Abstract
Cancer-related cognitive impairment (CRCI) is an important clinical problem for cancer patients and survivors. In this review, we summarize studies investigating the occurrence of impaired cognition in patients with haematological malignancies. Most published studies focus on survivors of childhood acute lymphoblastic leukaemia and primary CNS lymphoma. We also discuss studies conducted in acute myeloid leukaemia, myelodysplastic syndromes, chronic myeloid leukaemia, Hodgkin lymphoma, non-Hodgkin lymphoma and chronic lymphocytic leukaemia. Although research in this area is still emerging, it appears that a subset of chemotherapy-treated haematological malignancy survivors experience CRCI. Future research should focus on expanding the literature reviewed here with larger studies appropriately powered to assess cognition via objective and subjective measures in a longitudinal fashion to tease apart the impact of disease and the various forms of cancer treatment.
Keywords: Chemotherapy-related cognitive impairment, Haematological malignancy, cancer, chemotherapy, cognition
Introduction
In 2015, an estimated 162,000 new cases of haematological malignancies were diagnosed (approximately 10% of all cancer diagnoses) and over 1.8 million haematological cancer survivors live in the United States (Howlader et al 2014). Improved diagnosis and treatment have markedly increased survival for many patients with haematological malignancies. Based on the most recent literature, current five-year survival rates are as follows: all leukaemia 60.3%, Hodgkin lymphoma (HL) 87.7% and non-Hodgkin lymphoma (NHL) 71.4% (Howlader et al 2014). Nearly all leukaemias and 69% of NHL are treated with chemotherapeutic agents (Rossi, et al 2015). Treatment-related side effects, including cognitive impairment, can decrease treatment compliance and ultimately impact quality of life; however, a deep understanding of the aetiology of these cognitive problems as a consequence of disease and/or treatment in haematological malignancies is still in its infancy.
Chemotherapy-related cognitive impairment (CRCI) is a collection of problems in memory, attention, concentration and executive functions that is associated with chemotherapy treatments in cancer patients. These problems can range from subtle to severe and last for months or years after treatment. CRCI affects an estimated 10 million cancer survivors in the United States. Based on data from all types of cancers, up to 30% of survivors experience cognitive impairment prior to therapy, 80% during therapy, and up to 35% may live with CRCI up to 20 years after treatment (Koppelmans, et al 2012). Decreased cognitive function is associated with poorer quality of life, inability to achieve work and educational goals, inability to drive or read, and decreased social connectedness (Bradley, et al 2005, Reid-Arndt, et al 2010, Wefel, et al 2004). To date, the CRCI literature is dominated by breast cancer and other solid tumours. Haematological malignancies are usually systemic, and often treated with chemotherapeutic agents that have been implicated in CRCI in solid tumours. The growing literature in this area suggests that cognition is an important predictor of survival in patients with haematological malignancies (Dubruille, et al 2015) and therefore, understanding factors that lead to CRCI in haematological malignancies warrants attention.
Research on cognitive function in most types of haematological malignancies is limited. However, studies of cognitive function in paediatric acute lymphoblastic leukaemia (ALL), indicates that cognitive impairment can persist for years after completion of treatment. Clearly, a subset of haematological malignancy survivors does experience CRCI. This review will summarize the available literature on cancer-related cognitive impairment in survivors of haematological malignancies focusing on chemotherapy-treated survivors (Table I).
Table I.
Available studies of cancer-related cognitive impairment in haematological malignancies.
| Study | Design | Treatment/Participants | Assessment Schedule | Cognitive Domains Measured | Summary of Findings |
|---|---|---|---|---|---|
|
| |||||
|
Acute myeloid leukaemia/myelodysplastic syndrome
| |||||
| Meyers et al (2005) | Longitudinal | CHEM | Pre-treatment, 1 month | Attention span, graphomotor speed, memory verbal fluency, visual-motor scanning speed, executive function, fine motor dexterity | Decline on Motor function, psychomotor speed, memory, executive function |
| (n=;54 mean age 60 years) | |||||
|
| |||||
| Alibhai et al (2009) | Longitudinal | CHEM | Pre-treatment, 1,4,6,9,12 months | Subjective: EORTC-QLQ30 | No decline |
| (n=20; mean age 73 years) | |||||
|
| |||||
| CML/MDS | |||||
|
| |||||
| Meadows et al (2013) | Longitudinal | CHEM (n=36) | Pre-treatment, 12, 18 months | Attention, executive function, memory, processing speed, language, motor speed | Increase in memory, attention, executive function |
| HSCT (n=70) | |||||
| Mean age 48.1 years | |||||
|
| |||||
|
Chronic lymphocytic leukaemia
| |||||
| Pamuk et al (2008) | Cross-sectional | CHEM (n=133) | Between 9 and 22 months post-diagnosis | Subjective: EORTC-QLQ30 | Worse than norms |
| RT (n=19) | |||||
| Mean age N/A | |||||
|
| |||||
| Else et al (2008) | Cross-sectional | No treatment | Pre-treatment | Subjective: EORTC-QLQ30 | Worse than norms |
| (n=431, median age 64 years) | |||||
|
| |||||
| Else et al (2012) | Longitudinal | CHEM | Pre-treatment, 5 years | Subjective: EORTC-QLQ30 | Decline |
| (n=306, mean age N/A) | |||||
|
| |||||
| Holzner et al (2004) | Cross-sectional | CHEM (n=33) | Assessed four times over one year. | Subjective: EORTC-QLQ30 | Worse than norms |
| No CHEM (n=46) | |||||
| Median age 68 years | |||||
|
| |||||
|
Hodgkin lymphoma
| |||||
| Joly et al (1996) | Cross-sectional | CHEM | Mean 10 years from diagnosis | Subjective: EORTC-QLQ30 | Worse than norms |
| (n=93, mean age 42 years) | |||||
|
| |||||
| Krull et al (2012) | Cross-sectional | CHEM | Mean 27 years post-treatment | Intelligence, attention, memory, processing speed, executive function. | Worse than controls on attention, memory, executive function, processing speed |
| (n=62; mean age 42 years) | |||||
|
| |||||
|
Non-Hodgkin lymphoma
| |||||
| Zimmer et al (2015) | Cross-sectional | CHEM | Within 3 months post-chemotherapy | Attention and executive function | Worse than controls on attention and executive function. |
| (n=30, mean age 63 years) | |||||
|
| |||||
| Van Der Poel et al (2014) | Cross-sectional | CHEM (n=291) | Mean 3.4 years post-treatment | Subjective: EORTC-QLQ30 | Worse than controls |
| RT (n=89) | |||||
| Mean age 63 years | |||||
|
| |||||
|
Lymphoma
| |||||
| Baudino et al (2012) | Cross-sectional | Pre-treatment | Before or after chemotherapy (mean 7 months) | Attention, memory, language | Worse than controls on attention and executive function |
| (n=18, mean age 57 years) | |||||
| CHEM | |||||
| (n=32, mean age 45 years) | |||||
|
| |||||
| Ahles et al (2002) | Cross-sectional | CHEM | At least 5 years post-diagnosis (range 9-14 years) | Attention, verbal learning and memory, visual memory, verbal ability, spatial ability, psychomotor function | Worse than norms on language, memory, psychomotor function, attention and spatial ability |
| (n=36, mean age 55 years) | |||||
| S/RT | |||||
| (n=22, mean age 11 years) | |||||
|
| |||||
|
Primary central nervous system lymphoma
| |||||
| Correa et al (2012) | Longitudinal | CHEM | Post-treatment and 14 months later | Attention, executive function, motor speed, memory | Increase on attention |
| (n=26, mean age 70 years) | |||||
| CHEM and RT | |||||
| (n=24, mean age 53 years) | |||||
|
| |||||
| Doolittle et al (2013a) | Cross-sectional | CHEM | Median 5 years from treatment (range 2 to 26) | Attention, executive function, verbal memory, motor skills | Worse than norms on all domains |
| (n=65, mean age 59 years) | |||||
| CHEM and RT | |||||
| (n=15, mean age 57 years) | |||||
|
| |||||
| Doolittle et al (2013b) | Longitudinal | CHEM | Pre-treatment to median 12 years post-treatment | Attention, executive function | Improvement on attention and executive function |
| (n=26, median age 50 years) | |||||
|
| |||||
| Fliessbach et al (2005) | Longitudinal | CHEM | Pre-treatment, 3 months post-treatment, to median 44 months post-treatment | Attention, executive function, memory, verbal fluency. | Improvement on all domains |
| (n=23, age N/A) | |||||
|
| |||||
| Juergens et al (2010) | Longitudinal | CHEM | Pre-treatment, 4 and 100 months post-treatment | Attention, executive function, memory, verbal fluency, psychomotor speed | Improvement on all domains |
| (n=19, mean age 62 years) | |||||
CHEM, chemotherapy; RT, radiotherapy; S/RT, surgery + radiotherapy; HSCT, haematopoietic stem cell transplantation; N/A, not available.
