), throat washings, semen, urine, and stool were obtained from each participant at 4 study visits (0, 4, 8 and 24 weeks) in order to evaluate levels of EBV, CMV, herpes simplex 1 and 2 (HSV1 and HSV2), and human herpesvirus 6 and 8 (HHV6 and HHV8) DNA. At the same timepoints, peripheral blood mononuclear cells (PBMC) and plasma were collected for immunologic assays.
Herpesvirus Serology Testing
To determine serostatus for each herpesvirus, a cryopreserved serum sample obtained from each participant from the MACS visit date prior to enrollment to this study was sent to a commercial clinical laboratory to determine the presence of immunoglobulin G (IgG) antibodies to CMV, EBV (viral capsid antigen and nuclear antigen), HSV1 and 2, HHV6, and HHV8 using enzyme linked immunosorbent assay (ELISA).
Nucleic Acid Extraction and Herpesvirus DNA Quantitation
Nucleic acid was extracted from each sample using the EasyMag (bioMérieux, Durham, NC) automated extractor following manufacturer’s protocol. Before extraction, each specimen was spiked with 11000 copies of a DNA phocine herpesvirus (PhHV-1) for quality control of the extraction and PCR amplification procedures.
The extracted DNA was quantitated for HSV-1, HSV-2, CMV, EBV, HHV-6, HHV-8 and PhHV-1 by real time Taqman qPCR with respective primer pairs and probes (see supplemental methods for assay specifics including the specific primers, probes, and limits of detection for each herpesvirus evaluated).[30–36] For each PCR run, negative control, serial dilutions of the plasmids containing virus specific target sequences or quantitated viral DNA were included for generating a standard curve for virus quantitation. The plasmid containing EBV amplicon-target fragment was provided by Dr. R. Wadowsky, Children’s Hospital of Pittsburgh. Quantitated HSV-1 and HSV-2 DNA were purchased from Advanced Biotechnologies (Columbia, MD). All real time PCR assays were performed using the same format of 5µl sample DNA or standard control and 15µl of reaction mix containing 10µl of Taqman 2X Gene Expression Master Mix (Applied Biosystems, Foster City, CA) and a final concentration of 1.2µM forward and reverse primers and 0.3µM probe. Cycle conditions were identical for all quantitated DNA real time PCR assays: stage 1, 50°C for 2 minutes: stage 2 (Taq inhibitor inactivation), 95°C for 10 min, and PCR amplification (45 cycles), 95°C for 15 seconds and 60°C for 1 min. Levels are expressed as copies per milliliter of specimen.
Measurement of Percent T cell Activation
Cryopreserved PBMCs from three timepoints (weeks 4, 8, 24) were thawed and stained with the following monoclonal antibodies (mAb): anti-CD3 APC-H7, anti-CD4 PE-CF594, anti-CD8 AF700, anti-HLA-DR PE, anti-CD38 APC, all BD Biosciences. Fixed samples were analyzed within 24h of staining using an LSR Fortessa (BD Biosciences) and FlowJo version 10.0.7 software (TreeStar, OR, USA). Anti-mouse Ig compensation beads stained with each appropriate fluorophore in the immune activation panel were used as compensation controls. FMO (fluorescence minus one) controls were used to set the gates for CD4+ and CD8+ T cells and immune activation markers.
Measurement of Soluble Marker Levels
Stored plasma samples from all 4 timepoints were thawed and analyzed in batch. Commercially available ELISA kits were used to determine the plasma concentrations of soluble CD14 (sCD14; R&D, Minneapolis, MN), soluble CD163 (sCD163; R&D), interleukin 6 (IL-6; R&D), interferon gamma-induced protein 10 (IP-10; R&D), and C-reactive protein (CRP; R&D) according to manufacturer’s instructions. Duplicates of 20% of the samples were included in each ELISA plate. Results were analyzed using an ELX808 ELISA reader (Biotek, Vinooski, VT) using Gen5 software v2.06.
Statistical Analysis
GraphPad Prism v.6.05 was used for data analysis. Viral load and soluble marker results are expressed as log-converted means unless otherwise specified. Data is presented as means with standard error of the mean unless otherwise specified. Kruskal-Wallis test with Dunn’s multiple comparison test were used to compare levels and rates of herpesvirus shedding and shedding in the different body compartments. Mann-Whitney test was used for nonparametric comparison of mean herpesvirus shedding rates, DNA levels, and mean levels of immune parameters between the two study groups. Differences with p values<0.05 were considered significant. Correlations were calculated using Spearman correlations. Only correlations with r values >0.5 (or < −0.5) and two-tailed p values<0.05 were considered significant.
RESULTS
Study Population and Herpesvirus Serologies
Table 1 shows the baseline demographics of the two study populations. The median age of study participants at enrollment was 43 years (range=26 to 50); the majority of participants were Caucasian. In the HIV(+) group, participants had been infected with HIV for a median of 9 years and had been virally suppressed on ART for a median of 7 years. They had median CD4+ T cell count of 803 cells/mm3 (range=555–1355) with a median CD4% of 36. Among the HIV(+) participants with available nadir CD4+ T cell counts (11/15), the median was 359 cells/mm3 (range=195–773), and none of them were diagnosed with any AIDS-defining opportunistic infections. None of the participants reported recent fever, rash, upper respiratory infection symptoms (e.g., sore throat, cough, nasal congestion) or oral or genital ulcers at any study visit. All participants from both groups were positive for IgG to CMV, EBV, and HHV6. Although the HIV(+) group had higher percentage of participants who had IgG to HSV1, HSV2, and HHV8, these were not significantly different from the HIV(−) group (p=0.66, p=0.18, and p=0.15, respectively).
TABLE 1.
Demographics of study participants
| Characteristics | HIV(+) [%] | HIV(−) [%] |
|---|---|---|
| N | 15 | 12 |
|
| ||
| Median Age in years (range) | 43 (27–50) | 43 (26–50) |
|
|
||
| Race | ||
|
|
||
| Caucasian | 12 | 11 |
|
|
||
| African-American | 3 | 1 |
|
|
||
| Median Years HIV-infected (range) | 9 (3–19) | |
|
| ||
| Median Years ART-Suppressed (range) | 7 (1–10) | |
|
| ||
| Median CD4+ T cell count (range) | 803 cells/mm3 (533–1355) | |
|
| ||
| Median CD4+ Percent (range) | 36% (27–62) | |
|
| ||
| Median CD4/CD8 ratio | 1 (0.5–1.6) | |
|
| ||
| Nadir CD4+ T cell count (range)* | 359 cells/mm3 (195–773) | |
|
| ||
| Median Pre-ART HIV-1 RNA (range)* | 16,343 cps/mL (2137–61426) | |
|
| ||
| HSV1 IgG(+) n[%] | 12 [80] | 8 [67] |
|
| ||
| HSV2 IgG(+) n[%] | 9 [60] | 5 [42] |
|
| ||
| CMV IgG(+) n[%] | 15 [100] | 12 [100] |
|
| ||
| EBV IgG(+) n[%] | 15 [100] | 12 [100] |
|
| ||
| HHV6 IgG(+) n[%] | 15 [100] | 12 [100] |
|
| ||
| HHV8 IgG(+) n[%] | 8 [53] | 3 [25] |
N=11; 4/15 HIV(+) study participants were already on antiretroviral therapy when they were enrolled in the MACS, and as such, nadir CD4+ T cell count and pre-ART plasma HIV-1 RNA could not be obtained
The table shows the baseline characteristics of the two study populations, HIV(+) and HIV(−). None of the HIV(+) participants had ever been diagnosed with an AIDS-defining condition. None were started on antiretroviral treatment during acute infection. Results of serologic testing for immunoglobulin G (IgG) antibodies of the 6 different herpesviruses (Herpes Simplex 1 and 2 [HSV1 and HSV2], Cytomegalovirus [CMV], Epstein Barr virus [EBV], and Human Herpesvirus 6 and 8 [HHV6 and HHV8]) are included. All study participants have IgG antibodies to CMV, EBV, and HHV6.
