Abstract
This review highlights recent advances in how the innate and adaptive immune systems control the blood brain and blood nerve barriers. Interferons and TAM receptors play key roles in innate immune control of blood brain barriers. Cells of the adaptive immune system, particularly CD4+ T cells, take distinct routes to enter neural tissues and mediate immune surveillance. Furthermore, the T cell-mediated opening of blood nerve barriers is critical to block replication of neurotropic viruses by allowing antibody access. Such novel insights gained from basic research provide key foundations for future design of therapeutic strategies; enabling antibody access to the brain may be key to cancer immunotherapy and to the use of vaccines against neurodegenerative conditions such as Alzheimer’s disease.
Keywords: Antibody, CD4 T, Neuroimmunology, Alzheimer’s Disease, Neurotropic Viruses, Degeneration, Autoimmunity, Blood Brain Barrier
Antibody Disparity: Not All Tissues Have Access to Antibodies
Antibodies provide protection against infectious microorganisms. They are the basis of protective immunity conferred by most, if not all, childhood vaccines. In addition to providing immunity to pathogens, antibodies mediate clearance of extracellular protein aggregates and cell debris. Antibodies consist of a variable Fab region (see Glossary) and a constant Fc region. The Fab portion recognizes a specific three-dimensional conformation (epitope) of antigens, while the Fc portion mediates effector functions, such as complement activation, phagocytosis and cytotoxic responses. The latter two functions are mediated by Fc receptors [1]. Antibodies can also ‘neutralize’ toxins and viruses by preventing their binding to host entry receptors. Different antibody isotypes (IgM, IgG1-IgG4, IgA and IgE) are specialized for different effector functions [1].
Antibodies are produced by plasma cells, primarily in the bone marrow, and secreted into the circulation. Circulating antibodies can access a variety of tissues through active or passive transport. In some tissues, epithelial cells are equipped with transporters that enable active translocation of certain immunoglobulin isotypes in mice and humans [2]. In the gut mucosa, plasma cells are present in the lamina propria, and epithelial cells express a polymeric Ig receptor (pIgR) that transports dimeric IgA from the basolateral to the luminal side of the gut epithelial cells through transcytosis. In the gut and other mucosal tissues, epithelial cells express neonatal Fc receptor (FcRn) to transport circulating IgG in both directions [3]. Maternal IgG can cross the placenta into fetal circulation by binding to FcRn, expressed by syncytiotrophoblasts in humans [4] and other mammals [3]. Thus, antibodies are delivered actively into the lumen of the gut mucosa as well as into the developing fetus to provide protective immunity [3].
In contrast, antibody access to other tissues is more restricted. These tissues, known as immunopriviledged sites, include the central and peripheral nervous systems, testes, uterus, fetus, placenta and the retina. Antibody access to these tissues is blocked by the barrier created by microvascular endothelial cells and other supportive cell types. This review focuses on the barriers that exist in neural tissues. The blood brain barrier (BBB) and the blood nerve barrier (BNB) protect the central nervous system (CNS) and the peripheral nervous system (PNS), respectively. These barriers ensure that neuronal tissues are protected from entry of autoreactive antibodies and T cells, as well as other toxic constituents of the plasma. However, these effective barriers might predispose the host to neurotropic pathogens, by preventing the access of adaptive immune cells and antibodies [5].
Recent studies have also shed light on the precise paths taken by CD4+ T cells in accessing neural tissues in steady state, as well as autoimmune conditions [6–9]. Other studies have revealed the mechanism by which CD4+ T cells enable antibody access to neuronal tissues to confer antiviral protection [10]. In addition, encouraging results from a Phase 1b passive immunization trial for Alzheimer’s disease (AD) [11] have revealed that a candidate vaccine targeting the fibrillary form of amyloid beta protein might be used to prevent progression of AD. The time is right to integrate the discoveries from a wide range of disciplines in order to better understand antibody access to immunoprivileged sites, so as to be able to control the spread and damage inflicted in infectious and neurodegenerative diseases. Moreover, an improved understanding of antibody access to neural tissues and its regulation by the immune system is necessary to develop therapeutic approaches in the prevention of disease progression in autoimmune neuropathologies, as in the case of multiple sclerosis and Guillain-Barré syndrome.
In this review, I highlight the most recent advances in our understanding of how the innate and adaptive immune responses regulate the BBB and BNB. I discuss studies that reveal the role of interferons (IFN) and the TAM receptor tyrosine kinases (Tyro3, Axl, and Mertk) in regulating BBB during viral infection and sterile injury. I review recent work on various paths taken by CD4+ T cells to enter the CNS for immune surveillance. I present evidence that antibodies can indeed provide protection against neurotropic viruses through access provided by virus-specific CD4+ T cells. However, when not properly regulated, destruction of vascular barriers by autoreactive CD4+ T cells can underlie autoantibody-mediated immunopathology and neuropathies (Key Figure, Figure 1). Finally, the need for controlled access by antibodies to the CNS for cancer immunotherapy and neurodegenerative diseases is also addressed.
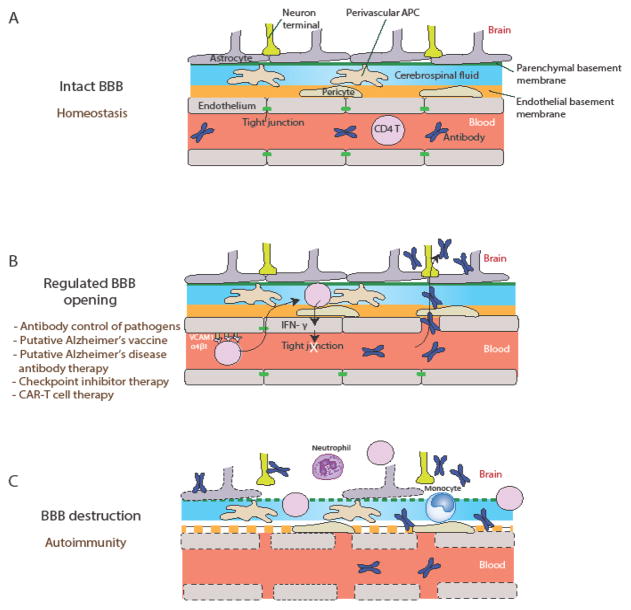
Key Figure, Figure 1. Blood Brain Barrier Regulation by CD4+ T Cells at Postcapillary Venules.
(A) During homeostasis, the blood brain barrier (BBB) forms an impermeable barrier to circulating leukocytes and antibodies. Microvascular endothelial cells (ECs) at postcapillary venules form tight junctions and the endothelial basement membrane surrounds blood vessels. Pericytes reside within the basement membrane and reinforce the barrier. Cerebral spinal fluid fills the perivascular space between the endothelial basement membrane and the parenchymal basement membrane, where antigen presenting cells (perivascular APCs) survey for pathogens. Astrocytes extend their endfeet to provide a further barrier. (B) A regulated opening of the BBB is necessary to enable antibody access to the parenchyma. This may be mediated by antigen-specific CD4+ T cells that recognize cognate antigen -- presented by perivascular APCs -- secrete IFN-γ, and reduce tight junctions between ECs. Circulating antibodies can access the brain parenchyma by crossing the BBB. Regulated BBB opening, if made possible, might be key to the success of antibody-based vaccines and therapies against neurotropic infections, neurodegenerative diseases and/or cancer immunotherapy. (C) Chronic inflammatory stimuli or CD4+ T cell engagement can result in BBB destruction. In this scenario, ECs are damaged, tight junctions are lost, basement membranes are compromised, and lymphocytes and leukocytes can enter the brain parenchyma. Plasma content, including antibodies, will also have free access to the parenchyma under these conditions, which might lead to triggering autoimmune diseases.
Blood Brain and Blood Nerve Barriers
Circulating antibodies are prevented from entry into the CNS and PNS through the action of BBB and BNB (Figure 2). Microvascular endothelial cells (EC) in the PNS and CNS form tight junctions, which consist of homophilic interactions of transmembrane proteins occludin, claudins, as well as junctional adhesion molecules (JAMs) [5] (Figure 2A). JAMs form an impermeable barrier to fluids and are connected to F-actin filaments by the zonula occludens proteins (ZO-1, ZO-2 and ZO-3) [5]. Adherens junctions are formed by homotypic binding of VE-Cadherin, platelet endothelial cell adhesion molecule (PECAM-1), and Nectin. Catenins link VE-cadherin to F-actin (component of microfilaments), while afadin links nectin to F-actin, which helps to coordinate cell surface adhesion and functions of the cytoskeleton (Figure 2A). These transmembrane proteins are associated with cytosolic adaptor protein complexes that couple them to the underlying cytoskeleton, and translate extracellular cues to intracellular actions, as in the case of cell contraction.
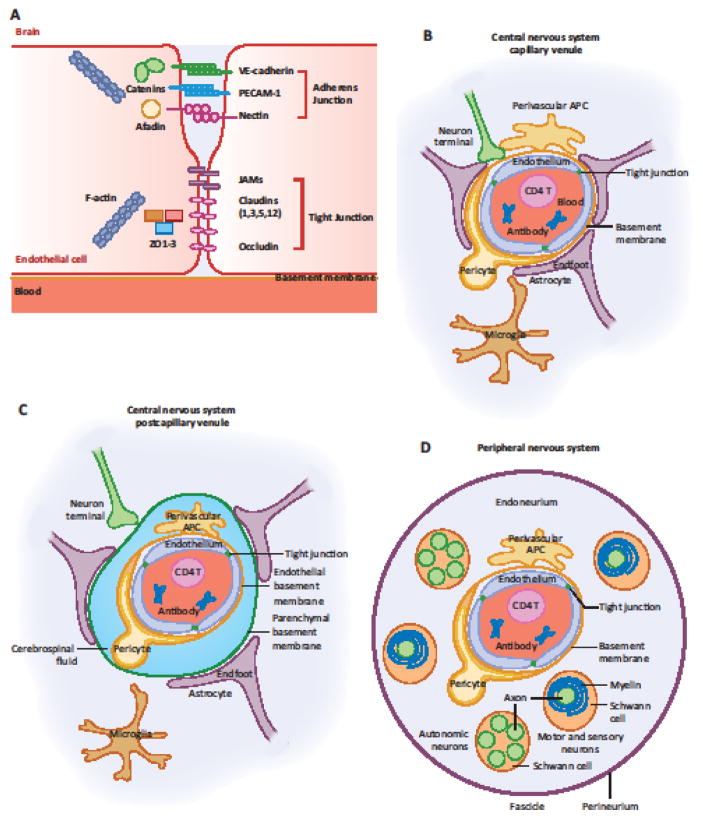
Figure 2. Cellular and Molecular Constituents of the Blood Brain Barrier and Blood Nerve Barrier.
(A) Capillary endothelial cells (ECs) of the CNS and PNS form tight junctions that form impermeable barrier to macromolecules and pathogens in circulation. Figure adapted from [5]. (B) In the CNS capillary microvasculature, the BBB consists of brain microvascular ECs that form tight junctions and pericytes surrounded by the basement membrane. Astrocyte endfeet wrap around the basement membrane forming an integral part of the BBB. Microglia and perivascular macrophages are also shown. (C) In the CNS postcapillary venule, the BBB is extended by the parenchymal basement membrane surrounding the perivascular space filled with CSF. (D) In the PNS, the BNB consists of endoneurial microvascular ECs that form tight junctions and pericytes surrounded by the endothelial basement membrane. Perineurium ensheathment surrounds the endoneurium containing thousands of nerve fibers. Schwann cells wrap myelin sheaths around motor neurons and some sensory neurons, while autonomic and small fiber sensory neurons are enclosed in cytoplasmic pockets.
