Abstract
Leaves are thought to be the primary carbon source for reproduction in plants, so a positive relationship between vegetative size and reproductive output is expected, establishing a trade-off between time to reproduction and reproductive output. A common response to higher temperatures due to climate changes is the induction of earlier transition into reproduction. Thus, in annual plants, earlier transition into flowering can potentially constrain plant size and reduce seed production. However, trade-offs between early reproduction and fitness are not always observed, suggesting mechanisms to escape the constraints of early flowering do exist. Here, we test whether inflorescence photosynthesis contribution to the reproductive output of Arabidopsis thaliana can offset the cost of early reproduction. We followed the development, growth rate and fitness of 15 accessions, and removed all rosette leaves at flowering (prior to the completion of inflorescence development or any fruit production) in half of the plants to determine the ability of inflorescences to maintain fitness in the absence of leaves. Although leaf removal significantly reduced fruit number, seed weight and plant height, even the most severely impacted accessions maintained 35% of their fitness with the inflorescence as the sole photosynthetic organ; and some accessions experienced no reduction in fitness. Differences between accessions in their ability to maintain fitness after leaf removal is best explained by earlier flowering time and the ability to maintain as many or more branches after leaf removal as in the control treatment. Although earlier flowering does constrain plant vegetative size, we found that inflorescence photosynthesis can significantly contribute to seed production, explaining why early flowering plants can maintain high fitness despite a reduction in vegetative size. Thus, plants can be released from the usually assumed trade-offs associated with earlier reproduction, and selection on inflorescence traits can mediate the impact of climate change on phenology.
Introduction
An organism’s life-history is shaped by its allocation to growth, maintenance (e.g. tissue repair and resistance to pathogens) and reproductive functions. Because resources are usually limited, life-history theory expects trade-offs between its allocation to different functions [1, 2]. Assuming all else remains the same, an earlier transition into reproduction is thought to restrict plant vegetative size and consequently the resources available for offspring production, reducing the quality and/or number of progeny [3,4,5,6]. Trade-offs between flowering time and vegetative size are particularly important for annual plants, because they have a single chance at maximizing fitness. There is concern that changes in the global climate may affect plant populations’ persistence and crop yield by causing many annual plant species to flower earlier [7,8,9,10]. Thus, it is important to determine whether there are mechanisms that can release annual plants from the expected trade-off between earlier flowering and fitness (yield).
Annual plants are a particularly good subject to investigate trade-offs between growth, time to reproduction, and fitness because they have distinctive vegetative and reproductive phases. A positive relationship between vegetative size and reproductive output is expected because the vegetative leaves are assumed to be the primary photosynthetic tissue responsible for carbon acquisition. Correlations between flowering time and vegetative size have been observed in a number of plants [11,12], as well as between vegetative size and reproductive output [12, 13]. However, many exceptions to the correlation between flowering time and reproductive output have also been observed [14, 15,16,17,18]. For example, Kover et al [19] compared ancestral and derived populations of Arabidopsis thaliana selected to flower earlier, and found that derived early flowering populations showed no reduction in fruit production.
A couple of mechanisms have been proposed to explain how reproductive output may be decoupled from time to reproduction. One hypothesis is that variation in the growth rate can remove the relationship between age of reproduction and plant size [2,18,20]. Thus, earlier flowering plants may also be larger if they have a larger growth rate (i.e. leaf production over time); and therefore, have more photosynthetic resources than plants that grow slowly and reproduce later. Genetic variation in growth rate has been previously observed within plant species [14, 18, 21, 22] but its correlation with flowering time and fitness in the context of understanding trade-offs or its absence has not been considered.
Alternatively, it is possible that the reproductive structure itself (inflorescence and flowers) contributes significantly to carbon acquisition. Green reproductive structures have previously been shown to be photosynthetically active and contribute to overall carbon gain [23,24,25,26,27,28]. If this is the case, an earlier transition to flowering can increase the resources available for future reproduction by increasing photosynthetic surfaces to support reproduction; releasing plants from the evolutionary constraints posed on flowering time evolution by the trade-off between age and size at reproduction. However, how much of a plant’s reproductive output can be maintained by the inflorescence photosynthetic ability is not well known.
Significant variation in flowering time, development rate and reproductive output has been previously observed in the annual plant A. thaliana [22,29]. The development of A. thaliana is divided into a vegetative phase where a laminar rosette grows in terms of leaf number and leaf area, and a reproductive phase characterised by an inflorescence developing vertically. It has been shown that the inflorescence of A. thaliana contributes significantly to carbon gain [27,28]. Earley et al. [27] also showed that the contribution of the inflorescence to carbon gain varies among natural accessions, and suggested that this variation may be an adaptive response to the accession’s climate of origin. They propose that switching to higher photosynthetic activity in the inflorescence may be favoured in warmer climates, where the increased ambient temperature at the soil surface makes photosynthesis in the rosette leaves less efficient.
Here, we investigate whether the inflorescence photosynthetic activity in A. thaliana can maintain seed production (fitness). We use a manipulative approach of removing all rosette leaves when plants transition into reproduction. This approach leaves the inflorescence as the sole remaining photosynthetic organ in the treated plants prior to the development of branches or fruits. Control plants are grown simultaneously, where leaves are kept throughout the plant’s life-cycle. Comparison of reproductive output in treatment and control plants of 15 natural accessions of A. thaliana from a wide geographical range and different flowering times allows the determination of whether there is natural variation in the ability of inflorescences to maintain fitness.