Subclinical Herpesvirus Shedding
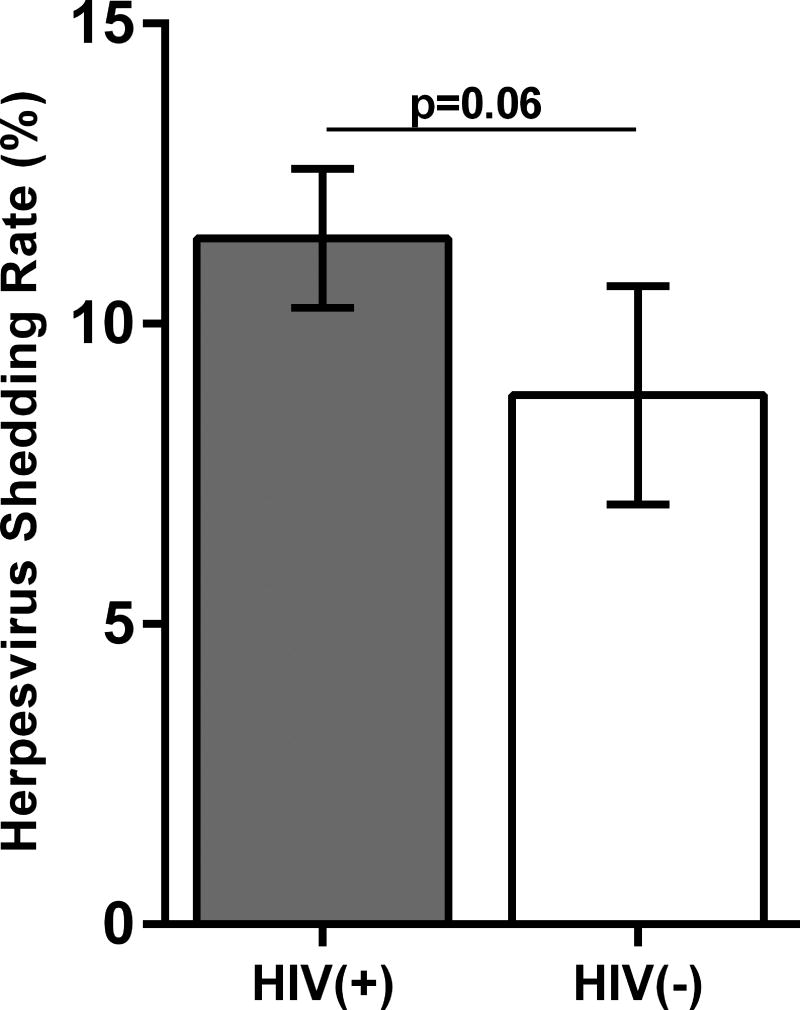
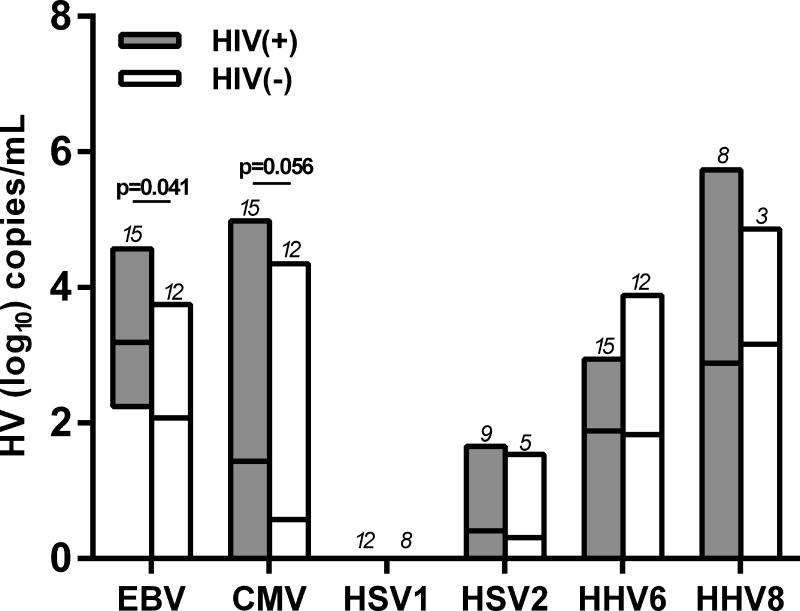
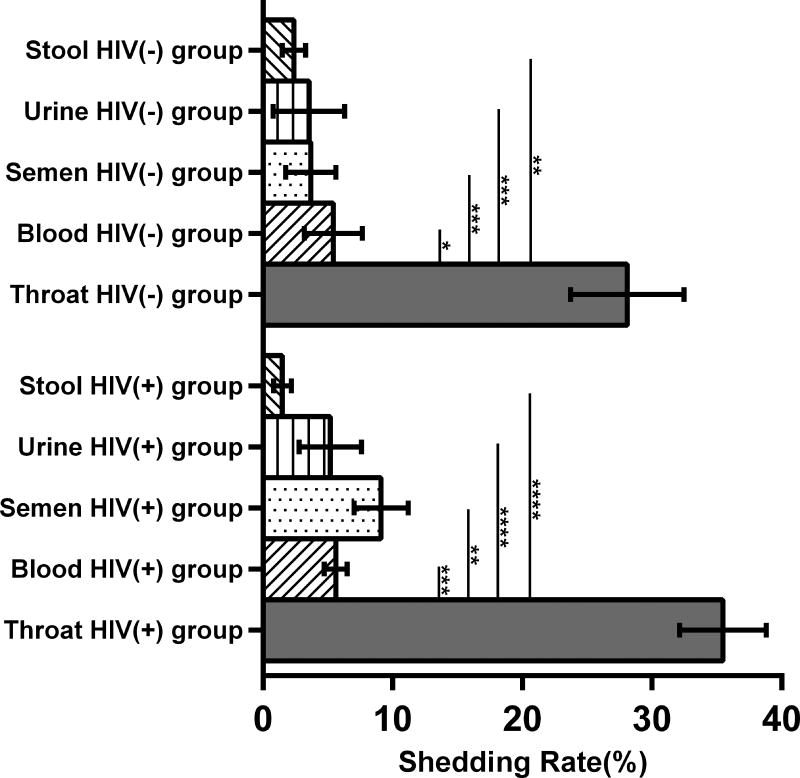
Figure 1A shows the means of the total herpesvirus shedding rate for the two study groups for the entire study. The shedding rate was calculated as the number of samples with (+)herpesvirus DNA over the total number of specimens tested throughout the 4 study visits (e.g. If a participant is IgG(+) for all the herpesviruses, the total number of samples tested for all the 6 herpeviruses=120 [5 specimens×4 timepoints×6 herpesviruses]). HIV(+) participants had a trend for a higher shedding rate compared to the HIV(−) group (mean of 11.4% vs 8.8%; p=0.06). Supplemental Figures 1A and 1B illustrate the levels of herpesvirus shedding for each body compartment at each study visit for all of the study participants. Compared to the other herpesviruses evaluated, HSV1 and HSV2 had the lowest shedding rates. Of the 12 HIV(+) and 8 HIV(−) participants with (+)IgG to HSV1, no HSV1 shedding was observed throughout the entire study period, and HSV2 shedding was infrequent [3/9 HIV(+) participants with positive HSV2 IgG and 1/5 HIV(−)]. The log-transformed mean levels of DNA shed of each herpesvirus for the two study groups are shown in Figure 1B. There were significantly higher mean levels of EBV DNA in the HIV(+) group (p=0.041), while there was a trend for higher mean CMV DNA levels (p=0.056). No differences between the two groups were observed the other herpesviruses. The rate of herpesvirus DNA shedding was highest in the throat washings compared to other body compartments in both HIV(+) and HIV(−) participants (Figure 1C). There were no differences in shedding rates among the other sampled compartments. Similarly, there was no difference in the shedding rates for each body compartment between the two study groups.
