Abstract
Background
Patients with colorectal adenoma polyps (PLP) are at higher risk of developing colorectal cancer (CRC). However, the development of improved and robust biomarkers to enable screening, surveillance and early detection of PLP and CRC continues to be a challenge. The aim of this study was to identify biomarkers of progression to CRC by metabolomic profiling of human serum samples using a multistage approach.
Methods
Metabolomic profiling was conducted using Metabolon platform for 30 PLP, 30 CRC and 30 control subjects followed by targeted validation of top metabolites in an additional set of 50 CRC, 50 PLP and 50 controls using liquid chromatography-tandem mass spectrometry (LC-MS/MS). Unconditional multivariable logistic regression models adjusted for covariates were used to evaluate association with PLP and CRC risks.
Results
For the discovery phase, 404 serum metabolites were detected with 50 metabolites showing differential levels between PLP, CRC and controls (P for trend<0.05). Following validation, three top metabolites (xanthine, hypoxanthine and D-mannose) were validated showing lower levels of xanthine and hypoxanthine and higher level of D-mannose in PLP and CRC cases compared to controls. Further exploratory analysis of metabolic pathways revealed key roles for urea cycle and caffeine metabolism associated with PLP and CRC risks. Additionally, joint effect of top metabolites with smoking and significant interaction with BMI were also observed. Analysis of hypoxanthine to xanthine levels as ratio indicated association with CRC progression.
Conclusions
Our results suggest the potential utility of circulating metabolites as novel biomarkers for early detection of CRC.
Keywords: metabolomic profiling, xanthine/hypoxanthine ratio, D-mannose, adenoma polyps, colorectal cancer
Introduction
Colorectal cancer (CRC) is one of the most prevalent and deadly types of cancer worldwide, and a major cause of human morbidity and mortality1. As the third most common type of cancer in the U.S. according to the American Cancer Society, it is estimated that CRC is responsible for over 136 000 new cases and 50 000 deaths in 20152. Several preventive screening and detection methods are suggested for CRC, including the fecal occult blood test (FOBT), fecal immunochemical test (FIT), colonoscopy, sigmoidoscopy, and family-history-based risk assessment3.
The development of improved and robust biomarkers to enable screening, surveillance, and early detection of CRC continues to be a challenge. Patients with colorectal adenoma polyps (PLP) are at higher risk of developing colon cancer; however, noninvasive methods to identify these patients with high sensitivity and specificity are not clinically available. Studies describing molecular phenotypes of patients with CRC and PLP can improve the understanding of the pathophysiology of CRC and offer strategies in the monitoring of the at-risk population for CRC.
Serum metabolites are emerging as promising minimally invasive biomarkers for the early detection and prognostication of cancers. Metabolomics, one of the “omic” sciences in systems biology, is the global assessment of endogenous small-molecule biochemical (metabolites) within a biologic system, providing advanced methods to identify changing metabolism and, in particular, metabolite levels, which has resulted in rapid progress in disease biomarker discovery over the past decade4. Perturbations in important metabolic pathways have therefore been the focus of many cancer studies5. Numerous studies have profiled the circulating or tissue metabolites in CRC patients to identify markers for prognosis, cancer progression monitoring, and early cancer screening and detection6-9. However, only few studies have focused on identifying metabolite biomarkers for PLP patients. For example, Daniel Raftery's group has investigated serum metabolic profile differences between colorectal polyp patients and healthy controls10.
The aim of this study was to identify circulating biomarkers of CRC disease progression by metabolomic profiling of human serum samples in a two-step global and targeted approach involving both discovery and validation phases, which may offer new avenues for risk prediction and early disease detection.
Materials and Methods
Patient population and study design
This is a retrospective project including two phase of case-control pairs which consisted of included 30 healthy controls (CTRL), 30 PLP and 30 CRC for metabolomics profile screening in discovery phase, 50 healthy controls (CTRL), 50 PLP and 50 CRC in validation phase. All PLP and CRC patients had no prior chemotherapy or radiotherapy treatment, and were histologically confirmed and recruited at the University of Texas MD Anderson Cancer Center between 2006 and 2013. In parallel, heathy individuals were recruited from the general population with no cancer history except non-melanoma skin cancer, and matched to cases by gender, age (+/-5 years), ethnicity and residence. The random digit dialing method was used to identify and recruit controls. BMI (kg/m2) was classified in accordance with World Health Organization standard classification (normal BMI<25; overweight BMI>=25). Individuals who never smoked or smoked less than 100 cigarettes in his or her lifetime were identified as never smokers. Individuals who smoked at least 100 cigarettes in his or her lifetime were considered ever smokers. The lifestyle and demographic information of cases and control were collected from the same self-administered questionnaire, and all study participants signed informed consent form according to institutional guidelines. This study was approved by the Institutional Review Board of MD Anderson Cancer Center.
Global metabolomic profiling and individual targeted metabolite validation
More detailed description is found in the Supplementary Methods file. In brief, blood samples were collected from patients and healthy controls. Whole blood specimens were immediately processed to obtain serum fraction and stored at -80°C until retrieval. All serum samples were thawed on ice before experiment. The metabolite detection was separated into discovery phase, which employed global metabolomic profiling, and validation phase, which measured candidate compounds from the discovery phase. Global metabolomics was performed by Metabolon (Durham, NC) using 200 μl of serum as previously described11. The validation experiments were conducted by employing liquid chromatography-tandem mass spectrometry (LC-MS/MS) platform for 100 ul of serum samples at Texas Southern University. The LC-MS/MS assays met standards of the Food and Drug Administration as indicated by “Guidance for Industry Bioanalytical Method Validation”. Absolute concentrations were determined as μg/mL or ng/μL unit. We chose three of the top metabolites of altered trend in global metabolic profiling phase to analyze by targeted detection in validation phase on the basis of biological relevance, significant expression levels and assay availability.