We also consider three specific hypotheses: 1) We hypothesize that if Early et al. [27] suggestion is correct, accessions from warmer climates should have inflorescences selected to contribute more to carbon gain, and therefore should be able to maintain a higher proportion of the fitness. 2) Alternatively, it is also possible that early flowering accessions would have been selected to have inflorescences with higher potential to photosynthesize. This could be because either they have to compensate for the smaller rosette size (if there is a positive relationship between flowering time and rosette size), or because ecological conditions that favour earlier flowering such as heat and drought will also favour inflorescence photosynthesis [27,30]. 3) Finally, it is also possible that variation in fitness reduction upon removal is a function of inflorescence size, since photosynthesis capability will increase with more branches and taller inflorescences. To test these hypotheses we will determine whether there is natural variation in the ability of inflorescences to maintain fitness, and whether this variation is correlated with average temperatures in the geographical locations of origin of the accessions used, flowering time, rosette or inflorescence size.
Materials and methods
Plant material and growing conditions
Seeds from the 15 natural accessions of A. thaliana listed on Table 1 were originally obtained from the Arabidopsis stock centre (www.arabidopsis.org), and selfed twice in our lab prior to use in these experiments. These accessions were chosen because of their wide geographic distribution, and large genetic variation [31]. Also, these accessions are among the parents of the MAGIC mapping lines [32].
Table 1. Rosette growth rate measured as number of rosette leaves per day, at 23 days after planting (when plants were between 18 ad 20 days), and at flowering time (when plants have completed their vegetative growth), averaged across 10 plants per accession.
| Accession | Stock # | Origin | Latitude | Spring T | Total Leaves | Growth rate @18–20 days | Growth rate @flowering | Days to Flowering | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SE | Mean | SE | Mean | SE | Mean | SE | |||||
| Bur-0 | CS6643 | Ireland | 53N | 4.5 | 25.9 | 1.64 | 0.64 | 0.01 | 0.86 | 0.05 | 35.6 | 0.34 |
| Col-0 | CS6673 | USA | 38N | 15.5 | 14.8 | 0.65 | 0.53 | 0.02 | 0.58 | 0.04 | 32.8 | 0.36 |
| Ct-1 | CS6674 | Italy | 37N | 13.5 | 14.2 | 0.84 | 0.54 | 0.03 | 0.58 | 0.04 | 32.6 | 0.50 |
| Hi-0 | CS6736 | Netherlands | 52N | 5.5 | 12.8 | 0.49 | 0.58 | 0.02 | 0.57 | 0.02 | 28.6 | 0.45 |
| Kn-0 | CS6762 | Lithuania | 54N | 3.5 | 16.4 | 0.83 | 0.60 | 0.02 | 0.65 | 0.03 | 32.5 | 0.67 |
| Ler-0 | CS20 | Germany | 52N | 3.5 | 10.6 | 0.37 | 0.50 | 0.02 | 0.48 | 0.02 | 28.0 | 0.40 |
| Mt-0 | CS1380 | Libya | 32N | 15.5 | 18.4 | 1.01 | 0.69 | 0.02 | 0.75 | 0.04 | 32.8 | 0.49 |
| No-0 | CS6805 | Germany | 51N | 5.5 | 12.1 | 0.48 | 0.52 | 0.03 | 0.50 | 0.02 | 31.2 | 0.53 |
| Oy-0 | CS6824 | Norway | 60N | 3.5 | 15.9 | 0.28 | 0.69 | 0.02 | 0.66 | 0.02 | 32.5 | 0.27 |
| Rsch-4 | CS6850 | Russia | 56N | 1 | 17.2 | 0.07 | 0.65 | 0.04 | 0.66 | 0.42 | 33.1 | 0.81 |
| Sf-2 | CS6857 | Spain | 41N | 11.5 | 32.0 | 4.19 | 0.58 | 0.02 | 1.0 | 0.11 | 37.8 | 1.86 |
| Tsu-0 | CS6874 | Japan | 34N | 9.5 | 26.2 | 1.01 | 0.71 | 0.2 | 0.93 | 0.04 | 35.8 | 0.51 |
| Wil-2 | CS6889 | Russia | 55N | 1 | 13.5 | 0.27 | 0.63 | 0.01 | 0.61 | 0.01 | 30.0 | 0.26 |
| Ws-0 | CS6891 | Russia | 52N | 3.5 | 24.0 | 1.97 | 0.67 | 0.01 | 0.86 | 0.04 | 34.3 | 1.35 |
| Wu-0 | CS6897 | Germany | 49N | 5.5 | 16.9 | 0.86 | 0.59 | 0.03 | 0.70 | 0.03 | 33.2 | 0.79 |
Ten replicates of each accession were grown individually in 5.5 cm diameter pots filled with Levingtons F2+S compost (Scotts, Marysville,OH). Pots were randomly allocated to trays and trays were rotated around the greenhouse at regular intervals to homogenize possible microenviromental effects. The experiment was carried out in the University of Bath greenhouse, set at 21°C day/18°C night and 16 hours of light/day. Seeds were suspended in a 0.015% agar solution at 4°C for 3 days to promote simultaneous germination, before being pipetted onto the pot’s surface for germination.
Half of the replicates of each accession were randomly assigned to either the “Removal” or “Control” group. The control group was left to grow normally, while the removal group had all rosette leaves removed using scissors on the day the first flower opened (prior to any seed being developed, and when the inflorescence was still small and unbranched). All removed leaves were attached to a piece of paper and scanned for later estimation of total rosette leaf area using ImageJ (http://rsbweb.nih.gov/ij/).
