Abstract
Purpose
Our purpose was to characterize the clinical influences, genetic risk factors, and gene mechanisms contributing to persistent cisplatin-induced peripheral neuropathy (CisIPN) in testicular cancer survivors (TCS).
Experimental Design
TCS given cisplatin-based therapy completed the validated EORTC QLQ-CIPN20 questionnaire. An ordinal CisIPN phenotype was derived and associations with age, smoking, excess drinking, hypertension, body mass index, diabetes, hypercholesterolemia, cumulative cisplatin dose, and self-reported health were examined for 680 TCS. Genotyping was performed on the Illumina HumanOmniExpressExome chip. Following quality control and imputation, 5.1 million SNPs in 680 genetically European TCS formed the input set. GWAS and PrediXcan were used to identify genetic variation and genetically-determined gene expression traits, respectively, contributing to CisIPN. We evaluated two independent datasets for replication: Vanderbilt’s electronic health database, BioVU and CALGB 90401 trial.
Results
Eight sensory items formed a subscale with good internal consistency (Cronbach α=0.88). Variables significantly associated with CisIPN included age at diagnosis (OR per year=1.06, p=2 × 10−9), smoking (OR=1.54, p=0.004), excess drinking (OR=1.83, p=0.007), and hypertension (OR=1.61, p=0.03). CisIPN was correlated with lower self-reported health (OR=0.56; p=2.6 × 10−9) and weight gain adjusted for years since treatment (OR per Δkg/m2=1.05, p=0.004). PrediXcan identified lower expressions of MIDN and RPRD1B, and higher THEM5 expression as associated with CisIPN (p-value for each < 5 × 10−6) with replication of RPRD1B meeting significance criteria (Fisher’s combined p=0.0089).
Conclusions
CisIPN is associated with age, modifiable risk factors and genetically-determined expression level of RPRD1B. Further study of implicated genes could elucidate the pathophysiologic underpinnings of CisIPN.
Keywords: cisplatin, peripheral neuropathy, genome wide association study, cancer survivorship, Platinum Study, young adult, testicular cancer, germ cell tumor, patient reported outcomes, pharmacogenetics
INTRODUCTION
Chemotherapy-induced peripheral neuropathy (CIPN) is one of the most common and often debilitating adverse effects of modern chemotherapy (1, 2). Platinum analogs, taxanes, vinca alkaloids, thalidomide, and epothilones are associated with CIPN, although with different clinical manifestations, which include sensory, motor and autonomic symptoms (1). Mechanisms underlying CIPN remain largely unclear (2). Unlike taxane-related CIPN, which can be reversible (2), cisplatin-induced peripheral neuropathy (CisIPN) can be cumulative and progressive and cause long-term effects on overall quality of life (QoL) and physical function (3). Studies have suggested that age, race, diabetes and obesity may be risk factors for CIPN (4, 5), but results have been conflicting (6–11).
No agents are currently available to prevent or treat CIPN (12). Management of CIPN is complicated by the lack of reliable means to identify at-risk patients. While genetic variants could address unexplained phenotypic variability, heterogeneity of treatment regimens and the lack of long-term patient follow-up complicate pharmacogenetic studies of chemotherapeutic toxicities, including CIPN. However, cisplatin-based chemotherapy for testicular cancer is relatively homogeneous, and is curative (13). Testicular cancer survivors (TCS) are typically young at diagnosis, and the period of survivorship can span upwards of 50 years, providing an ideal model to investigate long-term adverse effects of cisplatin-based chemotherapy. These include hearing impairment, tinnitus, renal dysfunction, cardiovascular disease, secondary malignancies, CisIPN, and others (14). CisIPN most commonly presents as a sensory neuropathy in a stocking-glove distribution with numbness, tingling, paresthesias, loss of proprioception, hyperalgesia and/or allodynia (3).
Although cisplatin is one of the most widely prescribed chemotherapeutics, there are few data available on genetic variants associated with CisIPN. Prior investigations assessed variants in a few candidate genes in small numbers of patients (range 104–238), who often also received other neurotoxic agents (15–18). In this study, we used the extensively validated EORTC-CIPN20 scale (19) and other patient-reported outcomes (PRO) to characterize CisIPN in a large number of TCS, the impact on self-reported health, and associated clinical and demographic variables. We additionally conducted, to our knowledge, the first genome-wide association study of CisIPN.
PATIENTS AND METHODS
Patients
All patients were enrolled in The Platinum Study, a cross sectional study that includes 8 cancer centers in the United States and Canada (14). Eligibility criteria included: men diagnosed with a histologically or serologically confirmed germ cell tumor (GCT), age <55 years at diagnosis and >18 years at enrollment, treatment with cisplatin-based chemotherapy for either initial GCT or recurrence, no antecedent chemotherapy for another primary cancer and no subsequent salvage chemotherapy. Of all eligible patients, 93% agreed to participate. Study procedures were approved by the Human Subjects Review Board at each institution. All patients provided written consent for participation in study procedures, including genetic analyses.
Data Collection
TCS visiting the clinic for follow-up and who provided written consent for participation underwent a brief physical examination, including measurement of height and weight. Body mass index (BMI) (kg/m2) was defined as normal, overweight, obese, or morbidly obese (<25, 25 to <30, 30 to <40, and ≥40 kg/m2, respectively). Figure S1 illustrates the data collection pipeline. Patients completed questionnaires with regard to neurotoxic symptoms, comorbidities, lifestyle behaviors, perceived health and medication use at the time of enrollment (range: 1–30 years after treatment completion). These included the EORTC-QLQ-CIPN20 questionnaire with questions listed in Table S1 (20). Data abstracted from medical records with standardized forms included all variables related to GCT diagnosis and treatment. For each administered cytotoxic drug, information was collected on name, dose, date(s) of administration, number of cycles, and cumulative dose.
Phenotype Analysis
We evaluated the frequency of sensory neuropathy using 9 items in the EORTC-CIPN20 (19). We assessed inter-item associations as well as relationships with motor and autonomic neuropathy by variance analysis using principal component analysis (PCA), following conversion of the Likert scale to a 0–3 numeric scale: 0 for “none”, 1 for “a little”, 2 for “quite a bit”, 3 for “very much”. We evaluated subscale internal consistency using Cronbach α (21). Following exclusion of the hearing impairment item, we created a summary statistic of CisIPN mathematically equivalent to the standard scoring algorithm (22): rather than summing item scores and scaling to 0–100, we took the mean of the scores, effectively summing scores and scaling to 0–3 and imputing missing data points from available ones (11 samples were missing one response, and two samples were missing three responses). The ceiling function was then used to place means into four ordinal groups reflecting the average severity across symptoms created as follows: none (0; mean = 0), a little (1; 0 < mean ≤ 1), quite a bit (2; 1 < mean ≤ 2), very much (3; 2 < mean ≤ 3). Groups 2 and 3 were combined due to low frequency.
Ordinal Regressions with Relevant Variables
We determined the statistical association between CisIPN and data abstracted from the medical records at the time of treatment and data collected at the time of clinical evaluation. Dependent variables abstracted from the medical records included GCT treatment regimen, cumulative cisplatin dose, age and BMI at the initiation of treatment. Data collected at clinical evaluation included age and BMI, self-reported smoking status, alcohol consumption, blood pressure, diabetes, and hypercholesterolemia. Age was analyzed as a continuous variable. Ever smokers were defined as those responding affirmatively to “have you ever smoked?”. Excessive drinkers were defined as those who reported consuming ≥ 2 drinks/day on average in the past year. Hypertensive patients were those who both indicated that they had received a diagnosis of high blood pressure and were taking prescription medication for it at the time of evaluation. Diabetic patients were those who indicated they had 1) diabetes requiring insulin or 2) diabetes requiring tablets or pills. We also took into consideration those patients currently taking prescription medication for high cholesterol in view of the small risk of neuropathy associated with statin therapy (23). TCS were also asked to rate their health as “excellent,” “very good,” “good,” “fair,” or “poor”, and to report the average time per week engaged in various physical activities during the past year (24). Total kilocalories per week were estimated by summing Metabolic Equivalent of Task (MET)-hours for each physical activity and multiplying by the participant’s weight (kilograms). Kilocalories per week were calculated and categorized as vigorous (≥6 METs), moderate (3 to <6 METs), and less than moderate (<3 METs) physical activity (25). Weight gain was determined as the difference in BMI between initiation of chemotherapy and study evaluation (Δkg/m2). Association with the CisIPN phenotype was evaluated in proportional odds ordered logistic regression models at α = 0.05. All univariate regressions were adjusted for age at diagnosis (covariate). A multi-variable regression model was constructed by using all significant variables in the age-adjusted univariate analysis.