Pathway analyses
The Kyoto Encyclopedia of Genes and Genomes (KEGG) database was utilized to identify the pathways of differential metabolites12. We identified the metabolic maps of significant CRC metabolites by using their KEGG compound ID. MetaboAnalyst 3.0, a comprehensive bioinformatic tool to examine the relationship between the identified metabolites and their corresponding mapped genes and pathways was used to observe the general biological processes in PLP and CRC (http://www.metaboanalyst.ca/)13.
Statistical methods
All statistical analyses were undertaken using Stata software (version 10.1; StataCorp, College Station, TX). We compared the difference between population subgroups by Pearson χ2 test for categorical variables while Student t test was used for continuous variables in both discovery and validation phases. The data of discovery set generated by Metabolon platform was shown as log2 transformed. During quality control, metabolites showing more than 20% of the samples with undetectable expression were removed. Comparison of metabolite overall mean value between different groups of subjects were analyzed by Wilcoxon rank sum test. Individual metabolites were assessed as categorical variables by setting cut-off points at median values in control group, dichotomizing all cases into high and low risk groups. We modeled the association between xanthine, hypoxanthine and D-Mannose with CRC progression risk by conducting logistic regression models. Odds ratio (OR) and corresponding 95% confidence interval (95% CI) were calculated using unconditional multivariable logistic regression models, adjusting for age, gender, smoking status, BMI and batch effect. Since smoking and BMI may influence CRC risk and metabolism, we add the interaction factors, smoking and BMI, into multivariable models to determine the interaction between the metabolite level, BMI and smoking in modulating CRC risk. All statistical tests were two sided with significance set at P<0.05.
Results
Population characteristics
The study population comprised of 80 CRC, 80 PLP and 80 CTRL subjects, who did not have prior history of cancer. Table 1 presented demographic characteristics of three groups in both discovery phase and validation phase including gender, age, mean of population BMI value, smoking status as well as BMI (overweight and normal categories) group. The mean age of CRC, PLP and CTRL individuals was 53.97, 51.87 and 55.23 years in the discovery phase, 58.66, 57.72 and 57.92 years in the validation phase, respectively. There were no significant differences between three groups in variables of gender, age, BMI value in either discovery set or validation set. However, overweight population (BMI≥25) in CRC and PLP groups had significantly higher proportion than CTRL individuals (73.91% vs. 67.86% vs. 50% in discovery set, 84.09% vs. 62.86% vs. 50% in validation set).
Table 1. CRC, PLP and CTRL characteristics of the study population in the discovery and validation phases.
| Variables | Discovery Phase | Validation Phase | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| CRC, N (%) | PLP, N (%) | CTRL, N (%) | P value | CRC, N (%) | PLP, N (%) | CTRL, N (%) | P value | |
| Age( Mean, SD) | 53.97(13.46) | 51.87(10.82) | 55.23(10.46) | 0.67 | 58.66(10.28) | 57.72( 9.21) | 57.92( 9.91) | 0.71 |
| BMI( Mean, SD) | 28.09( 5.20) | 27.92( 5.77) | 28.32( 6.18) | 0.87 | 30.25( 5.61) | 27.43( 5.58) | 28.37( 8.67) | 0.21 |
| Sex | ||||||||
| Male | 18(60.00) | 18(60.00) | 18(60.00) | 23(46.00) | 23(46.00) | 23(46.00) | ||
| Female | 12(40.00) | 12(40.00) | 12(40.00) | 1 | 27(54.00) | 27(54.00) | 27(54.00) | 1 |
| Smoking status | ||||||||
| Never | 16(53.33) | 20(66.67) | 13(43.33) | 31(62.00) | 28(56.00) | 30(60.00) | ||
| Former | 10(33.33) | 8(26.67) | 7(23.33) | 15(30.00) | 19(38.00) | 15(30.00) | ||
| Current | 4(13.33) | 2(6.67) | 10(33.33) | 0.07 | 4(8.00) | 3(6.00) | 5(10.00) | 0.86 |
| Smoker | ||||||||
| Ever | 14(46.67) | 10(33.33) | 17(56.67) | 19(38.00) | 22(44.00) | 20(40.00) | ||
| Never | 16(53.33) | 20(66.67) | 13(43.33) | 0.19 | 31(62.00) | 28(56.00) | 30(60.00) | 0.82 |
| BMI | ||||||||
| <25 | 6(26.09) | 9(32.14) | 15(50.00) | 7(15.91) | 13(37.14) | 25(50.00) | ||
| ≥25 | 17(73.91) | 19(67.86) | 15(50.00) | 0.16 | 37(84.09) | 22(62.86) | 25(50.00) | <0.01 |
CRC, colorectal cancer; PLP, adenoma polyp; CTRL, control; SD, standard deviation; BMI, body mass index
Differentially expressed metabolites in discovery phase and mapped in pathway analysis
We utilized Metabolon Inc. metabolomic profiling platform to identify 404 metabolites in serum of matched 30 PLP, 30 CRC and 30 healthy CTRL subjects in the discovery phase (Table 1). Prior to the statistics analysis, we performed quality control to exclude metabolites with missing values in more than 20% of samples (Supplementary Figure S1). Of the remaining 301 metabolites, 50 compounds displayed significant trend of differential expression between PLP, CRC patients and controls using Wilcoxon rank-sum test analysis. Twenty metabolites showed increasing levels in CRC and PLP patients comparing with controls and 30 metabolites with decreasing levels (Supplementary Table S1). Table 2 displayed top ten metabolites with differential levels between controls, PLP and CRC patients.