Methods of Study Selection
We conducted a PubMed search for studies published in English using the following search terms: cognition, cognitive impairment, cancer, hematological malignancy, leukemia, and lymphoma. Separate searches were done for each disease type and the words cognition or cognitive impairment. We included studies that reported on cognition in those treated with chemotherapy only for a haematological malignancy. In other words, studies were excluded if they only reported results of radiotherapy. In some malignancies (e.g. HL) only studies reporting on combination radiotherapy and chemotherapy were available and thus included. The search terms yielded 539 papers of which 75 were relevant to this review and abstracts were reviewed. Ultimately, 18 manuscripts met the inclusion criteria and are reviewed here. Of note, a large number of studies (n=23) examining CRCI in ALL are well characterized in a recent systematic review (Cheung & Krull 2015) and, therefore, these are only briefly summarized in this review.
Detection and Management of Cancer-Related Cognitive Impairment
The multi-faceted nature of cancer-related cognitive impairment creates challenges in both study design and the interpretation of available literature (Vardy, et al 2008). Cognitive function can be assessed using subjective or objective measures, and several objective neuropsychological tests exist for each cognitive domain. While recommendations exist, there is no standardized assessment of cognitive function in cancer survivors, making it difficult to compare results across studies. The International Cancer and Cognition Task Force (ICCTF) has suggested the use of three neuropsychological tests to increase comparability across studies (the Trail Making Test, Hopkins Verbal Learning Test-Revised and the Controlled Word Association test) (Wefel, et al 2011).
In addition to the ICCTF recommendations, many studies also focus on subjective measures of cognitive function. Subjective self-report measures, or patient-reported outcomes, are important because they reflect the person’s perceived current cognitive state (Phillips and Bernhard 2003) but are only moderately correlated with more precise concurrent objective measures, such as neuropsychological testing (Vardy, et al 2008). Nonetheless, subjective measures allow us to assess a period of time. For example, we can ask participants if they feel they have experienced a cognitive decline over the past week, which can capture CRCI symptoms that may not be occurring at the time of neuropsychological testing.
The majority of studies reviewed here used the European Organization for the Research and Treatment of Cancer-Quality of Life Questionnaire 30 (EORTC-QLQ30) to assess subjective cognitive function. The EORTC-QLQ30 is a validated quality of life questionnaire; it consists of five functional subscales, including one that subjectively assesses cognitive function. However, this subscale is based on only two line items within the EORTC-QLQ30 and may not accurately capture the cognitive impairment suffered by these patients (Alibhai, et al 2009). The Functional Assessment after Cancer Cognition (FACT-Cog) is a newer assessment tool that aims to gain a deeper understanding of cognitive problems and could be useful in studies of patients with haematological malignancies. The FACT-Cog is a well-validated Likert scale-based questionnaire of 37 line items (Wagner et al 2009). Participants are asked multidimensional questions regarding their perceived cognitive impairment, comments from others, perceived cognitive abilities and impact on their quality of life (Joly, et al 2012).
Objective measures of cognitive function can help to tease out which cognitive domains are most affected in cancer survivors. Despite the ICCTF recommendations, not all studies include the same neuropsychological tests. Further, within each scientific study of cognition, different domains are evaluated using different tests, and in any given domain of cognition anywhere from one to upwards of five tests or more can be used to evaluate a participant. Further, a significant association may be observed with only one of many tests within a domain, making it extremely difficult to compare results within and across studies (Vardy, et al 2008). Administering a comprehensive battery of these tests may take hours and can be burdensome to the participant (Matsuda, et al 2005). This is particularly a challenge for studies that involve children; care must be taken to select assessments that are appropriate for their age range, minimize time and distractions, allow for appropriate breaks, and are not influenced by motor-skill deficiencies (Weintraub, et al 2013). The lack of a standardized method to evaluate cognitive function contributes to inconsistent results between studies (Hess and Insel 2007). For example, if one study reports a decrease in executive function after chemotherapy but another does not, this discrepancy makes the results difficult to interpret.
The absence of a standardized grading of cognitive impairment adds to the challenges in obtaining consistent and comparable measurement of cognitive function. Researchers commonly use from 1.5 to 2 standard deviations below the normative mean to define mild cognitive impairment on objective assessments although recent research has suggested that even changes of less than 1-2 standard deviations may be meaningful (Joly, et al 2015). In addition, researchers may list the percentage of subjects impaired in each domain or consider global definitions with varying requirements (e.g. impaired on at least one test; impaired on at least two tests) making a comparison of the rate of impairment across studies difficult (Wefel, et al 2015).
Regardless of the challenges involved in the measurement and detection of cancer-related cognitive impairment, it is clear that a subset of cancer survivors have CRCI. Research has begun to focus on interventions to alleviate or prevent CRCI including: cognitive behavioural therapy, cognitive brain training, mindfulness-based stress reduction and physical activity (Janelsins, et al 2014, Joly, et al 2015). Cognitive behavioural therapy has been used in two pilot studies of breast cancer patients demonstrating improvements on objective and subjective measures of cognitive function (Ferguson, et al 2007, Ferguson, et al 2012). Cognitive brain-training studies suggest improvement in executive functions (Kesler, et al 2013) and yoga may reduce perceived memory problems (Janelsins, et al 2015). However, some of these studies were done in small populations or have other limitations and larger clinical trials are required to definitively test these interventions.
Clinical and Demographic Factors Influencing CRCI
Many non-therapy-related factors can influence CRCI in patients with a haematological malignancy (Figure 1). These include demographics (e.g. age, intelligence/education, gender, race), factors associated with a cancer diagnosis and treatment (e.g. anxiety, depression, fatigue) and comorbidities. It is estimated that, by 2020, two thirds of all cancer survivors will be at least 65 years of age (Stouten-Kemperman, et al 2015). Cancer and its treatment may exacerbate normal aging processes and increase cognitive impairment in these older cancer survivors (Mandelblatt, et al 2014). A recent National Health and Nutrition Examination Survey (NHANES) analysis showed that survivors aged 60 years and older scored worse on a test of processing speed, attention, working memory and executive function domains compared to non-cancer survivors (Williams, et al 2015). These findings justify additional research on cognitive function in patients with haematological malignancies, many of which are more common in older patients.
Figure 1.