Genotyping and Imputation
DNA extraction was performed from peripheral blood. SNPs were genotyped on the Illumina HumanOmniExpressExome chip (Illumina, San Diego, CA) at the RIKEN Center for Integrative Medical Science (Yokohama, Japan). Figure S2 illustrates the CONSORT flow diagram for the TCS. Quality control (QC) was performed as previously described (26). Individuals with pairwise identity by descent (IBD) > 0.125 and excess heterozygosity (F inbreeding coefficient >6 standard deviations from the mean) were excluded, leaving 827 individuals. PCA was performed on the genotype data to quantify genomic ancestry using SMARTPCA (27), revealing 713 genetically European individuals. Out of those, 680 had complete phenotypic data (CIPN20, cisplatin dose, demographics), and composed the study sample. SNPs with call rate < 0.99 and Hardy-Weinberg equilibrium (HWE) P < 1×10−6 were excluded, leaving 930,450 SNPs. These comprised the input set of genotype imputation with SHAPEIT phasing, which we performed on the University of Michigan Imputation Server using the EUR (European) population in 1000G Phase 1v3 ShapeIt2 (no singletons) reference panel (28). 5,068,489 SNPs with minor allele frequency (MAF) > 0.05, imputation R2 > 0.8 and INFO score 0.6–1.05 were retained for further analysis. No genetic PC was associated with CisIPN in univariate or multivariate analyses investigating the first 10 PCs.
Independent SNP Analysis
We performed independent SNP association tests with CisIPN by proportional odds ordinal logistic regression adjusted for covariates and assuming linear additive SNP effects. These did not include heritable covariates significantly associated with CisIPN, because they can bias effect estimates (29). R version 3.2.0 was used.
GCTA and PrediXcan Analysis
We estimated narrow-sense heritability using a variance-component model with a genetic relationship matrix (GRM) estimated from genotypes using GCTA (30). SNPs with HWE P > 0.05 and MAF > 0.05 and one of a pair of individuals with estimated relatedness >0.025 were included, leaving 4,897,434 SNPs and 623 individuals. We performed gene-based associations using PrediXcan (31). Briefly, PrediXcan uses reference transcriptome data to generate models ‘imputing’ gene expression in patients from their genotype data by leveraging the effects of SNPs predicting expression. In this study, we used models derived from the application of elastic net (α = 0.5). Genome-wide expression was predicted using reference transcriptome panels in four candidate tissues, chosen for their representation of the nervous system and the microenvironment of peripheral nerves and endings: whole blood using Depression Genes and Networks (DGN) (32), and three tissues from GTEx (33): tibial nerve, skin (not exposed to sun), and cerebral cortex. DGN (instead of GTEx) was used as a reference set for whole blood due to the larger sample size (n = 922 vs. 338). Genes with prediction R2 > 0.01 and prediction p < 0.05 were included. Genes with low-variance predicted expression were excluded (σ2 < 0.001). Associations were obtained by ordinal regression as described, with predicted expression as the independent variable and age as a covariate. Genome-wide significance was set at α = 0.05 with Bonferroni correction for the number of genes tested within the tissue. Experiment-wide significance was set conservatively with Bonferroni correction for the total number of tests performed in all four tissues.
Replication
To replicate our findings, we used two additional independent datasets, Vanderbilt’s electronic health records (EHR) BioVU (34, 35) and the CALGB 90401 trial (36). The BioVu cohort was composed of 18,620 genotyped individuals that were linked to EHR (34) and given a PheWAS code (ICD9-derived codes) for polyneuropathy due to drugs (n=20, ICD-9 code 316.1). Association with predicted gene expression was evaluated by one-tailed logistic regression in the direction of the discovery. For CALGB 90401, pre-imputation genotype quality control was performed with 543,834 SNPs and 623 genetically European individuals as described (36). Genotypes were imputed using IMPUTE2 software and the 1000 Genomes phase 3 EUR reference population. 3,031,145 SNPs with MAF > 0.05, IMPUTE2 INFO score > 0.3 were retained for further analysis. We evaluated the association of predicted gene expression with cumulative docetaxel dose (adjusted for competing-risks) at the time of occurrence of grade 3 or higher neuropathy using one-tailed Cox proportional hazards regression (36). Replication significance was assessed with Fisher’s combined p-value method using the one-tailed p-values of the two independent replications at α = 0.05.
RESULTS
Cohort Characteristics
The clinical and demographic characteristics of the genetically European subjects (n = 680) that passed all QC and had full phenotypic data are shown in Table 1 (additional diagnosis and treatment characteristic are listed in Table S2). Median age at testicular cancer diagnosis was 31 years (range: 15–50) with a median of 4.8 years (range: 0.4–30) between treatment completion and clinical evaluation. Chemotherapy regimens consisted largely of bleomycin, etoposide, and cisplatin (62.9%) or etoposide and cisplatin (29.9%). At the time of enrollment, one in three patients (33.6%) were either obese (BMI: 30 to 39) or morbidly obese (BMI ≥40) and only 16.8% participants reported having excellent health (Table S2 and Table 1). Current use of prescription medications for hypertension, hypercholesterolemia, and diabetes was reported by 12.8%, 11.0%, and 3.1% of participants, respectively. Approximately 42% of patients are former (34.4%) or current smokers (7.5%). For those, the median number of years smoking is 5, and the mean is 8.6.
Table 1.
Demographic features and clinical characteristics of 680 genetically European male germ cell tumor (GCT) survivors at the time of clinical evaluation
| Characteristic | Number (%) |
|---|---|
| Total patients | 680 |
| Age at clinical evaluation (years) | |
| Median (range) | 38 (18–68) |
| <20 y | 5 (0.7%) |
| 20–29 y | 124 (18.2%) |
| 30–39 y | 251 (36.9%) |
| 40–49 y | 186 (27.4%) |
| ≥50 y | 114 (16.8%) |
| Self-reported race | |
| White | 660 (97.1%) |
| Non-white | 20 (2.9%) |
| Chemotherapy regimen | |
| Cisplatin, bleomycin, etoposide: totala | 428 (62.9%) |
| ≤2 cycles | 13 (1.9%) |
| 3 cycles | 282 (41.5%) |
| 4 cycles | 124 (18.2%) |
| ≥5 cycles | 9 (1.3%) |
| Cisplatin, etoposide: totalb | 203 (29.9%) |
| ≤3 cycles | 2 (0.3%) |
| 4 cycles | 196 (28.8%) |
| ≥5 cycles | 5 (0.7%) |
| Other cisplatin-based regimen: totalc | 49 (7.2%) |
| 3 cycles | 6 (0.9%) |
| 4 cycles | 39 (5.7%) |
| ≥5 cycles | 4 (0.6%) |
| Cumulative dose of cisplatin (mg/m2), all patientsd | |
| <300 | 36 (5.3%) |
| 300 | 258 (37.9%) |
| 301–399 | 28 (4.1%) |
| 400 | 328 (48.2%) |
| >400 | 30 (4.4%) |
| Time from completion of chemotherapy to clinical evaluation, (years) | |
| Median (range) | 4.8 (0.4–29.9) |
| <2 | 146 (21.5%) |
| 2–5 | 250 (36.8%) |
| 6–9 | 127 (18.7%) |
| ≥10 | 156 (22.9%) |
| Not available | 1 (0.1%) |
| Hypertension and on prescription medicatione | |
| Yes | 87 (12.8%) |
| Nof | 593 (87.2%) |
| Diabetes and on prescription medicationg | |
| Yes | 21 (3.1%) |
| Noh | 659 (96.9%) |
| Hypercholesterolemia and on prescription medicationi | |
| Yes | 75 (11.0%) |
| Noj | 605 (89.0%) |
| Smoking status | |
| Current smoker | 51 (7.5%) |
| Former smoker | 234 (34.4%) |
| Never smoker | 395 (58.1%) |
| Average number of alcoholic drinks in past year | |
| <2 drinks per day | 593 (87.2 %) |
| ≥2 drinks per day | 84 (12.4 %) |
| Not indicated | 3 (0.4%) |
| Self-rating of healthk | |
| Excellent | 114 (16.8%) |
| Very good | 286 (42.1%) |
| Good | 249 (36.6%) |
| Poor/Fairl | 30 (4.4%) |
Median cumulative cisplatin dose for BEP-treated patients was 300 mg/m2 (range: 200–800): 300 mg/m2 (range: 272–400) among those receiving 3 cycles, and 400mg/m2 (range: 344–653) among those receiving 4 cycles. 317 received the standard doses for each cycle (i.e., bleomycin 30 units IV weekly, etoposide 100 mg/m2 IV daily × 5 days, cisplatin 20 mg/m2 IV daily × 5 days) and 111 received a modified dose for at least one cycle.
