Gekara previews studies published in Nature and Human Molecular Genetics identifying the subcellular localization where DNA lesions activate the innate immune response.
Abstract
DNA damage–induced activation of the cytoplasmic DNA sensor cGAS influences the outcome of infections, autoinflammation, and cancer. Recent studies by Harding et al. (2017. Nature. http://dx.doi.org/10.1038/nature23470), Mackenzie et al. (2017. Nature. http://dx.doi.org/10.1038/nature23449), and Bartsch et al. (2017. Human Molecular Genetics. https://doi.org/10.1093/hmg/ddx283) demonstrate a role for micronuclei formation in DNA damage–induced immune activation.
The innate immune recognition of and response to infection or tissue injury is mediated by conserved pattern recognition receptors, which detect microbial patterns or self-molecules altered or misplaced as a result of cellular stress or death. Pattern recognition receptors include the cytoplasmic protein cyclic GMP-AMP synthase (cGAS). Upon binding DNA, cGAS catalyzes the formation of the second messenger molecule cyclic GMP-AMP (cGAMP) from ATP and GTP. cGAMP in turn binds to the adaptor Stimulator of Interferon Genes (STING), triggering the formation of a signaling complex, leading to the transcription of inflammatory genes (Gao et al., 2013).
To minimize the misfiring of the immune system, in healthy cells, DNA is restricted to the nucleus or mitochondria. However, in response to genomic stress, DNA accumulates in the cytoplasm, thereby triggering the cGAS–STING pathway (Härtlova et al., 2015). While priming the immune system for an enhanced antiviral immunity, this DNA damage–induced activation of the cGAS–STING pathway has been linked to autoinflammatory diseases and cancer. But how does DNA, normally sequestered in the nucleus, gain access to the innate immune sensors in the cytoplasm upon DNA damage? That is the question addressed by Mackenzie et al. (2017), Harding et al. (2017), and Bartsch et al. (2017).
The integrity of the nuclear envelope is critical for nuclear compartmentalization and for the regulated exchange of molecules between the nucleus and the cytoplasm. In higher eukaryotes, cell division involves “open” mitosis, meaning that the nuclear envelope completely disassembles and then reassembles as the cell segregates the replicated DNA into daughter cells. In the course of these events, whole or broken chromosome fragments can miss-segregate from the main chromatin mass. During mitotic exit, lagging chromosome fragments can recruit nuclear envelope components to form micronuclei that are compartmentally separated from the primary nucleus. In recent studies, Mackenzie et al. (2017), Harding et al. (2017), and Bartsch et al. (2017) have linked micronuclei formation with genome instability-associated innate immune activation.
Initial studies had shown that genome instability caused by excessive exogenous genotoxic insults or spontaneous accumulation of DNA lesions caused by mutation in components of the DNA repair machinery cause cGAS–STING activation (Härtlova et al., 2015; Mackenzie et al., 2016; Pokatayev et al., 2016). One such component is the nuclease Rnase H2. Rnase H2 deficiency causes an autoimmune disorder called Aicardi-Goutières syndrome. While studying cells from Rnase H2–deficient (Rnase H2−/−) mice, Mackenzie et al. (2017) and Bartsch et al. (2017) observed that Rnase H2−/−cells had a high frequency of cytoplasmic DNA aggregates resembling micronuclei. Consistently, by fluorescence microscopic analyses, they found that these cytoplasmic DNA structures were surrounded by nuclear envelopes and contained proteins markers of damaged DNA. Further, they found that a substantial fraction of the micronuclei were enriched in cGAS and that ablation of cGAS or STING effectively eliminated the spontaneous inflammatory cytokine response in Rnase H2−/− cells.
Micronuclei are surrounded by envelope. So how is cGAS able to access DNA within such compartments? It has previously been demonstrated that because of defective nuclear lamina organization, micronuclei are prone to irreversible nuclear envelope collapse, hence loss of compartmentalization (Hatch et al., 2013). By using fluorescence time-lapse microscopy, Mackenzie et al. (2017) elegantly demonstrate that rupture of the micronuclear envelope is essential for the recruitment of cGAS into micronuclei. In accordance with the importance of cell division, they show that cells under cell cycle arrest neither form micronuclei nor exhibit inflammatory cytokine expression. This means that both DNA breaks and loss of nuclear compartmentalization are critical for DNA damage–induced innate immune activation. Further, using live-cell laser microdissection with single cell transcriptomics, Mackenzie et al. (2017) could show that the inflammatory cytokine gene induction was mainly occurring in micronucleated cells.
Harding et al. (2017) started their investigation from a different angle. DNA damage–induced immune activation contributes to the efficacy of genotoxic cancer therapy. However, in contrast to the acute DNA damage responses that occur within minutes to hours, DNA damage–induced inflammation has a delayed onset (days), suggesting the involvement of additional rate-limiting steps. While investigating these dichotomous responses, Harding et al. (2017) noted that DNA damage–induced expression of inflammatory markers coincided with the appearance of abnormally shaped nuclei and micronuclei. Given that micronuclei are by-products of cell cycle progression, they then considered whether DNA damage–induced inflammation was dependent on cell division.