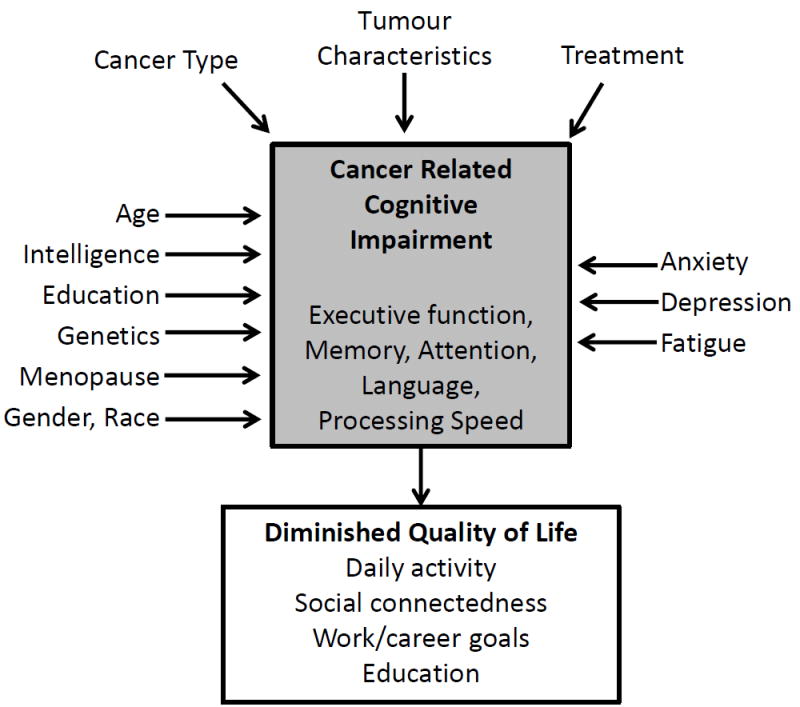
Factors Influencing Cancer Related Cognitive Impairment.
Anxiety, depression and fatigue may be key factors contributing to cognitive impairment pre- and post-chemotherapy. These factors are often highly correlated with subjective cognitive measures and, in non-cancer populations, anxiety and depression disorders have been associated with poorer performance on objective tests of executive function (Schagen, et al 2014). However, some studies in cancer survivors have not shown an association between anxiety, depression or fatigue and performance on objective tests of cognitive function, indicating they are one part of a multifaceted framework that contributes to CRCI (Figure 1) (Biglia, et al 2012). Research in this area is relatively new, and more data is needed to determine the role these factors play in CRCI.
Proposed Mechanisms of Chemotherapy Related Cognitive Impairment
Several biological mechanisms have been proposed that could explain the association between cancer and cognitive function, including neurotoxicity, inflammation, hormonal factors and a decrease in brain volume and metabolism (Phillips and Bernhard 2003). Most of the literature has hypothesized that the neurotoxic effects of chemotherapy cause extensive damage and inflammation that can persist for the rest of the survivor’s life (Ahles and Saykin 2001, Ahles and Saykin 2007, Dutta 2011, Janelsins, et al 2014). Conventional chemotherapy effects are generally not targeted, and some of these agents can cross an intact blood-brain barrier (Hess and Insel 2007). Further, genetic variability in blood-brain barrier proteins could potentially increase concentrations of chemotherapy in the brain and thus cause neurotoxicity (Ahles and Saykin 2007). These toxic effects can include altered brain metabolism, DNA damage and telomere shortening, resulting in long-lasting and irreversible decreases in cognitive function (Ahles and Saykin 2007, Dutta 2011, Koppelmans, et al 2013).
Chemotherapy can also indirectly influence cognitive function through vascular injury, inflammatory processes, oxidative stress, and mitochondrial toxicity. Chemotherapy-induced vascular injury can cause cerebral ischaemia and cognitive impairment (Ahles and Saykin 2001). Cancer and chemotherapy-induced inflammation can increase levels of cytokines, such as interleukins 6 and 8 and monocyte chemoattractant protein 1 (Janelsins, et al 2011). Cytokines can enter the brain and stimulate cells to produce other proinflammatory cytokines that interfere with neuronal growth. This cytokine deregulation can lead to an increase in oxidative stress and induce DNA damage (Ahles and Saykin 2007), which may help to explain why cognitive damage can occur in patients receiving treatments not expected to directly affect their brain (Asher 2011). Over time, this increase in oxidative stress can increase glucocorticoid levels resulting in structural and functional damage to the hippocampus, causing cell death, and decrease neuronal growth leading to defective learning and memory (Dutta 2011), which has been shown to persist in animal models (Koppelmans, et al 2013). Mitochondrial damage has been associated with cognitive impairment in traumatic brain injury and neurodegenerative disorders such as Alzheimer’s disease. Growing evidence suggests that chemotherapy and perhaps the tumour itself can alter mitochondrial function and affect cognition by a mechanism that is poorly understood (Vichaya, et al 2015).
The aetiology of CRCI is thus likely to be multifactorial and include both direct and indirect mechanisms. However, many of these hypothesized mechanisms are not well understood and require further evaluation. The following sections review the available literature on cognitive function in haematological malignancy survivors with a focus on those treated with chemotherapy.
Studies Encompassing all Haematological Malignancies
There is limited published data on cognitive impairment across all haematological malignancies. A study of 140 haematological malignancy survivors, most of whom were inpatients with active disease, self-reported worse cognitive functioning compared to published norms on the EORTC-QLQ30 (Pamuk, et al 2008). The EORTC-QLQ30 is a validated quality of life questionnaire used frequently in cancer survivorship studies, including a cognitive subscale based on only two line items that may not be representative of the true cognitive burden in these patients (Alibhai, et al 2009).
Another longitudinal series of studies in haematological and gastrointestinal malignancy survivors found that those treated with chemotherapy performed worse on tests of memory and verbal learning. When re-evaluated six months later, the chemotherapy-treated patients improved on these domains, implicating neurotoxic effects of chemotherapy (Eberhardt, et al 2006a, Eberhardt, et al 2006b). However, these results were not stratified by disease site (haematological or gastrointestinal) and cancers were not uniformly treated.
Acute Lymphoblastic Leukaemia
ALL is the most common leukaemia below age 20 years and is the most prevalent (26%) childhood cancer. In the United States, the 2014 estimated annual incidence was 6,000 cases with a prevalence of 68,728 (Howlader et al 2014). Historically, ALL treatment included cranial radiation, which has been associated with cognitive impairment; however, clinical trials demonstrated that improved systemic therapy combined with intrathecal chemotherapy could effectively replace cranial radiation (Gaynon, et al 2010). This paradigm shift has increased the importance of understanding the cognitive effects of chemotherapy in ALL.
There is considerable published data on CRCI in survivors of paediatric ALL treated without radiation as recently reviewed by Cheung and Krull (2015). Studies were included if they examined ALL survivors at least 5 years from diagnosis or two years from completion of treatment, and at least one study arm must have been treated with chemotherapy only (no cranial radiation). Twenty-three studies were included, four of which were longitudinal. Sample sizes of the included studies were small, ranging between 10 and 50 ALL survivors. The authors concluded that survivors treated with chemotherapy only regimens had less cognitive impairment compared to those who received cranial radiation. However, survivors treated with chemotherapy performed worse than healthy participants on tests of executive function, attention, memory and motor function. Those who received high-dose methotrexate suffered more cognitive impairment compared to those that received low-dose methotrexate (Cheung and Krull 2015).
Acute Myeloid Leukaemia (AML)
AML is more commonly diagnosed in older adults with an annual incidence of 18,860 and prevalence of over 37,000 in the U.S. (Howlader et al 2014). Many AML patients receive intensive and extended chemotherapy treatments, but there are only two small longitudinal published studies on CRCI in this population.
Meyers et al (2005) evaluated 54 AML and myelodysplastic syndrome (MDS) survivors pre-treatment and reassessed 26 of these participants one month after therapy completion (54% achieved a complete response). All patients were treated with liposomal daunorubicin plus cyclophosphamide or topotecan, plus or minus thalidomide. Prior to treatment, the patients performed worse than published norms on tests of memory, verbal fluency, processing speed, executive function and fine motor function. At one month after therapy completion, only fine motor function significantly worsened, with 37% of patients impaired at baseline and 54% impaired at follow-up. An increased percentage of patients had impairment on tests of psychomotor speed, memory, verbal fluency and executive function at follow-up, but these differences were not statistical significant. However, this study was limited because only half the patients were available for re-evaluation. The authors attribute this large loss to follow-up to logistical problems; however, selection bias cannot be ruled out. Patients included in the second evaluation were more likely to have achieved a complete remission and many participants who achieved a partial remission or had no response were not re-evaluated, suggesting the results may underestimate cognitive decline in this population. Unfortunately, these study results are not stratified by disease type or treatment regimen to further examine the cognitive effects of disease and chemotherapy (Meyers, et al 2005) .