Median cumulative cisplatin dose for EP-treated patients was 400 mg/m2 among patients receiving 4 cycles (range: 400–600), and among all patients (range: 200 – 600). Of patients receiving EP, 129 received standard dose (etoposide 100 mg/m2 IV daily × 5 days, cisplatin 20 mg/m2 IV daily × 5 days) and 74 received a modified dose for at least one cycle.
Of 49 patients, 24 received cisplatin, etoposide, ifosfamide regimen (17 standard, 7 modified), 14 received combination chemotherapy consisting of cisplatin and ifosfamide; 3 received cisplatin, bleomycin, etoposide, and ifosfamide, 1 patient received VelP, 1 patient received PVB, and 1 patient received unknown cisplatin-based regimen. For the remaining 5 patients, other combinations of cisplatin-based chemotherapy were applied.
Median cumulative dose of cisplatin among all patients was 400 mg/m2 (range: 200–800).
Includes patients who answered “Yes” to 1) have you ever been diagnosed with high blood pressure and “Yes, current” to 2) have you ever taken prescription medications for high blood pressure (including current use).
Includes 5 patients for whom status of either hypertension diagnosis or current prescription medication use was not reported.
Includes patients who answered “yes” to either of the following questions: 1) diabetes requiring insulin or 2) diabetes requiring tablets or pills.
Includes 13 patients for whom status of diabetes and current prescription medication use was not reported.
Includes patients who answered “yes, current” to the following question: have you ever taken prescription medications for high cholesterol.
Includes 3 patients for whom status of hypercholesterolemia and on prescription medication use was not reported.
Self-rating of health was not indicated by one patient.
Out of 30 patients, 25 assigned fair and 5 assigned poor to their self-rating of health.
Phenotype Analysis
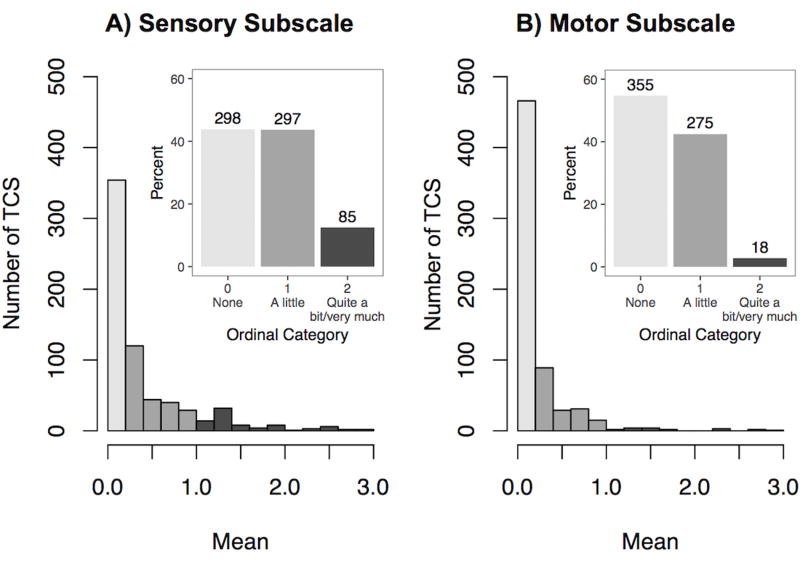
The EORTC-CIPN20 scale questionnaire (20) included nine sensory, eight motor and three autonomic items (Table S1). More than one third (36–38%) of patients reported experiencing some degree of tingling or numbness in hands and/or feet within the four weeks preceding clinical evaluation, with a smaller proportion (8–14%) reporting shooting pain, difficulty distinguishing hot and cold water, and problems feeling the ground (Figure S3). PCA indicated that items generally clustered by symptom type on the first two Principal Components (PCs), except for hearing loss (Figure S4). In the third and fourth PC, sensory and motor items clustered by limb (i.e., fingers/hands vs. toes/feet). Cumulatively, the four PCs explained 60% of the total phenotypic variance. Autonomic items and the hearing loss item formed a distinct cluster on both plots. We have previously comprehensively evaluated hearing loss in this cohort with quantitative audiometry (37) and assessed genetic variants associated with cisplatin-induced ototoxicity (26). Therefore, we excluded hearing loss item from the sensory subscale. The eight remaining items indicated high levels of internal consistency (Cronbach α = 0.88). We created a summary statistic of CisIPN using those items as described. Overall, more than half (56.2%) of patients reported at least some degree of sensory neuropathy, while motor symptoms were less common overall with 45.2% experiencing some form of motor neuropathy and 2.8% with quite a bit/very much (Figure 1) consistent with the observation that cisplatin predominantly causes sensory neuropathy (3).
Figure 1. Distributions of summary statistics for A) the sensory subscale (eight items), and B) the motor items (eight items).
Outset: Following the conversion of the Likert “none”-“very much” scale to a 0–3 numeric scale, each individual was attributed a summary statistic for the sensory subscale (Cronbach α = 0.88) and the motor subscale (α = 0.78) by taking the mean of the responses in the subscale: none (0; mean = 0), a little (1; 0 < mean ≤ 1), quite a bit (2; 1 < mean ≤ 2), very much (3; 2 < mean ≤ 3). Inset: Percent of patients in each group (top of column lists actual number of patients) with groups 2 and 3 combined due to low frequency.
CisIPN Association with Diagnosis and Treatment Characteristics
Summaries of statistical associations between evaluated variables and CisIPN are presented in Table 2A. CisIPN was associated with age at diagnosis (OR = 1.06, p = 2 × 10−9). BMI at evaluation did not correlate with CisIPN. Type of chemotherapy regimen (EP vs. BEP [data not shown]) and cumulative cisplatin dose (range: 100–800 mg/m2) were not significantly associated with CisIPN. Importantly, the number of years since therapy did not correlate negatively with CisIPN (p = 0.43) in age-adjusted analyses, suggesting there is no attenuation of symptoms over time several years following chemotherapy (data not shown). A longitudinal study or one with baseline data remains more appropriate to assess symptom progression.
Table 2.
Association of treatment and other variables with cisplatin-induced peripheral neuropathy. Bolded variables are significantly associated at α = 0.05.
| A. Treatment Variables | |||
|
| |||
| Model | Variable | OR (95% CI) | P-value |
|
| |||
| CisIPN ~ Variable | Age at diagnosis | 1.06 (1.05–1.07) | 2 × 10−9 |
|
| |||
| CisIPN ~ Variable (+Agediagnosis) | BMI at treatment | 1.00 (0.97–1.03) | 0.96 |
|
| |||
| Cisplatin dosea (mg/m2) | 1.00 (0.99–1.00) | 0.39 | |
|
| |||
| B. Variables at Clinical Evaluation | |||
|
| |||
| CisIPN ~ Variable | Age at evaluation | 1.04 (1.03–1.06) | 2 × 10−8 |
|
| |||
| CisIPN ~ Variable (+Agediagnosis) | Smokingb | 1.54 (1.15–2.07) | 0.004 |
|
| |||
| Excess drinkingc | 1.83 (1.18–2.84) | 0.007 | |
|
| |||
| Hypertensiond | 1.61 (1.04–2.50) | 0.03 | |
|
| |||
| BMI at evaluation | 1.03 (1.00–1.05) | 0.051 | |
|
| |||
| Diabetese | 1.27 (0.54–2.95) | 0.59 | |
|
| |||
| Hypercholesterolemiaf | 0.92 (0.57–1.48) | 0.72 | |
|
| |||
| C. Multivariate model | |||
|
| |||
| CisIPN ~ Significant Variables | Agediagnosis | 1.04 (1.02–1.06) | 3 × 10−6 |
| Excess drinking | 1.82 (1.16–2.86) | 0.009 | |
| Smoking | 1.56 (1.15–2.10) | 0.004 | |
| Hypertension | 1.54 (0.97–2.42) | 0.065 | |
Dose group was an ordinal variable created from cumulative cisplatin doses (in mg/m2) classified as follows: <300, 300–400 (excluding 400), 400–500 (excluding 500), and ≥500. OR is based on ordinal group.
Includes patients who answered yes to the question “have you ever smoked cigarettes?”
Includes patients who answered answered ≥ 2 drinks/day to the question “during the past year, how many drinks of alcoholic beverage have you consumed?”