Table 2. Top 10 metabolites differentially expressed between CRC, PLP and CTRL groups in the discovery set.
| Metabolite | CRC | PLP | CTRL | P value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||||
| N | Mean (SD) | N | Mean (SD) | N | Mean (SD) | CTRL vs PLP | CTRL vs CRC | PLP vs CRC | P for trend | |
| Hypoxanthine | 30 | 13.29( 0.49) | 30 | 13.63( 0.67) | 30 | 15.53( 1.33) | <0.01 | <0.01 | 0.06 | <0.01 |
| D-Mannose | 30 | 22.44( 0.57) | 30 | 22.16( 0.75) | 30 | 21.62( 0.83) | <0.01 | <0.01 | 0.21 | <0.01 |
| DSGEGDFXAEGGGVR* | 30 | 18.87( 1.90) | 30 | 17.50( 1.64) | 30 | 16.81( 2.11) | 0.09 | <0.01 | <0.01 | <0.01 |
| Xanthine | 30 | 15.79( 0.51) | 30 | 16.12( 0.42) | 30 | 16.41( 0.70) | 0.11 | <0.01 | 0.01 | <0.01 |
| Glutamate | 30 | 22.14( 0.70) | 30 | 22.44( 0.81) | 30 | 22.73( 0.76) | 0.1 | <0.01 | 0.07 | <0.01 |
| Tryptophylglutamate | 30 | 12.99( 1.11) | 30 | 13.28( 0.77) | 30 | 13.83( 1.16) | 0.07 | <0.01 | 0.08 | <0.01 |
| 3-methyl-2-oxobutyrate | 30 | 14.65( 0.38) | 30 | 14.89( 0.33) | 30 | 14.26( 0.40) | <0.01 | <0.01 | <0.01 | <0.01 |
| 5-oxoproline | 30 | 15.89( 0.36) | 30 | 16.12( 0.59) | 30 | 16.31( 0.63) | 0.12 | <0.01 | 0.08 | <0.01 |
| Lactate | 30 | 28.58( 0.64) | 30 | 28.83( 0.50) | 30 | 29.02( 0.71) | 0.09 | <0.01 | 0.09 | <0.01 |
| 1,3-dipalmitoylglycerol | 30 | 15.09( 0.88) | 30 | 15.63( 0.75) | 30 | 15.75( 0.92) | 0.51 | <0.01 | 0.03 | <0.01 |
Mean level in ng/μL or μg/mL; CRC, colorectal cancer; PLP, adenoma polyp; CTRL, control; SD, standard deviation
Boldface metabolites were selected for further validation.
Small peptide not officially multiplexed by Metabolon based on a standard
To probe the biological relevance of these metabolites, the global metabolite pathways were analyzed. There were thirty-two subpathways identified from the significant metabolites (Supplementary Table S2). Further analysis indicated the differential metabolites were mapped to five superpathways that characterized homeostasis metabolism activity in protein biosynthesis, purine metabolism and energy. Notably, the result pointed to urea cycle and caffeine metabolism as the main differential pathways in colorectal cancer progression. (Supplementary Table S3, Supplementary Figure S2)
Individual targeted validation of selected metabolites
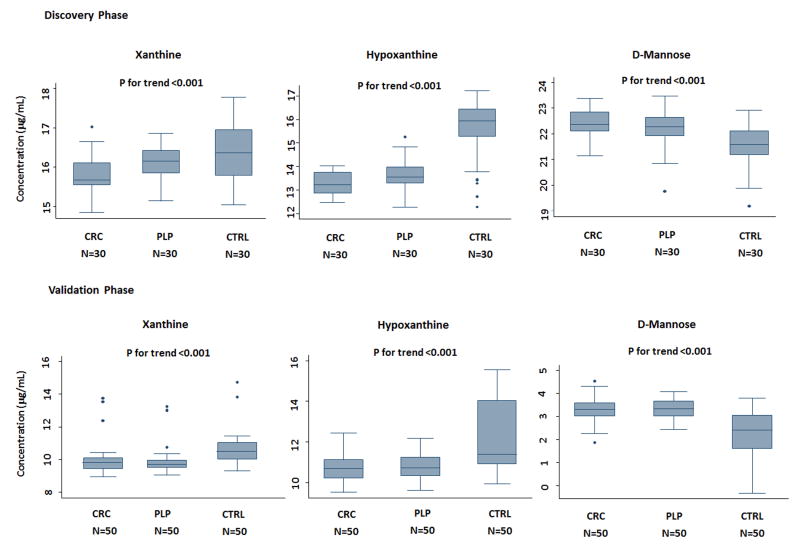
Metabolites showing a significant trend in serum levels from controls to PLP and CRC patients can be potential biomarkers for early detection of colorectal malignant disease. Of the sixty-five promising biomarkers, we chose top three metabolites, xanthine, hypoxanthine and D-mannose for further validation, according to their statistical significance as well as scientific impact from previous literature, by performing standard LC/MS-MS assays. One of the top metabolite was a small peptide which was not officially multiplexed in the Metabolon platform; therefore, we removed it from further consideration (Table 2). We measured the levels of selected metabolites in a group of participants in the validation phase, consisting with 50 healthy individuals, 50 PLP patients and 50 CRC patients (Table 1). The expression levels of these compounds all exhibited significant trends from controls to PLP and CRC patients both in discovery and validation sets (P<0.01) (Figure 1). In the validation phase, levels of xanthine and hypoxanthine in CRC and PLP were lower than controls, while D-mannose of CRC and PLP groups displayed higher levels than controls (P<0.01), consistent with discovery set. We also conducted stratified analyses by gender, age, smoking status and BMI (Supplementary Table S4) to see whether these host factors influence metabolite levels in cases and controls. In general, no dramatic changes were seen; however, the differential effects were more significant in ever smokers, younger and overweight/obese subjects.