A second study evaluated subjective cognitive function in 20 AML patients pre-treatment, with follow-up evaluations at 1, 4, 6, 9 and 12 months post-treatment (Alibhai et al 2009). The majority of patients received induction chemotherapy consisting of daunorubicin and cytarabine; those who did not achieve a complete remission were re-induced with mitoxantrone, etoposide, and cytarabine. Patients who achieved a complete remission received consolidation therapy using the same chemotherapeutic agents as induction and a second consolidation cycle with mitoxantrone and etoposide. Participants scored similarly to published norms at all time points on the cognitive subscale of the EORTC-QLQ30, and the authors report no clinically meaningful difference from baseline to 1 year.
Myelodysplastic Syndromes (MDS)
The annual incidence of MDS in the U.S. is over 14,500 cases and median overall survival ranges from less than one year in patients with very high risk MDS to over 8 years in the low risk groups (Howlader et al 2014). Current management of MDS is often supportive, but disease-directed therapies include chemotherapy and allogeneic haematopoietic stem cell transplantation (alloHSCT).
MDS patients were included in the two studies of myeloid malignancies. One study of patients with MDS or AML patients reported increased cognitive deficits prior to any treatment and more dysfunction up to one month after therapy (Meyers, et al 2005) . Another study of patients with MDS or chronic myeloid leukaemia (CML) reported high rates of impairment at baseline and improvements up to 18 months after treatment (Meadows, et al 2013). However, neither of these studies was able to stratify analyses on disease type due to small sample sizes.
Chronic Myeloid Leukaemia (CML)
There are over 6,000 new cases of CML diagnosed each year, accounting for 15% of all leukaemias, and 34,000 people living with CML in the U.S. The introduction of oral tyrosine kinase inhibitors doubled five-year survival to 60% by 2010 (Siegel, et al 2016). All but one study examining cognitive impairment in CML have focused on alloHSCT treatment for CML.
Meadows et al (2013) followed 91 CML and 11 MDS survivors who had either been diagnosed within the past year or had a treatment plan that included alloHSCT (34%). Most (85%) of the CML patients who did not receive alloHSCT were treated with imatinib mesylate. Participants were evaluated a median of 6 months after diagnosis (baseline), and then one year and 18 months after the first assessment. At baseline, at least 80% of the sample was impaired on at least one neuropsychological test and this decreased to 64% by 18 months (defined as ≥1.5 standard deviations below the mean). However, as a group, they performed similar to published norms in all domains at baseline, and the authors reported significant increases in memory, attention and executive function from baseline to 18 months. Unfortunately, results stratified by alloHSCT are not available for this study, making it difficult to isolate the effects of therapy on cognition. Conditioning regimens for alloHSCT can include total body irradiation, with its well-described effects on cognition, and graft-versus-host disease can induce inflammation, which is a possible cause of CRCI. These data are thus not sufficiently informative on the effect of CML and its targeted therapy on cognitive function (Meadows, et al 2013).
Chronic Lymphocytic Leukaemia (CLL)
CLL, including the small lymphocytic lymphoma (SLL) variant, is the most prevalent lymphoid malignancy in the U.S. with an estimated 120,000 - 140,000 Americans having the disease. Approximately 20,000 people are diagnosed with CLL each year and ~5,000 people will die from it annually (National Cancer Institute 2014). Chemoimmunotherapy is very effective in treating CLL but is non-curative. The introduction of monoclonal antibody and small molecule targeted therapy has increased treatment efficacy and survival.
To our knowledge only three studies have evaluated cognition in CLL patients, all using the EORTC QLQ-30. In a study of all haematological malignancies (Pamuk, et al 2008), CLL patients had a mean score of 63 on the cognitive functioning subdomain, which is significantly lower than published normative means of >85 (Hjermstad, et al 1998). However, this study included only 25 CLL patients, the majority of whom were inpatients with active disease and thus not representative of the general CLL population (Pamuk, et al 2008).
In the British-led Leukaemia Research Fund 4 (LRF4) study, 431 patients with Binet stage A CLL and progressive disease were assessed using the EORTC-QLQ30 before randomization. The patients scored lower than publish norms; however, this difference was not clinically meaningful (<5 points difference). Patients reporting B symptoms performed 13 points worse than other patients in the study, a difference that was both statistically and clinically significant (Else, et al 2008), suggesting that patients with more symptomatic disease could have more severe cognitive dysfunction. These patients were reassessed five years later. Approximately 19% of patients who received chlorambucil and 38% who received fludarabine and chlorambucil had worsened self-reported cognitive functioning (absolute measures not reported, p=0.005). These changes in cognitive function are probably underestimates, as patients who were older, had more advanced stage disease or had early disease progression were less likely to survive to be included in the five-year follow-up (Else, et al 2012). The complex nature of CRCI is highlighted by the difference in changes in cognitive function decline between the two reported study groups. Factors that could have contributed to these differences include treatment toxicity and the depth and duration of response, which were not analysed in detail.
A study of 76 CLL survivors and 152 age- and gender-matched non-cancer participants found that CLL survivors scored lower on the cognition domain of the EORTC QLQ-30 than healthy participants (74.4 vs. 80.4, p<0.1) and that patients previously treated with chemotherapy performed better than previously untreated patients (77.1 vs. 72.5 p>0.05) (Holzner, et al 2004). While this data is informative, all of these studies are based on self-report measures designed to capture quality of life where only two questions assess cognition. Objective assessments in CLL survivors are warranted to determine which cognitive domains are affected (if any).
Hodgkin Lymphoma (HL)
Over 9,000 new cases of HL will be diagnosed each year and there are currently over 177,000 HL survivors in the U.S. (Howlader et al 2014). HL treatment modalities include chemotherapy, radiation, conjugated monoclonal antibodies and targeted immune modulator agents.
Two cross-sectional studies of long-term HL survivors have reported objective and subjective cognitive deficits persisting for years after completion of treatment. One of the earliest studies on CRCI evaluated 93 HL survivors at an average of 10 years from diagnosis, and 186 sex-, age- and residency-matched healthy participants using the EORTC-QLQ30. HL survivors reported significantly lower cognitive functioning compared to healthy subjects (79.5 vs. 89.8, p=0.03) (Joly, et al 1996). Another study evaluated 62 HL survivors at least 15 years post-therapy with combination chemotherapy including an anthracycline, who were treated with either a higher (≥30 Gy) or lower (<30 Gy) dose of thoracic radiation. Up to 27% of survivors were impaired (>1 standard deviation below the normative mean) on tests of attention, 45% on tests of memory, 43% on tests of processing speed and 25% on tests of executive function without any differences according to radiation dose (Krull, et al 2012).
Because HL therapy often includes radiation, it is unclear what proportion of cognitive impairment is attributable to chemotherapy. Although Krull et al (2012) did not find significant differences in the effects on cognition of radiation dose, they hypothesized that thoracic radiation is associated with cardiopulmonary morbidities that may result in central nervous system (CNS) morbidities that ultimately result in cognitive impairment. Anthracyclines and other chemotherapies used in HL have been associated with cognitive impairment when used to treat other malignancies, and it’s possible that thoracic radiation magnifies cognitive impairment in this population (Krull, et al 2012).
Two studies have examined groups composed of both HL and non-Hodgkin lymphoma (NHL) survivors.Baudino et al (2012) compared 14 patients prior to chemotherapy to 14 age- and sex-matched patients who had finished chemotherapy between one week and three years previously. Patients who had received chemotherapy were treated with either ABVD (doxorubicin, bleomycin, vinblastine, dacarbazine) or CHOP (cyclophosphamide, doxorubicin, vincristine, prednisolone). The authors reported no significant differences on tests of language; however participants did perform worse on measures of attention and verbal fluency although these differences were not significant (Baudino, et al 2012). Ahles et al (2002) also enrolled HL and NHL survivors at least five years after diagnosis who had received systemic chemotherapy (n=36) or surgery and radiation (n=22). Among those receiving chemotherapy the most common regimen was CHOP. Lymphoma survivors performed worse on tests of language, memory, psychomotor function, attention and spatial ability; however, these differences were not statistically significant (Ahles, et al 2002).