Includes patients who answered yes to both of the following: 1) “have you ever been diagnosed with high blood pressure?” and 2) “are you currently taking prescription medication for high blood pressure?”
Includes those patients who answered “yes” to either of the following questions: 1) “diabetes requiring insulin?” or 2) “diabetes requiring tablets or pills?”.
Includes those patients who are currently taking prescription medications for high cholesterol.
CisIPN Association with Clinical and Behavioral Characteristics at Evaluation
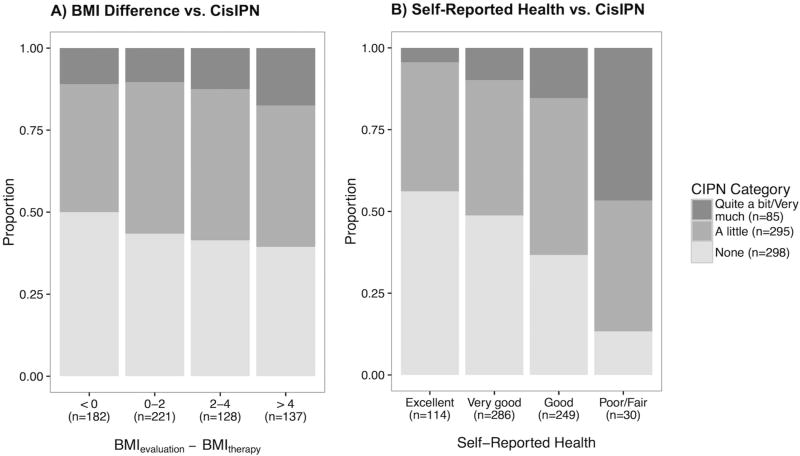
Age at evaluation significantly correlated with CisIPN (OR = 1.04, p = 2 × 10−8) but displayed a slightly smaller effect size than at diagnosis (Table 2B). Age at diagnosis and evaluation were highly correlated (R2 = 0.8), and therefore, subsequent analyses were adjusted for age at diagnosis only. Smoking status (‘ever smoker’) (OR = 1.54; p= 0.004) as well as the self-reported number of years smoking (OR = 1.04 per year, p = 0.0002 [data not shown]) and excess drinking (OR = 1.83; p = 0.007) were significantly associated with CisIPN in univariate, age-adjusted analyses. CisIPN was also negatively correlated with physical activity level (OR = 0.72; p = 0.02; data not shown). Hypertension displayed a correlation (OR = 1.61, p = 0.03) with CisIPN, but not hypercholesterolemia (OR = 0.92, p = 0.72) or diabetes (OR = 1.27, p = 0.59), although the sample size of the latter was small (n=21). In the multivariate model, hypertension was marginally associated with CisIPN while age, smoking status, and excessive drinking displayed consistent effect sizes and statistical significance (Table 2C). BMI at evaluation was marginally associated with CisIPN (OR = 1.03, p = 0.051). Weight gain as measured by the absolute difference in BMI at evaluation and BMI at treatment initiation showed a stronger and more significant association with CisIPN (OR per Δkg/m2= 1.05; p = 0.004, adjusted for years since treatment, Figure 2A).
Figure 2. Relationship between weight gain and self-reported health, and severity of CisIPN.
Barplots of CisIPN vs A. Weight gain (measured as the BMI difference between evaluation and therapy) and B. Self-reported health. BMI difference positively correlated with CisIPN (OR = 1.05) after adjusting for age (p = 0.009) or number of years since treatment (p = 0.004). Self-reported health (poor-excellent) strongly negatively correlated with CisIPN (OR = 0.56, p = 2.6 × 10−9), but not with age.
CisIPN Association with Self-Reported Health
In a univariate analysis, we found a strong negative correlation of CisIPN with self-reported health (OR = 0.56; p = 2.6 × 10−9) (Figure 2B). A multivariate model with self-reported health as the dependent variable revealed a negative correlation with CisIPN (OR = 0.59, p < 0.0001) and with weight gain since treatment (OR = 0.91, p < 0.0001), and a positive correlation with physical activity (25) (OR = 1.92, p < 0.0001). Considering additional variables, self-reported health negatively associated with smoking (OR = 0.64, p= 0.003) but not hypertension (OR = 0.69, p = 0.096) or excess drinking (OR = 0.70, p = 0.12). CisIPN (OR = 0.64, p < 0.0001), weight gain (OR per Δkg/m2= 0.91, p < 0.0001), and exercise (OR = 1.97, p < 0.0001) remained the most significantly associated variables in a model including hypertension and smoking. We compared these findings in CisIPN to ototoxicity on the subset of TCS (n = 511) with quantitative audiometry included in a prior report (37), using a rank normalized geometric mean of audiogram thresholds in linear regression, adjusted for age. Although cisplatin-associated hearing loss and CisIPN were correlated (β = 0.14, p = 0.008), there was no relationship between ototoxicity and weight gain, physical activity, or self-reported health (data not shown, p > 0.05).
GWAS of CisIPN
The results of the GWAS using the 5,068,489 SNPs with age as a covariate are shown in Figure S5, and the top 100 SNPs in Table S3. No SNP met genome-wide significance (p < 5 × 10−8). None of the four SNPs in GSTP1 (18, 38), ABCG2 (39), XPC (39), and ERCC1 (15) previously identified in candidate gene studies of peripheral neuropathy following platinum-based regimens were replicated (Table S4). Out of the four SNPs meeting genome-wide significance in previous GWAS of CIPN due to other neurotoxic chemotherapies (Table S5), one SNP (rs7349683 in EPHA5) met replication significance (p = 0.05) with a concordant direction of effect. A SNP in ATP8A2 (rs1326116) that was of borderline significance (p=1.8 × 10−6) in a GWAS of docetaxel-induced neuropathy (36) showed the same direction of effect and met replication significance (p= 0.01).
GCTA and PrediXcan Analysis
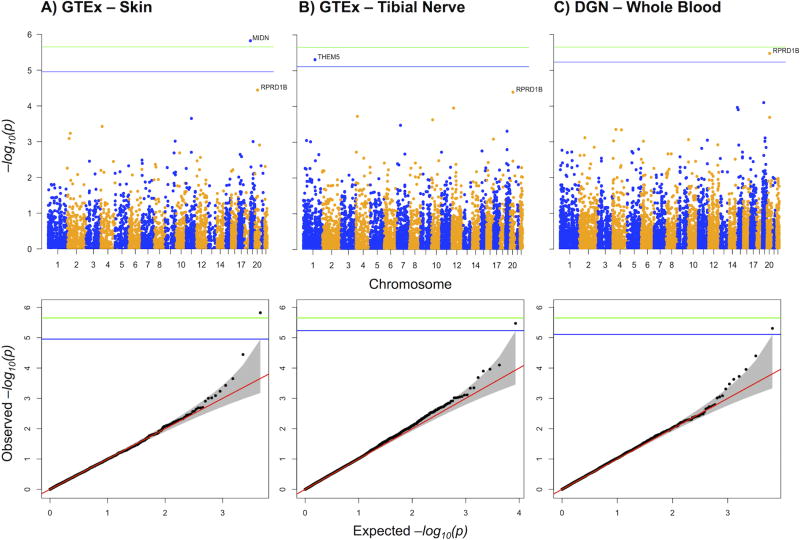
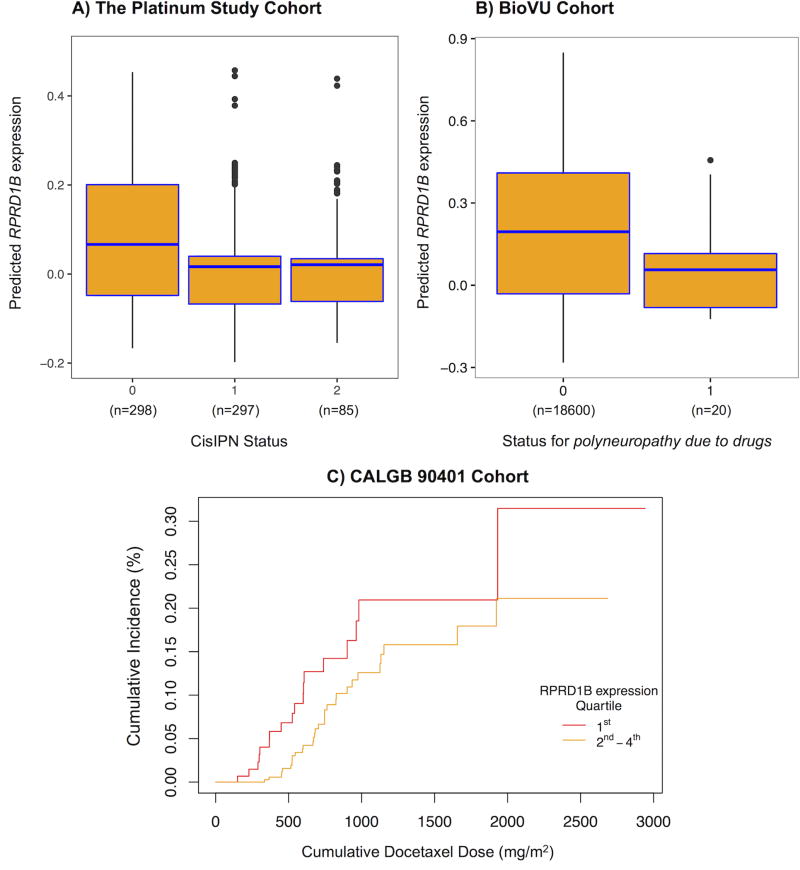
Using GCTA (30), we found CisIPN to be highly heritable, with common variants explaining up to 74% of phenotypic variability (h2 = 0.74, SE = 0.48; p = 0.03). This finding suggests a polygenic architecture of CisIPN. We used PrediXcan, a gene based test (31), to associate genetically regulated components of gene expression with CisIPN (Figure 3). This method increases power to explain the heritability by aggregating the effects of SNPs associated with gene expression. The most significant (p <0.001) results are listed in Table S6. One gene met experiment-wide significance (MIDN in skin, p = 1.4 × 10−6) and two genes met genome-wide significance (RPRD1B in whole blood, p = 3.4 × 10−6 and THEM5 in tibial nerve, p = 4.9 × 10−6). RPRD1B revealed a protective effect, i.e. lower RPRD1B gene expression is associated with CisIPN (Figure 4A). MIDN had the same direction of effect, while THEM5 displayed the opposite. Although RPRD1B was discovered using DGN, it was the second most significant hit in both GTEx skin and tibial nerve tissues.