In addition to tight junctions and adherens junctions between microvascular EC, other supportive structures limit CNS neurons from access by circulating leukocytes and various plasma contents including antibodies (Figure 2B). At the capillary, the BBB consists of the end feet of astrocytes, pericytes and the basement membrane surrounding the blood vessel (Figure 2B). In post-capillary venules, the BBB additionally incorporates the perivascular space filled with cerebrospinal fluid (CSF) with an additional parenchymal basement membrane [12] (Figure 2C).
PNS neurons are protected by the BNB through a space between the proximal root attachment zone of the spinal nerves and the distal sensory and motor end organs [13]. The BNB consists of endoneureal vascular endothelium that forms tight junctions, pericytes, and the perineurium -- the connective tissue ensheathment that encloses around many nerve bundles (Figure 2D). Axons of motor neurons and some sensory neurons are surrounded by the myelin sheath of Schwann cells, which are encased inside the endoneurium. Axons of the autonomic neuron and of the small diameter sensory neuron are unmyelinated, but are encased within the folds of Schwann cells [14] (Figure 2D). Perineurium surrounds multiple nerve fibers, blood microvasculature and endoneurium (Figure 2D). Together, the PNS neurons are protected from the contents of circulating blood by the BNB.
The BBB and BNB enable transport of nutrients and oxygen into the CNS and PNS while blocking diffusion of plasma contents, blood cells and pathogens. In addition to antibodies, plasma contains many other neurotoxic substances, including albumin that induces edema; plasminogen that can be converted into plasmin to degrade the extracellular matrix; fibrinogen that can activate microglia to promote neuroinflammation and demyelination; and red blood cells that can release hemoglobin and Fe+, resulting in reactive oxygen species production and neural damage [5].
In the developing embryo, BBB formation begins with angiogenesis when preexisting vessels sprout into the embryonic neuroectoderm [15]. Acquisition of barrier function in ECs is aided by accessory cells, including pericytes (embryonic) followed by astrocytes (postnatal), in mice [16]. In mice, the majority of the BBB in the hind and mid brain is functional before embryonic day 13.5 (E13.5) while the cerebral cortex BBB becomes functional at E15.5 [17]. Differentiation of astrocytes and the encirclement of capillaries occur in the first 3 weeks of postnatal life [18]. A recent study indicates that the microbiota play an important role in establishing and maintaining BBB integrity [19]. Indeed, germ-free mice exhibit increased BBB leakiness during fetal, post-natal and adult life throughout, with impaired expression of occludin and claudin-5, the components of tight junctions between brain microvascular endothelial cells (BMEC) [19] (Fig. 2A). These data suggest that BBB is controlled by cues coming from distal (gut) microbiota. It will be indeed important to determine the molecular mechanisms underlying microbiome regulation of the BBB.
Innate Immune Regulation of BBB
The BBB is a dynamically regulated barrier. One of the best studied factors that open or close the BBB are cytokines. On the one hand, proinflammatory cytokines released as a result of innate recognition of pathogens through pattern recognition receptors on various leukocytes can bind to BMECs and weaken the BBB [20]; these include TNF-α, IL-1 and IL-6. On the other hand, type I and type III IFNs are induced in response to viral infections and are now known to stabilize the BBB and prevent entry of vascular contents into the CNS [21, 22]. In addition to cytokines, signaling through TAM receptor tyrosine kinases (Tyro3, Axl, and Mertk) on ECs has been shown to enhance BBB integrity in mice and humans [23, 24].
Proinflamatory cytokines secreted by innate immune cells, such as TNF-α, IL-1β, and IL-6 break down the BBB by reducing the expression of tight junction proteins (occludin, claudin-5 and ZO-1) and adherens junction proteins (VE-Cadherin) [5]. The mechanism may include both transcriptional and post-translational down regulation of the genes that encode for these proteins. The source of cytokines can either be systemic (e.g., in sepsis), or local (microglial cells) [20]. These processes have been studied for decades, and I defer the readers to excellent reviews on this topic [20, 25].
Type I and type III IFNs are secreted by cells that detect the presence of viral infections through pattern recognition receptors, and constitute potent antiviral cytokines that can block viral replication in neighboring cells [26]. Recent studies have revealed that type I and type III IFNs enhance BBB by non-redundant mechanisms. For example, West Nile Virus (WNV) infection results in the induction of type I and type III IFNs, as well as in TNF-α and IL-1β secretion [21]. One study in mice has shown that the BBB is enhanced during WNV infection in an IFNAR-dependent [21]. Indeed, WNV infection of isolated mouse BMEC induced the production of type I IFNs by triggering IFNAR signaling in the ECs, thus preventing the vascular permeability imposed by TNF-α and IL-1β secretion [21]. IFNAR signaling can activate cytoskeletal regulator GTPase Rac1 and suppress RhoA to enhance tight junctions and block transendothelial permeability [21]. Similarly, another study reported that type III IFNs, IFN-λs, stabilizes the BBB during WNV infection [22]. Of note, type I IFNs and signaling through IFNAR are required in part, to induce type III IFNs by BMEC, which in turn act on BMECs through the IFN-λ receptor (IFNLR1), thus mediating BBB enhancement [22]. Notably, both type I and type III IFNs mediate BBB stabilization via a non-canonical STAT-1 independent pathway in BMECs [22]. Thus, type I and type III IFNs act in a non-redundant manner on BMEC to enhance and stabilize the BBB against the barrier-reducing effects of TNF-α and IL-1β [21, 22]. In addition to the direct impact of IFN-β on microvascular ECs, IFN-β also blocks neutrophil invasion through the BBB by reducing the neutrophil levels of matrix metalloproteinase (MMP) 9, an enzyme that degrades the basement membrane of the blood vessel endothelium [27]. Overall, an important consequence of enhancing the BBB via type I and type III IFNs is to be able to prevent viral entry into the CNS, and thus, block neutrophil infiltration.
TAM receptor tyrosine kinases, Tyro3, Axl, and Mertk, bind to their ligands, Gas6 and Protein S bound to phosphatidyl serine on the surface of apoptotic cells [28]. Tyro3 and Mertk bind to both Gas6 and Protein S, while Axl binds only to Gas6 [29]. TAMs control a variety of biological processes, including regulation of innate immune signaling [28]. TAM receptor signaling requires IFNAR and STAT1 in mice [30]. Recent studies have revealed the importance of TAM signaling on ECs to stabilize the BBB. For instance, following infection with neurotropic viruses, such as WNV or La Cross virus, mice lacking Axl or Mertk -- but not Tyro3 -- succumb to infection due to an impaired BBB [23]. Axl and Mertk likely play a compensatory role, as Axl/Mertk double knockout (KO) mice suffer from an even more rapid viral dissemination in the CNS followed by death compared to single KO mice (of either gene/protein) [23]. Type I IFNs and TAMs trigger non-redundant signaling pathways to enforce the BBB, as stimulation of BMEC with the combination of IFN-β (IFNAR ligand) and Gas6 (TAM ligand) synergizes to enhance the barrier provided by BMECs [23]. During ischemic BBB disruption in a transient middle cerebral artery occlusion model, administration of a TAM ligand, recombinant Protein S, was shown to protect mice from BBB breakdown [24]. However, this protective effect was lost in Tyro3−/−, but not Mertk−/− or Axl−/−, mice. This study also showed that Protein S treatment mediated Rac1-dependent barrier enhancement in a human BMEC monolayer in vitro, as evidenced by the fact that in BMEC monolayers transduced with a dominant negative Rac1 adenovirus, Protein S failed to improve barrier permeability[24]. Protein S-dependent enhancement in barrier function depended on Tyro3, as knockdown of TYRO3 in human BMEC or BMEC isolated from Tyro3 KO mice resulted in loss of enhanced transendothelial resistance conferred by Protein S [24]. Taken together, these studies reveal a role for each of these TAM receptors in promoting BBB integrity at the level of BMECs. Depending on whether the insult constitutes viral infection or ischemic injury, different TAMs are engaged to provide vasculoprotection in the brain. Whether TAM agonists can be used to promote BBB integrity and protect against autoimmune CNS diseases, however, remains to be seen. Thus, it is intriguing that an association between Mertk polymorphisms and susceptibility to MS has been found in large-scale genome-wide association studies [31–33].
T Cell Regulation of BBB and BNB
In addition to innate proinflammatory cytokines, T cells, particularly CD4+ T cells, enable BBB opening to neuronal tissues by secretion of cytokines such as IFN-γ [34, 35] and IL-17A [36]. Indeed, recent studies have shed light on the coordinated antiviral responses that are mediated by CD4+ T cells and antibodies. CD4+ T cells first enter the neural tissue as “pioneers” [37] to mediate the access of other T cells (both CD4+ and CD8+ T cells), as well as antibodies (discussed below). A pioneering role of CD8+ T cells has not been reported.
T Cell Entry into the CNS
T cells must undergo a series of steps, namely, rolling, adhesion and diapedesis, to enter a tissue [38, 39]. In postcapillary venules, T cells tether and roll on the activated EC surface. The rolling step involves selectins expressed by ECs and selectin ligands expressed by T cells. Integrin α4β1 (also known as very late antigen 4 or VLA-4) can also serve as rolling receptors. The next step involves activation of lymphocytes by chemokines presented by glycosaminoglycans on ECs. Chemokine binding to chemokine receptors on lymphocytes triggers Gαi-dependent “inside-out” signaling leading to activation of integrins on T cells [38]. Activated integrins bind tightly to their ligands on ECs, allowing lymphocytes to arrest on the EC surface. Finally, lymphocytes enter the tissue through a series of interactions between molecules expressed on ECs to accomplish diapedesis [40].
Activated, but not naïve, CD4+ T cells can access the parenchyma of CNS through three separate routes [41] (Figure 3A). The first two routes involve migration of activated T cell from the blood to the cerebrospinal fluid (CSF). CSF entry does not require antigen specificity by activated CD4+ T cells [37]. It occurs in the absence of inflammation, and likely explains the constitutive low presence of polyclonal CD4+ T cells in the CSF. In the first route (➀), activated T cells migrate through the fenestrated endothelium of the choroid plexus, interact with epithelial cells of the choroid plexus, and enter the CSF by crossing the epithelial layer (Figure 3B). The choroid plexus epithelial cells form tight junctions, which must be crossed by the T cells. This requires the expression of chemokine receptor CCR6 on CD4+ T cells, which responds to CCL20, constitutively secreted by choroid plexus epithelial cells [9] (Figure 3B). Once inside the CSF, T cells survey the environment by disseminating to the meningeal and perivascular spaces, aided by the flow of the CSF [6].
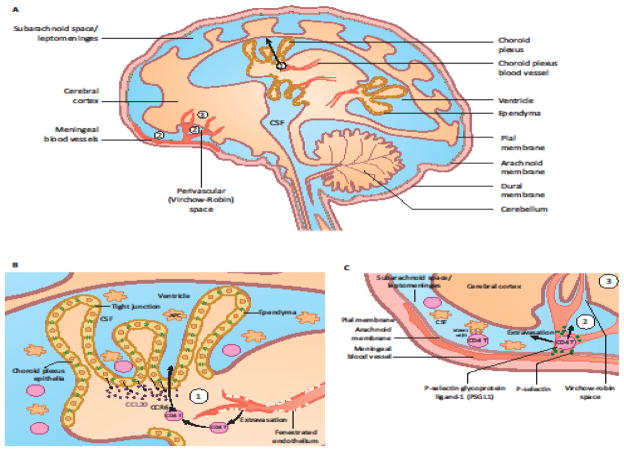
Figure 3. Three Routes of CD4+ T Cell Entry into the CNS.