Non-Hodgkin Lymphomas (NHL)
Approximately 70,800 cases of NHL are diagnosed annually in the U.S. with approximately 585,000 survivors living in the U.S. (Howlader et al 2014). NHL is a heterogeneous group of diseases with diverse treatments including radiation, immunotherapy and chemotherapy.
A study of 30 B-cell NHL survivors 1-3 months post-therapy with either bendamustine and rituximab or CHOP plus rituximab found more self-reported cognitive problems on the EORTC-QLQ30 test compared to healthy controls (N=10) (88.3% vs. 64.4% %, p=0.013) (Zimmer, et al 2015). NHL survivors also performed significantly worse on tests of executive function and attention (p=0.003). However, this study was small and only evaluated executive function and attention (Zimmer, et al 2015).
Another study comparing 307 diffuse large B-cell lymphoma survivors (mean of 3.4 years from treatment) with 596 age- and sex-matched non-cancer controls examined subjective cognitive impairment using the EORTC-QLQ30 (van der Poel, et al 2014). Almost all of the survivors had received chemotherapy (95%, type not specified) and 29% also received radiation. The lymphoma survivors self-reported more cognitive problems than healthy participants, with a larger effect size in survivors aged 18 to 59 than in 60- to 85-year-olds (18 to 59 group difference 13 points, p<0.01) (van der Poel, et al 2014).
Primary CNS Lymphoma (PCNSL)
Up to 1,600 cases of PCNSL are diagnosed in the U.S. each year (Villano, et al 2011). Treatment for PCNSL with whole-brain irradiation results in a median overall survival of 12-18 months but high dose chemotherapy (e.g. high dose methotrexate with leucovorin rescue) increases median survival to 60 months or more (Schafer, et al 2012).
We reviewed five published studies of CRCI in PCNSL survivors treated without whole brain radiotherapy, four of which were longitudinal (Correa, et al 2012, Doolittle, et al 2013a, Doolittle, et al 2013b, Fliessbach, et al 2005, Juergens, et al 2010). The longitudinal studies reported that compared to pre-treatment cognition, there was stable or increased cognitive function months or years post-treatment. However, two of these studies reported that up to 60% of survivors were still impaired compared to published norms for the domains of attention, executive function, verbal fluency, memory and psychomotor function 5 or more years after treatment (Doolittle, et al 2013b, Fliessbach, et al 2005).
Cognitive Impairment with Treatment Modalities Other than Chemotherapy
Radiation Therapy
The neurotoxicity of whole brain radiation has been well documented, especially in ALL and PCNSL, and new treatment regimens avoid or limit its use (Cheung and Krull 2015) (Doolittle, et al 2013b). CRCI is also associated with radiotherapy to other parts of the body, although the effects do not seem to be as severe as with chemotherapy (Janelsins, et al 2011).
HSCT
There are several studies showing patient-reported cognitive impairment before and after HSCT (Andrykowski, et al 1999, Booth-Jones, et al 2005). However, objective assessments of cognition in these studies were less consistent, and studies frequently did not distinguish between allogeneic and autologous HSCT. Phillips et al (2013) published a systematic review and meta-analysis of all studies examining cognition in HSCT survivors prior to 2011. Seventeen articles were reviewed and 11 were included in the meta-analysis (n=404 subjects). The authors concluded that there was consistent evidence for pre-HSCT cognitive impairment in the domains of memory, attention, verbal fluency, executive functioning and visuospatial functioning. However, given that HSCT is not typically a first line therapy, the majority of patients would have already received treatment for their disease . The longitudinal studies reviewed provided mixed evidence for cognitive decline from pre- to post-HSCT. The meta-analysis showed declines in attention, information processing and motor speed (effect sizes of -0.10, -0.21, -0.17 respectively) but these failed to reach statistical significance, leading the authors to conclude there is not sufficient evidence to suggest cognitive decline after HSCT (Phillips et al 2013).
The HSCT CRCI literature is limited by small studies with extreme sample heterogeneity. In addition to including patients with both allogeneic and autologous donors, the studies did not stratify for conditioning regimens (and especially use of radiotherapy), disease entities and prior therapies. In studies comparing haematological malignancy survivors treated with or without HSCT, HSCT was associated with worse psychomotor function (n=106, and 40 respectively) (Chang, et al 2009, Harder, et al 2007). However, only one of these studies found that HSCT was associated with worse function on tests of attention and executive function (20 months post-treatment) (Harder, et al 2007).
Targeted small molecule inhibitors
Small molecule inhibitors and other targeted therapies have emerged as effective and less toxic treatments for haematological malignancies. There are no published data on the effects of these targeted treatments on cancer-related cognition. As the role of these new drugs expands, these data will become more important in planning patient management.
Clinical Implications of CRCI
Accurate clinical evaluation and description of cognitive impairments is important because cognitive impairment can lead to poor treatment compliance, decreased quality of life, inability to work, drive and read (especially in older adults), and difficulty returning to work or school (Myers 2012, Reid-Arndt, et al 2010). In a study of 226 severely ill patients, 80% said they would not have chosen a treatment if they had known it would affect their cognitive ability (Fried, et al 2002), and cancer survivors who self-report cognitive difficulties are often significantly bothered by them (Schagen, et al 2008). Thus, concerns about cognitive problems should be evaluated and addressed by the treating physician and psychologists.
An improved understanding of CRCI could improve treatment decisions and interventions. For example, more research is need in indolent lymphoid malignancies, such as CLL, where there can be a long time between diagnosis and initial treatment, to determine if disease progression impairs cognitive function before patients meet the current criteria for initiation of treatment and if successful therapy exacerbates or decreases cognitive dysfunction. Additionally, learning more about the interrelationships between cognitive dysfunction and specific malignancies and their treatments will be informative for developing interventions that could improve treatment outcomes.
Lastly, baseline cognitive function has been correlated with survival. A longitudinal study of haematological malignancy survivors found that those with any cognitive impairment had a one-year overall survival of 63% compared to 83% for those with no cognitive impairment (hazard ratio 3.2 85% confidence interval 1.04-10.19) (Dubruille et al 2015). However, the independent value of these findings needs to be confirmed in studies that control for confounding variables including comorbidities.
The management of cognitive impairment in haematological malignancy survivors should be individualized depending on the patient’s specific circumstances and concerns. Physicians may consider addressing anxiety, depression and fatigue as well as stressors, and suggest coping strategies for managing cognitive complaints. Cognitive impairment is one of many adverse events that cancer survivors experience; increased physician and patient awareness of treatment history and associated risks will help to identify those patients most at risk for early and late effects including CRCI and provide individualized care (Robison and Hudson 2014).
Limitations of Current Research and Future Directions
The available haematological malignancy CRCI data are limited by small sample sizes, heterogeneous patient populations, cross-sectional design and few (or no) studies in each malignancy. Future research should focus on haematological malignancies that have the least data. For example, there are many studies of childhood ALL survivors but sparse literature on patients with CLL and other NHL subtypes. Future studies should utilize multiple study sites to increase sample size and focus on patients with the same disease and treatment whenever possible. Longitudinal studies with pre-treatment cognitive function assessments are needed to differentiate between the effects of disease and treatment on cognitive function.
The studies reviewed used a variety of neuropsychological assessments of many cognitive domains, making it difficult to compare results. Future studies should employ the neuropsychological assessments recommended by the ICCTF (Wefel, et al 2011). Most studies that examined subjective measures of cognitive function used the cognitive subdomain of the EORTCQLQ-30. This validated questionnaire was developed to assess quality of life and conclusions on cognitive function are based on only two questions. Future research should employ a validated questionnaire designed to assess cognitive function, such as the Functional Assessment after Cancer Cognition (FAT-Cog).