Figure 3. Genome-wide PrediXcan analysis of CisIPN in TCS.
Manhattan and Q-Q plots of associations with PrediXcan expressions in 3 out of 4 candidate tissues tested, including A. GTEx skin – not exposed to sun, B. GTEx tibial nerve, C. DGN whole blood. Genes that passed the following criteria were included: prediction R2 > 0.01, prediction p < 0.05, predicted expression variance > 0.001. Blue lines indicate significance threshold within the tissue, −log10 (0.05/ntissue) where ntissue is the number of genes tested in the tissue. The green line indicates experiment-wide significance threshold, −log10 (0.05/ntotal) where ntotal is the total number of tests performed.
Figure 4. Association of lower RPRD1B expression and CisIPN.
Box and cumulative incidence plots of PrediXcan-predicted RPRD1B expression by neuropathy status reveal that lower expression correlates with neuropathy in A. The Platinum Study’s TCS cohort (p = 3.6 × 10−6), and B. Vanderbilt’s BioVU cohort (one tailed p = 0.021) and C. the CALGB 90401 docetaxel trial (one tailed p = 0.055). In plots A and B: The centers of the boxplots indicate means, the hinges indicate interquartile regions (IQR), the whiskers indicate points within 1.5 × IQR. Data beyond the end of the whiskers are outliers plotted as points. In plot B: Logistic regression was performed in BioVU to assess the association between PrediXcan expression and the code for polyneuropathy due to drugs – 1 for cases (n = 20), 0 for controls (n = 18,600). In plot C: Cox proportional hazards regression was performed to assess the association between PrediXcan expression and a dose-to-grade 3 or higher neuropathy event in the CALGB. An arbitrary cutoff is used to illustrate the association between the continuous gene expression variable and the dose-to-event phenotype: Individuals were ranked according to gene expression as determined by PrediXcan and the 1st quartile refers to the 25% with the lowest genetically determined RPRD1B expression and 2nd to 4th refers to the remaining individuals. Replication was assessed by Fisher’s combined p-value of both replications (BioVu and CALGB) and met significance (p = 0.0089).
In efforts to replicate, we interrogated a BioVu cohort of 18,620 genotyped individuals that were linked to EHR (34, 35) with the phenotype “polyneuropathy due to drugs” and found lower PrediXcan-predicted RPRD1B expression was associated with this phenotype (Figure 4B, one-tailed p = 0.021, 20 cases, 18600 controls). We further investigated the association in the Cancer and Leukemia Group B (CALGB) 90401 cohort, an independent dataset assessing docetaxel-induced peripheral neuropathy and found it nominally significant (Figure 4C, HR = 0.4, one-tailed p = 0.055). We assessed replication significance by computing the combined p-value using Fisher’s method and found replication to be significant (p = 0.0089).
DISCUSSION
Although cisplatin is one of the most commonly used cytotoxic drugs worldwide, to our knowledge, this study represents the first and largest investigation comprehensively evaluating clinical and genome-wide associations with long-term CisIPN in TCS, an ideal population for long-term pharmacogenetic studies of cisplatin toxicities. The validated EORTC-CIPN20 was applied in this large series to capture a broad spectrum of neurological damage from the patient’s perspective that might not be apparent on formal neurologic testing (40), and confounded by interpretation in physician-graded scales (41). We found sensory CisIPN symptoms to be common, and significantly correlated with weight gain since treatment, and inversely correlated with self-reported health and physical activity. In univariate models, we identified age, smoking status, excess drinking, and hypertension as significantly associated with CisIPN. As these variables are non-independent, we assessed their associations in a multivariate model and found some of the effect of hypertension to be explained by the other variables. The cross-sectional design prevents conclusions as to whether these associated variables are risk factors or consequences of CisIPN which would require a prospective design. We found significant heritability, indicating that genetic variants could explain a large proportion of variability in the phenotype. Aggregating SNP effects using PrediXcan (31), a gene-level test imputing genetically regulated expression from genotypes, revealed lower expression of MIDN in skin and RPRD1B in whole blood, as well as higher expression of THEM5 in tibial nerve as genome-wide significant. Using two independent datasets, we replicated the finding that lower RPRD1B predicted expression is associated with drug-induced neuropathy using predictors from whole blood, investigating a docetaxel-induced neuropathy phenotype and a broader drug-induced polyneuropathy phenotype in EHR.
PrediXcan
PrediXcan is a recently developed gene-based method that tests the genetically determined component of gene expression in a given tissue for association with a phenotype (31). The use of genetically determined gene expression to identify trait-associated genes is supported by evidence that SNPs associated with chemotherapeutic drug susceptibility (42) and complex traits (43) are more likely to be sites that associate with gene expression. Further, an observed association with the genetic component proposes a causal direction of effect since the phenotype does not alter the germline genetic profile. We theorized that tibial nerve, skin, and whole blood best represent neuronal tissue and the microenvironment surrounding nerves and their endings, a potentially important source of variability in CIPN. Since genetic regulation of gene expression can be non-tissue specific, the Bonferroni correction (correcting for the total number of tests across the tissues) is conservative. In three of the four tissues tested, lower expression of RPRD1B is associated with CisIPN at p < 10−4, illustrating substantial tissue-shared genetic regulation. Vanderbilt’s BioVU identified lower predicted gene expression of RPRD1B in the discovery tissue (DGN whole blood) as associated with drug-induced polyneuropathy. However, the analysis could not distinguish drug classes. Further suggestive association of neuropathy with lower expression of RPRD1B in the CALGB 90401 may indicate that the mechanism for the association is not drug-specific, as that trial investigated neuropathy induced by docetaxel, another neurotoxic chemotherapeutic agent with a distinct mechanism of cytotoxicity. Therefore, RPRD1B might have broad implications in CIPN.
RPRD1B
Twenty SNPs in four distinct linkage disequilibrium blocks (R2 < 0.5) 36–37.5 kilobases into chromosome 20 predict RPRD1B expression. RPRD1B codes for Kub5-Hera, a protein regulating the binding of RNA polymerase II to CCND1 gene (cyclin D1), and regulating the transcription of several genes involved in the cell cycle (44). Emerging data indicate that RPRD1B plays an important role in several DNA repair mechanisms, including double-strand breaks (DSB) repair through the association with core non-homologous end joining (NHEJ) proteins (45) and mismatch repair (46). Defects in RPRD1B expression or knockdown cause a deficiency in DNA repair mechanisms known to be critical in resolving cisplatin-induced lesions (47). Consistent with our data indicating low levels of RPRD1B being associated with CisIPN, knockdown of this gene in a breast cancer cell line, MDA-123 results in increased sensitivity to cisplatin (45). However, the suggestive association of RPRD1B with docetaxel-induced neuropathy, indicates that other mechanisms may be important. RPRD1B functions as a co-activator of the β-catenin-TCF complex to enhance the transcriptional activity of Wnt signaling (48). Wnt signaling is critical for initial neural cell-fate determination, patterning and synapse formation of sensory neurons of the dorsal root ganglia. This signaling pathway is also active in adult sensory neurons and modulates sensitivity to nociceptive stimuli (49, 50). Mechanistic insights into the function of RPRD1B are warranted to assess its role in the pathophysiology of neurotoxicity, possibly revealing novel targets.