Using a variety of pharmacological agents, Harding et al. (2017) found that inhibition of cell cycle progression blocked micronuclei generation and the concomitant expression of inflammatory molecules in cancer cells upon irradiation. In absence of cGAS and STING, the researchers observed no inflammatory induction in cancer cells upon irradiation. Moreover, much like the approach by Mackenzie et al. (2017) using fluorescence live cell microscopy, Harding et al. (2017) provided data supporting the collapse of the micronuclear envelope as a mechanism by which cGAS gains access to damaged DNA. Interestingly, and similar to Mackenzie et al. (2017) and the recent work from the Chen laboratory (Yang et al., 2017), they found that cGAS transiently binds nuclear chromatin upon disassembly of the nuclear envelope during mitosis. Migration of cells through narrow spaces can cause mechanical DNA breaks and nuclear envelope rupture. Indeed, Harding et al. (2017) detected cGAS localization into both micronuclei and the primary nucleus upon migration of cells through narrow pores. This implies that loss of nuclear compartmentalization, irrespective of how it occurs, leads to cGAS-mediated innate immune activation. This raises interesting questions. Does the nuclear membrane rupture associated with squeezing through tight interstitial spaces prime immune cells during transmigration from the blood to injured/infected sites?
What is the significance of DNA damage–induced activation of the cGAS–STING pathway in cancer therapy? It has been known for decades that localized radiation of a tumor causes shrinking of both the treated tumor as well as tumors distant from the site of irradiation (Mole, 1953). This phenomenon, termed abscopal effect, from the Latin ab (away from) and scopus (target), has long been thought to be a result of the systemic immune response evoked by DNA damage. Using a mouse model of melanoma, Harding et al. (2017) compellingly show that shrinking of abscopal tumors upon irradiation involves the cGAS–STING pathway.
How does the cell cope with the accumulation of micronuclei? Autophagy (“self-eating”) is a cellular process by which cytoplasmic waste is engulfed in a double-membrane vesicle and delivered to the lysosomes for breakdown and eventual recycling. Using microscopic analysis, Bartsch et al. (2017) observed that a preponderance of micronuclei that accumulate in Rnase H2−/− cells were associated with autophagosome markers. Using genetic and pharmacological approaches, they found that blocking autophagy resulted in elevated micronuclei accumulation and inflammatory gene induction in Rnase H2−/− cells. In contrast, activation of autophagy had the reverse effect, implicating the role of autophagy in the control of DNA damage–induced immune activation.
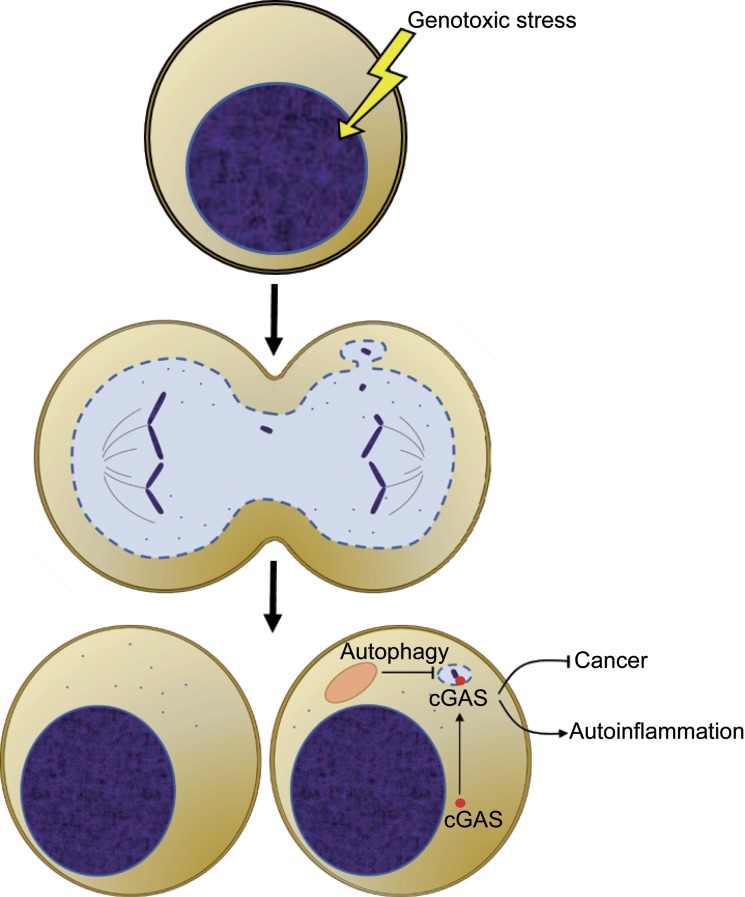
Finally, are micronuclei the only access route for nuclear DNA to the cytoplasm? As acknowledged by Mackenzie et al. (2017) and Harding et al. (2017), other additional mechanisms may play a role. Small DNA fragments are generated during repair of damaged replication forks or double-strand breaks. Conceivably, passive diffusion of these fragments or release into the cytoplasm upon mitotic nuclear envelope disruption could in part account for cGAS–STING–dependent responses. Moreover, DNA fragments aside, as observed by Mackenzie et al. (2017), Harding et al. (2017), and Yang et al. (2017), cGAS can bind to nuclear chromosomes during mitosis. Although transcriptional silencing during mitosis mitigates against cGAS-mediated gene induction, there might be the possibility of cGAS activating TBK1 (TANK-binding kinase 1) and IRF3 (interferon regulatory factor 3) to modulate cellular processes such as autophagy or apoptosis independently of transcription. Regardless and collectively, the studies by Mackenzie et al. (2017), Harding et al. (2017), and Bartsch et al. (2017) do make a persuasive case that micronuclei are a substantial source of immunostimulatory DNA during genotoxic stress (Fig. 1). Importantly, they suggest possibilities for manipulating the innate immune system in the context of autoinflammatory diseases or DNA damage–based therapies.