Summary
The limited CRCI literature in haematological malignancy survivors is currently dominated by studies of childhood ALL survivors and PCNSL. Many of the published studies in other haematological malignancies are limited by small sample size, cross-sectional design and heterogeneous patient populations. Nevertheless, it is apparent that a subset of chemotherapy-treated haematological malignancy survivors experience cognitive impairment. Future research should focus on understudied malignancies (NHL; CLL) using homogenous samples with similar treatment experiences, and take advantage of multiple sites to enrol large populations in studies powered to detect subtle cognitive changes.
Acknowledgments
This manuscript was funded by NCI K07 CA168886.
Footnotes
Competing Interests: The authors have no competing interests.
Author contributions: All authors contributed to the design and writing of this paper.
References
- Ahles TA, Saykin A. Cognitive effects of standard-dose chemotherapy in patients with cancer. Cancer Invest. 2001;19:812–820. doi: 10.1081/cnv-100107743. [DOI] [PubMed] [Google Scholar]
- Ahles TA, Saykin AJ. Candidate mechanisms for chemotherapy-induced cognitive changes. Nat Rev Cancer. 2007;7:192–201. doi: 10.1038/nrc2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahles TA, Saykin AJ, Furstenberg CT, Cole B, Mott LA, Skalla K, Whedon MB, Bivens S, Mitchell T, Greenberg ER, Silberfarb PM. Neuropsychologic impact of standard-dose systemic chemotherapy in long-term survivors of breast cancer and lymphoma. J Clin Oncol. 2002;20:485–493. doi: 10.1200/JCO.2002.20.2.485. [DOI] [PubMed] [Google Scholar]
- Alibhai SM, Leach M, Gupta V, Tomlinson GA, Brandwein JM, Saiz FS, Minden MD. Quality of life beyond 6 months after diagnosis in older adults with acute myeloid leukemia. Crit Rev Oncol Hematol. 2009;69:168–174. doi: 10.1016/j.critrevonc.2008.07.015. [DOI] [PubMed] [Google Scholar]
- Andrykowski MA, Cordova MJ, Hann DM, Jacobsen PB, Fields KK, Phillips G. Patients’ psychosocial concerns following stem cell transplantation. Bone Marrow Transplant. 1999;24:1121–1129. doi: 10.1038/sj.bmt.1702022. [DOI] [PubMed] [Google Scholar]
- Asher A. Cognitive dysfunction among cancer survivors. Am J Phys Med Rehabil. 2011;90:S16–26. doi: 10.1097/PHM.0b013e31820be463. [DOI] [PubMed] [Google Scholar]
- Baudino B, D’Agata F, Caroppo P, Castellano G, Cauda S, Manfredi M, Geda E, Castelli L, Mortara P, Orsi L, Cauda F, Sacco K, Ardito RB, Pinessi L, Geminiani G, Torta R, Bisi G. The chemotherapy long-term effect on cognitive functions and brain metabolism in lymphoma patients. Q J Nucl Med Mol Imaging. 2012;56:559–568. [PubMed] [Google Scholar]
- Biglia N, Bounous VE, Malabaila A, Palmisano D, Torta DM, D’Alonzo M, Sismondi P, Torta R. Objective and self-reported cognitive dysfunction in breast cancer women treated with chemotherapy: a prospective study. Eur J Cancer Care (Engl) 2012;21:485–492. doi: 10.1111/j.1365-2354.2011.01320.x. [DOI] [PubMed] [Google Scholar]
- Booth-Jones M, Jacobsen PB, Ransom S, Soety E. Characteristics and correlates of cognitive functioning following bone marrow transplantation. Bone Marrow Transplant. 2005;36:695–702. doi: 10.1038/sj.bmt.1705108. [DOI] [PubMed] [Google Scholar]
- Bradley CJ, Neumark D, Bednarek HL, Schenk M. Short-term effects of breast cancer on labor market attachment: results from a longitudinal study. J Health Econ. 2005;24:137–160. doi: 10.1016/j.jhealeco.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Chang G, Meadows ME, Orav EJ, Antin JH. Mental status changes after hematopoietic stem cell transplantation. Cancer. 2009;115:4625–4635. doi: 10.1002/cncr.24496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung YT, Krull KR. Neurocognitive outcomes in long-term survivors of childhood acute lymphoblastic leukemia treated on contemporary treatment protocols: A systematic review. Neurosci Biobehav Rev. 2015;53:108–120. doi: 10.1016/j.neubiorev.2015.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa DD, Shi W, Abrey LE, Deangelis LM, Omuro AM, Deutsch MB, Thaler HT. Cognitive functions in primary CNS lymphoma after single or combined modality regimens. Neuro Oncol. 2012;14:101–108. doi: 10.1093/neuonc/nor186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doolittle ND, Dosa E, Fu R, Muldoon LL, Maron LM, Lubow MA, Tyson RM, Lacy CA, Kraemer DF, Butler RW, Neuwelt EA. Preservation of cognitive function in primary CNS lymphoma survivors a median of 12 years after enhanced chemotherapy delivery. J Clin Oncol. 2013a;31:4026–4027. doi: 10.1200/JCO.2013.52.7747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doolittle ND, Korfel A, Lubow MA, Schorb E, Schlegel U, Rogowski S, Fu R, Dosa E, Illerhaus G, Kraemer DF, Muldoon LL, Calabrese P, Hedrick N, Tyson RM, Jahnke K, Maron LM, Butler RW, Neuwelt EA. Long-term cognitive function, neuroimaging, and quality of life in primary CNS lymphoma. Neurology. 2013b;81:84–92. doi: 10.1212/WNL.0b013e318297eeba. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubruille S, Libert Y, Roos M, Vandenbossche S, Collard A, Meuleman N, Maerevoet M, Etienne AM, Reynaert C, Razavi D, Bron D. Identification of clinical parameters predictive of one-year survival using two geriatric tools in clinically fit older patients with hematological malignancies: Major impact of cognition. J Geriatr Oncol. 2015;6:362–369. doi: 10.1016/j.jgo.2015.07.006. [DOI] [PubMed] [Google Scholar]
- Dutta V. Chemotherapy, neurotoxicity, and cognitive changes in breast cancer. J Cancer Res Ther. 2011;7:264–269. doi: 10.4103/0973-1482.87008. [DOI] [PubMed] [Google Scholar]
- Eberhardt B, Dilger S, Musial F, Wedding U, Weiss T, Miltner WH. Medium-term effects of chemotherapy in older cancer patients. Support Care Cancer. 2006a;14:216–222. doi: 10.1007/s00520-005-0894-4. [DOI] [PubMed] [Google Scholar]
- Eberhardt B, Dilger S, Musial F, Wedding U, Weiss T, Miltner WH. Short-term monitoring of cognitive functions before and during the first course of treatment. J Cancer Res Clin Oncol. 2006b;132:234–240. doi: 10.1007/s00432-005-0070-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Else M, Smith AG, Cocks K, Richards SM, Crofts S, Wade R, Catovsky D. Patients’ experience of chronic lymphocytic leukaemia: baseline health-related quality of life results from the LRF CLL4 trial. Br J Haematol. 2008;143:690–697. doi: 10.1111/j.1365-2141.2008.07407.x. [DOI] [PubMed] [Google Scholar]
- Else M, Cocks K, Crofts S, Wade R, Richards SM, Catovsky D, Smith AG. Quality of life in chronic lymphocytic leukemia: 5-year results from the multicenter randomized LRF CLL4 trial. Leuk Lymphoma. 2012;53:1289–1298. doi: 10.3109/10428194.2011.649479. [DOI] [PubMed] [Google Scholar]