Figure 1. Herpesvirus DNA shedding levels and frequencies.
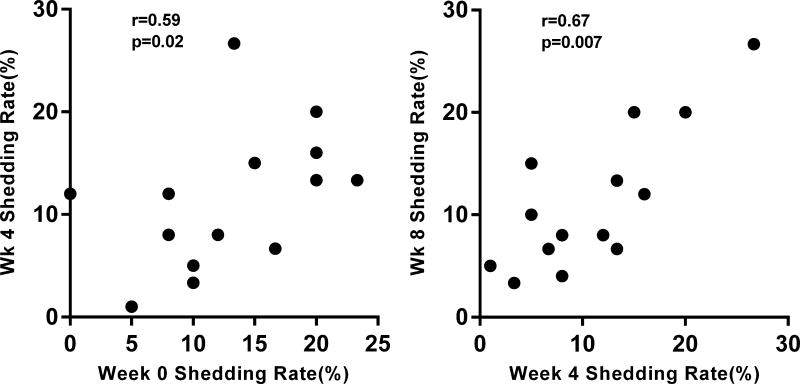
(A) Bar graphs compare the mean (with standard error of the mean; SEM) herpesvirus shedding rate between the two study groups, and show a trend for higher shedding rates among HIV(+) participants (mean= 11.4% vs 8.8%; p=0.06) (B) Floating bar graphs show the average minimum and maximum log-transformed DNA levels (copies/mL) with the mean (middle line) for each herpesvirus in the two study groups. The numbers above each bar graph correspond to the number of study participants with positive IgG for that specific herpesvirus. (C) Bar graphs compare the mean (with SEM) herpesvirus shedding rates in the different body compartments of HIV(+) and HIV(−) groups. Herpesvirus DNA shedding was highest in throat washings of the both groups (*- p<0.05; **-p<0.01; ***-p<0.001; ****-P<0.0001; Kruskal-Wallis with Dunn’s multiple comparison test) (D) Among HIV(+) participants, the herpesvirus shedding rate at one time point significantly correlated with the rate in the another time points only if they were within 4 weeks of each other (week 0 to week 4 and week 4 to week 8; Spearman correlation). These correlations were not observed in the HIV(−) group.
Next, we compared whether herpesvirus shedding rates were similar across all study visits. Among the HIV(+) groups, the shedding rates between visits that are within 4 weeks of each other (i.e., week 0 to week 4 and week 4 to week 8; Figure 1D) were significantly correlated. However, for visits that are more than a month apart, no correlation was observed (Supplemental Fig 2A). In the HIV(−) group, shedding rates between the different study visits were not correlated, although there was a trend for a positive correlated between week 4 and week 8 (r=0.55; p=0.07; Supplemental Fig 2B).
EBV, CMV, and HHV6 Shedding
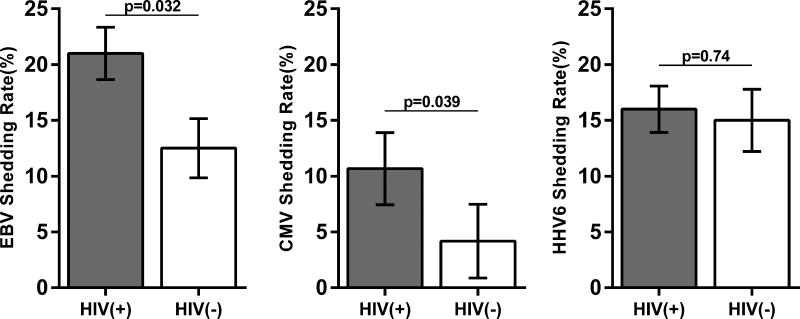
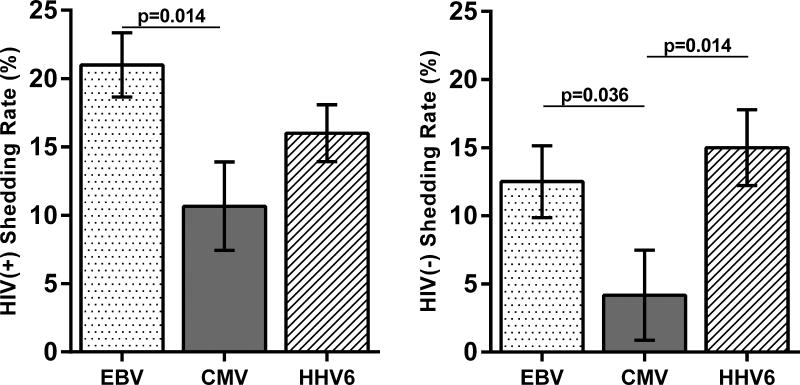
Since all participants were IgG(+) for EBV, CMV, and HHV6, we evaluated differences in reactivation of these herpesviruses among the two study groups. Figure 2A compares the shedding rates for EBV, CMV, and HHV6 DNA between the two groups. The HIV(+) group had a higher EBV DNA shedding rate (mean of 21.0% vs 12.5%; p=0.032). EBV was detected in the throat washings of all HIV(+) participants (albeit not at every timepoint) whereas it was detected in 7/12 HIV(−) participants (Supplemental Fig 1). Similarly, the HIV(+) group had a higher CMV DNA shedding rate (mean of 10.7% vs 4.2%; p=0.039). CMV was mostly detected in semen (6/15) followed by urine and throat washings (both 4/15), whereas HHV6 was mostly detected in throat washings (14/15). There was no difference in the HHV6 shedding rate between the two groups. Figure 2B compares the shedding rates of the three herpesviruses in the two study groups. Among HIV(+) participants, EBV shedding rates are higher than CMV, but are not different from HHV6, whereas among the HIV(−) group, both EBV and HHV6 had significantly higher shedding rates than CMV.