(A) CD4+ T cells can enter the CNS through at least three separate routes. (B) The first route (➀) involves T cell migration out of the fenestrated endothelial blood vessels of the choroid plexus. T cells then cross the choroid plexus epithelial layer, forming tight junctions in a chemokine-dependent manner to enter the CSF. Choroid plexus epithelial cells secrete CCL20 constitutively, which can recruit CCR6+ CD4+ T cells to the CSF. From there, T cells might disseminate to the meningeal and perivascular spaces. (C) In the second route (➁), T cells can access the CNS from blood to the subarachnoid space, and the Virchow-Robin perivascular space at the pial surface of the brain. In the third route (A, ➂), T cells access the parenchyma by directly crossing the microvascular endothelium at the BBB. This only occurs under inflammatory conditions. These routes are originally described in the review [41].
In the second route (➁), activated T cells access the CSF from blood to subarachnoid space/leptomeninges and the Virchow-Robin perivascular space at the pial surface of the brain (Figure 3C) [42]. Both brain-reactive (myelin basic protein; MBP)-specific CD4+ T cells and anti-CD3 antibody activated non-specific splenic CD4+ T cells enter the leptomeninges, as early as 2 hours post injection into mice [37]. This early migration of activated CD4+ T cells requires P-selectin, constitutively expressed by the meningeal endothelial cells, but not VLA-4 [37] (Figure 3C). However, VLA-4 is necessary for subsequent T cell persistence within the leptomeninges and ultimate entry of reactive T cells into the parenchyma [43, 44]. Recent studies in Lewis rats using intravital imaging revealed that MBP-specific CD4+ T cells enter the CSF via the leptomeninges, and adhere to perivascular macrophages through VLA-4 and LFA-1 interactions (Figure 3C), as well as chemokine signaling via CCR5 and CXCR3 [6]. CD4+ T cells with weaker interactions with CSF macrophages are flushed away by the fluid flow of the CSF, but can reattach to another area of the leptomeninges and ultimately, invade the parenchyma [6]. These results indicate that the leptomeninges is an important checkpoint for T-cell infiltration into the CNS during autoimmune inflammation or immune surveillance. Thus, once CD4+ T cells enter the CSF through route ➀ or ➁, they will likely survey the CSF environment for cognate antigen presented by perivascular APCs by attaching and reattaching to different areas within the ventricle and leptomeninges, respectively [6].
In contrast to the first two routes, in the third route (➂), T cells access the brain parenchyma by directly crossing the microvascular endothelium at the BBB [12]. This occurs following induction of chemokines and other inflammatory adhesion molecules by ‘pioneering’ CD4+ T cells that enter the CSF using route ➀ or ➁ at early time points [9, 37]. This requires engagement of the T cell receptor (TCR) by antigen presenting cells (APCs) in the CSF, along with induction of chemokines enabling T cell entry into the parenchyma [12]. For example, MBP-specific but not polyclonal activated CD4+ T cells secrete IFN-γ in the leptomeningeal space, and enable other circulating CD4+ T cells to enter the CNS parenchyma following 48 hours of infusion in mice [37]. Upon inflammation, BMECs with elevated expression of P-selectins and adhesion molecules enable T cells to roll and slow down in the vasculature. T cells, recognizing chemokines such as CXCL9 and CXCL10 induced by secreted IFN-γ from pioneering T cells [9], activate their integrins such as LFA-1 or VLA-4, which subsequently bind to ICAM-1 or VCAM-1, respectively on the endothelium, thus enabling T cell arrest (Figure 4A) [38]. ICAM-1 and VCAM-1 are not normally expressed by the BMEC but can be induced in response to inflammation [37, 45]. Blocking antibodies to VLA-4 are highly effective in blocking the entry of effector CD4+ T cells into the CNS via this route [43]. Even after having extravasated to the abluminal side of BMECs, T cells do not have a direct access to the neural parenchyma. Instead, they are collected in the perivascular space created by retracted astrocyte endfeet and the basement membrane surrounding the blood vessels (Figure 4C) [12]. Within this perivascular space, T cells might interact with perivascular APCs to become activated. In pathogenic conditions such as MS, with the breakdown of astrocyte endfeet and basement membrane, T cells can then migrate into the parenchyma (Figure 4D) [12].
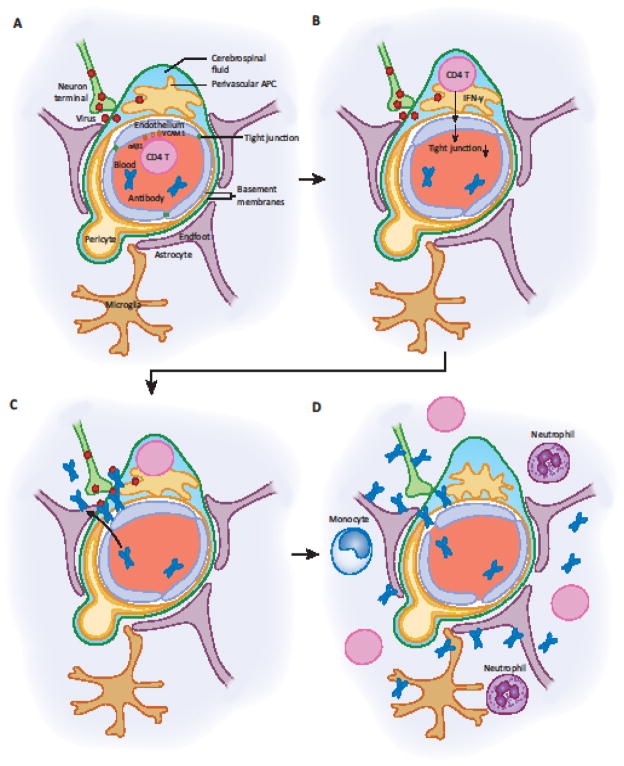
Figure 4. Proposed Mechanism of CD4+ T cell-mediated BBB Aperture for Antibody Access.
(A) Neurotropic viruses can replicate in neurons and are shed from axonal termini near blood vessels in the CNS or PNS. This induces activation of vascular ECs (ECs) to increase expression of VCAM-1, which enables memory CD4+ T cells to exit the blood via VLA-4 engagement. (B) CD4+ T cells enter the perivascular space, and are stimulated by perivascular APCs presenting viral antigen to secrete IFN-γ. IFN-γ acts on vascular ECs and downregulates tight junction proteins. (C) Loss of tight junctions allows circulating antibodies to access the tissue at the site of infection, and virus spread is controlled by antibodies. (D) If this condition persists, it may result in destruction of ECs, a compromised basement membrane, invasion by CD4+ T cells and leukocytes into the parenchyma. Inflammatory conditions lead to activation of perivascular APCs and microglia, leading to the amplification of immune responses, and priming of T and B cell responses to autoantigens within the CNS or PNS.
T Cells in the CNS Can Open the BBB for Antibody Access
Studies in the 1980’s showed that co-injection of MBP-specific T cells and antibody to myeloid oligodendrocyte glycoprotein (MOG) into Lewis rats led to a marked demyelination of CNS neurons [46]. However, injection of MBP-reactive T cell lines or injection of MBP-primed splenocytes alone, or anti-MOG antibody alone, resulted in minimal demyelination, suggesting that T cells that were specific to the myelin sheath could promote antibody access to neuronal tissues, thus leading to myelin destruction [46]. A later study showed that CD4+ T cells, specific to the S100β antigen, not associated with the myelin sheath, (another CNS antigen expressed by astrocytes) also promoted BBB opening in Lewis rats [47]. These studies led to the hypothesis that CNS antigen-specific CD4+ T cells could mediate BBB opening. However, a later study showed that entry of T cells with any specificity could open the BBB, as long as these were restimulated within neuronal tissues [48]. For instance, ovalbumin (OVA)-specific CD4+ T cells injected into Lewis rats could migrate into the CNS after injection of OVA into the thoracic dorsal column [48]. A simultaneous injection of MOG-specific antibody then led to demyelination in these rats, indicating that T cells of any specificity, as long as they were recruited to the CNS, could lead to BBB aperture, thus allowing antibody passage [48]. Why such a system exists has remained unclear until recent work revealed the importance of such a mechanism in immune defense against neurotropic viruses.
Antibody-Mediated Protection against Neurotropic Viruses
Many viruses are known to infect the CNS and PNS, including rabies, measles, poliovirus, WNV, varicella zoster virus (VZV), herpes simplex viruses (HSV), Japanese encephalitis virus (JEV), Zika virus, cytomegalovirus (CMV) and others (Table 1) [49]. Neurotropic viruses use various means to enter the nervous system [50]. Some enter by directly infecting microvascular ECs and budding into the CNS through the basolateral side (e.g., measles virus) [51], or through the infection of the olfactory bulb (e.g., vesicular stomatitis virus) [52]. In addition, viruses such as WNV enter the CNS by hijacking leukocytes [53] and T cells [54] that migrate into the CNS, subsequently seeding the brain. Once within the CNS, WNV infection results in the production of high levels of proinflammatory cytokines [55], leading to a breach in the BBB and further entry of the virus coming from the circulation [21]. Others, such as HSV, circumvent the BBB and enter the CNS through retrograde axonal transport within the pseudounipolar sensory neurons of the PNS ganglia [56]. From the infected sensory neuronal cell body, HSV can spread from the PNS to the CNS through anterograde axonal transport of progeny virions toward the spinal cord [50]. Rabies virus (RABV) and poliovirus enter the CNS neurons from neuromuscular junctions from muscles into somatic motor neurons in the spinal cord [57].
Table 1.