Figure 1. Levels of validated serum metabolites showing differential levels in discovery and validation groups.
Box plots displayed the median levels of xanthine, hypoxanthine and D-mannose according to colorectal cancer (CRC), adenoma polyp (PLP), and control (CTRL) groups in both discovery and validation phases. Line in the middle of the box indicates median value and the box defines values from 25th percentile to 75th percentile. The upper and lower bars mark boundaries of metabolite levels within 95% confidence interval. P value is generated from Wilcoxon rank sum test.
Associations of xanthine, hypoxanthine and D-mannose with PLP and CRC risk
The differential levels of xanthine, hypoxanthine, and D-mannose prompted us to examine the association of these metabolites with PLP and CRC risks. We dichotomized the case levels of metabolites on the basis of median levels in controls. As shown in Table 3, lower level of xanthine and hypoxanthine were associated with higher risks of PLP and CRC in both discovery and validation sets (OR ranges from 1.8 to 38.8). In contrast, higher level of D-Mannose was associated with increased risks of PLP and CRC (OR ranges from 8 to 16). Some association analyses could not be carried out due to absence of subjects in the subgroups.
Table 3. Association of top selected metabolites with PLP and CRC risk.
| PLP, N (%) | Control, N (%) | OR* (95% CI)a | P value | CRC, N (%) | OR* (95% CI) a | P value | |
|---|---|---|---|---|---|---|---|
| Discovery | |||||||
| Xanthine | |||||||
| High | 10(33.33) | 15(50.00) | 1(reference) | 5(16.67) | 1(reference) | ||
| Low | 20(66.67) | 15(50.00) | 1.78(0.53-5.88) | 0.3 | 25(83.33) | 5.00(1.45-16.66) | 0.01 |
| Hypoxanthine | |||||||
| High | 0 | 15(50.00) | 1(reference) | 0 | 1(reference) | ||
| Low | 30(100.00) | 15(50.00) | NA | NA | 30(100.00) | NA | NA |
| D-Mannose | |||||||
| Low | 5(16.67) | 15(50.00) | 1 (reference) | 3(10.00) | 1(reference) | ||
| High | 25(83.33) | 15(50.00) | 7.98 (1.94-32.82) | <0.01 | 27(90.00) | 14.02(3.00-65.45) | <0.01 |
| Validation | |||||||
| Xanthine | |||||||
| High | 3(6.00) | 25(50.00) | 1(reference) | 3(6.00) | 1(reference) | ||
| Low | 47(94.00) | 25(50.00) | 10.47(2.63-41.63) | <0.01 | 47(94.00) | 38.76(6.58-228.51) | <0.01 |
| Hypoxanthine | |||||||
| High | 5(10.00) | 25(50.00) | 1(reference) | 5(10.00) | 1(reference) | ||
| Low | 45(90.00) | 25(50.00) | 6.50(1.98-21.24) | <0.01 | 45(90.00) | 11.19(3.28-38.21) | <0.01 |
| D-Mannose | |||||||
| Low | 0 | 25(50.00) | 1(reference) | 4(8.00) | 1(reference) | ||
| High | 50(100.0) | 25(50.00) | NA | NA | 46(92.00) | 15.99(4.07-62.88) | <0.01 |
Risk estimate for each metabolite predisposing to adenoma polyp (PLP) or colorectal cancer (CRC) comparing “High” to “Low” group using median value of control (CTRL) group as cutoff.
OR = Odds Ratio; CI = confidence interval; NA = Not able to estimate.
Adjusted by age, gender, smoking status and BMI
Joint effects with smoking and BMI
Previous studies have revealed that obesity and smoking are risk factors for CRC. We analyzed the joint effects of the three metabolic markers with smoking and BMI on PLP and CRC risk in the validation set. The results indicated that in ever smokers low level of xanthine and low level of hypoxanthine showed joint effect with smoking on PLP risk (OR=12.46; 95% CI, 2.04-75.95, P<0.01 and OR=7.27; 95% CI, 1.57-33.68, P=0.01 respectively)(Supplementary Table S5). Similarly, low level of xanthine or hypoxanthine displayed joint effect with smoking in modifying the risk of CRC (OR=29.23; 95% CI, 3.94-217.01, P<0.01 and OR=14.20; 95% CI, 1.39-84.26, P<0.01, respectively). We also found that overweight/obese subjects (BMI≥25) with low level of xanthine or hypoxanthine had elevated PLP risk (OR=19.1; 95% CI, 1.98-185, P<0.05 and OR=10.1; 95% CI, 1.78-57.5, P<0.01, respectively)(Supplementary Table S6). High levels of D-Mannose showed joint effects with smoking on CRC risk (OR=27.91; 95% CI, 2.98-261.33, P<0.01)(Supplementary Tables S5). Notably, there was significant interaction between BMI and D-mannose level in modifying CRC risk (P interaction <0.001)(Supplementary Tables S6).