- Ferguson RJ, Ahles TA, Saykin AJ, McDonald BC, Furstenberg CT, Cole BF, Mott LA. Cognitive-behavioral management of chemotherapy-related cognitive change. Psychooncology. 2007;16:772–777. doi: 10.1002/pon.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson RJ, McDonald BC, Rocque MA, Furstenberg CT, Horrigan S, Ahles TA, Saykin AJ. Development of CBT for chemotherapy-related cognitive change: results of a waitlist control trial. Psychooncology. 2012;21:176–186. doi: 10.1002/pon.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliessbach K, Helmstaedter C, Urbach H, Althaus A, Pels H, Linnebank M, Juergens A, Glasmacher A, Schmidt-Wolf IG, Klockgether T, Schlegel U. Neuropsychological outcome after chemotherapy for primary CNS lymphoma: a prospective study. Neurology. 2005;64:1184–1188. doi: 10.1212/01.WNL.0000156350.49336.E2. [DOI] [PubMed] [Google Scholar]
- Fried TR, Bradley EH, Towle VR, Allore H. Understanding the treatment preferences of seriously ill patients. N Engl J Med. 2002;346:1061–1066. doi: 10.1056/NEJMsa012528. [DOI] [PubMed] [Google Scholar]
- Gaynon PS, Angiolillo AL, Carroll WL, Nachman JB, Trigg ME, Sather HN, Hunger SP, Devidas M. Long-term results of the children’s cancer group studies for childhood acute lymphoblastic leukemia 1983-2002: a Children’s Oncology Group Report. Leukemia. 2010;24:285–297. doi: 10.1038/leu.2009.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder H, Van Gool AR, Duivenvoorden HJ, Cornelissen JJ, Eijkenboom WM, Barge RM, van den Bent MJ. Case-referent comparison of cognitive functions in patients receiving haematopoietic stem-cell transplantation for haematological malignancies: two-year follow-up results. Eur J Cancer. 2007;43:2052–2059. doi: 10.1016/j.ejca.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Hess LM, Insel KC. Chemotherapy-related change in cognitive function: a conceptual model. Oncol Nurs Forum. 2007;34:981–994. doi: 10.1188/07.ONF.981-994. [DOI] [PubMed] [Google Scholar]
- Hjermstad MJ, Fayers PM, Bjordal K, Kaasa S. Using reference data on quality of life--the importance of adjusting for age and gender, exemplified by the EORTC QLQ-C30 (+3) Eur J Cancer. 1998;34:1381–1389. doi: 10.1016/s0959-8049(98)00136-1. [DOI] [PubMed] [Google Scholar]
- Holzner B, Kemmler G, Kopp M, Nguyen-Van-Tam D, Sperner-Unterweger B, Greil R. Quality of life of patients with chronic lymphocytic leukemia: results of a longitudinal investigation over 1 yr. Eur J Haematol. 2004;72:381–389. doi: 10.1111/j.1600-0609.2004.00233.x. [DOI] [PubMed] [Google Scholar]
- Howlader N, Noone AM, Krapcho M, Garshell J, Miller D, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA, editors. National Cancer Institute; Bethesda, MD: 2014. SEER Cancer Statistics Review 1975-2011. http://seer.cancer.gov/csr/1975_2011/, based on November 2013 SEER data submission, posted to the SEER web site, April 2014. [Google Scholar]
- Janelsins MC, Kohli S, Mohile SG, Usuki K, Ahles TA, Morrow GR. An update on cancer- and chemotherapy-related cognitive dysfunction: current status. Semin Oncol. 2011;38:431–438. doi: 10.1053/j.seminoncol.2011.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janelsins MC, Kesler SR, Ahles TA, Morrow GR. Prevalence, mechanisms, and management of cancer-related cognitive impairment. Int Rev Psychiatry. 2014;26:102–113. doi: 10.3109/09540261.2013.864260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janelsins MC, Peppone LJ, Heckler CE, Kesler SR, Sprod LK, Atkins J, Melnik M, Kamen C, Giguere J, Messino MJ, Mohile SG, Mustian KM. YOCAS(c)(R) Yoga Reduces Self-reported Memory Difficulty in Cancer Survivors in a Nationwide Randomized Clinical Trial: Investigating Relationships Between Memory and Sleep. Integr Cancer Ther. 2015 doi: 10.1177/1534735415617021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joly F, Henry-Amar M, Arveux P, Reman O, Tanguy A, Peny AM, Lebailly P, Mace-Lesec’h J, Vie B, Genot JY, Busson A, Troussard X, Leporrier M. Late psychosocial sequelae in Hodgkin’s disease survivors: a French population-based case-control study. J Clin Oncol. 1996;14:2444–2453. doi: 10.1200/JCO.1996.14.9.2444. [DOI] [PubMed] [Google Scholar]
- Joly F, Lange M, Rigal O, Correia H, Giffard B, Beaumont JL, Clisant S, Wagner L. French version of the Functional Assessment of Cancer Therapy-Cognitive Function (FACT-Cog) version 3. Support Care Cancer. 2012;20:3297–3305. doi: 10.1007/s00520-012-1439-2. [DOI] [PubMed] [Google Scholar]
- Joly F, Giffard B, Rigal O, De Ruiter MB, Small BJ, Dubois M, LeFel J, Schagen SB, Ahles TA, Wefel JS, Vardy JL, Pancre V, Lange M, Castel H. Impact of Cancer and Its Treatments on Cognitive Function: Advances in Research From the Paris International Cognition and Cancer Task Force Symposium and Update Since 2012. J Pain Symptom Manage. 2015;50:830–841. doi: 10.1016/j.jpainsymman.2015.06.019. [DOI] [PubMed] [Google Scholar]
- Juergens A, Pels H, Rogowski S, Fliessbach K, Glasmacher A, Engert A, Reiser M, Diehl V, Vogt-Schaden M, Egerer G, Schackert G, Reichmann H, Kroschinsky F, Bode U, Herrlinger U, Linnebank M, Deckert M, Fimmers R, Schmidt-Wolf IG, Schlegel U. Long-term survival with favorable cognitive outcome after chemotherapy in primary central nervous system lymphoma. Ann Neurol. 2010;67:182–189. doi: 10.1002/ana.21824. [DOI] [PubMed] [Google Scholar]
- Kesler S, Hadi Hosseini SM, Heckler C, Janelsins M, Palesh O, Mustian K, Morrow G. Cognitive training for improving executive function in chemotherapy-treated breast cancer survivors. Clin Breast Cancer. 2013;13:299–306. doi: 10.1016/j.clbc.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppelmans V, Breteler MM, Boogerd W, Seynaeve C, Gundy C, Schagen SB. Neuropsychological performance in survivors of breast cancer more than 20 years after adjuvant chemotherapy. J Clin Oncol. 2012;30:1080–1086. doi: 10.1200/JCO.2011.37.0189. [DOI] [PubMed] [Google Scholar]
- Koppelmans V, Breteler MM, Boogerd W, Seynaeve C, Schagen SB. Late effects of adjuvant chemotherapy for adult onset non-CNS cancer; cognitive impairment, brain structure and risk of dementia. Crit Rev Oncol Hematol. 2013;88:87–101. doi: 10.1016/j.critrevonc.2013.04.002. [DOI] [PubMed] [Google Scholar]
- Krull KR, Sabin ND, Reddick WE, Zhu L, Armstrong GT, Green DM, Arevalo AR, Krasin MJ, Srivastava DK, Robison LL, Hudson MM. Neurocognitive function and CNS integrity in adult survivors of childhood hodgkin lymphoma. J Clin Oncol. 2012;30:3618–3624. doi: 10.1200/JCO.2012.42.6841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandelblatt JS, Jacobsen PB, Ahles T. Cognitive effects of cancer systemic therapy: implications for the care of older patients and survivors. J Clin Oncol. 2014;32:2617–2626. doi: 10.1200/JCO.2014.55.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda T, Takayama T, Tashiro M, Nakamura Y, Ohashi Y, Shimozuma K. Mild cognitive impairment after adjuvant chemotherapy in breast cancer patients--evaluation of appropriate research design and methodology to measure symptoms. Breast Cancer. 2005;12:279–287. doi: 10.2325/jbcs.12.279. [DOI] [PubMed] [Google Scholar]