Demographic and clinical factors and health behaviors
Our study identified age, smoking, excess alcohol use, and hypertension as associated with CisIPN. The relationship with age is consistent with several previous reports of taxane-induced neuropathy, including ECOG 5103 (4, 8, 36). In contrast, a number of studies of oxaliplatin-treated patients did not find an association with age (6, 7).
Tobacco and alcohol use
Few studies have addressed the role of tobacco use in CIPN. The authors of one study that found a correlation between smoking and CIPN postulated that long-term heavy smoking reduces peripheral blood flow, likely exacerbating paclitaxel-induced neuropathy (5). Conversely, a study of 730 TCS given platinum-based chemotherapy (9) and another of 62 colon cancer patients receiving oxaliplatin reported no association (7). Among 169 patients given oxaliplatin-based chemotherapy, alcohol consumption was associated with neuropathy (6). In contrast, two studies in breast cancer patients found no such relation of CIPN (10, 11). Alcohol-related peripheral neuropathy is a complication of alcoholism affecting up to half of these individuals (51). Excess alcohol use also plays a role in the development and progression of diabetic neuropathy (52).
Hypertension
In our study, the association between hypertension and CIPN was of borderline significance in multivariate analyses. Hypertension was not significantly associated with CIPN in the studies by Hershman et al. (4) and Glendenning et al. (9). One could postulate that microvascular complications associated with hypertension may contribute to CIPN, but our findings remain to be confirmed in other studies.
Lower physical activity and weight gain
We found CisIPN was associated with lower physical activity levels and greater weight gain since chemotherapy completion. While a longitudinal study design would be best for establishing causal inferences, it is possible that CIPN symptoms could deter from physical activity and thereby promote weight gain. Known downstream effects of weight gain include obesity, diabetes and other medical complications. Another long-term study in TCS has shown other co-morbidities are associated with CIPN, including neuroticism (53), further emphasizing the clinical impact of CIPN symptoms and their effects on patient health.
Strengths and limitations
A major strength of our study consists of its comprehensive assessment of a variety of clinical and genetic factors associated with CisIPN. Other strengths include the homogeneity of cisplatin-based chemotherapy without the administration of other neurotoxic drugs, detailed data on cisplatin dose, and the high patient participation rate (93%). Patient reported outcomes were carefully considered, and for the CisIPN phenotype, the validated EORTC QLQ-CIPN20 questionnaire (20) was applied. Although a number of studies of CIPN (reviewed above) considered the influence of one or a few covariates on CIPN, the present investigation considered all variables taken together. In addition, to our knowledge, no other study has considered the impact of CIPN on self-reported health or reported associations with weight gain and physical activity. PrediXcan (31), a state-of-the-art gene based method implicated RPRD1B, a gene known to play an important role in DNA repair mechanisms involved in cisplatin-induced damage, and shown to increase cisplatin sensitivity upon knockdown (45). Predictions are most helpful for variables known prior to treatment, yet as in previous studies (15, 18, 38, 39), baseline data before treatment were not collected. Any cross-sectional study design has potential inherent limitations, and does not allow us to infer causation of other evaluated risk factors for CisIPN, but rather to report important associations.
Another limitation in this study is the underpowered statistical analyses, as only 85 out of 680 reported severe CisIPN, although a larger proportion reported moderate symptoms (297 of 680). A larger sample size would allow better estimation of the effects of clinical factors and SNPs, and would enable making meaningful inferences about phenotype heritability which is limited by large standard errors here. Last, in replicating our study in a large set of EHR, we found lower predicted gene expression of RPRD1B to be associated with drug-induced polyneuropathy; however this phenotype was not able to distinguish specific drug classes.
Conclusions
In view of the significant associations we found between smoking, excess alcohol use, decreased physical activity, and weight gain with CisIPN, health care providers should promote a healthy lifestyle among patients with CisIPN. The borderline significant association we observed for hypertension and CisIPN remains to be confirmed in other studies, but nonetheless, in view of the highly significant relationship we previously reported between hypertension and hearing loss in TCS (37), health care providers should carefully monitor blood pressure.
There are currently no agents available to prevent or treat CIPN (12), which in our population and as reported by others (9) persists long-term for cisplatin. This observation underscores the importance of identifying at-risk patients prior to the administration of chemotherapy, which is currently not possible. Significant heritability indicates that a large proportion of variability in toxicity could be explained by genetic variants, but larger samples are needed to reduce the standard error before variants can be used in clinical prediction. Future research efforts should continue to elucidate the genetic underpinnings of CIPN, with our investigation now showing the importance of using functional genomic information such as expression data in genome-wide analyses to improve power and provide mechanistic explanations. Such approaches are more robust than reporting single polymorphisms with small-to-moderate effect sizes, which require larger datasets and are less clinically impactful. In addition, future research efforts should continue to provide the underpinnings for the eventual development of risk prediction models for CIPN that take into account not only genetic influences, but also clinical, demographic, and other important covariates. Our study provides one such example of a comprehensive approach. Future efforts will focus on independent replication in a similarly characterized TCS cohort, and evaluation of the predictive power of the variables associated in this study to potentially translate models of CisIPN prediction into the clinic.
Supplementary Material
Translational Relevance.
Cisplatin is one of the most commonly prescribed chemotherapeutics worldwide, but is associated with multiple toxicities that adversely impact survivors’ quality of life. One such toxicity, CisIPN often persists and can be debilitating. We identified strong effects of long-term CisIPN on self-reported health and showed associations of CisIPN with age, hypertension and modifiable risk factors such as smoking, excess drinking and weight gain since treatment. We found CisIPN to be highly heritable, indicating that genetic variants could explain phenotypic variability. We implicated lower (genetically determined) expression of RPRD1B and MIDN and higher expression of THEM5 as associated with CisIPN. RPRD1B was replicated in an independent cohort of patients who developed drug-induced polyneuropathy and was of borderline significance in a clinical trial of docetaxel-induced neuropathy. RPRD1B functions in DNA repair, transcription, and cell cycle control and may be a target for drug development. Our data indicate the importance of both clinical characteristics and genetic variation in the development of long-term CisIPN.
Acknowledgments
FUNDING: This work was supported by the National Institutes of Health, National Cancer Institute (1 R01 CA157823 to LBT). In addition, support was provided by Pharmacogenomics Research Network (PGRN)-RIKEN Global Alliance (GM115370), the Riken Center for Integrative Medical Science, Japan; the University of Chicago Cancer Research Foundation Women’s Board (to MED), the GTEx Project (R01 MH090937), and the Conte Center for Neuropsychiatric Genomics (P50 MH094267). The Genotype-Tissue Expression (GTEx) Project was supported by the Common Fund of the Office of the Director of the National Institutes of Health. Additional funds were provided by the NCI, NHGRI, NHLBI, NIDA, NIMH, and NINDS. Donors were enrolled at Biospecimen Source Sites funded by NCI\SAIC-Frederick, Inc. (SAIC-F) subcontracts to the National Disease Research Interchange (10XS170), Roswell Park Cancer Institute (10XS171), and Science Care, Inc. (X10S172). The Laboratory, Data Analysis, and Coordinating Center (LDACC) was funded through a contract (HHSN268201000029C) to The Broad Institute, Inc. Biorepository operations were funded through an SAIC-F subcontract to Van Andel Institute (10ST1035). Additional data repository and project management were provided by SAIC-F (HHSN261200800001E). The Brain Bank was supported by a supplement to University of Miami grants DA006227 & DA033684 and to contract N01MH000028. Statistical Methods development grants were made to the University of Geneva (MH090941 & MH101814), the University of Chicago (MH090951, MH090937, MH101820, MH101825), the University of North Carolina - Chapel Hill (MH090936 & MH101819), Harvard University (MH090948), Stanford University (MH101782), Washington University St Louis (MH101810), and the University of Pennsylvania (MH101822). The GTEx datasets used in this manuscript were obtained from dbGaP through accession number phs000424.v6.p1. The data set used for the CALGB 90401 - Genome-wide Pharmacogenomic Study of docetaxel and bevacizumab in Hormone Refractory Prostate Cancer described in this manuscript were obtained from dbGaP at (https://www.ncbi.nlm.nih.gov/gap) through dbGaP study accession (phs001002.v1.p1).