Plants were inspected every couple of days and the timing of germination, flowering (day first flower opened) and senescence were noted. Senescence was defined as the day when no more open flowers were visible. Prior to removal of leaves, rosette leaves were counted on plants assigned to both treatments every couple of days until flowering (starting 10 days after planting). After the plants had senesced the inflorescence height was measured and the number of branches (derived from the inflorescence or rosette) and fruits per plant were recorded. To assess seed weight and seed number per fruit, 3 fruits between the 6th and 10th fruit on the main stem were collected. A Nikon SMZ 1500 dissecting microscope with a Nikon Digital Sight DS-U1 camera was used to capture images of seeds to determine seed number per fruit. Average seed weight per seed was measured by weighing seeds on a Mettler UMT2 Ultra Microbalance. Total fitness was estimated as the average seed number per fruit multiplied by total fruit number produced per plant. All raw data is available in Appendix 1.
Statistical analysis
All analyses were carried out in SPSS version 23 (IBM Corp.). A one-way ANOVA was used to test whether accessions differ significantly in days to flower, leaf number at flowering and growth rate. Growth rate was measured as the number of rosette leaves at bolting divided by the number of days between germination and bolting. As growth rate varies throughout rosette development, we also compared growth rate 19 days after planting. Since all of these variables were measured prior to the removal treatment, and a two-way ANOVA confirmed that none of them were affected by treatment (see below), all 10 replicates/accession were included in this set of analysis.
A two-way ANOVA was employed to analyse the effects of accession (random variable) and treatment (fixed variable) on inflorescence traits (height and number of branches), fitness components (average seed weight, average seed number per fruit, total number of fruits produced per plant, and total fitness), and senescence.
We used the ratio of the average total fitness for plants in the removal group relative to the control group for each accession, to estimate the contribution of inflorescence photosynthetic activity to fitness (from here on called “fitness maintenance”). For example, accessions that can maintain 100% of the fitness observed in control plants, despite having their leaves removed, would obtain a fitness maintenance value of 1. To investigate which factor(s) better explain variation in fitness maintained among accessions, we carried out a number of multiple regression models with fitness maintenance as the dependent variable. The independent variables included the average spring temperature in the location of origin of each accession, total leaf area, flowering time, inflorescence height, and branch number.
A caveat of our experimental design is that if rosette leaves significantly contribute to the fitness output of a plant, removal of larger rosettes might increase the cost of the leaf removal treatment. This could lead to some of the variation in fitness maintenance being due to variation in the cost of the leaf removal treatment. To address this caveat, we estimated the Pearson’s bivariate correlation between all variables measured among individual plants in the control and removal treatments, as well as correlations among accession average trait values. If the removal of leaf size affected fitness maintenance we expect that rosette size would be positively correlated with fitness under control condition, but negatively correlated in the removal treatment.
Results
Natural variation in vegetative growth and time to reproduction
There is extensive variation among the accessions studied in terms of their rate of vegetative growth, rosette size and time to reproduction (Fig 1, Table 1). Significant differences in growth rate across the 15 accessions were observed when measured across the entire vegetative stage (F14,142 = 14.7, p<0.001), or during the first 19 days of development (F14,142 = 9.1, p<0.001). Accessions also significantly differ in the number of days they take to transition into the reproductive phase (F14,142 = 11.6, p<0.001) and in final vegetative size (measured in terms of total leaf number at transition to reproduction, F14,142 = 17.8, p<0.001).
Fig 1. Natural variation among 15 accessions (10 replicates/accession) in vegetative growth curve as measured in terms of leaf number over time.
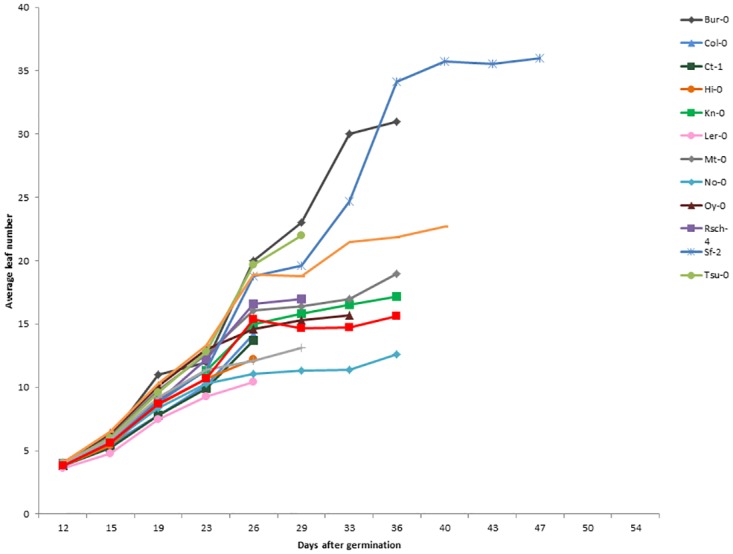
Early flowering does constrain vegetative growth, since days to flowering is strongly and positively correlated with final vegetative size measured as total rosette leaf number (Table 2). We found no evidence of faster growth rate to compensate for earlier flowering. On the contrary, the tendency is for growth rate to be positively associated with flowering time (Table 2). In other words, accessions that flower earlier also tend to have slower growth rate; e.g. Ler-0 has the lowest growth rate and earliest flowering time, as shown in Table 1.
Table 2. Bivariate Pearson’s correlation among all variables measured.