The effects of hypoxanthine/xanthine ratio on PLP and CRC risk
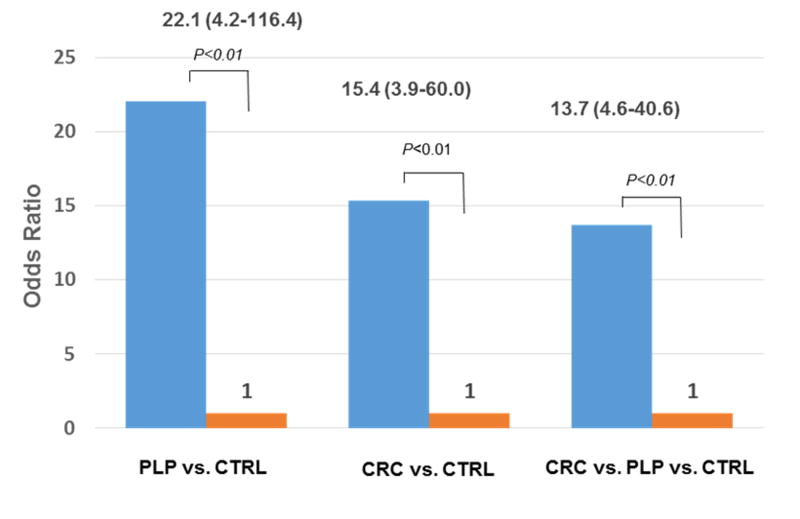
Hypoxanthine and xanthine are metabolites found in the same biological pathway involving purine metabolism, and low levels of these metabolites increased the risk of PLP and CRC. To evaluate the potential effect of both metabolites on susceptibility to PLP and CRC, we combined two markers by analyzing the association of hypoxanthine/xanthine ratio (purine metabolite ratio) with PLP and CRC risk via the unconditional multivariate logistic regression analysis method. Figure 2 demonstrated the association of purine metabolite ratio with the risks of PLP, CRC, and CRC progression. Compared with controls, 96% of PLP and 90% of CRC cases displayed low ratios. Low purine metabolite ratio was associated with increased PLP risk (OR=22.08; 95% CI, 4.18-116.41, P<0.001). Similar effect was also seen with low ratio and elevated CRC risk (OR= 15.38; 95% CI, 3.94-60.00, P<0.001) and risk of CRC progression (OR=13.73; 95% CI, 4.64-40.59, P<0.001). We also explored the joint effect of smoking, BMI and purine metabolite ratio on PLP and CRC risk in the validation set (table not shown). Smoking could result in increasing PLP and CRC risk in subjects with low ratios (OR=17.31; 95% CI, 2.57-108.92, P<0.001; OR=15.30; 95% CI, 2.56-91.39, P<0.001, respectively). Additionally, overweight/obese patients with low ratio showed high PLP risk (OR=24.04; 95% CI, 2.59-222.77, P<0.01). Moreover, BMI and metabolite ratio displayed significant interaction effect on CRC risk (P<0.001).
Figure 2. Association between hypoxanthine/xanthine ratio and risk of PLP, CRC, and CRC progression.
Bar chart displayed high hypoxanthine/xanthine ratio was associated with 22.1-fold, 15.4-fold, and 13.7-fold increased risk of PLP, CRC, and CRC progression, respectively, compared to controls.
Discussion
Dysregulation of the metabolic pathways has been implicated in neoplastic development and is characterized as a hallmark of cancer14. Tumor cells require large quantities of different energy sources to support their high rate of proliferation with increased energy consumption, which would lead to alterations of key metabolic pathways15.
In this study, we aimed to identify and validate the serum metabolite biomarkers for the noninvasive early detection of colorectal polyps and cancer and as risk factors for cancer progression on the basis of a two-phase approach using independent detection platforms. Our findings showed that 50 metabolites were significantly altered in PLP and CRC patients in the discovery phase using metabolomic profiling, which were mapped to eight superpathways (nucleotide, carbohydrate, peptide, amino acid, lipid, energy, carnitine metabolism, and xenobiotics) and thirty-one subpathways (Supplementary Table S2) associated with metabolism. Sample size was increased in the validation phase to verify the candidate metabolites as predictors of PLP and CRC risks. Specifically, we found significantly altered expression of xanthine, hypoxanthine and D-Mannose in both discovery and validation sets. In addition, smoking and obesity factors, which are known risk factors for CRC, were found to modify the association with PLP and CRC risks.