Figure 1.
Micronuclei serve as a source of immunostimulatory DNA. Cell division after DNA damage leads to micronuclei formation. cGAS sensing of micronuclei DNA triggers gene activation, which may lead to autoinflammation or antitumor immunity. Micronuclei can be cleared via autophagy.
Acknowledgments
I apologize to those colleagues whose work could not be cited because of length restrictions.
The work in Gekara’s laboratory is supported by the Laboratory for Molecular Infection Medicine Sweden and Vetenskapsrådet grants (reference numbers 2015-02857 and 2016-00890).
The author declares no competing financial interests.
References
- Bartsch K., Knittler K., Borowski C., Rudnik S., Damme M., Aden K., Spehlmann M.E., Frey N., Saftig P., Chalaris A., and Rabe B.. 2017. Absence of RNase H2 triggers generation of immunogenic micronuclei removed by autophagy. Human Molecular Genetics . 10.1093/hmg/ddx283. [DOI] [PubMed]
- Gao D., Wu J., Wu Y.T., Du F., Aroh C., Yan N., Sun L., and Chen Z.J.. 2013. Cyclic GMP-AMP synthase is an innate immune sensor of HIV and other retroviruses. Science. 341:903–906. 10.1126/science.1240933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding S.M., Benci J.L., Irianto J., Discher D.E., Minn A.J., and Greenberg R.A.. 2017. Mitotic progression following DNA damage enables pattern recognition within micronuclei. Nature. 548:466–470. 10.1038/nature23470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Härtlova A., Erttmann S.F., Raffi F.A., Schmalz A.M., Resch U., Anugula S., Lienenklaus S., Nilsson L.M., Kröger A., Nilsson J.A., et al. . 2015. DNA damage primes the type I interferon system via the cytosolic DNA sensor STING to promote anti-microbial innate immunity. Immunity. 42:332–343. 10.1016/j.immuni.2015.01.012 [DOI] [PubMed] [Google Scholar]
- Hatch E.M., Fischer A.H., Deerinck T.J., and Hetzer M.W.. 2013. Catastrophic nuclear envelope collapse in cancer cell micronuclei. Cell. 154:47–60. 10.1016/j.cell.2013.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie K.J., Carroll P., Lettice L., Tarnauskaitė Ž., Reddy K., Dix F., Revuelta A., Abbondati E., Rigby R.E., Rabe B., et al. . 2016. Ribonuclease H2 mutations induce a cGAS/STING-dependent innate immune response. EMBO J. 35:831–844. 10.15252/embj.201593339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie K.J., Carroll P., Martin C.A., Murina O., Fluteau A., Simpson D.J., Olova N., Sutcliffe H., Rainger J.K., Leitch A., et al. . 2017. cGAS surveillance of micronuclei links genome instability to innate immunity. Nature. 548:461–465. 10.1038/nature23449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mole R.H. 1953. Whole body irradiation; radiobiology or medicine? Br. J. Radiol. 26:234–241. 10.1259/0007-1285-26-305-234 [DOI] [PubMed] [Google Scholar]
- Pokatayev V., Hasin N., Chon H., Cerritelli S.M., Sakhuja K., Ward J.M., Morris H.D., Yan N., and Crouch R.J.. 2016. RNase H2 catalytic core Aicardi-Goutières syndrome-related mutant invokes cGAS-STING innate immune-sensing pathway in mice. J. Exp. Med. 213:329–336. 10.1084/jem.20151464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H., Wang H., Ren J., Chen Q., and Chen Z.J.. 2017. cGAS is essential for cellular senescence. Proc. Natl. Acad. Sci. USA. 114:E4612–E4620. 10.1073/pnas.1705499114 [DOI] [PMC free article] [PubMed] [Google Scholar]