Figure 2. EBV, CMV, and HHV6 reactivation.
(A) The bar graphs of the mean (with standard error of the mean; SEM) EBV (left), CMV (center), HHV6 (right) shedding rates for the two study groups. EBV and CMV shedding rates were significantly higher among HIV(+) participants. (Mann-Whitney). (B) The bar graphs compare the mean (with SEM) shedding rates of EBV, CMV, and HHV6 among HIV(+) (left) and HIV(−) (right) participants (Kruskal-Wallis with Dunn’s multiple comparison test).
Subclinical Herpesvirus Reactivation, Immune Activation, and Inflammation in HIV
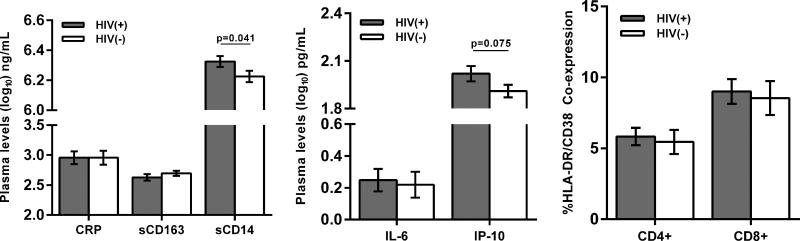
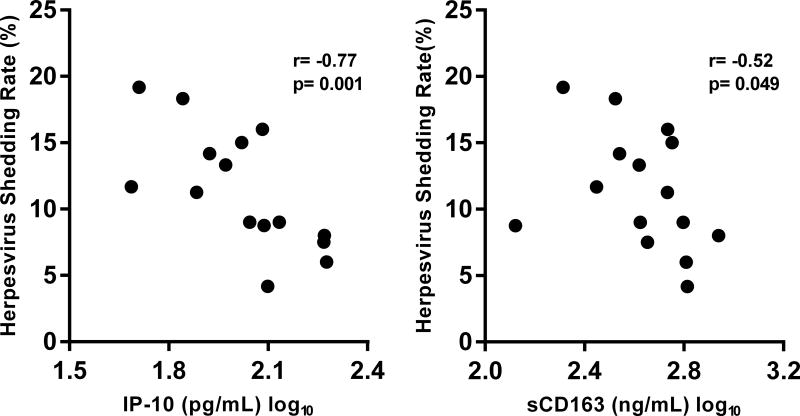
Since it is hypothesized that herpesvirus reactivation contributes to the increased levels of inflammation among treated HIV(+) individuals, we evaluated associations between subclinical herpesvirus DNA shedding and the levels of immune activation and inflammation in the HIV(+) study group. Using plasma and PBMC obtained at each timepoint we determined the average levels of monocyte (sCD14) and macrophage (sCD163) activation, systemic inflammation (IL-6, CRP, and IP-10), and T cell activation (CD4+ HLA-DR+ CD38+ or CD8+ HLA-DR+ CD38+) for each participant and compared the values between the two groups (Figure 3A). The soluble biomarkers chosen have been shown in previous studies as persistently elevated despite HIV suppression[37] or have been associated with non-AIDS chronic diseases or mortality in HIV-infected individuals.[7, 8, 38] T cell activation is also associated with HIV disease progression[39] as well as impaired vascular health among virally suppressed individuals.[40] Plasma levels of sCD14 remained elevated in the HIV(+) group despite viral suppression and CD4+ T cell reconstitution. There was also a trend for higher plasma IP-10 levels in the HIV(+) group. No differences were observed in the levels of sCD163, IL-6, and CRP. The CD4+ and CD8+ T cell activation levels for the 3 time points were also similar between the two groups. Of these different immunologic parameters, only IP-10 and sCD163 had significant associations with the herpesvirus shedding rates. The log-transformed mean plasma levels of IP-10 (r= −0.77; p=0.0001) as well as sCD163 (r= −0.52; p=0.049) inversely correlated with the mean herpesvirus shedding rate (Figure 3B). These correlations were not observed in the HIV(−) group.
Figure 3. Inflammation, immune activation, and herpesvirus shedding.
(A) Bar graphs show mean (with standard error of the mean, SEM) plasma levels of 5 soluble biomarkers (C-reactive protein [CRP], soluble CD163 [sCD163] and CD14 [sCD14], interleukin 6 [IL-6], and interferon gamma inducible protein 10 [IP-10], and soluble CD163 [sCD163]) and frequencies of CD8+ and CD4+ T cell activation (%co-expression of HLA-DR and CD38) in the HIV(+) and HIV(−) groups. HIV(+) participants had significantly higher levels of sCD14 and had a trend for higher IP-10 levels (Mann-Whitney). (B) Plasma levels of IP-10 and sCD163 negatively correlated with average herpesvirus shedding rate (Spearman correlation).