Human Disease Manifestation of Neurotropic Viruses
| Virus | Type | Transmission | Route of entry into the nervous system | Disease manifestations |
|---|---|---|---|---|
| Cytomegalovirus | DNA, Herpesviridae | Contact with urine or saliva, sexual contact, breast milk, mother to child during pregnancy | In vertical transmission, possibly through placenta to fetal brain neural stem progenitor (NSP) cells [89]. | Fever, sore throat, fatigue. Vertical transmission – microcephaly, eye disease |
| Dengue virus | RNA, Flaviviridae | Mosquitoes (Aedes) | BBB breakdown [90] Retrograde axonal transport [91] |
Fever, sore throat, headache, vomiting, fatigue, skin rash. In rare cases, cerebrospinal fluid viremia, encephalitis, meningoencephalitis |
| Herpes simplex viruses | DNA, Herpesviridae | Oral and genital mucosae, sexual transmission | Retrograde axonal transport in sensory neurons to dorsal root ganglia [56] | Mucocutaneous blisters, encephalitis. Vertical transmission – microcephaly, eye disease |
| Japanese encephalitis virus | RNA, Flaviviridae | Mosquitoes (Culex) | CNS infection occurs without BBB breakdown [55] | Fever, headache, vomiting. In rare cases, paralysis, seizures, mental retardation. |
| Measles virus | Negative sense RNA, Paramyxoviridae | Infection of BMEC and budding into the CNS [51] | Fever, cough, rash, conjunctivitis, pneumonia, encephalitis | |
| Rabies virus | Negative sense RNA, Rhabdoviridae | Bites by infected animals | Neuromuscular junctions from muscles into somatic motor neurons in the spinal cord [57] | Fever, headache, cerebral dysfunction, anxiety, confusion, agitation, delirium, abnormal behavior, hallucinations, and insomnia – once the symptoms begin, almost always fatal in humans. |
| Poliovirus | Positive sense RNA, Picornaviridae | Oral fecal | Neuromuscular junctions from muscles into somatic motor neurons in the spinal cord [57] | Fever, sore throat, headache, vomiting, fatigue, stiffness. In rare cases, paralytic polio ensues (loss of reflexes, severe muscle aches, weakness, and loose limbs). |
| Varicella zoster virus (chicken pox, shingles) | DNA, Herpesviridae | Contact, inhalation of viral particles from blisters. | Infected T cells deliver the virus to the skin, where it enters sensory ganglia and through retrograde transport enters the neuronal nuclei [92] | Primary infection – chicken pox; pain, itching, vesicular rash. Reactivation of latent virus – shingles; additional symptoms include postherpetic neuralgia, motor weakness, and sensory loss. |
| West Nile virus | Positive sense RNA, Flaviviridae | Mosquitoes (Culex) | Carried into the CNS via leukocytes [53, 54], retrograde axonal transport [93], BBB breakdown by cytokines followed by transendothelial transmission at the BMEC [21]. | Fever, body aches, joint pains, vomiting, diarrhea, or rash. In rare cases, encephalitis, meningitis, paralysis, cutaneous disease. |
| Zika virus | Positive sense RNA, Flaviviridae | Mosquitoes (Aedes), sexual transmission | In vertical transmission, possibly through placenta [94] to fetal brain neural progenitor cells (NPC) [95, 96, 97–99].. In sexual transmission, ascending infection of the fetal brain may occur [100]. In adult IRF3/5/7 knockout mice, NPC infection is seen [101]. In human brain tissues, radial glial cells and neuroepithelial stem (NES) were targeted by Zika [102] | Fever, joint pain, rash, conjunctivitis. In rare cases, Guillain-Barré syndrome, meningoencephalitis. Vertical transmission - microcephaly and other birth defects in fetus, eye disease |
Based on the route of entry, antibodies can block the spread of neurotropic viruses at multiple stages. For example, HSV-1 and HSV-2 enter the female genital mucosa to establish infection in the host [58]. Antibodies that are secreted into the mucosa can block HSV viral entry into host cells and provide sterilizing immunity [59, 60]. The efficacy of oral poliovirus vaccine (Sabin) thus likely depends on the robust secretion of IgA into the lumen of the gastrointestinal tract, blocking the virus from entering host cells [61]. Even after the virus enters host cells, the subsequent spread of the virus can be prevented by antibodies in the interstitial space, as evidenced by the effective treatment of RABV infection with antisera before the virus enters the CNS [50]. However, once the virus enters neurons and establishes infection, do antibodies have any role in protection? If so, how do antibodies cross the BBB and BNB?
Transient BBB Breakdown is Necessary for Immune Protection Against Neurotropic Viruses
Emerging evidence indicates that antibody blockade of neurotropic viruses requires CD4+ T cell dependent opening of the BBB. Indeed, in the mouse model of genital herpes infection, B cells are required for protection against genital HSV-2 challenge conferred by immunization with attenuated HSV-2 [10]. However, passive transfer of immune serum has been shown to confer protection in B cell-deficient mice, only when previously immunized with attenuated HSV-2 [10, 62]. These results suggest that non-B cell memory - presumably T cell memory, appears to be required for antibody-mediated protection against secondary HSV-2 challenge in mice. Indeed, depletion of CD4+ T cells or blockade of IFN-γ at the time of wild type (WT) HSV-2 challenge was shown to abrogate protective immunity without affecting circulating levels of antiviral IgG [10]. Depletion of CD8+ T cells or NK cells however, had no impact on protection conferred by intranasal immunization [63]. These results collectively indicate that antibody-dependent protection against HSV-2 requires IFN-γ secretion by CD4+ T cells. Circulating memory CD4+ T cells express VLA-4, and upon interaction with VCAM-1 on the activated endothelium, enter the infected dorsal root ganglia and spinal cord (Figure 4A). After crossing the endothelial barrier, they likely recognize viral antigen presented by APCs in the perivascular space near microvascular ECs and induce a robust secretion of IFN-γ (Figure 4B) [10]. IFN-γ induces endocytosis of tight junction proteins upon activation of Rho GTPase and up-regulation of Rho associated kinase [34, 35]. This enables circulating antibodies and other plasma proteins to gain access to infected neuronal tissues. Moreover, IFN-γ can further enhance antigen presentation, VCAM-1 expression, thus providing a positive feedback loop to amplify antiviral responses [64].
In addition to HSV-2 infection, CD4+ T cell-dependent antibody access to the CNS is likely required for protection against RABV. Specifically, intranasal infection of mice with the laboratory-attenuated strain of RABV -- but not the WT RABV-- results in transient infection that is cleared by the immune system [65]. Both B cells and IFN-γR have been found to be necessary for clearance of attenuated RABV from the brain, but antiviral antibodies alone are not sufficient for clearance [65]. This was shown in studies of IFN-γR KO mice, which despite being able to produce elevated neutralizing antibodies against RABV, were unable to clear the virus from the brain [65]. Infection with the attenuated strain was also accompanied by CD4+ T cell entry into the CNS through the microvasculature, concomitant with increased IFN-γ levels in the brain and a breakdown of the BBB [66]. Aperture of the BBB following RABV has been associated with enhanced clearance of virus from the CNS, which is likely mediated by the action of antiviral antibodies that access the tissue [66]. The WT RABV can somehow evade immune clearance by inhibiting BBB breakdown; therefore, WT RABV can establish chronic infection in the CNS of the host. In addition to RABV, antibody access to the CNS following intranasal VSV infection requires VLA-4-mediated CD4+ T cell entry into the CNS. Indeed, depletion of CD4+ T cells or antibody blockade of VLA-4 prior to intranasal VSV challenge in VSV-immunized mice results in a block of antibody access to the brain [10]. Collectively, these studies support the idea that CD4+ T cell entry into the CNS and PNS may be a prerequisite for antibody access to such restricted tissues, and once inside, antibodies are capable of blocking viral replication and spread (Figure 4C).
This mode of antibody access to an otherwise immunoprivileged tissue might be advantageous over the systemic opening of the BBB and BNB, presumably because, i) antibody access is restricted to the virus-infected tissue, given that CD4+ T cells will only be stimulated in response to local viral antigens; and ii) it provides transient tissue access to antibody, as CD4+ T cells will cease to provide antibody access once viral antigens are cleared. The transient and reversible opening of the BBB is needed to prevent immune damage that occurs with chronic damage to the BBB (Figure 4D). A clinical implication of these findings is that for neurotropic viruses that have already entered neural tissues, monotherapy with passive transfer of virus-specific antibody may not work, unless it can be made to cross the BBB. I propose that the regulation of BBB opening which is mediated by CD4+ T cells within the perivascular space (Figure 4C) is critical for delivery of antibodies and drugs to the CNS and PNS without causing immunopathology (Figure 4D).
Disease Consequences of the Loss of the BBB
In contrast to the ‘regulated’ BBB opening mediated by CD4+ T cells (Figure 4C), prolonged BBB opening and CD4+ T cell entry into the parenchyma (Figure 4D) play a detrimental role in the pathogenesis of autoimmune diseases, such as multiple sclerosis (MS) and Guillain–Barré syndrome [67, 68]. In the majority of cases, the Guillain–Barré syndrome occurs up to 4 weeks subsequent to a minor gastrointestinal or respiratory infection with bacteria or viruses [69]. In some cases, infections may also precede and contribute to the onset of MS [70]. This is consistent with the possibility that pathogen mimicry of autoantigens occurs. It is also consistent with the entry of activated T cells into the CNS and the opening of the BBB for a prolonged period of time (Figure 4D) [70].
In the experimental autoimmune encephalomyelitis (EAE) model of MS, infiltration of pathogenic autoreactive Th17 CD4+ T cells into the CNS occurs through two portals, namely, through the choroid plexus – CSF (Figure 3, route ➀) or through the blood to the CNS (Figure 3, route ➂) in the spinal cord. In the EAE model where mice are immunized with MOG(33–55) peptide in complete Freund’s adjuvant (CFA) followed by intravenous (i.v.) injection of pertussis toxin (PTX), CCR6+ Th17 cells are generated, and migrate into the CNS through the choroid plexus that expresses CCL20 around day 13 post immunization [9]. Th17 cells that enter the CSF can then disseminate at the pial surface and in the perivascular Virchow-Robin spaces, where they may recognize self-antigens presented by APCs and secrete IL-17 [9]. In the inflamed brain of MS patients, high expression of CCL20 has also been found in astrocytes in the parenchyma, infiltrated by CD4+ T cells [9]. This study indicated that CCR6+ Th17 cells could infiltrate the brain through the choroid plexus, into the CSF, and be activated by local antigens. Notably, the first wave of Th17 cell entry was followed by a second wave of Th1 cell infiltration into CNS, showing that Th17 cells paved the way for CCR6− Th1 cell entry via local chemokine induction of Th17 cells [9]. A recent study using single-cell RNA sequencing, demonstrated that pathogenic CD4+ T cells in the CNS using the EAE model, presented features of both Th17 and Th1 cells, such as the co-expression of Hif1a, Fosl2, Stat4, and Rel, suggesting that Th17 cells that have entered the CNS may undergo further differentiation into a Th17/Th1 hybrid phenotype [71].
In another study, autoreactive pathogenic Th17 cells were generated in a similar manner as the former example (MOG(35–55) in CFA plus PTX), and then adoptively transferred into naïve WT mice [7]; five days after the transfer, pathogenic Th17 cells migrated into the CNS at the fifth lumbar cord of the spine [7]. Intriguingly, this study found that the frequent stimulation of the soleus muscles in response to a gravitational stimulus (such as by standing on hind legs) was required to induce - via sensory nerves – local IL-6 production by non-immune cells. This in turn, induced CCL20 expression in blood vessels of the fifth lumbar cord [7]. When the mice were suspended via the tail (no sensory neurons being stimulated by the soleus muscles), reduced CCL20 expression was observed along with an improved clinical outcome of EAE disease [7]. In addition to gravitational sensing, mice underwent a relapse in EAE disease following stimulation of sensory neurons by various pain triggers [8]. Pain-mediated neural signals induced chemokine CX3CL1, which recruited myeloid APCs expressing the receptor CX3CR1, eliciting in turn the recruitment of pathogenic CD4+ T cells to the fifth lumbar cord [8]. These results suggest that autoreactive T cells (Th1) need an ‘access pass’ to enter the CNS, and that access is initiated by pioneering Th17/Th1 cells that recognize specific antigen within the CSF. This process is facilitated by systemic PTX injection [9], gravitational stimulus inducing IL-6 [7], or pain-sensing triggering the recruitment of myeloid cells [8]; such pathways converge to induce chemokines that can elicit the recruitment of pioneering T cells into the CNS. The implications of these findings are that we might actually have an opportunity to interfere with this process prior to the entry of autoreactive T cells into the brain parenchyma in human MS disease, so long as we can identify the nature of such an ‘access pass’for T cells.