Values above the diagonal are for plants in the control group and values bellow the diagonal are for plants in the removal group. The statistical significance of the correlation is indicated by asterisks: * indicates p<0.05; and ** indicates p<0.01.
| Days to flower | Growth@ 18–20 days | Growth @ flowering | Total nr. leaves | Total leaf area | Branches | Inflorescence height | Senescence | Number of fruits | Average seed weight | Nr. Seeds/pod | Total seed nr. | |
| Days to flower | .150 | .514** | .679** | NA | 0.069 | .239* | .489** | -.231* | .282* | -.299* | -.326** | |
| Growth@ 18–20 days | .045 | .567** | .400** | NA | 0.169 | .381** | .147 | .076 | .135 | -0.173 | -0.020 | |
| Growth @ flowering | .572** | .582** | .951** | NA | .274* | .400** | .327** | -0.02 | .337** | -.238* | -0.112 | |
| Total nr. leaves | .733** | .367** | .941** | NA | 0.224 | .362** | .491** | -0.075 | .417** | -.334** | -0.214 | |
| Total leaf area | .690** | .431** | .793** | .837** | NA | NA | NA | NA | NA | NA | NA | |
| Branches | 0.057 | .449** | .439** | .252* | .484** | .301** | -0.152 | .466** | -0.048 | .254* | .487** | |
| Inflorescence height | 0.079 | .260* | .246* | 0.145 | .352** | .374** | 0.152 | .447** | 0.028 | 0.005 | .324** | |
| Senescence | .661** | -0.063 | .464** | .657** | .527** | -0.077 | -0.052 | 0.095 | .289* | -.407** | -0.184 | |
| Number of fruits | -.400** | 0.108 | -0.156 | -.274* | -0.129 | .358** | .484** | -.285* | -0.126 | 0.084 | .791** | |
| Average seed weight | .249* | 0.171 | .282* | .327** | .438** | 0.172 | 0.08 | .455** | -0.054 | -.314** | -.255* | |
| Nr. Seeds/pod | -.244* | -0.001 | -.242* | -.326** | -0.191 | 0.199 | 0.218 | -.482** | .302* | -.376** | .647** | |
| Total seed nr. | -.345** | 0.122 | -0.19 | -.314** | -0.140 | .383** | .460** | -.417** | .866** | -0.21 | .713** |
Effect of rosette removal
On average, removal of rosette leaves at flowering time had a negative impact on reproductive output (Table 3). Across all accessions, rosette removal significantly reduced inflorescence height (reduced on average by 12%), number of fruits per plant (reduced on average by 38%), and total fitness (reduced on average by 40%), as shown by statistically significant effects of treatment on a two-way ANOVA (Table 3).
Table 3. Comparison of estimated mean and standard error for each measured trait in control and treated (leaves removed at flowering) plants.
Last three columns show the F statistic and their associated probability (in brackets) from the two-way ANOVA to assess the effect of rosette removal (Treatment) and Accession on measured traits. Treatment is a fixed variable and accession is a random variable. Values in Bold indicate statistically significant effects (i.e. probability <0.05).
| Control | Removal | Treatment | Accession | Treatment*Accession | |||
|---|---|---|---|---|---|---|---|
| Mean | SE Mean | Mean | SE Mean | ||||
| Flowering | 32.4 | 0.35 | 32.9 | 0.49 | 1.4 (0.232) | 11.6 (<0.001) | 0.5 (0.955) |
| Growth | 0.69 | 0.02 | 0.68 | 0.02 | 1.0 (0.327) | 15.1 (<0.001) | 0.9 (0.553) |
| Height | 42.7 | 0.58 | 37.0 | 0.61 | 39.1(<0.001) | 13.9 (<0.001) | 2.1 (0.019) |
| Nodes | 4.3 | 0.14 | 4.2 | 0.15 | 0.2 (0.665) | 12.1 (<0.001) | 0.5 (0.925) |
| Branches | 5.3 | 0.14 | 4.9 | 0.15 | 3.0 (0.087) | 5.3 (<0.001) | 2.6 (0.003) |
| Senescence | 52.3 | 0.32 | 49.2 | 0.34 | 1.5 (0.225) | 5.1 (<0.001) | 1.0 (0.514) |
| Fruit number | 164.2 | 4.42 | 102.4 | 4.66 | 93.7 (<0.001) | 4.3 (<0.001) | 2.7 (0.002) |
| Seeds/pod | 52.1 | 1.18 | 49.2 | 1.24 | 2.9 (0.092) | 4.9 (<0.001) | 0.9 (0.529) |
| Seed weight | 22.4 | 0.46 | 21.2 | 0.48 | 3.1 (0.079) | 8.7 (<0.001) | 0.8 (0.637) |
| Total fitness | 8587.6 | 268.92 | 5116.5 | 283.32 | 79.0 (<0.001) | 6.4 (<0.001) | 2.3 (0.009) |
Although there is only a marginally significant effect of rosette removal on branch number, a significant interaction between treatment and accession was observed, indicating that the effect of rosette removal on reducing branch number depended on the specific accession. An interaction between accession and treatment was also observed for inflorescence height, fruit number and total fitness. Such interaction can be clearly seen in Fig 2, where the majority of accessions displayed a large reduction in fitness with rosettes removed, but Ct-1 and Hi-0 treated plants produced almost as many or more seeds than control plants. Given that treatment plants relied solely on inflorescence photosynthesis, the ability of the inflorescence to maintain normal levels of fitness varied among accessions.
Fig 2. Average fitness for control (light grey) and treated (dark grey) plants for each accession.
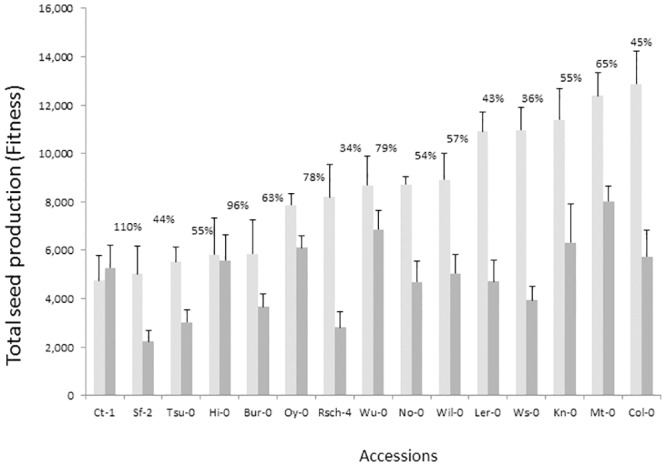
Accessions are listed in the X axis in order of their total seed production under control conditions. Numbers on the top of the bars indicate percentage of fitness maintained by inflorescence photosynthesis only (fitness maintenance).