To our knowledge, there are few studies that investigated the metabolite shifts in CRC development8. In the current study, we observed a variety of serum metabolites with altered levels in PLP and CRC subjects, which suggested a step-wise progression. Notably, we observed two metabolites of the purine metabolism pathway, xanthine and hypoxanthine, being associated with PLP and CRC risk. Xanthine is a metabolite intermediate in the degradation of adenosine monophosphate to uric acid, whereas hypoxanthine is oxidized to xanthine by xanthine oxidase (XO), a form of xanthine oxidoreductase16 (Supplementary Figure S3). XO is the last catalyzing enzyme of purine metabolism, so it plays critical role in nucleic acid degradation. Cancer and polyps are often accompanied by low expression levels of xanthine and hypoxanthine likely due to increased DNA synthesis (adenine utilization) in the hyper-proliferative tissues. Xanthine can be produced by all human cells, as well as specific plants and animals. Interestingly, caffeine is one of several types of xanthine derivatives and may affect circulating xanthine level. Indeed, pathway analysis of metabolites indicated compounds of caffeine metabolism altered significantly from controls to PLP and CRC. Some studies reported that caffeine inhibited colon cancer cell proliferation and reduced colorectal cancer risk17,18. Decreased hypoxanthine level was detected in the urine of patients with Non-Hodgkin lymphoma19, and the same trend of reduced level was observed in gastric cancer patients compared to normal individuals20. Hypoxanthine level showed reduction in the serum of advanced rectal cancer patients when it was compared between different stages of tumor progression21, which is consistent with our findings. Lower levels of xanthine were identified in glioblastoma cases compared to controls in a study by Benny Björkblom, et al22. As the termination product of xanthine and hypoxanthine degradation, the level of uric acid is affected by xanthine and hypoxanthine levels (Supplementary Figure S3). Findings from previous studies have raised the viewpoint that uric acid might contribute to cancer risk, recurrence and mortality23, thus pointing to the importance of this metabolic pathway in influencing neoplastic development and outcome.
We also analyzed a novel measure, the ratio of hypoxanthine/xanthine levels. This is the first study to report the association of hypoxanthine/xanthine ratio with PLP and CRC risk. Converted from hypoxanthine, xanthine level is dependent on the activity of hypoxanthine metabolism. Thus, hypoxanthine/xanthine ratio demonstrates the relative metabolic rate of hypoxanthine and xanthine degradation. It is shown in our study that low ratio was associated with increased risk, which suggested that PLP and CRC patients have higher hypoxanthine turnover likely due to elevated XO activity. Our findings are consistent with the previous study of Linder et al., which indicated that the expression of XO was elevated during the progression of human colon carcinoma cells in in vitro experiments24. Circulating OX level was also reported higher in head and neck carcinoma25 as well as lung cancer patients26, and its activity was observed to be significantly increased in human cancerous colorectal tissues compared to non-cancerous adjacent tissues27.
In contrast to the decrease of xanthine and hypoxanthine levels associated with increased PLP and CRC risks, low D-Mannose level was protective in our study. An early literature reported that high mannose oligosaccharides were more prevalent on tumor cells surface compared to normal cells28. Mannose, an intermediate metabolite of galactose metabolism pathway, plays an essential role in N-linked glycosylation, which modified certain proteins at the post-translational step. It was reported that high mannose glycans were increased in breast cancer progression29 and glycans took part in dominant cancer progression phases, such as cancer cell proliferative signaling, activating invasion and metastasis, and tumor promoting inflammation30. Our previous study observed that D-Mannose was elevated in late stage esophageal cancer patients31, consistent with the finding of higher levels of D-Mannose in PLP and CRC cases compared to controls.
Another discovery of our current study is the joint effect of metabolites, BMI and smoking status on CRC and PLP risk. It is known that smoking and obesity are risk factors for CRC31-34. We observed that in ever smokers the risk of PLP and CRC significantly increased about two to three fold in subjects with low xanthine or hypoxanthine level or high D-mannose level compared to never smokers. Similar joint effect is seen with high BMI modifying the risk of PLP in subjects with low xanthine or hypoxanthine level or high D-mannose level compared to low BMI individuals. It is notable that for the BMI analysis there was significant interaction with D-mannose in modifying CRC risk. One limitation of these analyses is that we were only able to analyze the validation set due to the limited sample size of the discovery group. Moreover, the usage of different metabolic profiling platforms precluded us from pooling the two sets of data for combined analysis. Nevertheless, these partial data may suggest the importance of linking lifestyle factors with specific metabolites in predicting the risk of PLP and CRC.
Besides the small sample size which might confer low statistical power for subgroup analysis, other potential limitations include the retrospective nature of the study, which allowed the possibility for recall bias and reverse causation. It should be cautioned that circulating level of hypoxanthine has been found to correlate with increased blood sample storage time at room temperature35. Thus, the metabolite may represent a good biomarker for sample quality. Since, our samples from cases and controls were collected and immediately processed in similar manner and time frame by laboratory personnel without knowledge of the case-control status, any confounding effect from time of sample storage may be negligible. Moreover, the small fold changes in metabolite levels between patients and controls may present challenges for translational application. Several demographic and personal factors such as gender, age, BMI, diet, and smoking status might influence the metabolomic landscape. Therefore, careful matching of known risk factors during study design and adjustment of covariates in the statistical analysis are necessary to minimize false discoveries. Nevertheless, the global profiling of circulating metabolites with targeted validation and comprehensive collection of epidemiologic information in a specialty hospital setting provide valuable resources to identify candidate metabolic biomarkers for clinical applications.