- Meadows ME, Chang G, Jones JA, Antin JR, Orav EJ. Predictors of neuropsychological change in patients with chronic myelogenous leukemia and myelodysplastic syndrome. Arch Clin Neuropsychol. 2013;28:363–374. doi: 10.1093/arclin/acs141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers CA, Albitar M, Estey E. Cognitive impairment, fatigue, and cytokine levels in patients with acute myelogenous leukemia or myelodysplastic syndrome. Cancer. 2005;104:788–793. doi: 10.1002/cncr.21234. [DOI] [PubMed] [Google Scholar]
- Myers JS. Chemotherapy-related cognitive impairment: the breast cancer experience. Oncol Nurs Forum. 2012;39:E31–40. doi: 10.1188/12.ONF.E31-E40. [DOI] [PubMed] [Google Scholar]
- National Cancer Institute. SEER Cancer Statistics Factsheets: Chronic Lymphocytic Leukemia. Bethesda, MD: 2014. [Google Scholar]
- Pamuk GE, Harmandar F, Ermantas N, Harmandar O, Turgut B, Demir M, Vural O. EORTC QLQ-C30 assessment in Turkish patients with hematological malignancies: association with anxiety and depression. Ann Hematol. 2008;87:305–310. doi: 10.1007/s00277-008-0445-4. [DOI] [PubMed] [Google Scholar]
- Phillips KA, Bernhard J. Adjuvant breast cancer treatment and cognitive function: current knowledge and research directions. J Natl Cancer Inst. 2003;95:190–197. doi: 10.1093/jnci/95.3.190. [DOI] [PubMed] [Google Scholar]
- Phillips KM, McGinty HL, Cessna J, Asvat Y, Gonzalez B, Cases MG, Small BJ, Jacobsen PB, Pidala J, Jim HS. A systematic review and meta-analysis of changes in cognitive functioning in adults undergoing hematopoietic cell transplantation. Bone Marrow Transplant. 2013;48:1350–1357. doi: 10.1038/bmt.2013.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid-Arndt SA, Hsieh C, Perry MC. Neuropsychological functioning and quality of life during the first year after completing chemotherapy for breast cancer. Psychooncology. 2010;19:535–544. doi: 10.1002/pon.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robison LL, Hudson MM. Survivors of childhood and adolescent cancer: life-long risks and responsibilities. Nat Rev Cancer. 2014;14:61–70. doi: 10.1038/nrc3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi D, Terzi-di-Bergamo L, De Paoli L, Cerri M, Ghilardi G, Chiarenza A, Bulian P, Visco C, Mauro FR, Morabito F, Cortelezzi A, Zaja F, Forconi F, Laurenti L, Del Giudice I, Gentile M, Vincelli I, Motta M, Coscia M, Rigolin GM, Tedeschi A, Neri A, Marasca R, Perbellini O, Moreno C, Del Poeta G, Massaia M, Zinzani PL, Montillo M, Cuneo A, Gattei V, Foa R, Gaidano G. Molecular prediction of durable remission after first-line fludarabine-cyclophosphamide-rituximab in chronic lymphocytic leukemia. Blood. 2015;126:1921–1924. doi: 10.1182/blood-2015-05-647925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer N, Glas M, Herrlinger U. Primary CNS lymphoma: a clinician’s guide. Expert Rev Neurother. 2012;12:1197–1206. doi: 10.1586/ern.12.120. [DOI] [PubMed] [Google Scholar]
- Schagen SB, Boogerd W, Muller MJ, Huinink WT, Moonen L, Meinhardt W, Van Dam FS. Cognitive complaints and cognitive impairment following BEP chemotherapy in patients with testicular cancer. Acta Oncol. 2008;47:63–70. doi: 10.1080/02841860701518058. [DOI] [PubMed] [Google Scholar]
- Schagen SB, Klein M, Reijneveld JC, Brain E, Deprez S, Joly F, Scherwath A, Schrauwen W, Wefel JS. Monitoring and optimising cognitive function in cancer patients: Present knowledge and future directions. EJC Suppl. 2014;12:29–40. doi: 10.1016/j.ejcsup.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- Stouten-Kemperman MM, de Ruiter MB, Koppelmans V, Boogerd W, Reneman L, Schagen SB. Neurotoxicity in breast cancer survivors >/=10 years post-treatment is dependent on treatment type. Brain Imaging Behav. 2015;9:275–284. doi: 10.1007/s11682-014-9305-0. [DOI] [PubMed] [Google Scholar]
- van der Poel MW, Oerlemans S, Schouten HC, Mols F, Pruijt JF, Maas H, van de Poll-Franse LV. Quality of life more impaired in younger than in older diffuse large B cell lymphoma survivors compared to a normative population: a study from the population-based PROFILES registry. Ann Hematol. 2014;93:811–819. doi: 10.1007/s00277-013-1980-1. [DOI] [PubMed] [Google Scholar]
- Vardy J, Wefel JS, Ahles T, Tannock IF, Schagen SB. Cancer and cancer-therapy related cognitive dysfunction: an international perspective from the Venice cognitive workshop. Ann Oncol. 2008;19:623–629. doi: 10.1093/annonc/mdm500. [DOI] [PubMed] [Google Scholar]
- Vichaya EG, Chiu GS, Krukowski K, Lacourt TE, Kavelaars A, Dantzer R, Heijnen CJ, Walker AK. Mechanisms of chemotherapy-induced behavioral toxicities. Front Neurosci. 2015;9:131. doi: 10.3389/fnins.2015.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villano JL, Koshy M, Shaikh H, Dolecek TA, McCarthy BJ. Age, gender, and racial differences in incidence and survival in primary CNS lymphoma. Br J Cancer. 2011;105:1414–1418. doi: 10.1038/bjc.2011.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner LI, Sweet JJ, Butt Z, Lai J, Cella D. Measuring Patient Self-Reported Cognitive Function: Development of the Functional Assessment of Cancer Therapy Cognitive Function Instrument. Journal of Supportive Oncology. 2009;7:W32–W39. [Google Scholar]
- Wefel JS, Lenzi R, Theriault RL, Davis RN, Meyers CA. The cognitive sequelae of standard-dose adjuvant chemotherapy in women with breast carcinoma: results of a prospective, randomized, longitudinal trial. Cancer. 2004;100:2292–2299. doi: 10.1002/cncr.20272. [DOI] [PubMed] [Google Scholar]
- Wefel JS, Vardy J, Ahles T, Schagen SB. International Cognition and Cancer Task Force recommendations to harmonise studies of cognitive function in patients with cancer. Lancet Oncol. 2011;12:703–708. doi: 10.1016/S1470-2045(10)70294-1. [DOI] [PubMed] [Google Scholar]
- Wefel JS, Kesler SR, Noll KR, Schagen SB. Clinical characteristics, pathophysiology, and management of noncentral nervous system cancer-related cognitive impairment in adults. CA Cancer J Clin. 2015;65:123–138. doi: 10.3322/caac.21258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub S, Bauer PJ, Zelazo PD, Wallner-Allen K, Dikmen SS, Heaton RK, Tulsky DS, Slotkin J, Blitz DL, Carlozzi NE, Havlik RJ, Beaumont JL, Mungas D, Manly JJ, Borosh BG, Nowinski CJ, Gershon RC. I. NIH Toolbox Cognition Battery (CB): introduction and pediatric data. Monogr Soc Res Child Dev. 2013;78:1–15. doi: 10.1111/mono.12031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams AM, Janelsins MC, van Wijngaarden E. Cognitive function in cancer survivors: analysis of the 1999-2002 National Health and Nutrition Examination Survey. Support Care Cancer. 2015;24:2155–2162. doi: 10.1007/s00520-015-2992-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer P, Mierau A, Bloch W, Struder HK, Hulsdunker T, Schenk A, Fiebig L, Baumann FT, Hahn M, Reinart N, Hallek M, Elter T. Post-chemotherapy cognitive impairment in patients with B-cell non-Hodgkin lymphoma: a first comprehensive approach to determine cognitive impairments after treatment with rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone or rituximab and bendamustine. Leuk Lymphoma. 2015;56:347–352. doi: 10.3109/10428194.2014.915546. [DOI] [PubMed] [Google Scholar]