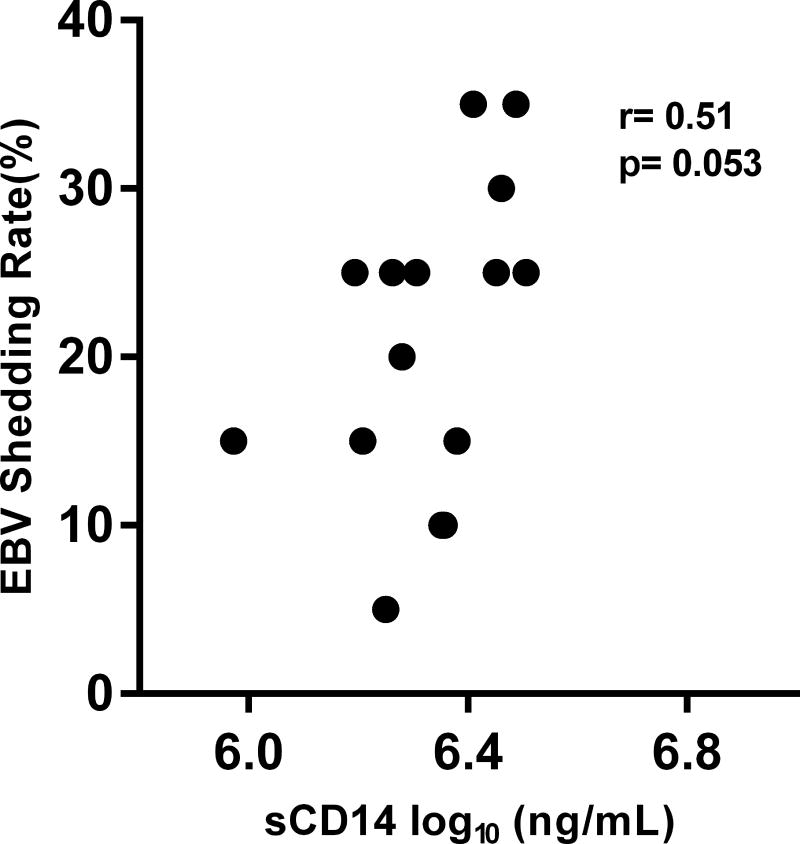
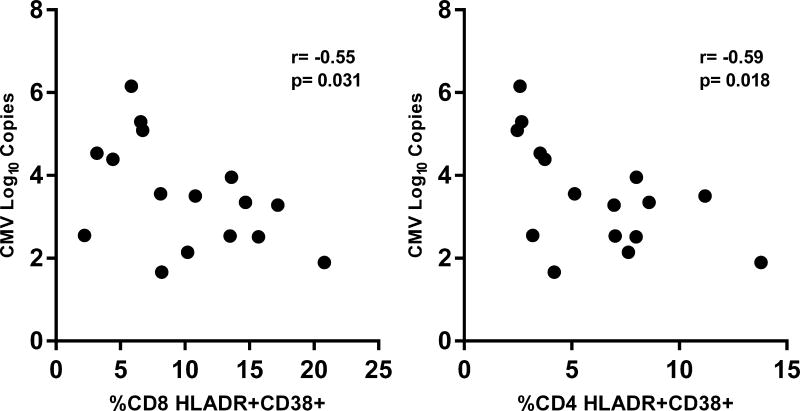
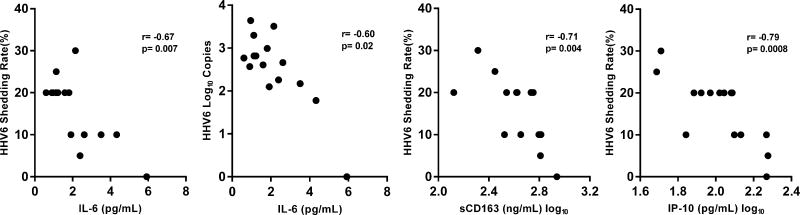
Next, we evaluated whether CMV, EBV, or HHV6 shedding rates among HIV(+) participants are associated with the different immunologic parameters. Figure 4A shows a trend towards a significant positive correlation between EBV DNA shedding rate and plasma levels of sCD14 (r=0.51; p=0.053; Figure 4A). In contrast, CMV shedding rates were not associated with any of the immune parameters. However, among HIV(+) participants who had subclinical CMV reactivation, the log-transformed levels of CMV DNA that reactivated at a particular time point negatively correlated with the frequencies of both CD8+ (r= −0.55; p=0.031) and CD4+ T cell activation (r= −0.59; p=0.018) at that corresponding time points (Figure 4B). Figure 4C shows that HHV6 DNA shedding rates negatively correlated with plasma levels of IL-6 (r= −0.67; p=0.007), IP-10 (r= −0.79; p=0.0008), and sCD163 (r= −0.71; p=0.004). In addition, the log-transformed mean HHV6 DNA copies shed negatively correlated with plasma IL-6 levels (r= −0.60; p=0.02). Shedding rates from these 3 herpesviruses did not correlate with the levels of T cell activation. These significant correlations were not observed in the HIV(−) group.
Figure 4. EBV, CMV, and HHV6 reactivation and inflammation in the HIV(+) group.
(A) There was a trend for a positive correlation between the average EBV shedding rate and the plasma levels of soluble CD14 (sCD14). (B) Among HIV(+) participants who had CMV reactivation, the levels of CMV DNA (in log10) copies shed at a particular timepoint negatively correlated with the frequencies of CD8+ (right panel) and CD4+ (left panel) T cell activation (% co-expression of HLA-DR and CD38) at the corresponding time point. (C) The average HHV6 shedding rates negatively correlated with plasma levels of interleukin-6 (IL-6; first panel), soluble CD163 (sCD163; third panel), and interferon gamma inducible protein 10 (IP-10; fourth panel). The log-transformed mean levels of HHV6 DNA also negatively correlated with the plasma IL-6 levels (second panel). All associations were tested using Spearman correlation.
DISCUSSION
The primary aim of this study was to define the characteristics of subclinical herpesvirus reactivation among ART-suppressed HIV(+) men with CD4+ T cell reconstitution throughout a 6-month period, and evaluate whether these characteristics correlate with levels of inflammation and immune activation. Our study confirms previous findings of asymptomatic herpesvirus replication in HIV-infected individuals who are suppressed on ART.[24, 41, 42] Although there was only a modest trend for higher mean shedding rates among HIV(+) participants compared to age-matched HIV(−) MSM, there are some significant findings among the HIV(+) group that will have important implications in further studies on HIV-herpesvirus co-infection. First, shedding rates at each of the four timepoints did not necessarily correlate with each other. Only timepoints that were within 4 weeks of each other showed significant positive correlations. These results highlight the importance of obtaining samples at multiple timepoints since herpesvirus DNA measured at a single timepoint may not represent the broader viral DNA shedding burden that occurs among ART-treated HIV(+) individuals.
Second, we showed that treated HIV(+) MSM not only have CMV shedding rates but also have higher EBV shedding rates and shed significantly higher EBV DNA levels compared to HIV(−) MSM. These results stress the importance of including EBV in studies looking into the role of herpesviruses in chronic HIV-associated inflammation, in order to have a better picture of how co-infection can affect HIV immunopathogenesis.
Third, we observed frequent asymptomatic HHV6 DNA shedding in our population of treated HIV(+) participants. HHV6 has been previously hypothesized to play a role in HIV disease progression among untreated individuals.[43] Although HHV6 shedding is common among immunocompetent individuals,[44] one previous study showed low levels of shedding among HIV(+) individuals.[45] Conversely, our findings show HHV6 DNA shedding was common among HIV(+) individuals, as DNA was detected in the throat washings of 14/15 HIV(+) participants, with shedding rates similar to the HIV(−) group. We believe these findings have not been previously shown among ART-treated HIV(+) individuals and could have important implications concerning persistent inflammation in treated HIV infection.
Fourth, comparison of herpesvirus DNA shedding within different body compartments showed that most episodes of shedding, specifically EBV and HHV6, were detected in throat washings rather than the other body compartments. Therefore, HIV-herpesvirus co-infection studies not evaluating oral viral reactivation are likely understating the rates of herpesvirus DNA shedding. In addition, episodes of CMV shedding among HIV(+) participants were higher in semen compared to blood. Consideration of where herpesviruses are most likely to reactivate can help in better designing studies that evaluate the relationship between herpesvirus co-infection and HIV persistence and levels of immune activation.