We are grateful to Drs. Chen Jiang, Kouros Owzar and Howard McLeod for intellectual contributions related to the CALGB90401 study. We are grateful to Ryan Cook and Eileen Johnson for technical assistance.
Abbreviations
- CIPN
chemotherapy-induced peripheral neuropathy
- CisIPN
cisplatin-induced peripheral neuropathy
- TCS
testicular cancer survivors
- EORTC QLQ-CIPN20
European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire Chemotherapy Induced Peripheral Neuropathy20
- QoL
quality of life
- GCT
germ cell tumor
- BMI
body mass index
- PCA
principal component analysis
- MET
Metabolic Equivalent of Task
- EUR
European
- IBD
identity by descent
- GTEx
Gene-Tissue Expression project
- DGN
Depression Genes and Networks
- OR
odds ratio
References
- 1.Argyriou AA, Bruna J, Marmiroli P, Cavaletti G. Chemotherapy-induced peripheral neurotoxicity (CIPN): an update. Crit Rev Oncol Hematol. 2012;82:51–77. doi: 10.1016/j.critrevonc.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 2.Brewer JR, Morrison G, Dolan ME, Fleming GF. Chemotherapy-induced peripheral neuropathy: Current status and progress. Gynecol Oncol. 2016;140:176–83. doi: 10.1016/j.ygyno.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Avan A, Postma TJ, Ceresa C, Avan A, Cavaletti G, Giovannetti E, et al. Platinum-induced neurotoxicity and preventive strategies: past, present, and future. Oncologist. 2015;20:411–32. doi: 10.1634/theoncologist.2014-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hershman DL, Till C, Wright JD, Awad D, Ramsey SD, Barlow WE, et al. Comorbidities and Risk of Chemotherapy-Induced Peripheral Neuropathy Among Participants 65 Years or Older in Southwest Oncology Group Clinical Trials. J Clin Oncol. 2016;34:3014–22. doi: 10.1200/JCO.2015.66.2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kawakami K, Tunoda T, Takiguchi T, Shibata K, Ohtani T, Kizu J, et al. Factors exacerbating peripheral neuropathy induced by paclitaxel plus carboplatin in non-small cell lung cancer. Oncol Res. 2012;20:179–85. doi: 10.3727/096504012x13522227232192. [DOI] [PubMed] [Google Scholar]
- 6.Vincenzi B, Frezza AM, Schiavon G, Spoto C, Silvestris N, Addeo R, et al. Identification of clinical predictive factors of oxaliplatin-induced chronic peripheral neuropathy in colorectal cancer patients treated with adjuvant Folfox IV. Support Care Cancer. 2013;21:1313–9. doi: 10.1007/s00520-012-1667-5. [DOI] [PubMed] [Google Scholar]
- 7.Uwah AN, Ackler J, Leighton JC, Jr, Pomerantz S, Tester W. The effect of diabetes on oxaliplatin-induced peripheral neuropathy. Clin Colorectal Cancer. 2012;11:275–9. doi: 10.1016/j.clcc.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 8.Schneider BP, Li L, Radovich M, Shen F, Miller KD, Flockhart DA, et al. Genome-Wide Association Studies for Taxane-Induced Peripheral Neuropathy in ECOG-5103 and ECOG-1199. Clin Cancer Res. 2015;21:5082–91. doi: 10.1158/1078-0432.CCR-15-0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glendenning JL, Barbachano Y, Norman AR, Dearnaley DP, Horwich A, Huddart RA. Long-term neurologic and peripheral vascular toxicity after chemotherapy treatment of testicular cancer. Cancer. 2010;116:2322–31. doi: 10.1002/cncr.24981. [DOI] [PubMed] [Google Scholar]
- 10.Pereira S, Fontes F, Sonin T, Dias T, Fragoso M, Castro-Lopes JM, et al. Chemotherapy-induced peripheral neuropathy after neoadjuvant or adjuvant treatment of breast cancer: a prospective cohort study. Support Care Cancer. 2016;24:1571–81. doi: 10.1007/s00520-015-2935-y. [DOI] [PubMed] [Google Scholar]
- 11.Eckhoff L, Feddersen S, Knoop AS, Ewertz M, Bergmann TK. Docetaxel-induced neuropathy: a pharmacogenetic case-control study of 150 women with early-stage breast cancer. Acta Oncol. 2015;54:530–7. doi: 10.3109/0284186X.2014.969846. [DOI] [PubMed] [Google Scholar]
- 12.Hershman DL, Lacchetti C, Dworkin RH, Lavoie Smith EM, Bleeker J, Cavaletti G, et al. Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2014;32:1941–67. doi: 10.1200/JCO.2013.54.0914. [DOI] [PubMed] [Google Scholar]
- 13.Einhorn LH. Curing metastatic testicular cancer. Proc Natl Acad Sci U S A. 2002;99:4592–5. doi: 10.1073/pnas.072067999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Travis LB, Fossa SD, Sesso HD, Frisina RD, Herrmann DN, Beard CJ, et al. Chemotherapy-induced peripheral neurotoxicity and ototoxicity: new paradigms for translational genomics. J Natl Cancer Inst. 2014;106 doi: 10.1093/jnci/dju044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim HS, Kim MK, Chung HH, Kim JW, Park NH, Song YS, et al. Genetic polymorphisms affecting clinical outcomes in epithelial ovarian cancer patients treated with taxanes and platinum compounds: a Korean population-based study. Gynecol Oncol. 2009;113:264–9. doi: 10.1016/j.ygyno.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 16.Khrunin AV, Moisseev A, Gorbunova V, Limborska S. Genetic polymorphisms and the efficacy and toxicity of cisplatin-based chemotherapy in ovarian cancer patients. Pharmacogenomics J. 2010;10:54–61. doi: 10.1038/tpj.2009.45. [DOI] [PubMed] [Google Scholar]
- 17.Khrunin AV, Khokhrin DV, Moisseev AA, Gorbunova VA, Limborska SA. Pharmacogenomic assessment of cisplatin-based chemotherapy outcomes in ovarian cancer. Pharmacogenomics. 2014;15:329–37. doi: 10.2217/pgs.13.237. [DOI] [PubMed] [Google Scholar]
- 18.Oldenburg J, Kraggerud SM, Brydoy M, Cvancarova M, Lothe RA, Fossa SD. Association between long-term neuro-toxicities in testicular cancer survivors and polymorphisms in glutathione-s-transferase-P1 and -M1, a retrospective cross sectional study. J Transl Med. 2007;5:70. doi: 10.1186/1479-5876-5-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cavaletti G, Cornblath DR, Merkies IS, Postma TJ, Rossi E, Frigeni B, et al. The chemotherapy-induced peripheral neuropathy outcome measures standardization study: from consensus to the first validity and reliability findings. Ann Oncol. 2013;24:454–62. doi: 10.1093/annonc/mds329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Postma TJ, Aaronson NK, Heimans JJ, Muller MJ, Hildebrand JG, Delattre JY, et al. The development of an EORTC quality of life questionnaire to assess chemotherapy-induced peripheral neuropathy: the QLQ-CIPN20. Eur J Cancer. 2005;41:1135–9. doi: 10.1016/j.ejca.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 21.Cronbach L. Coefficient alpha and the internal structure of tests. Psychometrika. 1951;16:297–334. [Google Scholar]
- 22.Leal AD, Qin R, Atherton PJ, Haluska P, Behrens RJ, Tiber CH, et al. North Central Cancer Treatment Group/Alliance trial N08CA-the use of glutathione for prevention of paclitaxel/carboplatin-induced peripheral neuropathy: a phase 3 randomized, double-blind, placebo-controlled study. Cancer. 2014;120:1890–7. doi: 10.1002/cncr.28654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gaist D, Jeppesen U, Andersen M, Garcia Rodriguez LA, Hallas J, Sindrup SH. Statins and risk of polyneuropathy: a case-control study. Neurology. 2002;58:1333–7. doi: 10.1212/wnl.58.9.1333. [DOI] [PubMed] [Google Scholar]
- 24.Taylor HL, Jacobs DR, Jr, Schucker B, Knudsen J, Leon AS, Debacker G. A questionnaire for the assessment of leisure time physical activities. J Chronic Dis. 1978;31:741–55. doi: 10.1016/0021-9681(78)90058-9. [DOI] [PubMed] [Google Scholar]
- 25.Chasan-Taber S, Rimm EB, Stampfer MJ, Spiegelman D, Colditz GA, Giovannucci E, et al. Reproducibility and validity of a self-administered physical activity questionnaire for male health professionals. Epidemiology. 1996;7:81–6. doi: 10.1097/00001648-199601000-00014. [DOI] [PubMed] [Google Scholar]