Possible explanations for variation in inflorescence contribution across accessions
Because the two-way ANOVA (Table 3) indicated that treatment affected the number of branches and the inflorescence height, the best estimate of the accession characteristic trait value is the average number of branches and height in the control treatment. In addition, the fact that inflorescence traits respond to the leaf removal treatment differently depending on the accession, open the possibility that fitness maintenance in our experiment is not just a function of inflorescence size, but also of the specific plastic response expressed by the different accessions in response to the treatment. Thus, we also included in our regression models the ratio of the means of inflorescence traits in the two treatments to estimate this plasticity.
Models that included both number of branches and inflorescence height had colinearity problems, so we used only branches as an estimate of inflorescence size in the following model: Fitness maintenance = Average spring temperature + Flowering time + Branch number in Control treatment + Ratio of branches (number of branches in removal treatment/ number of branches in the control treatment). The full version of this model (Table 4) shows a significant effect of flowering time and branch ratio on fitness maintenance. Running forward or backward selection does not affect the model outcome. The standardized regression coefficients indicate that branch ratio has the biggest impact, with accessions that maintain or increase the number of branches after the removal of leaves having higher fitness maintenance. Flowering time has a negative coefficient, indicating that early flowering plants maintain higher fitness after leaf removal. No evidence was found that variation in fitness maintenance can be explained by the mean spring temperature of the accession’s place of origin (Table 4 and S1 Table). A model using height as an indicator of inflorescence size show similar results but those models have a lower adjusted R than the ones using branches (see S1 and S3 Tables, Supporting Information).
Table 4. Multiple linear regression model for fitness maintenance after leaf removal.
“Spring Temperature” is the average spring temperature in the place of origin of each accession, “Control branches” is the average number of branches produced per accession in the control treatment (an indicator of inflorescence size), and branch ratio is the average number of branches in the removal treatment divided by the number in the control treatment. “Std β” stands for standardized regression coefficient, “p” for probability.
| Enter Full Model | ||
|---|---|---|
| R2 = 0.82; p<0.001 | ||
| Std β | p | |
| Spring Temperature | 0.10 | 0.51 |
| Flowering Time | -0.34 | 0.03 |
| Control Branches | 0.26 | 0.21 |
| Branch Ratio | 1.01 | >0.01 |
A possible caveat of our experimental design is that leaf removal might introduce a negative impact of rosette size. This is because if plants with bigger rosettes have more resources for seed production, it follows that the removal of bigger rosettes might cause larger reduction in fitness. Although a significant negative correlation between fitness and leaf area is observed among plants in the removal treatment (see Table 2), there is also evidence for a negative correlation between rosette size and fitness on the control treatment. Leaf area was only measured on the removal treatment because it requires destructive sampling. However, leaf area is strongly and positively correlated with leaf number (0.941, Table 2), and leaf number is negatively correlated with fitness as strongly in the control as in the removal treatment. In addition, accession level correlations are negative and non-significant between leaf area and both control and removal treatment fitness (Pearson’s correlation = -0.26 and -0.33, respectively). Thus, it is unlikely that the negative impact of rosette size on fitness is only due to the removal treatment.
Discussion
Here we show that flowering time does constrain vegetative growth, but that such constraint does not affect reproductive output in a predictable way. The observed decoupling between vegetative size and reproductive output is likely due to a significant contribution of inflorescence photosynthesis, which was able to maintain a significant proportion of fitness in the plants with leaves removed. It is unlikely that carbon storage could explain the results because carbon storage in A. thaliana occurs mostly in leaves, and is mainly transitory, to cope with night time lack of irradiance for photosynthesis [33]. Thus, the significant contribution of the inflorescence photosynthetic activity to the plant’s reproductive output can potentially decouple the commonly assumed trade-off between age of reproduction and fitness [3,4,5,6].
Variation in growth rate has also been suggested as a possible mechanism to decouple size, age at reproduction and reproductive output [2,18,20]. Although we did find variation in growth rate among the accessions studied (from 0.5 to 0.7 leaves per day, Table 1), we find that flowering time still correlates strongly with final rosette size. This result suggests that variation in growth rate was not sufficient to break down the constraint exerted by flowering time on vegetative size. The maintenance of the correlation between flowering time and rosette size, despite variation in growth rate occurs because the variation in growth rate is not associated in a way that allows compensation for earlier flowering; on the contrary, our results reveal earlier flowering plants tend to have a slower growth rate (Fig 1, Table 1). Our results are in agreement with Weis et al. [34], who found that growth rate variation had little effect on the shape of selection on flowering time.