Lastly, we found significant correlations between several immune parameters of inflammation and immune activation and the frequency as well as the level of herpesvirus DNA shedding. When taken as a group, we observed negative correlations between herpesvirus shedding rates and some immunologic measures, i.e., herpesvirus DNA loads were lower in participants with higher levels of inflammation. It is possible that lower levels of sCD163 and IP-10 could be allowing herpesviruses to reactivate rather than the reactivation driving the high levels of these soluble markers. Therefore the presence residual immune dysfunction in treated HIV infection could be allowing increased reactivation of these herpesviruses. With CMV, although there were no correlations between the shedding rate and the immune parameters, the levels of CMV DNA among those with reactivation negatively correlated with the level T cell activation at that timepoint. We hypothesize that in these participants, the higher levels of CMV DNA being shed resulted in increased expression of checkpoint inhibitors[46] such as the programmed death receptor 1 (PD-1), thereby leading to an exhausted T cell phenotype and less immune activation. In contrast, we observed a modest trend for a positive correlation between EBV shedding rate and levels of plasma sCD14. EBV infection has been shown to play a role in inflammasome activation in monocytes,[47] so it is possible that higher rates of EBV shedding can lead to more activated monocytes. Indeed, our results showed the HIV(+) group had higher levels of sCD14 as well as higher rates of EBV shedding. Further studies should determine the specific mechanisms by which EBV reactivation could contribute to higher levels of monocyte activation in treated HIV(+) individuals, and subsequently higher systemic inflammation. We also report, for the first time, strong negative correlations between HHV6 DNA shedding rates and levels of IL-6, sCD163, and IP-10. This may be explained by the ability of HHV6 to modulate the immune system, since HHV6 has been shown impair the phenotype and function of antigen presenting cells, down-modulate the CD3+/T cell receptor complex, and expand regulatory T cells.[48, 49] As such, HHV6 DNA shedding can result in a more anti-inflammatory environment. Interestingly, these correlations were not observed among the age-matched EBV/CMV/HHV6(+) HIV(−) participants, pointing to the possibility of HIV-specific interactions with these herpesviruses affecting different immunologic parameters. It will therefore be important to determine the pathways by which HIV immune pathogenesis affects herpesvirus reactivation and vice versa so as to develop intervention strategies to lower levels in inflammation in treated HIV infection.
There are several limitations to our study. First is the small number of participants enrolled in each group. Also, since we are only assessing the MSM population, differences in the frequency and level of shedding as well as the correlations with the immune parameters are possible with HIV(+) women and thus further studies are needed for comparison. Furthermore, due to limited clinical information, it is possible the significant confounding variables such as other co-morbidities and tobacco use, may have influenced the associations we describe. Third, we were not able to include two other herpesviruses, VZV and HHV7, both of which may have asymptomatic shedding and may contribute to the levels of systemic inflammation. Despite these limitations, our results underscore important factors that should be considered in future analyses of herpesvirus shedding to fully understand how co-infection with these viruses affect HIV pathogenesis. Additional studies should also be conducted to evaluate how the episodes of reactivation as well as the levels of herpesvirus DNA being shed correlate with markers of HIV persistence.
Supplementary Material
Primary columns represent the 6 herpesviruses (EBV, CMV, HSV1 and 2, HHV6, and HHV8) studied. Subcolumns represent the body compartments sampled (T-throat washing; B-blood, Se-semen, U-urine, St-stool). Primary rows represent each study participant while subrows represent the time point when the specimens were obtained (0, 4, 8, 24 weeks). Different colors represent different levels herpesvirus DNA for a given body compartment and time point. Study participants who have no immunoglobulin G antibodies detected for a specific herpesvirus are shaded light blue. We removed HIV(−) participant 1 HHV6 results in the analyses as the presence high levels of HHV6 DNA shed in multiple body compartments is indicative of chromosomally integrated HHV6 and does not represent reactivating virus.
Among HIV(+) participants, shedding rates at one timepoint significantly correlated with another timepoint if these timepoints are within 4 weeks of each other (week 0 to 4, Suppl Fig 2A top panel left and week 4 to 8, bottom panel left). Among HIV(−) participants, a trend for a positive correlation was only observed between week 4 and week 8 shedding rates (Suppl Fig 2B, bottom panel left). All associations were tested using Spearman correlation.
Acknowledgments
This study was supported by NIH U01 AI35041 and internal funding from the Department of Medicine, University of Pittsburgh School of Medicine. The authors would like to thank Susan McQuiston, Jay Hayes, Aarika Yates, Angel Anthony, Eric Fialkovich, and Janet McLaughlin from the Rinaldo Lab, Christopher Shoff and Peter Nam from the Macatangay Lab, Jeffrey Toth and Alyssa Abebe from the Pittsburgh MACS, Nancy McCarthy, Angel Pappalardo, Patricia Peters, Renee Weinman, and most especially, the study participants of the Multicenter AIDS Cohort Study without whom this study would not be possible.
References
- 1.Islam FM, Wu J, Jansson J, Wilson DP. Relative risk of cardiovascular disease among people living with HIV: a systematic review and meta-analysis. HIV medicine. 2012;13(8):453–468. doi: 10.1111/j.1468-1293.2012.00996.x. [DOI] [PubMed] [Google Scholar]
- 2.Gibellini D, De Crignis E, Ponti C, Cimatti L, Borderi M, Tschon M, et al. HIV-1 triggers apoptosis in primary osteoblasts and HOBIT cells through TNFalpha activation. Journal of medical virology. 2008;80(9):1507–1514. doi: 10.1002/jmv.21266. [DOI] [PubMed] [Google Scholar]
- 3.Althoff KN, McGinnis KA, Wyatt CM, Freiberg MS, Gilbert C, Oursler KK, et al. Comparison of risk and age at diagnosis of myocardial infarction, end-stage renal disease, and non-AIDS-defining cancer in HIV-infected versus uninfected adults. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2015;60(4):627–638. doi: 10.1093/cid/ciu869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Freiberg MS, Chang CC, Kuller LH, Skanderson M, Lowy E, Kraemer KL, et al. HIV infection and the risk of acute myocardial infarction. JAMA internal medicine. 2013;173(8):614–622. doi: 10.1001/jamainternmed.2013.3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smits HA, Boven LA, Pereira CF, Verhoef J, Nottet HS. Role of macrophage activation in the pathogenesis of Alzheimer's disease and human immunodeficiency virus type 1-associated dementia. European journal of clinical investigation. 2000;30(6):526–535. doi: 10.1046/j.1365-2362.2000.00661.x. [DOI] [PubMed] [Google Scholar]