- 26.Wheeler HE, Gamazon ER, Frisina RD, Perez-Cervantes C, El Charif O, Mapes B, et al. Variants in WFS1 and other Mendelian deafness genes are associated with cisplatin-associated ototoxicity. Clin Cancer Research. 2016 doi: 10.1158/1078-0432.CCR-16-2809. Under Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patterson N, Price AL, Reich D. Population structure and eigenanalysis. PLoS Genet. 2006;2:e190. doi: 10.1371/journal.pgen.0020190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Howie B, Fuchsberger C, Stephens M, Marchini J, Abecasis GR. Fast and accurate genotype imputation in genome-wide association studies through pre-phasing. Nat Genet. 2012;44:955–9. doi: 10.1038/ng.2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aschard H, Vilhjalmsson BJ, Joshi AD, Price AL, Kraft P. Adjusting for heritable covariates can bias effect estimates in genome-wide association studies. Am J Hum Genet. 2015;96:329–39. doi: 10.1016/j.ajhg.2014.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang J, Lee SH, Goddard ME, Visscher PM. GCTA: a tool for genome-wide complex trait analysis. Am J Hum Genet. 2011;88:76–82. doi: 10.1016/j.ajhg.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gamazon ER, Wheeler HE, Shah KP, Mozaffari SV, Aquino-Michaels K, Carroll RJ, et al. A gene-based association method for mapping traits using reference transcriptome data. Nat Genet. 2015;47:1091–8. doi: 10.1038/ng.3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Battle A, Mostafavi S, Zhu X, Potash JB, Weissman MM, McCormick C, et al. Characterizing the genetic basis of transcriptome diversity through RNA-sequencing of 922 individuals. Genome Res. 2014;24:14–24. doi: 10.1101/gr.155192.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Consortium GT. The Genotype-Tissue Expression (GTEx) project. Nat Genet. 2013;45:580–5. doi: 10.1038/ng.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Denny JC, Ritchie MD, Basford MA, Pulley JM, Bastarache L, Brown-Gentry K, et al. PheWAS: demonstrating the feasibility of a phenome-wide scan to discover gene-disease associations. Bioinformatics. 2010;26:1205–10. doi: 10.1093/bioinformatics/btq126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Denny JC, Bastarache L, Ritchie MD, Carroll RJ, Zink R, Mosley JD, et al. Systematic comparison of phenome-wide association study of electronic medical record data and genome-wide association study data. Nat Biotechnol. 2013;31:1102–10. doi: 10.1038/nbt.2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hertz DL, Owzar K, Lessans S, Wing C, Jiang C, Kelly WK, et al. Pharmacogenetic Discovery in CALGB (Alliance) 90401 and Mechanistic Validation of a VAC14 Polymorphism that Increases Risk of Docetaxel-Induced Neuropathy. Clin Cancer Res. 2016;22:4890–900. doi: 10.1158/1078-0432.CCR-15-2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Frisina RD, Wheeler HE, Fossa SD, Kerns SL, Fung C, Sesso HD, et al. Comprehensive Audiometric Analysis of Hearing Impairment and Tinnitus After Cisplatin-Based Chemotherapy in Survivors of Adult-Onset Cancer. J Clin Oncol. 2016;34:2712–20. doi: 10.1200/JCO.2016.66.8822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goekkurt E, Al-Batran SE, Hartmann JT, Mogck U, Schuch G, Kramer M, et al. Pharmacogenetic analyses of a phase III trial in metastatic gastroesophageal adenocarcinoma with fluorouracil and leucovorin plus either oxaliplatin or cisplatin: a study of the arbeitsgemeinschaft internistische onkologie. J Clin Oncol. 2009;27:2863–73. doi: 10.1200/JCO.2008.19.1718. [DOI] [PubMed] [Google Scholar]
- 39.Lamba JK, Fridley BL, Ghosh TM, Yu Q, Mehta G, Gupta P. Genetic variation in platinating agent and taxane pathway genes as predictors of outcome and toxicity in advanced non-small-cell lung cancer. Pharmacogenomics. 2014;15:1565–74. doi: 10.2217/pgs.14.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hershman DL, Weimer LH, Wang A, Kranwinkel G, Brafman L, Fuentes D, et al. Association between patient reported outcomes and quantitative sensory tests for measuring long-term neurotoxicity in breast cancer survivors treated with adjuvant paclitaxel chemotherapy. Breast Cancer Res Treat. 2011;125:767–74. doi: 10.1007/s10549-010-1278-0. [DOI] [PubMed] [Google Scholar]
- 41.Wolf SL, Barton DL, Qin R, Wos EJ, Sloan JA, Liu H, et al. The relationship between numbness, tingling, and shooting/burning pain in patients with chemotherapy-induced peripheral neuropathy (CIPN) as measured by the EORTC QLQ-CIPN20 instrument, N06CA. Support Care Cancer. 2012;20:625–32. doi: 10.1007/s00520-011-1141-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gamazon ER, Huang RS, Cox NJ, Dolan ME. Chemotherapeutic drug susceptibility associated SNPs are enriched in expression quantitative trait loci. Proc Natl Acad Sci U S A. 2010;107:9287–92. doi: 10.1073/pnas.1001827107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nicolae DL, Gamazon E, Zhang W, Duan S, Dolan ME, Cox NJ. Trait-associated SNPs are more likely to be eQTLs: annotation to enhance discovery from GWAS. PLoS Genet. 2010;6:e1000888. doi: 10.1371/journal.pgen.1000888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ni Z, Olsen JB, Guo X, Zhong G, Ruan ED, Marcon E, et al. Control of the RNA polymerase II phosphorylation state in promoter regions by CTD interaction domain-containing proteins RPRD1A and RPRD1B. Transcription. 2011;2:237–42. doi: 10.4161/trns.2.5.17803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morales JC, Richard P, Rommel A, Fattah FJ, Motea EA, Patidar PL, et al. Kub5-Hera, the human Rtt103 homolog, plays dual functional roles in transcription termination and DNA repair. Nucleic Acids Res. 2014;42:4996–5006. doi: 10.1093/nar/gku160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Patidar PL, Motea EA, Fattah FJ, Zhou Y, Morales JC, Xie Y, et al. The Kub5-Hera/RPRD1B interactome: a novel role in preserving genetic stability by regulating DNA mismatch repair. Nucleic Acids Res. 2016;44:1718–31. doi: 10.1093/nar/gkv1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sawant A, Kothandapani A, Zhitkovich A, Sobol RW, Patrick SM. Role of mismatch repair proteins in the processing of cisplatin interstrand cross-links. DNA Repair (Amst) 2015;35:126–36. doi: 10.1016/j.dnarep.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang Y, Liu C, Duan X, Ren F, Li S, Jin Z, et al. CREPT/RPRD1B, a recently identified novel protein highly expressed in tumors, enhances the beta-catenin.TCF4 transcriptional activity in response to Wnt signaling. J Biol Chem. 2014;289:22589–99. doi: 10.1074/jbc.M114.560979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ille F, Sommer L. Wnt signaling: multiple functions in neural development. Cell Mol Life Sci. 2005;62:1100–8. doi: 10.1007/s00018-005-4552-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Simonetti M, Agarwal N, Stosser S, Bali KK, Karaulanov E, Kamble R, et al. Wnt-Fzd signaling sensitizes peripheral sensory neurons via distinct noncanonical pathways. Neuron. 2014;83:104–21. doi: 10.1016/j.neuron.2014.05.037. [DOI] [PubMed] [Google Scholar]
- 51.Mellion M, Gilchrist JM, de la Monte S. Alcohol-related peripheral neuropathy: nutritional, toxic, or both? Muscle Nerve. 2011;43:309–16. doi: 10.1002/mus.21946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ziegler D. Current concepts in the management of diabetic polyneuropathy. Curr Diabetes Rev. 2011;7:208–20. doi: 10.2174/157339911795843113. [DOI] [PubMed] [Google Scholar]
- 53.Grov EK, Fossa SD, Bremnes RM, Dahl O, Klepp O, Wist E, et al. The personality trait of neuroticism is strongly associated with long-term morbidity in testicular cancer survivors. Acta Oncol. 2009;48:842–9. doi: 10.1080/02841860902795232. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.