We find that inflorescence photosynthesis contribute considerably to fitness (on average 60%), with some accessions showing no reduction in fitness despite the removal of leaves. It is noteworthy that fitness reduction was not accompanied by a significant reduction in the average seed weight (Table 3); confirming the hypothesis that seed size is highly canalized [35] despite being heritable among genotypes (Gnan et al, 2014). While previous studies have shown that reproductive structures in plants are photosynthetically active [23,26,27,36], a cost of early reproduction is still implicit in most models of life-history evolution [25,34,37,38,39]; and trade-offs between size and age of reproduction are commonly used as an explanation for lack of response to selection for larger vegetative size [40] or the maintenance of variation in flowering time [8]. Our results show that despite the constraint exercised by earlier flowering on plant vegetative size, a cost of early reproduction should not always be assumed, since reproductive structures can sustain significant proportions of the reproductive output. Field experiments imposing artificial herbivory in A. thaliana found that herbivory after flowering affects seed production less than before flowering [41], which might be due to the inflorescence significantly contributing to reproductive output, as found in this study. In addition, a field experiment with Brassica rapa has shown that the commonly observed selection for early flowering, is mediated by the Julian calendar not the developmental age of the plant [38]. This suggests that independent of size, plants that flower at a certain time of the year have higher fitness. Thus, smaller vegetative size does not seem to constraint fitness of B. rapa under field conditions either.
The ability to maintain fitness while relying solely on the inflorescence photosynthetic activity varies among accessions. After leaf removal, accessions maintained between 34% and 110% of the fitness of control plants (Fig 2). Earley et al. [27] suggested that variation among accessions in inflorescence photosynthetic activity might be adaptive. They hypothesized that shifting carbon acquisition to inflorescences may be beneficial for accessions in warmer climates, because with increasing temperatures, photosynthesis would be more efficient away from the ground surface. We addressed this possibility by testing whether the average spring temperatures from the accession’s place of origin or flowering time, was associated with fitness maintenance. While we found no evidence that spring average temperatures in their place of origin explain the variation in fitness maintenance (Table 4), we recognize that there are many other climate variables that might also be relevant and were not tested (e.g. precipitation). Flowering time in A. thaliana has been previously shown to be correlated with climate gradients and can serve as a good indicator of general ecological conditions experienced during past evolution [30,42,43]. Furthermore, Earley et al [27] hypothesis specifically suggest that environmental conditions unfavourable for photosynthesis at the soil surface (where rosette leaves are located) should select for earlier transition into reproduction to provide photosynthesis under cooler air temperatures. Thus, the fact that flowering time explains some of the variation in fitness maintenance is compatible with the idea that earlier reproduction can be an adaptive strategy to better cope with stressful climatic conditions by shifting much of the carbon fixation towards the inflorescence. The fact that rosette leaf area is strongly and negatively correlated with flowering time (Table 2), and that models that include leaf area instead of flowering time find that accessions with smaller rosettes maintain more of their fitness after leaf removal (S2 Table), indicates that it is also possible that early flowering accessions have inflorescences that contribute more to fitness because they have been selected to overcompensate for small rosettes.
Variance in fitness maintenance was also shown to be explained by inflorescence size plasticity (Table 4). Some accessions (e.g Ct-1) produced inflorescences with the same height and more branches after leaf removal than in the control treatment; while most (e.g. Ler) show reduced height and number of branches after leaf removal. The reduction in inflorescence size can be explained by the fact that resources were significantly reduced by the leaf removal treatment during inflorescence development. The accessions that maintained or improved their inflorescence size were the ones that also maintained most of their fitness. The mechanism that allows some accession to grow the same size inflorescences despite the lack of leaves is unknown, but the inflorescence of accession Mt-0 has been shown to contribute 93% of the carbon gain during the plant lifetime [27]. In agreement, Mt-0 is one of the accessions that maintained the same size infloresecence in the removal or control treatments. Thus, it is possible that accession with inflorescence with more efficient photosynthetic rates will be able to maintain their inflorescence size better. It is also possible that our manipulative approach may have triggered some stress responses akin to responses to herbivory, and that accessions from locations with higher herbivory recovered better. Genetic variation in tolerance to herbivory have been previously observed [44,45], and a relationship between inflorescence architecture and tolerance to herbivory has been documented across a number of species [46]. Unfortunately, to the best of our knowledge, a mechanism through which such stress could be measured has not been characterized, and a previous study that attempted to identify quantitative trait locus underlying tolerance to herbivory was unsuccessful [44].
In summary, our manipulative approach shows unequivocally that the photosynthetic activity of the inflorescence of A. thaliana is capable of maintaining a high proportion of the plant’s fitness. While similar experiments need to be carried out in more species to confirm the generality of our results, evidence in Lolium perenne [40] and B. rapa [34] support the conclusion that assuming an inherent cost of early reproduction due to exclusive contribution of vegetative tissues to resource acquisition might not be warranted. On the contrary, our results suggest that earlier transition into reproduction can diversify life-history strategies, and might be favoured depending on environmental conditions, such as locations where soil temperatures are less optimal for photosynthesis, or where herbivory or competition compromise the ability to maintain high carbon fixation through rosettes.
Supporting information
(CSV)
(XLSX)
“Std β” stands for standardized regression coefficient, and “p” is the probability associated with each factor. Bold p values indicate significant results.
(DOCX)
“Std β” stands for standardized regression coefficient, and “p” is the probability associated with each factor. Bold p values indicate significant results.
(DOCX)
“Std β” stands for standardized regression coefficient, and “p” is the probability associated with each factor. Bold p values indicate significant results.