In this regard, a monoclonal antibody against the α4 integrin, Natalizumab, has been successfully used to treat MS [72]. An unfortunate side effect of Natalizumab is that in rare cases, it causes progressive multifocal leukoencephalopathy (PML) as a result of an opportunistic brain infection caused by the JC virus [73] (Box 1). This side effect reveals the importance of immunosurveillance of the JC virus by circulating lymphocytes that enter the CNS to prevent their reactivation. Despite being transiently pulled off the market between 2005–2006 due to PML complications, Natalizumab was returned to the market due to its significant clinical benefits, and remains approved for the treatment of MS in USA and Europe.
Box 1. Progressive Multifocal Leukoencephalopathy.
PML is caused by infection of the brain with a polyomavirus, JC virus. JC virus is commonly acquired during childhood by 70–90% of humans. JC can cross the BBB and enter the CNS to infect oligodendrocytes and astrocytes and remain latent in healthy humans. The JC virus that causes PML has different promoter sequences compared to that found in healthy humans [87]. The risk of acquiring PML following Natalizumab treatment is higher in those that have preexisting antibodies to JC (i.e., prior exposure to the virus), prior use of immunosuppressants, and prolonged use of Natalizumab [73]. A safe use of Natalizumab may require vaccination of patients against JC virus prior to treatment. Currently there are no vaccines against JC virus. In addition, a therapeutic antiviral treatment for JC virus is warranted as JC virus causes opportunistic and often lethal infections in immunocompromised patients.
In addition to MS, T cell-mediated BBB breakdown may underlie the pathogenesis of antibody-mediated CNS and PNS diseases. Autoantibodies against neural antigens are known to drive several types of CNS diseases and PNS autoimmune neuropathies. In the CNS, autoantibodies are thought to contribute to MS [74]. In the PNS, autoantibodies to glycoproteins mediate motor and sensory neuropathies [75]. Autoantibodies mediate the pathogenesis of a neuropathy caused by monoclonal IgM antibodies to myelin-associated glycoprotein (MAG), i.e. anti-MAG neuropathy, as well as the multifocal motor neuropathy (MMN), characterized by anti-GM1 ganglioside autoantibodies [75]. While these diseases are treated with rituximab (anti-CD20 antibody against B cells for anti-MAG neuropathy), or intravenous immunoglobulin (IVIG for MMN), I speculate that the blockade of autoreactive CD4+ T cell entry by Natalizumab (anti-α4 integrin) might reduce the access of autoreactive antibodies in the CNS and PNS. However, as stated above, the use of Natalizumab should take into account the accompanying risk for developing PML.
Immunotherapies for Brain Tumors and Neurodegenerative Diseases
In addition to defense against neurotropic viruses, there are several instances where controlled antibody access to the CNS is warranted. For instance, monoclonal antibodies or chimeric antigen receptor (CAR) T cells targeting tumor-associated antigens on primary brain tumors, including glioblastoma multiforme and meningiomas, must gain access to tumor cells within the CNS. Similarly, suppression of tumor associated T cells can only be lifted by checkpoint blockade antibodies if such antibodies are able to enter the tumor tissue environment in the CNS. However, antibody penetration into the CSF following systemic injection of monoclonal antibodies -- for example, rituximab --is extremely limited due to the BBB, only reaching 0.1% of the concentrations found in the serum of patients [76]. In addition, pharmacokinetic analysis suggests that antibody clearance from the CSF is rapid, with a terminal half-life of 4.96 hours [76]. Thus, BBB appears to constitute an obstacle to brain tumor immunotherapy.
Another example where antibody access to the CNS is crucial is the case of vaccines against neurodegenerative diseases. Key drivers of Alzheimer’s disease (AD) include the amyloid beta (Aβ) peptide that can form fibrils (aggregates) and neuritic plaques in the parenchyma and blood vessels of the brain, and hyperphosphorylation of the tau protein, which in addition to forming aggregates, destabilizes microtubules, thus causing impairments in axonal transport and neuronal dysfunction [77]. Vaccines and therapeutics targeting key drivers of AD, such as Aβ or tau, have been tested in humans with mixed results. For instance, antibodies against Aβ have been shown to promote clearance of Aβ deposits and to prevent AD in the amyloid precursor protein (APP) transgenic mouse model (reviewed in [78]). However, the first AD vaccine, AN-1792 (fibrillar Aβ42 formulated in QS21 adjuvant) was halted in 2002 when 6% of the trial subjects receiving the vaccine developed some degree of aseptic meningoencephalitis [79]. This was associated with brain infiltration of autoreactive T cells [80–82]. The outcome is an important reminder of the caution that must be taken in the development of vaccines against CNS antigens: the CD4+ T cell response must be present in the CNS to allow antibody access (Figure 4C) but must be tightly controlled to avoid immunopathology (Figure 4D). Currently, there are several ongoing active AD vaccine clinical trials in various phases with acceptable safety and tolerability [83]. Indeed, it will be exciting to follow the results from these trials. Furthermore, their outcome might inform future vaccine designs for other neurodegenerative diseases.
Unlike active vaccines, passive immunization with monoclonal antibodies avoids CD4+ T cell-mediated neurotoxicity following opening of the BBB. However, because BBB prevents passive diffusion of antibodies, passive immunization requires repeated injection of antibody, and such antibodies must somehow cross the BBB to clear the target antigen. Recent results from four multicenter randomized double-blind, placebo-controlled, phase 3 trials of bapineuzumab (humanized monoclonal IgG1 anti-Aβ42 antibody (Aβ1–5)) showed no efficacy in treatment of AD disease [84, 85]. The phase 3 solanezumab (humanized monoclonal IgG1 anti-Aβ42 antibody (Aβ13–28)) trials demonstrated acceptable safety, with subjects presenting a slowing down of cognitive decline in approximately 34% of cases, and a slowing in functional decline in approximately 18% of cases, compared to placebo controls [86]. However, the trials did not meet the primary endpoints.
Encouraging results with passive immunization using another human monoclonal antibody specific to the aggregated form of Aβ, aducanumab, were reported in 2016 [11]. Aducanumab enters the CNS and was shown to reduce soluble oligomers and insoluble aggregates of Aβ in an APP transgenic (Tg) mouse model of AD [11]. In addition, in patients with prodromal or mild AD, one year of monthly intravenous infusions of aducanumab reduced brain Aβ in a dose- and time-dependent manner, and delayed clinical AD progression [11]. Moreover, in APP Tg mice, treatment with the mouse version of aducanumab, a murine IgG2a/κ chimeric analog, resulted in reduction of Aβ deposits, accompanied by an increased recruitment of microglia [11]. These data indicate that an antibody against aggregated Aβ can somehow enter the CNS and bind to oligomeric and fibrillar Aβ, thus leading to Aβ clearance and improved clinical outcome. While these results are encouraging, offering a possibility of antibody therapy against AD and potentially other neurodegenerative diseases, a phase 3 clinical trial outcome will be needed to determine whether monoclonal antibody-based therapy of AD is possible. Specifically, it is unknown how/if aducanumab crosses the BBB in patients, and whether preexisting endothelial dysfunction in AD (Box 2) promotes or precludes the access of injected antibodies. Finally, whether antibodies to other AD-driver antigens, such as tau, also have the ability to interfere with clinical progression of AD, awaits future studies.
Box 2. Endothelial Dysfunction in Alzheimer’s Disease.
Endothelial dysfunction may contribute to the pathogenesis of neurodegenerative diseases. Multiple studies indicate that endothelial dysfunction occurs during the course of neurodegenerative diseases, resulting in progressive synaptic and neuronal dysfunction and loss, thus leading to the proposal of the neurovascular hypothesis [5]:
Aβ clearance from the CNS requires active transport across the BBB via transendothelial transport
Aβ binds to low density lipoprotein receptor related protein-1 (LRP-1). LRP1 is expressed on the abluminal side of ECs which are taken up via clathrin-mediated endocytosis [5]
Aβ is released into circulation after transcytosis into the blood luminal side of the endothelium
An antisense knockdown of LRP-1 in mice has been shown to reduce the clearance of Aβ, increase brain levels of Aβ, and impair learning ability and recognition memory in mice [88]
Impaired clearance of oligomeric or fibrillar Aβ is thought to drive inflammation, leading to synaptic failure and neuronal loss.
Thus, the success of antibody-based therapy of AD depends on whether antibody delivery to the CNS can occur in the face of, or because of, a preexisting vascular dysfunction.
Concluding Remarks
Emerging evidence indicates that CD4+ T cells can pave the way to antibody access in immunoprivileged tissues by reducing the BBB and BNB. On the one hand, transient access by antibodies is necessary for protection against neurotropic viruses. On the other hand, chronic access by antibodies to neural tissues can drive autoimmune diseases (see Box 3). We can leverage this information for the design of rational vaccines and antibody-mediated immunotherapy against neurotropic viruses and brain tumors. For instance, vaccines against neurotropic viruses might be more effective when incorporating both antibody as well as CD4+ T cell epitopes expressed by viruses within the nervous system. In immunized individuals, preexisiting antiviral CD4+ T cells might open the BBB or BNB only at the site of infection to enable virus-specific antibodies to flood in and block further viral replication. Similarly, immunotherapy for brain tumors might benefit from having coincident neurotropic CD4+ T cell responses that can be introduced transiently. CAR-T cells might exploit the increased access to the CNS to kill cancer cells with the help of CD4+ T cells capable of recognizing antigen presented within the perivascular space surrounding the BMEC (Figure 4C). Moreover, monoclonal antibodies targeting brain cancer cells might also require the transient opening of the BBB. And lastly, vaccines designed to clear Aβ or tau aggregates may benefit from CD4+ T cells that allow antibody access to lesion sites. The key challenge areas in this regard are to identify the differences, if any, between the CD4+ T cells that open the BBB for antiviral antibody access vs. those that result in recruitment of autoimmune lymphocytes and cause immunopathology. Based on such an understanding, it may be possible to design vaccine strategies to induce CD4+ T cells that transiently open the BBB but do not cause chronic inflammation. It may also be possible to design a delivery strategy that allows monoclonal therapeutic antibody access to the CNS, regardless of the status of the BBB and BNB (see Outstanding Questions). As such, research that promotes a basic understanding of the immunological control of BBB access may guide future prophylactic and therapeutic approaches in the areas of infectious diseases, neurodegeneration and cancer.
Box 3. Clinician’s Corner.
Circulating antibodies provide an efficient mechanism of protection against infectious agents, toxins, and mediate clearance of debris. While antibodies are actively transported to the gut mucosa and to the developing fetus through specific receptors, other organs rely on diffusion through capillary vessels.
In immunoprivileged organs, including the brain, spinal cord and peripheral nerves, antibody access is restricted by barriers imposed by ECs and other accessory cell types, including astrocytes (in the CNS), pericytes and basement membrane cells.
Antibodies are capable of controlling virus replication in neural tissues only after accessing the tissue. The opening of the BBB and BNB for antibody access requires entry of antigen-specific CD4+ T cells and induction of vascular permeability upon secretion of cytokines such as IFN-γ or IL-17A.
Vaccines against neurotropic viruses, neurodegenerative diseases and immunotherapy against brain tumors might benefit from the inclusion of CD4+ T cell epitopes to enable the opening of the BBB. However, a mechanism must be set in place to terminate the prolonged activation of CD4+ T cell responses in order to avoid encephalitis.
Indeed, excessive activation of CD4+ T cells in the CNS and PNS may result in autoimmune neuropathies. Treatment of the so-called antibody mediated neuropathies might potentially benefit from blocking autoreactive CD4+ T cell entry into neural tissues using antibodies against α4 integrin. However, patients should be screened for prior exposure to polyomavirus in order to avoid the potential onset of progressive multifocal leukoencephalopathy.