- 6.Longenecker CT, Jiang Y, Orringer CE, Gilkeson RC, Debanne S, Funderburg NT, et al. Soluble CD14 is independently associated with coronary calcification and extent of subclinical vascular disease in treated HIV infection. Aids. 2014;28(7):969–977. doi: 10.1097/QAD.0000000000000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mooney S, Tracy R, Osler T, Grace C. Elevated Biomarkers of Inflammation and Coagulation in Patients with HIV Are Associated with Higher Framingham and VACS Risk Index Scores. PloS one. 2015;10(12):e0144312. doi: 10.1371/journal.pone.0144312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hsu DC, Ma YF, Hur S, Li D, Rupert A, Scherzer R, et al. Plasma IL-6 levels are independently associated with atherosclerosis and mortality in HIV-infected individuals on suppressive antiretroviral therapy. Aids. 2016;30(13):2065–2074. doi: 10.1097/QAD.0000000000001149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nature medicine. 2006;12(12):1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 10.Douek D. HIV disease progression: immune activation, microbes, and a leaky gut. Topics in HIV medicine : a publication of the International AIDS Society, USA. 2007;15(4):114–117. [PubMed] [Google Scholar]
- 11.Papagno L, Spina CA, Marchant A, Salio M, Rufer N, Little S, et al. Immune activation and CD8+ T-cell differentiation towards senescence in HIV-1 infection. PLoS biology. 2004;2(2):E20. doi: 10.1371/journal.pbio.0020020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gianella S, Massanella M, Richman DD, Little SJ, Spina CA, Vargas MV, et al. Cytomegalovirus replication in semen is associated with higher levels of proviral HIV DNA and CD4+ T cell activation during antiretroviral treatment. Journal of virology. 2014;88(14):7818–7827. doi: 10.1128/JVI.00831-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Freeman ML, Mudd JC, Shive CL, Younes SA, Panigrahi S, Sieg SF, et al. CD8 T-Cell Expansion and Inflammation Linked to CMV Coinfection in ART-treated HIV Infection. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2016;62(3):392–396. doi: 10.1093/cid/civ840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maidji E, Somsouk M, Rivera JM, Hunt PW, Stoddart CA. Replication of CMV in the gut of HIV-infected individuals and epithelial barrier dysfunction. PLoS Pathog. 2017;13(2):e1006202. doi: 10.1371/journal.ppat.1006202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Freeman ML, Lederman MM, Gianella S. Partners in Crime: The Role of CMV in Immune Dysregulation and Clinical Outcome During HIV Infection. Curr HIV/AIDS Rep. 2016;13(1):10–19. doi: 10.1007/s11904-016-0297-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Catalfamo M, Wilhelm C, Tcheung L, Proschan M, Friesen T, Park JH, et al. CD4 and CD8 T cell immune activation during chronic HIV infection: roles of homeostasis, HIV, type I IFN, and IL-7. Journal of immunology. 2011;186(4):2106–2116. doi: 10.4049/jimmunol.1002000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schuler PJ, Macatangay BJ, Saze Z, Jackson EK, Riddler SA, Buchanan WG, et al. CD4(+)CD73(+) T cells are associated with lower T-cell activation and C reactive protein levels and are depleted in HIV-1 infection regardless of viral suppression. Aids. 2013;27(10):1545–1555. doi: 10.1097/QAD.0b013e328360c7f3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rahman MA, Kingsley LA, Breinig MK, Ho M, Armstrong JA, Atchison RW, et al. Enhanced antibody responses to Epstein-Barr virus in HIV-infected homosexual men. The Journal of infectious diseases. 1989;159(3):472–479. doi: 10.1093/infdis/159.3.472. [DOI] [PubMed] [Google Scholar]
- 19.Brunner S, Herndler-Brandstetter D, Weinberger B, Grubeck-Loebenstein B. Persistent viral infections and immune aging. Ageing research reviews. 2011;10(3):362–369. doi: 10.1016/j.arr.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 20.Naeger DM, Martin JN, Sinclair E, Hunt PW, Bangsberg DR, Hecht F, et al. Cytomegalovirus-specific T cells persist at very high levels during long-term antiretroviral treatment of HIV disease. PloS one. 2010;5(1):e8886. doi: 10.1371/journal.pone.0008886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Derhovanessian E, Larbi A, Pawelec G. Biomarkers of human immunosenescence: impact of Cytomegalovirus infection. Current opinion in immunology. 2009;21(4):440–445. doi: 10.1016/j.coi.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 22.Hunt PW, Martin JN, Sinclair E, Epling L, Teague J, Jacobson MA, et al. Valganciclovir reduces T cell activation in HIV-infected individuals with incomplete CD4+ T cell recovery on antiretroviral therapy. The Journal of infectious diseases. 2011;203(10):1474–1483. doi: 10.1093/infdis/jir060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gianella S, Smith DM, Vargas MV, Little SJ, Richman DD, Daar ES, et al. Shedding of HIV and human herpesviruses in the semen of effectively treated HIV-1-infected men who have sex with men. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2013;57(3):441–447. doi: 10.1093/cid/cit252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gianella S, Anderson CM, Var SR, Oliveira MF, Lada SM, Vargas MV, et al. Replication of Human Herpesviruses Is Associated with Higher HIV DNA Levels during Antiretroviral Therapy Started at Early Phases of HIV Infection. Journal of virology. 2016;90(8):3944–3952. doi: 10.1128/JVI.02638-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lisco A, Munawwar A, Introini A, Vanpouille C, Saba E, Feng X, et al. Semen of HIV-1-infected individuals: local shedding of herpesviruses and reprogrammed cytokine network. The Journal of infectious diseases. 2012;205(1):97–105. doi: 10.1093/infdis/jir700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pinheiro Rdos S, Ferreira Dde C, Nobrega F, Santos NS, Souza IP, Castro GF. Current status of herpesvirus identification in the oral cavity of HIV-infected children. Rev Soc Bras Med Trop. 2013;46(1):15–19. doi: 10.1590/0037-868217172013. [DOI] [PubMed] [Google Scholar]
- 27.Handley SA, Thackray LB, Zhao G, Presti R, Miller AD, Droit L, et al. Pathogenic simian immunodeficiency virus infection is associated with expansion of the enteric virome. Cell. 2012;151(2):253–266. doi: 10.1016/j.cell.2012.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Monaco CL, Gootenberg DB, Zhao G, Handley SA, Ghebremichael MS, Lim ES, et al. Altered Virome and Bacterial Microbiome in Human Immunodeficiency Virus-Associated Acquired Immunodeficiency Syndrome. Cell Host Microbe. 2016;19(3):311–322. doi: 10.1016/j.chom.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaslow RA, Ostrow DG, Detels R, Phair JP, Polk BF, Rinaldo CR., Jr The Multicenter AIDS Cohort Study: rationale, organization, and selected characteristics of the participants. Am J Epidemiol. 1987;126(2):310–318. doi: 10.1093/aje/126.2.310. [DOI] [PubMed] [Google Scholar]