(DOCX)
Acknowledgments
We acknowledge useful discussions with Byron Marks and Jason Wolf. We thank the two anonymous reviewers for many constructive suggestions.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Stearns SC (1976) Life-History Tactics: A Review of the Ideas. The Quarterly Review of Biology 51: 3–47. [DOI] [PubMed] [Google Scholar]
- 2.Roff (2000) Trade-offs between growth and reproduction: an analysis of the quantitative genetic evidence. Journal of Evolutionary Biology 13: 434–445. [Google Scholar]
- 3.Obeso JR (2002) The costs of reproduction in plants. New Phytologist 155: 321–348. [DOI] [PubMed] [Google Scholar]
- 4.Gauslaa Y (2006) Trade-off between reproduction and growth in the foliose old forest lichen Lobaria pulmonaria. Basic and Applied Ecology 7: 455–460. [Google Scholar]
- 5.Colautti RI, Barrett SCH (2013) Rapid adaptation to climate facilitates range expansion of an invasive plant. Science 342: 364–366. doi: 10.1126/science.1242121 [DOI] [PubMed] [Google Scholar]
- 6.Remington DL, Leinonen PH, Leppälä J, Savolainen O (2013) Complex genetic effects on early vegetative development shape resource allocation differences between Arabidopsis lyrata Populations. Genetics 195: 1087–1102. doi: 10.1534/genetics.113.151803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Craufurd PQ, Wheeler TR (2009) Climate change and the flowering time of annual crops. Journal of Experimental Botany 60: 2529–2539. doi: 10.1093/jxb/erp196 [DOI] [PubMed] [Google Scholar]
- 8.Johansson J, Bolmgren K, Jonzén N (2013) Climate change and the optimal flowering time of annual plants in seasonal environments. Global Change Biology 19: 197–207. doi: 10.1111/gcb.12006 [DOI] [PubMed] [Google Scholar]
- 9.Haro RJ, Baldessari J, Otegui ME (2015) Genetic improvement of peanut in Argentina between 1948 and 2004: Links between phenology and grain yield determinants. Field Crops Research 174: 12–19. [Google Scholar]
- 10.Anwar MR, Li Liu D, Farquharson R, Macadam I, Abadi A, et al. (2015) Climate change impacts on phenology and yields of five broadacre crops at four climatologically distinct locations in Australia. Agricultural Systems 132: 133–144. [Google Scholar]
- 11.Mitchell-Olds T (1996) Genetic constraints on life-history evolution: quantitative-trait loci influencing growth and flowering in Arabidopsis thaliana. Evolution 50: 140–145. doi: 10.1111/j.1558-5646.1996.tb04480.x [DOI] [PubMed] [Google Scholar]
- 12.Neytcheva MS, Aarssen LW (2008) More plant biomass results in more offspring production in annuals, or does it? Oikos 117: 1298–1307. [Google Scholar]
- 13.Clauss M, Aarssen LW (1994) Phenotypic plasticity of size-fecundity relationships in Arabidopsis thaliana. The Journal of Ecology 82: 447–455. [Google Scholar]
- 14.Koornneef M, Hanhart CJ, van der Veen JH (1991) A genetic and physiological analysis of late flowering mutants in Arabidopsis thaliana. Molecular and General Genetics MGG 229: 57–66. [DOI] [PubMed] [Google Scholar]
- 15.Galen C (1993) Cost of Reproduction in Polemonium viscosum: Phenotypic and Genetic Approaches. Evolution 47: 1073–1079. doi: 10.1111/j.1558-5646.1993.tb02136.x [DOI] [PubMed] [Google Scholar]
- 16.Franco M, Silvertown J (1996) Life History Variation in Plants: An Exploration of the Fast-Slow Continuum Hypothesis. Philosophical Transactions of the Royal Society of London Series B: Biological Sciences 351: 1341–1348. [Google Scholar]
- 17.Anderson JT, Lee C-R, Mitchell-Olds T (2011) Life-history QTLs and natural selection on flowering time in Boechera stricta, a perennial relative of Arabidopsis. Evolution 65: 771–787. doi: 10.1111/j.1558-5646.2010.01175.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haselhorst MSH, Edwards CE, Rubin MJ, Weinig C (2011) Genetic architecture of life history traits and environment-specific trade-offs. Molecular Ecology 20: 4042–4058. doi: 10.1111/j.1365-294X.2011.05227.x [DOI] [PubMed] [Google Scholar]
- 19.Kover PX, Rowntree J, Scarcelli N, Savriama Y, Eldridge T, Scaal B (2009) Pleiotropic effects of environment-specific adaptation in Arabidopsis thaliana. New Phytologist 183: 816–825. doi: 10.1111/j.1469-8137.2009.02943.x [DOI] [PubMed] [Google Scholar]
- 20.Roff DA, Fairbairn DJ (2007) The evolution of trade-offs: where are we? Journal of Evolutionary Biology 20: 433–447. doi: 10.1111/j.1420-9101.2006.01255.x [DOI] [PubMed] [Google Scholar]
- 21.Stinchcombe JR, Izem R, Heschel MS, McGoey BV, Schmitt J (2010) Across environment gnetic correlations and the frequency of selective environments shape the evolutionary dynamics of growth rate in Impatiens capensis. Evolution 64: 2887–2903. doi: 10.1111/j.1558-5646.2010.01060.x [DOI] [PubMed] [Google Scholar]
- 22.Zhang X, Hause RJ, Borevitz JO (2012) Natural genetic variation for growth and development revealed by high-throughput phenotyping in Arabidopsis thaliana. G3: Genes|Genomes|Genetics 2: 29–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bazzaz F, Carlson R, Harper J (1979) Contribution to reproductive effort by photosynthesis of flowers and fruits. Nature 279: 554–555. [Google Scholar]
- 24.Watson MA, Casper BB (1984) Morphogenetic constraints on patterns of carbon distribution in plants. Annual Review of Ecology and Systematics 15: 233–258. [Google Scholar]
- 25.Reekie EG, Avila-Sakar G (2005) The shape of the trade-off function between reproduction and growth. Reproductive allocation in plants: 189–214. [Google Scholar]