Outstanding questions box.
How can we open the BBB or the BNB in a site-specific manner for therapeutic purposes?
What are the tissue reparative mechanisms that can restore the BBB or BNB?
What antigen-presenting cells restimulate the CD4+ T cells that cross the BBB or BNB?
What effector mechanisms are employed for pathogen clearance by antibodies that reach the CNS or PNS?
What effector mechanisms are employed to remove aggregate antigens by antibodies that reach the CNS?
Trends box.
The blood brain barrier (BBB) and blood nerve barrier (BNB) limit antibody access to the brain and peripheral nervous system.
The integrity of the BBB and BNB is enforced by type I and type III interferons, and is reduced by TNF-α, IL-1, IL-6, type II interferon (IFN-γ) and IL-17A.
CD4+ T cells of the Th1 and Th17 subtypes can open the BBB and BNB upon entry into neuronal tissues.
CD4+ T cell opening of the BBB is required for antibody-mediated block of neurotropic virus replication.
CD4+ T cell-mediated regulation of the BBB has important implications for cancer immunotherapy and vaccine design to treat conditions such as Alzheimer’s disease.
Acknowledgments
I wish to thank the members of my laboratory for their work that inspired me to think about how adaptive immunity is coordinated against viral pathogens. Work in my laboratory is supported by funding from the National Institutes of Health (R01AI054359, R21AI131284, R56AI125504, R41AI120269) and the Howard Hughes Medical Institute.
Glossary
- Abluminal
endothelial side that faces away from the lumen and into the parenchyma.
- Anti-MAG neuropathy
in the PNS; mediated by monoclonal IgM against myelin-associated glycoprotein (MAG), which damages Schwann cells.
- Astrocytes
star-shaped glial (non-neuronal) cells in the brain and spinal cord. Astrocyte endfeet surround the brain microvascular ECs to form the BBB.
- Autoimmune neuropathies
caused by autoreactive T and B cells in the PNS.
- Blood brain barrier (BBB)
Impermeable barrier that prevents large molecules (antibodies, albumin, and other plasma contents), leukocytes and pathogens from entering the CNS. It consists of ECs that form tight junctions, pericytes, basement membrane and astrocyte endfeet.
- Blood nerve barrier (BNB)
impermeable barrier that prevents plasma contents, leukocytes and pathogens from entering the PNS. It consists of ECs that form tight junctions, pericytes, basement membrane, Schwann cells, and endoneurium.
- Brain microvascular endothelial cells (BMEC)
brain capillary ECs that serve as a key component of BBB, forming tight junctions and an impermeable barrier in the brain.
- CCL20
chemokine constitutively secreted by epithelial cells of the choroid plexus; attracts cells bearing its receptor, CCR6. It is also expressed by epithelial cells overlying the dome region of the Peyer’s patches in the terminal ileum.
- CCR6
chemokine receptor recognizing the chemokine CCL20. CCR6 is expressed by Th17 cells, and is needed for their entry into the choroid plexus.
- Cerebrospinal fluid (CSF)
produced by the choroid plexus of the brain ventricles. It fills the subarachnoid space and the ventricular system inside the brain and spinal cord.
- Checkpoint blockade antibodies
antibodies targeted to inhibitory receptors that serve as inhibitory checkpoints for T cell activation. The antibody targets inhibitory receptors (e.g., CTLA4, PD1) expressed by T cells or their ligands on other cells. Examples of checkpoint blockade antibodies in use to treat cancer patients include anti-CTLA4, anti-PD1 and anti-PDL-1 antibodies.
- Chimeric antigen receptor T (CAR T) cells
T cells transduced with a retrovirus encoding a chimeric antigen receptor constituting a single chain Fv derived from a monoclonal antibody targeting a tumor surface antigen, and the CD3ζ chain intracellular domain. Autologous CAR T cells are adoptively transferred into patients with specific tumors expressing a surface antigen. Next generation CAR T cells combine the CD3 ζ chain, CD28 and 41BB or OX40 in the intracellular domain, to deliver better signaling.
- Choroid plexus
specialized ependymal cells in the brain ventricles that produce the CSF. The epithelial cells of the choroid plexus form tight junctions, and provide the blood-CSF barrier.
- Complement
system consisting of a number of proteins found in the blood circulating as inactive precursors. When triggered, proteases in the system cleave specific substrate proteins, amplifying a cascade of further cleavage of downstream substrates. The end product of complement activation is the formation of the membrane attack complex that drills a hole through cell membranes, killing microbial pathogens.
- Demyelination
damage to the protective covering (myelin sheath) that surrounds nerve fibers of the brain, spinal cord and peripheral neurons. When the myelin sheath is damaged, nerve impulses slow or stop, causing neurological problems.
- Diapedesis
migration of circulating cells out of the bloodstream into peripheral tissues across EC junctions.
- Experimental autoimmune encephalomyelitis (EAE) model of multiple sclerosis (MS)
a rodent model of brain inflammation that mimics MS. EAE is elicited by immunization of rodents with an antigen associated with myelin in complete Freund’s adjuvant. Systemic injection of pertussis toxin is required to cause inflammatory demyelinating disease of the CNS.
- Endoneurium
connective tissue surrounding myelinated and unmyelinated nerve fibers of the PNS. It is covered by the perinuerium ensheathment.
- Epitope
antigenic entity recognized by T and B cell receptors or antibodies.
- Fab region
fragment antigen-binding (Fab); region of an antibody that binds antigens.
- Fc region
fragment crystallizable (Fc); region of an antibody that consists of the constant regions (C2 and beyond) of the heavy chain. It dictates the function of an antibody by interacting with cell surface receptors known as Fc receptors and proteins of the complement system.
- Glia limitans
thin barrier of astrocyte foot processes associated with the parenchymal basal lamina surrounding the brain and spinal cord.
- Guillain–Barré syndrome
a potentially life-threatening disease characterized by rapidly progressive, symmetrical weakness of the extremities. Approximately 25% of patients develop respiratory insufficiency and many show signs of autonomic dysfunction. The immune response against myelin sheath and its underlying axon mediates damage to PNS neurons.
- Immunoprivileged sites
classically defined as organs of the body within which tissue grafts can survive for extended periods of time without rejection. They include the CNS, PNS, eye, testis, placenta, fetus, and the uterus.
- Inside-out signaling
intracellular signals mediated by G-protein-couple receptors that, through their action on integrin cytoplasmic domains, induce conformational changes in integrin extracellular domains, resulting in an increased affinity for a ligand.
- Intercellular adhesion molecule-1 (ICAM-1)
transmembrane protein expressed on ECs. ICAM-1 is a ligand for LFA-1 expressed on lymphocytes and leukocytes, and helps transendothelial migration of these circulating cells. ICAM-1 is also expressed by antigen presenting cells and mediates activation of lymphocytes expressing LFA-1.
- Lamina propria
thin layer of loose connective tissue that lies beneath the epithelium.
- Lymphocyte function associated antigen 1 (LFA-1)
αLβ2 integrin expressed by lymphocytes, macrophages and neutrophils. LFA-1 binds to its ligand ICAM-1 expressed on endothelial cells enabling transendothelial migration of cells.
- Middle cerebral artery occlusion model
experimental animal model to study ischemic injury and BBB disruption occurring during stroke. The middle cerebral artery is occluded by an inserted nylon filament, which is either removed after 0.5 - 2 hours (transient model) or left in place for 24 hours (permanent model). It is a widely used method to mimic permanent and transient focal cerebral ischemia in rodents.
- Multifocal motor neuropathy
characterized by motor deficits in the distribution of single nerves without associated sensory loss. Patients with MMN present monoclonal IgM antibodies to GM1 ganglioside.
- Multiple sclerosis (MS)
autoimmune neurodegenerative disorder where the immune system attacks the myelin that protects and insulates the neurons. Breakdown in the BBB results in entry of autoreactive CD4+ T cells in the CNS and PNS, leading to MS attacks.
- Neonatal Fc receptor (FcRn)
receptor for IgG expressed on the surface of ECs and epithelial cells, including syncytiotrophoblasts. It enables transport of IgG across these cell types. FcRn also prolongs the half-life of circulating IgG by protecting the antibody from degradation.
- Neurotropic pathogens
pathogens capable of infecting neural tissues.
- Passive transfer
conferring protection against pathogens by transfer of lymphocytes or antibodies into a naïve individual. By contrast, in active immunization, T and B cell responses are generated de novo upon vaccination.
- Pericytes
contractile cells that wrap around ECs of capillaries and venules throughout the body. They reside within the endothelial basement membrane.
- Perineurium
protective connective tissue sheath enclosing nerve bundles and endoneurium of PNS neurons.
- Pial surface
boundary between grey matter and CSF.
- Plasma cells
terminally differentiated B cells secreting immunoglobulins.
- Prodromal
the period between the appearance of initial symptoms and the full development of a disease.
- Progressive multifocal leukoencephalopathy (PML)
disease caused by a polyomavirus JC virus infection targeting oligodendrocytes in the CNS.
- Protein S
Protein plasma protein with a role in the anti-coagulation pathway. It can bind to negatively charged phospholipids via the Gla domain. This property allows Protein S to function in the removal of apoptotic cells. It is a ligand for TAM receptors.
- Retrograde axonal transport
movement of molecules/organelles/pathogens away from axon termini toward the cell body.
- Schwann cell
principal glial cells that support the function of neurons in the PNS.
- Subarachnoid space
or leptomeningeal space. Space between the arachnoid membrane and the pia membrane, occupied by delicate connective tissue trabeculae and intercommunicating channels containing CSF.
- Syncytiotrophoblasts
multinuclear epithelial cells; layer covering the highly vascular embryonic placental villi, which invades the wall of the uterus to establish nutrient circulation between the embryo and the mother.
- TAM receptor tyrosine kinases
three receptor tyrosine kinases, Tyro3, Axl, and Mertk, by binding to their ligands—Gas6 and Protein S—are essential for the efficient phagocytosis of apoptotic cells and membranes and which act as inhibitors of the innate inflammatory response to pathogens.
- Th1 cells
differentiated CD4+ T cells that secrete IFN-γ. Th1 cells are critical for defense against intracellular bacteria and viruses.
- Th17 cells
differentiated CD4+ T cells that secrete IL-17. Th17 cells are critical for defense against extracellular bacteria and fungi.
- Tight junction
junction between two cells composed of homotypic interactions of transmembrane proteins, claudins, occludins and JAMs, the form an impermeable barrier. Tight junctions can form between epithelial cells or between ECs.
- Transcytosis
transcellular transport in which various macromolecules are transported across the interior of a cell (e.g. epithelial cells). Macromolecules are captured in vesicles on one side of the cell, drawn across the cell, and ejected on the other side without being modified.
- Vascular cell adhesion molecule 1 (VCAM-1)
transmembrane protein expressed on inflamed ECs. VCAM-1 is a ligand for VLA-4 expressed on effector and memory T cells and monocytes.
- Virchow-Robin perivascular space
it is filled with CSF. Perivascular spaces surrounding arteries in the cerebral cortex and the basal ganglia are separated from the subpial space by one or two layers of leptomeninges, respectively, as well as the pia mater.