In conclusion, our results supported the notion that serum metabolic detection might be utilized as a non-invasive approach to assess the risk of CRC progression. We determined that the analysis of circulating xanthine, hypoxanthine and D-Mannose levels has the potential to distinguish patients with PLP and CRC from those without cancer. The hypoxanthine/xanthine ratio, which showed plausible biological and clinical significance, may provide a new assessment tool to gauge clinical risk of CRC progression. In addition, the analyses of lifestyle factors contributing to the effects of serum metabolites and PLP and CRC risks suggested that smoking and obesity might jointly affect cancer progression by altering metabolic pathways. Taken together, global metabolic changes measurable in blood samples might present early indications of CRC development, which could be leveraged as clinical screening tool to enhance present diagnostic method. Future prospective validation in independent cohorts is warranted to confirm our observations, and further investigation is needed to determine the potential clinical utility of these findings.
Supplementary Material
Acknowledgments
Funding support: This work was supported in part by funding provided by the Premalignant Genome Atlas program of the Duncan Family Institute for Cancer Prevention and Risk Assessment. Additional funding provided by MD Anderson's Center for Translational and Public Health Genomics and by NIH through MD Anderson's Cancer Center Support Grant CA016672.
Footnotes
Conflict of interest: No conflicts of interests are declared.
Author contributions: Xifeng Wu obtained funding and contributed to study design and supervision; Yin Long analyzed the data and drafted the manuscript; Beatriz Sanchez-Espiridion contributed to methodology, analyzed and interpreted the data; Dong Liang and Lyndsey White were involved in data acquisition for validation phase; Yuanqing Ye performed statistical analysis; Moubin Lin and David W. Chang analyzed the data and revised the manuscript for important intellectual content; Lopa Mishra, Gottumakkala S. Raju, Scott Kopetz, Cathy Eng and Michelle A.T. Hildebrandt provided clinical resources and/or technical support.
References
- 1.Favoriti P, Carbone G, Greco M, et al. Worldwide burden of colorectal cancer: a review. Updates Surg. 2016;68:7–11. doi: 10.1007/s13304-016-0359-y. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 3.Schreuders EH, Grobbee EJ, Spaander MC, Kuipers EJ. Advances in Fecal Tests for Colorectal Cancer Screening. Curr Treat Options Gastroenterol. 2016;14:152–162. doi: 10.1007/s11938-016-0076-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zamboni N, Saghatelian A, Patti GJ. Defining the metabolome: size, flux, and regulation. Mol Cel l. 2015;58:699–706. doi: 10.1016/j.molcel.2015.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kishton RJ, Rathmell JC. Novel therapeutic targets of tumor metabolism. Cancer J. 2015;21:62–69. doi: 10.1097/PPO.0000000000000099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu J, Djukovic D, Deng L, et al. Targeted serum metabolite profiling and sequential metabolite ratio analysis for colorectal cancer progression monitoring. Anal Bioanal Chem. 2015;407:7857–7863. doi: 10.1007/s00216-015-8984-8. [DOI] [PubMed] [Google Scholar]
- 7.Qiu Y, Cai G, Zhou B, et al. A distinct metabolic signature of human colorectal cancer with prognostic potential. Clin Cancer Res. 2014;20:2136–2146. doi: 10.1158/1078-0432.CCR-13-1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu J, Djukovic D, Deng L, et al. Colorectal cancer detection using targeted serum metabolic profiling. J Proteome Res. 2014;13:4120–4130. doi: 10.1021/pr500494u. [DOI] [PubMed] [Google Scholar]
- 9.Ritchie SA, Ahiahonu PW, Jayasinghe D, et al. Reduced levels of hydroxylated, polyunsaturated ultra long-chain fatty acids in the serum of colorectal cancer patients: implications for early screening and detection. BMC Med. 2010;8:13. doi: 10.1186/1741-7015-8-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen C, Deng L, Wei S, et al. Exploring Metabolic Profile Differences between Colorectal Polyp Patients and Controls Using Seemingly Unrelated Regression. J Proteome Res. 2015;14:2492–2499. doi: 10.1021/acs.jproteome.5b00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lawton KA, Berger A, Mitchell M, et al. Analysis of the adult human plasma metabolome. Pharmacogenomics. 2008;9:383–397. doi: 10.2217/14622416.9.4.383. [DOI] [PubMed] [Google Scholar]
- 12.Kanehisa M. The KEGG database. Novartis Found Symp. 2002;247:91–101. discussion 101-103, 119-128, 244-152. [PubMed] [Google Scholar]