- 26.Aschan G, Pfanz H (2003) Non-foliar photosynthesis–a strategy of additional carbon acquisition. Flora-Morphology, Distribution, Functional Ecology of Plants 198: 81–97. [Google Scholar]
- 27.Earley EJ, Ingland B, Winkler J, Tonsor SJ (2009) Inflorescences contribute more than rosettes to lifetime carbon gain in Arabidopsis thaliana (Brassicaceae). American Journal of Botany 96: 786–792. doi: 10.3732/ajb.0800149 [DOI] [PubMed] [Google Scholar]
- 28.Leonardos ED, Rauf SA, Weraduwage SM, Marillia E-F, Taylor DC, Micallef BJ, et al. (2014) Photosynthetic capacity of the inflorescence is a major contributor to daily-C-gain and the responsiveness of growth to elevated CO2 in Arabidopsis thaliana with repressed expression of mitochondrial-pyruvate-dehydrogenase-kinase. Environmental and Experimental Botany 107: 84–97. [Google Scholar]
- 29.Weigel D (2012) Natural variation in Arabidopsis thaliana: from molecular genetics to ecological genomics. Plant Physiology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Montesinos-Navarro A, Wig J, Xavier Pico F, Tonsor SJ (2011) Arabidopsis thaliana populations show clinal variation in a climatic gradient associated with altitude. New Phytologist 189: 282–294. doi: 10.1111/j.1469-8137.2010.03479.x [DOI] [PubMed] [Google Scholar]
- 31.Gan X, Stegle O, Behr J, Steffen JG, Drewe P, Hildebrand KL, et al. (2011) Multiple reference genomes and transcriptomes for Arabidopsis thaliana. Nature 477: 419 doi: 10.1038/nature10414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kover PX, Valdar W, Trakalo J, Scarcelli N, Ehrenreich IM, Purugganan MC, et al. (2009) A multiparent advanced generation inter-cross to fine-map quantitative traits in Arabidopsis thaliana. PLoS genetics 5: e1000551 doi: 10.1371/journal.pgen.1000551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zeeman SC, Tiessen A, Pilling E, Kato KL, Donald AM, Smith AM (2002) Starch synthesis in Arabidopsis. Granule synthesis, composition, and structure. Plant Physiology 129: 516–529. doi: 10.1104/pp.003756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weis AE, Wadgymar SM, Sekor M, Franks SJ (2014) The shape of selection: using alternative fitness functions to test predictions for selection on flowering time. Evolutionary ecology 28: 885–904. [Google Scholar]
- 35.Silvertown J (1989) The paradox of seed size and adaptation. Trends in Ecology & Evolution 4: 24–26. [DOI] [PubMed] [Google Scholar]
- 36.Laporte MM, Delph LF (1996) Sex-specific physiology and source-sink relations in the dioecious plant Silene latifolia. Oecologia 106: 63–72. doi: 10.1007/BF00334408 [DOI] [PubMed] [Google Scholar]
- 37.Araújo R, Serrão EA, Sousa-Pinto I, Arenas F, Monteiro CA, Toth G, et al. (2015) Trade-offs between life-history traits at range-edge and central locations. Journal of phycology 51: 808–818. doi: 10.1111/jpy.12321 [DOI] [PubMed] [Google Scholar]
- 38.Austen EJ, Weis AE (2015) What drives selection on flowering time? An experimental manipulation of the inherent correlation between genotype and environment. Evolution 69: 2018–2033. doi: 10.1111/evo.12709 [DOI] [PubMed] [Google Scholar]
- 39.Vermeulen PJ (2015) On selection for flowering time plasticity in response to density. New Phytologist 205: 429–439. doi: 10.1111/nph.12984 [DOI] [PubMed] [Google Scholar]
- 40.Thiele J, Jørgensen RB, Hauser TP (2009) Flowering does not decrease vegetative competitiveness of Lolium perenne. Basic and Applied Ecology 10: 340–348. [Google Scholar]
- 41.Akiyama R, Ågren J (2012) Magnitude and timing of leaf damage affect seed production in a natural population of Arabidopsis thaliana (Brassicaceae). PLoS One 7: e30015 doi: 10.1371/journal.pone.0030015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Banta JA, Ehrenreich IM, Gerard S, Chou L, Wilczek A, Schmitt J, et al. (2012) Climate envelope modelling reveals intraspecific relationships among flowering phenology, niche breadth and potential range size in Arabidopsis thaliana. Ecology Letters 15: 769–777. doi: 10.1111/j.1461-0248.2012.01796.x [DOI] [PubMed] [Google Scholar]
- 43.Debieu M, Tang C, Stich B, Sikosek T, Effgen S, Josephs E, et al. (2013) Co-variation between seed dormancy, growth rate and flowering time changes with latitude in Arabidopsis thaliana. PLoS One 8: e61075 doi: 10.1371/journal.pone.0061075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weinig C, Stinchcombe JR, Schmitt J (2003) Evolutionary genetics of resistance and tolerance to natural herbivory in Arabidopsis thaliana. Evolution 57: 1270–1280. [DOI] [PubMed] [Google Scholar]
- 45.Tucker C, Avila-Sakar G (2010) Ontogenetic changes in tolerance to herbivory in Arabidopsis. Oecologia 164: 1005–1015. doi: 10.1007/s00442-010-1738-6 [DOI] [PubMed] [Google Scholar]
- 46.Carmona D, Lajeunesse MJ, Johnson MT (2011) Plant traits that predict resistance to herbivores. Functional Ecology 25: 358–367. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(CSV)
(XLSX)
“Std β” stands for standardized regression coefficient, and “p” is the probability associated with each factor. Bold p values indicate significant results.
(DOCX)
“Std β” stands for standardized regression coefficient, and “p” is the probability associated with each factor. Bold p values indicate significant results.
(DOCX)
“Std β” stands for standardized regression coefficient, and “p” is the probability associated with each factor. Bold p values indicate significant results.
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.