- 30.Locatelli G, Santoro F, Veglia F, Gobbi A, Lusso P, Malnati MS. Real-time quantitative PCR for human herpesvirus 6 DNA. J Clin Microbiol. 2000;38(11):4042–4048. doi: 10.1128/jcm.38.11.4042-4048.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Niesters HG. Clinical virology in real time. Journal of clinical virology : the official publication of the Pan American Society for Clinical Virology. 2002;25(Suppl 3):S3–12. doi: 10.1016/s1386-6532(02)00197-x. [DOI] [PubMed] [Google Scholar]
- 32.Qu L, Triulzi DJ, Rowe DT, Jenkins FJ. Detection of HHV-8 (human herpesvirus-8) genomes in induced peripheral blood mononuclear cells (PBMCs) from US blood donors. Vox Sang. 2011;100(3):267–271. doi: 10.1111/j.1423-0410.2010.01404.x. [DOI] [PubMed] [Google Scholar]
- 33.Sanghavi SK, Abu-Elmagd K, Keightley MC, St George K, Lewandowski K, Boes SS, et al. Relationship of cytomegalovirus load assessed by real-time PCR to pp65 antigenemia in organ transplant recipients. Journal of clinical virology : the official publication of the Pan American Society for Clinical Virology. 2008;42(4):335–342. doi: 10.1016/j.jcv.2008.03.031. [DOI] [PubMed] [Google Scholar]
- 34.Sanghavi SK, Bullotta A, Husain S, Rinaldo CR. Clinical evaluation of multiplex real-time PCR panels for rapid detection of respiratory viral infections. Journal of medical virology. 2012;84(1):162–169. doi: 10.1002/jmv.22186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wadowsky RM, Laus S, Green M, Webber SA, Rowe D. Measurement of Epstein-Barr virus DNA loads in whole blood and plasma by TaqMan PCR and in peripheral blood lymphocytes by competitive PCR. J Clin Microbiol. 2003;41(11):5245–5249. doi: 10.1128/JCM.41.11.5245-5249.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weidmann M, Meyer-Konig U, Hufert FT. Rapid detection of herpes simplex virus and varicella-zoster virus infections by real-time PCR. J Clin Microbiol. 2003;41(4):1565–1568. doi: 10.1128/JCM.41.4.1565-1568.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wada NI, Jacobson LP, Margolick JB, Breen EC, Macatangay B, Penugonda S, et al. The effect of HAART-induced HIV suppression on circulating markers of inflammation and immune activation. Aids. 2015;29(4):463–471. doi: 10.1097/QAD.0000000000000545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Knudsen TB, Ertner G, Petersen J, Moller HJ, Moestrup SK, Eugen-Olsen J, et al. Plasma Soluble CD163 Level Independently Predicts All-Cause Mortality in HIV-1-Infected Individuals. The Journal of infectious diseases. 2016;214(8):1198–1204. doi: 10.1093/infdis/jiw263. [DOI] [PubMed] [Google Scholar]
- 39.Giorgi JV, Hultin LE, McKeating JA, Johnson TD, Owens B, Jacobson LP, et al. Shorter survival in advanced human immunodeficiency virus type 1 infection is more closely associated with T lymphocyte activation than with plasma virus burden or virus chemokine coreceptor usage. The Journal of infectious diseases. 1999;179(4):859–870. doi: 10.1086/314660. [DOI] [PubMed] [Google Scholar]
- 40.Grome HN, Barnett L, Hagar CC, Harrison DG, Kalams SA, Koethe JR. Association of T Cell and Macrophage Activation with Arterial Vascular Health in HIV. AIDS Res Hum Retroviruses. 2017;33(2):181–186. doi: 10.1089/aid.2016.0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scaggiante R, Andreis S, Basso M, Franchin E, Franzetti M, Del Vecchio C, et al. Epstein-Barr and cytomegalovirus DNA salivary shedding correlate with long-term plasma HIV RNA detection in HIV-infected men who have sex with men. Journal of medical virology. 2016;88(7):1211–1221. doi: 10.1002/jmv.24441. [DOI] [PubMed] [Google Scholar]
- 42.Pere H, Rascanu A, LeGoff J, Matta M, Bois F, Lortholary O, et al. Herpes simplex virus type 2 (HSV-2) genital shedding in HSV-2-/HIV-1-co-infected women receiving effective combination antiretroviral therapy. Int J STD AIDS. 2016;27(3):178–185. doi: 10.1177/0956462415577727. [DOI] [PubMed] [Google Scholar]
- 43.Munawwar A, Singh S. Human Herpesviruses as Copathogens of HIV Infection, Their Role in HIV Transmission, and Disease Progression. J Lab Physicians. 2016;8(1):5–18. doi: 10.4103/0974-2727.176228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cone RW, Huang ML, Ashley R, Corey L. Human herpesvirus 6 DNA in peripheral blood cells and saliva from immunocompetent individuals. J Clin Microbiol. 1993;31(5):1262–1267. doi: 10.1128/jcm.31.5.1262-1267.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lucht E, Brytting M, Bjerregaard L, Julander I, Linde A. Shedding of cytomegalovirus and herpesviruses 6, 7, and 8 in saliva of human immunodeficiency virus type 1-infected patients and healthy controls. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 1998;27(1):137–141. doi: 10.1086/514604. [DOI] [PubMed] [Google Scholar]
- 46.Dan JM, Massanella M, Smith DM, Spina CA, Schrier R, Daar ES, et al. Brief Report: Effect of CMV and HIV Transcription on CD57 and PD-1 T-Cell Expression During Suppressive ART. J Acquir Immune Defic Syndr. 2016;72(2):133–137. doi: 10.1097/QAI.0000000000000936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Torii Y, Kawada JI, Murata T, Yoshiyama H, Kimura H, Ito Y. Epstein-Barr virus infection-induced inflammasome activation in human monocytes. PloS one. 2017;12(4):e0175053. doi: 10.1371/journal.pone.0175053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lusso P. HHV-6 and the immune system: mechanisms of immunomodulation and viral escape. Journal of clinical virology : the official publication of the Pan American Society for Clinical Virology. 2006;37(Suppl 1):S4–10. doi: 10.1016/S1386-6532(06)70004-X. [DOI] [PubMed] [Google Scholar]
- 49.Wang F, Yao K, Yin QZ, Zhou F, Ding CL, Peng GY, et al. Human herpesvirus-6-specific interleukin 10-producing CD4+ T cells suppress the CD4+ T-cell response in infected individuals. Microbiol Immunol. 2006;50(10):787–803. doi: 10.1111/j.1348-0421.2006.tb03855.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Primary columns represent the 6 herpesviruses (EBV, CMV, HSV1 and 2, HHV6, and HHV8) studied. Subcolumns represent the body compartments sampled (T-throat washing; B-blood, Se-semen, U-urine, St-stool). Primary rows represent each study participant while subrows represent the time point when the specimens were obtained (0, 4, 8, 24 weeks). Different colors represent different levels herpesvirus DNA for a given body compartment and time point. Study participants who have no immunoglobulin G antibodies detected for a specific herpesvirus are shaded light blue. We removed HIV(−) participant 1 HHV6 results in the analyses as the presence high levels of HHV6 DNA shed in multiple body compartments is indicative of chromosomally integrated HHV6 and does not represent reactivating virus.
Among HIV(+) participants, shedding rates at one timepoint significantly correlated with another timepoint if these timepoints are within 4 weeks of each other (week 0 to 4, Suppl Fig 2A top panel left and week 4 to 8, bottom panel left). Among HIV(−) participants, a trend for a positive correlation was only observed between week 4 and week 8 shedding rates (Suppl Fig 2B, bottom panel left). All associations were tested using Spearman correlation.