- Very late antigen 4 (VLA-4)
α4β1 integrin expressed on effector and memory T cells, as well as monocytes. VLA-4 binds to its ligand VCAM-1 expressed by inflamed ECs and mediates transendothelial migration.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gunn BM, Alter G. Modulating Antibody Functionality in Infectious Disease and Vaccination. Trends Mol Med. 2016 doi: 10.1016/j.molmed.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iwasaki A. Exploiting Mucosal Immunity for Antiviral Vaccines. Annu Rev Immunol. 2016;34:575–608. doi: 10.1146/annurev-immunol-032414-112315. [DOI] [PubMed] [Google Scholar]
- 3.Roopenian DC, Akilesh S. FcRn: the neonatal Fc receptor comes of age. Nat Rev Immunol. 2007;7(9):715–25. doi: 10.1038/nri2155. [DOI] [PubMed] [Google Scholar]
- 4.Story CM, et al. A major histocompatibility complex class I-like Fc receptor cloned from human placenta: possible role in transfer of immunoglobulin G from mother to fetus. J Exp Med. 1994;180(6):2377–81. doi: 10.1084/jem.180.6.2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao Z, et al. Establishment and Dysfunction of the Blood-Brain Barrier. Cell. 2015;163(5):1064–78. doi: 10.1016/j.cell.2015.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schlager C, et al. Effector T-cell trafficking between the leptomeninges and the cerebrospinal fluid. Nature. 2016;530(7590):349–53. doi: 10.1038/nature16939. [DOI] [PubMed] [Google Scholar]
- 7.Arima Y, et al. Regional neural activation defines a gateway for autoreactive T cells to cross the blood-brain barrier. Cell. 2012;148(3):447–57. doi: 10.1016/j.cell.2012.01.022. [DOI] [PubMed] [Google Scholar]
- 8.Arima Y, et al. A pain-mediated neural signal induces relapse in murine autoimmune encephalomyelitis, a multiple sclerosis model. Elife. 2015:4. doi: 10.7554/eLife.08733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reboldi A, et al. C-C chemokine receptor 6-regulated entry of TH-17 cells into the CNS through the choroid plexus is required for the initiation of EAE. Nat Immunol. 2009;10(5):514–23. doi: 10.1038/ni.1716. [DOI] [PubMed] [Google Scholar]
- 10.Iijima N, Iwasaki A. Access of protective antiviral antibody to neuronal tissues requires CD4+ T-cell help. Nature. 2016;533(7604):552–6. doi: 10.1038/nature17979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sevigny J, et al. The antibody aducanumab reduces Abeta plaques in Alzheimer’s disease. Nature. 2016;537(7618):50–6. doi: 10.1038/nature19323. [DOI] [PubMed] [Google Scholar]
- 12.Engelhardt B, Ransohoff RM. Capture, crawl, cross: the T cell code to breach the blood-brain barriers. Trends Immunol. 2012;33(12):579–89. doi: 10.1016/j.it.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 13.Weerasuriya A, Mizisin AP. The blood-nerve barrier: structure and functional significance. Methods Mol Biol. 2011;686:149–73. doi: 10.1007/978-1-60761-938-3_6. [DOI] [PubMed] [Google Scholar]
- 14.Corfas G, et al. Mechanisms and roles of axon-Schwann cell interactions. J Neurosci. 2004;24(42):9250–60. doi: 10.1523/JNEUROSCI.3649-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Obermeier B, et al. Development, maintenance and disruption of the blood-brain barrier. Nat Med. 2013;19(12):1584–96. doi: 10.1038/nm.3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Daneman R, et al. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature. 2010;468(7323):562–6. doi: 10.1038/nature09513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ben-Zvi A, et al. Mfsd2a is critical for the formation and function of the blood-brain barrier. Nature. 2014;509(7501):507–11. doi: 10.1038/nature13324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saunders NR, et al. Barrier mechanisms in the developing brain. Front Pharmacol. 2012;3:46. doi: 10.3389/fphar.2012.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Braniste V, et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci Transl Med. 2014;6(263):263ra158. doi: 10.1126/scitranslmed.3009759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rochfort KD, Cummins PM. The blood-brain barrier endothelium: a target for pro-inflammatory cytokines. Biochem Soc Trans. 2015;43(4):702–6. doi: 10.1042/BST20140319. [DOI] [PubMed] [Google Scholar]
- 21.Daniels BP, et al. Viral pathogen-associated molecular patterns regulate blood-brain barrier integrity via competing innate cytokine signals. MBio. 2014;5(5):e01476–14. doi: 10.1128/mBio.01476-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lazear HM, et al. Interferon-lambda restricts West Nile virus neuroinvasion by tightening the blood-brain barrier. Sci Transl Med. 2015;7(284):284ra59. doi: 10.1126/scitranslmed.aaa4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miner JJ, et al. The TAM receptor Mertk protects against neuroinvasive viral infection by maintaining blood-brain barrier integrity. Nat Med. 2015;21(12):1464–72. doi: 10.1038/nm.3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu D, et al. Protein S controls hypoxic/ischemic blood-brain barrier disruption through the TAM receptor Tyro3 and sphingosine 1-phosphate receptor. Blood. 2010;115(23):4963–72. doi: 10.1182/blood-2010-01-262386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ransohoff RM, Brown MA. Innate immunity in the central nervous system. J Clin Invest. 2012;122(4):1164–71. doi: 10.1172/JCI58644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schoggins JW, Rice CM. Interferon-stimulated genes and their antiviral effector functions. Curr Opin Virol. 2011;1(6):519–25. doi: 10.1016/j.coviro.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Veldhuis WB, et al. Interferon-beta prevents cytokine-induced neutrophil infiltration and attenuates blood-brain barrier disruption. J Cereb Blood Flow Metab. 2003;23(9):1060–9. doi: 10.1097/01.WCB.0000080701.47016.24. [DOI] [PubMed] [Google Scholar]
- 28.Lemke G, Rothlin CV. Immunobiology of the TAM receptors. Nat Rev Immunol. 2008;8(5):327–36. doi: 10.1038/nri2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lew ED, et al. Differential TAM receptor-ligand-phospholipid interactions delimit differential TAM bioactivities. Elife. 2014:3. doi: 10.7554/eLife.03385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rothlin CV, et al. TAM receptors are pleiotropic inhibitors of the innate immune response. Cell. 2007;131(6):1124–36. doi: 10.1016/j.cell.2007.10.034. [DOI] [PubMed] [Google Scholar]
- 31.Ma GZ, et al. Polymorphisms in the receptor tyrosine kinase MERTK gene are associated with multiple sclerosis susceptibility. PLoS One. 2011;6(2):e16964. doi: 10.1371/journal.pone.0016964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sawcer S, et al. Genetic risk and a primary role for cell-mediated immune mechanisms in multiple sclerosis. Nature. 2011;476(7359):214–9. doi: 10.1038/nature10251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Genome-wide association study identifies new multiple sclerosis susceptibility loci on chromosomes 12 and 20. Nat Genet. 2009;41(7):824–8. doi: 10.1038/ng.396. [DOI] [PubMed] [Google Scholar]
- 34.Bruewer M, et al. Interferon-gamma induces internalization of epithelial tight junction proteins via a macropinocytosis-like process. Faseb j. 2005;19(8):923–33. doi: 10.1096/fj.04-3260com. [DOI] [PubMed] [Google Scholar]
- 35.Utech M, et al. Mechanism of IFN-gamma-induced endocytosis of tight junction proteins: myosin II-dependent vacuolarization of the apical plasma membrane. Mol Biol Cell. 2005;16(10):5040–52. doi: 10.1091/mbc.E05-03-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kebir H, et al. Human TH17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nat Med. 2007;13(10):1173–5. doi: 10.1038/nm1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carrithers MD, et al. Differential adhesion molecule requirements for immune surveillance and inflammatory recruitment. Brain. 2000;123(Pt 6):1092–101. doi: 10.1093/brain/123.6.1092. [DOI] [PubMed] [Google Scholar]
- 38.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76(2):301–14. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 39.Pober JS, Sessa WC. Evolving functions of endothelial cells in inflammation. Nat Rev Immunol. 2007;7(10):803–15. doi: 10.1038/nri2171. [DOI] [PubMed] [Google Scholar]
- 40.Muller WA. How endothelial cells regulate transmigration of leukocytes in the inflammatory response. Am J Pathol. 2014;184(4):886–96. doi: 10.1016/j.ajpath.2013.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ransohoff RM, et al. Three or more routes for leukocyte migration into the central nervous system. Nat Rev Immunol. 2003;3(7):569–81. doi: 10.1038/nri1130. [DOI] [PubMed] [Google Scholar]
- 42.Kivisakk P, et al. Human cerebrospinal fluid central memory CD4+ + T cells: evidence for trafficking through choroid plexus and meninges via P-selectin. Proc Natl Acad Sci U S A. 2003;100(14):8389–94. doi: 10.1073/pnas.1433000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baron JL, et al. Surface expression of alpha 4 integrin by CD4+ T cells is required for their entry into brain parenchyma. J Exp Med. 1993;177(1):57–68. doi: 10.1084/jem.177.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baron JL, et al. The pathogenesis of adoptive murine autoimmune diabetes requires an interaction between alpha 4-integrins and vascular cell adhesion molecule-1. J Clin Invest. 1994;93(4):1700–8. doi: 10.1172/JCI117153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee SJ, Benveniste EN. Adhesion molecule expression and regulation on cells of the central nervous system. J Neuroimmunol. 1999;98(2):77–88. doi: 10.1016/s0165-5728(99)00084-3. [DOI] [PubMed] [Google Scholar]
- 46.Linington C, et al. Augmentation of demyelination in rat acute allergic encephalomyelitis by circulating mouse monoclonal antibodies directed against a myelin/oligodendrocyte glycoprotein. Am J Pathol. 1988;130(3):443–54. [PMC free article] [PubMed] [Google Scholar]
- 47.Kojima K, et al. Experimental autoimmune panencephalitis and uveoretinitis transferred to the Lewis rat by T lymphocytes specific for the S100 beta molecule, a calcium binding protein of astroglia. J Exp Med. 1994;180(3):817–29. doi: 10.1084/jem.180.3.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Westland KW, et al. Activated non-neural specific T cells open the blood-brain barrier to circulating antibodies. Brain. 1999;122(Pt 7):1283–91. doi: 10.1093/brain/122.7.1283. [DOI] [PubMed] [Google Scholar]
- 49.Miner JJ, Diamond MS. Mechanisms of restriction of viral neuroinvasion at the blood-brain barrier. Curr Opin Immunol. 2016;38:18–23. doi: 10.1016/j.coi.2015.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koyuncu OO, et al. Virus infections in the nervous system. Cell Host Microbe. 2013;13(4):379–93. doi: 10.1016/j.chom.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dittmar S, et al. Measles virus-induced block of transendothelial migration of T lymphocytes and infection-mediated virus spread across endothelial cell barriers. J Virol. 2008;82(22):11273–82. doi: 10.1128/JVI.00775-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Plakhov IV, et al. The earliest events in vesicular stomatitis virus infection of the murine olfactory neuroepithelium and entry of the central nervous system. Virology. 1995;209(1):257–62. doi: 10.1006/viro.1995.1252. [DOI] [PubMed] [Google Scholar]