- 13.Xia J, Sinelnikov IV, Han B, Wishart DS. MetaboAnalyst 3.0--making metabolomics more meaningful. Nucleic Acids Res. 2015;43:W251–257. doi: 10.1093/nar/gkv380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Benjamin DI, Cravatt BF, Nomura DK. Global profiling strategies for mapping dysregulated metabolic pathways in cancer. Cell Metab. 2012;16:565–577. doi: 10.1016/j.cmet.2012.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lucarelli G, Galleggiante V, Rutigliano M, et al. Metabolomic profile of glycolysis and the pentose phosphate pathway identifies the central role of glucose-6-phosphate dehydrogenase in clear cell-renal cell carcinoma. Oncotarget. 2015;6:13371–13386. doi: 10.18632/oncotarget.3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hille R. Molybdenum-containing hydroxylases. Arch Biochem Biophys. 2005;433:107–116. doi: 10.1016/j.abb.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 17.Guertin KA, Loftfield E, Boca SM, et al. Serum biomarkers of habitual coffee consumption may provide insight into the mechanism underlying the association between coffee consumption and colorectal cancer. Am J Clin Nutr. 2015;101:1000–1011. doi: 10.3945/ajcn.114.096099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Merighi S, Benini A, Mirandola P, et al. Caffeine inhibits adenosine-induced accumulation of hypoxia-inducible factor-1alpha, vascular endothelial growth factor, and interleukin-8 expression in hypoxic human colon cancer cells. Mol Pharmacol. 2007;72:395–406. doi: 10.1124/mol.106.032920. [DOI] [PubMed] [Google Scholar]
- 19.Yoo BC, Kong SY, Jang SG, et al. Identification of hypoxanthine as a urine marker for non-Hodgkin lymphoma by low-mass-ion profiling. BMC Cancer. 2010;10:55. doi: 10.1186/1471-2407-10-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jung J, Jung Y, Bang EJ, et al. Noninvasive diagnosis and evaluation of curative surgery for gastric cancer by using NMR-based metabolomic profiling. Ann Surg Oncol. 2014;21(Suppl 4):S736–742. doi: 10.1245/s10434-014-3886-0. [DOI] [PubMed] [Google Scholar]
- 21.Kim K, Yeo SG, Yoo BC. Identification of hypoxanthine and phosphoenolpyruvic Acid as serum markers of chemoradiotherapy response in locally advanced rectal cancer. Cancer Res Treat. 2015;47:78–89. doi: 10.4143/crt.2013.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bjorkblom B, Wibom C, Jonsson P, et al. Metabolomic screening of pre-diagnostic serum samples identifies association between alpha- and gamma-tocopherols and glioblastoma risk. Oncotarget. 2016;7:37043–37053. doi: 10.18632/oncotarget.9242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fini MA, Elias A, Johnson RJ, Wright RM. Contribution of uric acid to cancer risk, recurrence, and mortality. Clin Transl Med. 2012;1:16. doi: 10.1186/2001-1326-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Linder N, Martelin E, Lundin M, et al. Xanthine oxidoreductase - clinical significance in colorectal cancer and in vitro expression of the protein in human colon cancer cells. Eur J Cancer. 2009;45:648–655. doi: 10.1016/j.ejca.2008.10.036. [DOI] [PubMed] [Google Scholar]
- 25.Kalcioglu MT, Kizilay A, Yilmaz HR, et al. Adenosine deaminase, xanthine oxidase, superoxide dismutase, glutathione peroxidase activities and malondialdehyde levels in the sera of patients with head and neck carcinoma. Kulak Burun Bogaz Ihtis Derg. 2004;12:16–22. [PubMed] [Google Scholar]
- 26.Kaynar H, Meral M, Turhan H, et al. Glutathione peroxidase, glutathione-S-transferase, catalase, xanthine oxidase, Cu-Zn superoxide dismutase activities, total glutathione, nitric oxide, and malondialdehyde levels in erythrocytes of patients with small cell and non-small cell lung cancer. Cancer Lett. 2005;227:133–139. doi: 10.1016/j.canlet.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 27.Ozturk HS, Karaayvaz M, Kacmaz M, et al. Activities of the enzymes participating in purine and free-radical metabolism in cancerous human colorectal tissues. Cancer Biochem Biophys. 1998;16:157–168. [PubMed] [Google Scholar]
- 28.Johns TG, Mellman I, Cartwright GA, et al. The antitumor monoclonal antibody 806 recognizes a high-mannose form of the EGF receptor that reaches the cell surface when cells over-express the receptor. Faseb J. 2005;19:780–782. doi: 10.1096/fj.04-1766fje. [DOI] [PubMed] [Google Scholar]
- 29.de Leoz ML, Young LJ, An HJ, et al. High-mannose glycans are elevated during breast cancer progression. Mol Cell Proteomics. 2011;10 doi: 10.1074/mcp.M110.002717. M110.002717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Munkley J, Elliott DJ. Hallmarks of glycosylation in cancer. Oncotarget. 2016 doi: 10.18632/oncotarget.8155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sanchez-Espiridion B, Liang D, Ajani JA, et al. Identification of Serum Markers of Esophageal Adenocarcinoma by Global and Targeted Metabolic Profiling. Clin Gastroenterol Hepatol. 2015;13:1730–1737.e1739. doi: 10.1016/j.cgh.2015.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parekh N, Lin Y, Vadiveloo M, et al. Metabolic dysregulation of the insulin-glucose axis and risk of obesity-related cancers in the Framingham heart study-offspring cohort (1971-2008) Cancer Epidemiol Biomarkers Prev. 2013;22:1825–1836. doi: 10.1158/1055-9965.EPI-13-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cross AJ, Boca S, Freedman ND, et al. Metabolites of tobacco smoking and colorectal cancer risk. Carcinogenesis. 2014;35:1516–1522. doi: 10.1093/carcin/bgu071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muc-Wierzgon M, Nowakowska-Zajdel E, Dziegielewska-Gesiak S, et al. Specific metabolic biomarkers as risk and prognostic factors in colorectal cancer. World J Gastroenterol. 2014;20:9759–9774. doi: 10.3748/wjg.v20.i29.9759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yin P, Peter A, Franken H, Zhao X, et al. Preanalytical aspects and sample quality assessment in metabolomics studies of human blood. Clin Chem. 2013;59:833–845. doi: 10.1373/clinchem.2012.199257. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.