- 53.Verma S, et al. West Nile virus infection modulates human brain microvascular endothelial cells tight junction proteins and cell adhesion molecules: Transmigration across the in vitro blood-brain barrier. Virology. 2009;385(2):425–33. doi: 10.1016/j.virol.2008.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang S, et al. Drak2 contributes to West Nile virus entry into the brain and lethal encephalitis. J Immunol. 2008;181(3):2084–91. doi: 10.4049/jimmunol.181.3.2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li F, et al. Viral Infection of the Central Nervous System and Neuroinflammation Precede Blood-Brain Barrier Disruption during Japanese Encephalitis Virus Infection. J Virol. 2015;89(10):5602–14. doi: 10.1128/JVI.00143-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cook ML, Stevens JG. Pathogenesis of herpetic neuritis and ganglionitis in mice: evidence for intra-axonal transport of infection. Infect Immun. 1973;7(2):272–88. doi: 10.1128/iai.7.2.272-288.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Racaniello VR. One hundred years of poliovirus pathogenesis. Virology. 2006;344(1):9–16. doi: 10.1016/j.virol.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 58.Koelle DM, Corey L. Herpes simplex: insights on pathogenesis and possible vaccines. Annu Rev Med. 2008;59:381–95. doi: 10.1146/annurev.med.59.061606.095540. [DOI] [PubMed] [Google Scholar]
- 59.Whaley KJ, et al. Passive immunization of the vagina protects mice against vaginal transmission of genital herpes infections. J Infect Dis. 1994;169(3):647–9. doi: 10.1093/infdis/169.3.647. [DOI] [PubMed] [Google Scholar]
- 60.Sherwood JK, et al. Controlled release of antibodies for long-term topical passive immunoprotection of female mice against genital herpes. Nat Biotechnol. 1996;14(4):468–71. doi: 10.1038/nbt0496-468. [DOI] [PubMed] [Google Scholar]
- 61.Faden H, et al. Comparative evaluation of immunization with live attenuated and enhanced-potency inactivated trivalent poliovirus vaccines in childhood: systemic and local immune responses. J Infect Dis. 1990;162(6):1291–7. doi: 10.1093/infdis/162.6.1291. [DOI] [PubMed] [Google Scholar]
- 62.Morrison LA, et al. Vaccine-induced serum immunoglobin contributes to protection from herpes simplex virus type 2 genital infection in the presence of immune T cells. J Virol. 2001;75(3):1195–204. doi: 10.1128/JVI.75.3.1195-1204.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sato A, et al. Vaginal memory T cells induced by intranasal vaccination are critical for protective T cell recruitment and prevention of genital HSV-2 disease. J Virol. 2014;88(23):13699–708. doi: 10.1128/JVI.02279-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Parr MB, Parr EL. Interferon-gamma up-regulates intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 and recruits lymphocytes into the vagina of immune mice challenged with herpes simplex virus-2. Immunology. 2000;99(4):540–5. doi: 10.1046/j.1365-2567.2000.00980.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hooper DC, et al. Collaboration of antibody and inflammation in clearance of rabies virus from the central nervous system. J Virol. 1998;72(5):3711–9. doi: 10.1128/jvi.72.5.3711-3719.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chai Q, et al. Enhancement of blood-brain barrier permeability and reduction of tight junction protein expression are modulated by chemokines/cytokines induced by rabies virus infection. J Virol. 2014;88(9):4698–710. doi: 10.1128/JVI.03149-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ortiz GG, et al. Role of the blood-brain barrier in multiple sclerosis. Arch Med Res. 2014;45(8):687–97. doi: 10.1016/j.arcmed.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 68.Brosnan CF, et al. Mechanisms of autoimmune neuropathies. Ann Neurol. 1990;27(Suppl):S75–9. doi: 10.1002/ana.410270719. [DOI] [PubMed] [Google Scholar]
- 69.van den Berg B, et al. Guillain-Barre syndrome: pathogenesis, diagnosis, treatment and prognosis. Nat Rev Neurol. 2014;10(8):469–82. doi: 10.1038/nrneurol.2014.121. [DOI] [PubMed] [Google Scholar]
- 70.Gilden DH. Infectious causes of multiple sclerosis. Lancet Neurol. 2005;4(3):195–202. doi: 10.1016/S1474-4422(05)01017-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gaublomme JT, et al. Single-Cell Genomics Unveils Critical Regulators of Th17 Cell Pathogenicity. Cell. 2015;163(6):1400–12. doi: 10.1016/j.cell.2015.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Polman CH, et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2006;354(9):899–910. doi: 10.1056/NEJMoa044397. [DOI] [PubMed] [Google Scholar]
- 73.Bloomgren G, et al. Risk of natalizumab-associated progressive multifocal leukoencephalopathy. N Engl J Med. 2012;366(20):1870–80. doi: 10.1056/NEJMoa1107829. [DOI] [PubMed] [Google Scholar]
- 74.Fraussen J, et al. B cells and antibodies in progressive multiple sclerosis: Contribution to neurodegeneration and progression. Autoimmun Rev. 2016;15(9):896–9. doi: 10.1016/j.autrev.2016.07.008. [DOI] [PubMed] [Google Scholar]
- 75.Latov N. Diagnosis and treatment of chronic acquired demyelinating polyneuropathies. Nat Rev Neurol. 2014;10(8):435–46. doi: 10.1038/nrneurol.2014.117. [DOI] [PubMed] [Google Scholar]
- 76.Rubenstein JL, et al. Rituximab therapy for CNS lymphomas: targeting the leptomeningeal compartment. Blood. 2003;101(2):466–8. doi: 10.1182/blood-2002-06-1636. [DOI] [PubMed] [Google Scholar]
- 77.Bloom GS. Amyloid-beta and tau: the trigger and bullet in Alzheimer disease pathogenesis. JAMA Neurol. 2014;71(4):505–8. doi: 10.1001/jamaneurol.2013.5847. [DOI] [PubMed] [Google Scholar]
- 78.Agadjanyan MG, et al. A fresh perspective from immunologists and vaccine researchers: active vaccination strategies to prevent and reverse Alzheimer’s disease. Alzheimers Dement. 2015;11(10):1246–59. doi: 10.1016/j.jalz.2015.06.1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schenk D. Amyloid-beta immunotherapy for Alzheimer’s disease: the end of the beginning. Nat Rev Neurosci. 2002;3(10):824–8. doi: 10.1038/nrn938. [DOI] [PubMed] [Google Scholar]
- 80.Nicoll JA, et al. Neuropathology of human Alzheimer disease after immunization with amyloid-beta peptide: a case report. Nat Med. 2003;9(4):448–52. doi: 10.1038/nm840. [DOI] [PubMed] [Google Scholar]
- 81.Orgogozo JM, et al. Subacute meningoencephalitis in a subset of patients with AD after Abeta42 immunization. Neurology. 2003;61(1):46–54. doi: 10.1212/01.wnl.0000073623.84147.a8. [DOI] [PubMed] [Google Scholar]
- 82.Ferrer I, et al. Neuropathology and pathogenesis of encephalitis following amyloid-beta immunization in Alzheimer’s disease. Brain Pathol. 2004;14(1):11–20. doi: 10.1111/j.1750-3639.2004.tb00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sterner RM, et al. Active Vaccines for Alzheimer Disease Treatment. J Am Med Dir Assoc. 2016;17(9):862e11–5. doi: 10.1016/j.jamda.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 84.Vandenberghe R, et al. Bapineuzumab for mild to moderate Alzheimer’s disease in two global, randomized, phase 3 trials. Alzheimers Res Ther. 2016;8(1):18. doi: 10.1186/s13195-016-0189-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Salloway S, et al. Two phase 3 trials of bapineuzumab in mild-to-moderate Alzheimer’s disease. N Engl J Med. 2014;370(4):322–33. doi: 10.1056/NEJMoa1304839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Siemers ER, et al. Phase 3 solanezumab trials: Secondary outcomes in mild Alzheimer’s disease patients. Alzheimers Dement. 2016;12(2):110–20. doi: 10.1016/j.jalz.2015.06.1893. [DOI] [PubMed] [Google Scholar]
- 87.Ferenczy MW, et al. Molecular biology, epidemiology, and pathogenesis of progressive multifocal leukoencephalopathy, the JC virus-induced demyelinating disease of the human brain. Clin Microbiol Rev. 2012;25(3):471–506. doi: 10.1128/CMR.05031-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jaeger LB, et al. Testing the neurovascular hypothesis of Alzheimer’s disease: LRP-1 antisense reduces blood-brain barrier clearance, increases brain levels of amyloid-beta protein, and impairs cognition. J Alzheimers Dis. 2009;17(3):553–70. doi: 10.3233/JAD-2009-1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tsutsui Y, et al. Roles of neural stem progenitor cells in cytomegalovirus infection of the brain in mouse models. Pathol Int. 2008;58(5):257–67. doi: 10.1111/j.1440-1827.2008.02221.x. [DOI] [PubMed] [Google Scholar]
- 90.Chaturvedi UC, et al. Breakdown of the blood-brain barrier during dengue virus infection of mice. J Gen Virol. 1991;72(Pt 4):859–66. doi: 10.1099/0022-1317-72-4-859. [DOI] [PubMed] [Google Scholar]
- 91.An J, et al. The pathogenesis of spinal cord involvement in dengue virus infection. Virchows Arch. 2003;442(5):472–81. doi: 10.1007/s00428-003-0785-3. [DOI] [PubMed] [Google Scholar]
- 92.Zerboni L, et al. Molecular mechanisms of varicella zoster virus pathogenesis. Nat Rev Microbiol. 2014;12(3):197–210. doi: 10.1038/nrmicro3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Roe K, et al. West Nile virus-induced disruption of the blood-brain barrier in mice is characterized by the degradation of the junctional complex proteins and increase in multiple matrix metalloproteinases. J Gen Virol. 2012;93(Pt 6):1193–203. doi: 10.1099/vir.0.040899-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Miner JJ, et al. Zika Virus Infection during Pregnancy in Mice Causes Placental Damage and Fetal Demise. Cell. 2016 doi: 10.1016/j.cell.2016.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li C, et al. Zika Virus Disrupts Neural Progenitor Development and Leads to Microcephaly in Mice. Cell Stem Cell. 2016;19(1):120–6. doi: 10.1016/j.stem.2016.04.017. [DOI] [PubMed] [Google Scholar]
- 96.Dang J, et al. Zika Virus Depletes Neural Progenitors in Human Cerebral Organoids through Activation of the Innate Immune Receptor TLR3. Cell Stem Cell. 2016;19(2):258–65. doi: 10.1016/j.stem.2016.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tang H, et al. Zika Virus Infects Human Cortical Neural Progenitors and Attenuates Their Growth. Cell Stem Cell. 2016;18(5):587–90. doi: 10.1016/j.stem.2016.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Garcez PP, et al. Zika virus impairs growth in human neurospheres and brain organoids. Science. 2016;352(6287):816–8. doi: 10.1126/science.aaf6116. [DOI] [PubMed] [Google Scholar]
- 99.Qian X, et al. Brain-Region-Specific Organoids Using Mini-bioreactors for Modeling ZIKV Exposure. Cell. 2016;165(5):1238–54. doi: 10.1016/j.cell.2016.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yockey LJ, et al. Vaginal Exposure to Zika Virus during Pregnancy Leads to Fetal Brain Infection. Cell. 2016;166(5):1247–1256. e4. doi: 10.1016/j.cell.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Li H, et al. Zika Virus Infects Neural Progenitors in the Adult Mouse Brain and Alters Proliferation. Cell Stem Cell. 2016;19(5):593–598. doi: 10.1016/j.stem.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Onorati M, et al. Zika Virus Disrupts Phospho-TBK1 Localization and Mitosis in Human Neuroepithelial Stem Cells and Radial Glia. Cell Rep. 2016;16(10):2576–92. doi: 10.1016/j.celrep.2016.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]