Abstract
Purpose
The TP53 tumor suppressor gene is mutated in >95% of high grade serous ovarian cancers. Detecting an autologous antibody response to TP53 might improve early detection.
Experimental design
An immunoassay was developed to measure TP53 autoantibody in sera from 378 cases of invasive epithelial ovarian cancer and in 944 age-matched healthy controls from the United States, Australia and the United Kingdom. Serial preclinical samples from cases and controls were also assayed from the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS).
Results
Using a cut-off of 78 U/mL to achieve a specificity of 97.4%, TP53 autoantibody were elevated in 30% of 50 cases from MD Anderson, 21.3% of 108 cases from the Australian Ovarian Cancer Study and 21% of 220 cases from the UKCTOCS. Among 164 cases with rising CA125 detected with the UKCTOCS risk of ovarian cancer algorithm (ROCA), 20.7% had elevated TP53 autoantibody. In cases missed by the ROCA, 16% of cases had elevated TP53 autoantibody. Of the 34 ovarian cancer cases detected with the ROCA, TP53 autoantibody titers were elevated 11.0 months prior to CA125. In the 9 cases missed by the ROCA, TP53 autoantibody was elevated 22.9 months before cancer diagnosis. Similar sensitivity was obtained using assays with specific mutant and wild-type TP53.
Conclusion
TP53 autoantibody levels provide a biomarker with clinically significant lead time over elevation of CA125 or an elevated ROCA value. Quantitative assessment of autoantibodies in combination with CA125 hold promise for earlier detection of invasive epithelial ovarian cancer.
Keywords: TP53 autoantibody, early detection, ovarian cancer, CA125, ROCA
Introduction
Ovarian cancer ranks fifth in cancer deaths among women in 2016 and is the leading cause of death from gynecologic malignancies (1). If detected while still confined to the ovaries (Stage I) or to the pelvis (Stage II), 5-year survival rates are 90% and 70% respectively. Once the disease has spread to the peritoneal cavity (Stage III) or beyond (Stage IV), the 5-year survival decreases sharply to less than 20% (2-4). Computer simulation suggests that earlier detection could reduce mortality by as much as 43% (5). Recent results from the United Kingdom Collaborative Trial of Ovarian Cancer Screening (UKCTOCS) document that screening for ovarian cancer using a multimodal (two-stage) strategy can lead to a stage shift and an estimated 20% reduction in mortality (6).
In the multimodal (two-stage) screening strategy utilized both in UKCTOCS (6) and in the Normal Risk Ovarian Screening Study (NROSS) (7), the level of the serum biomarker CA125 (MUC16) was determined annually in postmenopausal women at average (general population) risk of developing ovarian cancer. A Bayesian statistical method, the Risk of Ovarian Cancer Algorithm (ROCA), was used to calculate each woman's risk of having ovarian cancer based on age specific incidence, dates of blood draws and longitudinal CA125 levels after every new CA125 test. ROCA assesses whether CA125 has significantly increased above an individual's CA125 baseline levels (8,9). If risk has not changed, women return in one year for the next CA125 determination. If the ROCA is strongly elevated, transvaginal ultrasound (TVS) is obtained and, if this suggests malignancy, an operation is performed. If a modest increase in risk is observed, CA125 is repeated in 3 months. This two-stage strategy (ROCA + TVS), where two independent tests are both positive improves the limited specificity of CA125 using a single threshold (e.g. 30 or 35 U/mL) as well as the limited specificity of TVS and results in only 3-4 operations (40% positive predictive value) for each case of ovarian cancer detected (6,7). It also increases the sensitivity for earlier detection of ovarian cancer to around 85% (10,11). A recent report suggesting that this approach may reduce disease specific mortality (6), adds impetus to the search for new markers that can complement CA125, especially in women who are not detected using CA125 alone.
About 80% of ovarian cancers express significant amounts of CA125 at a tissue level (12). However, only 50-60% of early stage cancers have elevated CA125 serum levels at presentation (13). This suggests that a panel of biomarkers will almost certainly be required to detect all early stage ovarian cancers. During the last two decades, more than 110 different markers have been studied to increase sensitivity of CA125 for detecting ovarian cancer. HE4 and CA72.4 can detect a fraction of cases missed by CA125 (14,15), but in studies with preclinical samples to date, no biomarker has been consistently elevated prior to CA125 before diagnosis of the disease.
The sensitivity of tumor-derived biomarkers for early detection is limited by the expression of a biomarker within a cancer, the rate of biomarker-shedding and the volume of early stage cancers. Small cancers may not shed sufficient amounts of biomarker(s) to raise serum levels, but could evoke an immune response (16). Small volumes of cancer with mutated, overexpressed or aberrantly compartmentalized tumor associated proteins could evoke a humoral immune response with readily detectable titers of specific autoantibody (17). Therefore, detecting an autologous immune response to tumor associated antigens might improve lead time and detect cancers that are too small to be detected by antigen assays.
The TP53 tumor suppressor gene is frequently mutated in a variety of human cancers. TP53 is mutated in virtually all high grade serous ovarian cancers (18). Approximately two thirds of TP53 mutations are missense that stabilize TP53 protein and increase TP53 accumulation (19). Autoantibody reactive with wild-type TP53 have been reported in sera from approximately 15% of women with ovarian cancers, but most reports have studied only a limited number of cases at the time of symptomatic clinical diagnosis (20-36).
The goals of this study are (1) to develop and validate a new and more sensitive immunoassay for autoantibodies against wild-type TP53 protein, (2) to determine the fraction of ovarian cancer patients with early stage disease who have elevated levels of TP53 autoantibody, (3) to discover whether TP53 autoantibody can provide lead time over CA125 and detect cases that do not have elevated CA125, and (4) to test whether autoantibodies against patient specific mutations of TP53 provide more sensitive assays. Availability of serial pre-clinical specimens from UKCTOCS has permitted measurement of TP53 autoantibody in large numbers of women who went on to develop ovarian cancer. We have tested for the first time whether TP53 autoantibody are elevated prior to elevated risk (based on the ROCA). In earlier studies, autoantibody has been detected to wild-type TP53 protein. With access to serum specimens obtained at clinical diagnosis from the Australian Ovarian Cancer Study (AOCS) for which TP53 sequences are known, we have tested for the first time whether greater sensitivity could be attained by detecting autoantibody to the patient's specific mutant TP53 protein rather than the wild-type TP53 protein.
Materials and Methods
MagPlex bead-based indirect serological assay development
The MagPlex/xMAP technology (Luminex Corp., Austin, TX) was used to develop an indirect serological assay for detecting human TP53 autoantibody. Coupling recombinant wild-type TP53 antigen to microspheres, confirmation of effective coupling and calibration of the assay were accomplished using protocols modified from the Luminex xMAP Cookbook. In brief, 1×106 microspheres were coupled with recombinant human wild-type TP53 protein using an xMAP antibody coupling kit. Coupling was completed by a carbodiimide reaction linking the primary amino groups on TP53 protein and the carboxyl groups on the microsphere surface. The antigen conjugation procedure was performed according to the manufacturer's instructions. Biotin-conjugated anti-human TP53 antibody was used to validate coupling efficiency. A TP53 autoantibody calibrator (10 U/mL) was used to establish a standard curve for quantitating the titer of TP53 autoantibody in human serum samples. The standard operating protocol for performing the assay was established during assay development.
TP53 autoantibody immunoassay performance
A new xMAP bead-based immunoassay for detecting TP53 autoantibody in human serum samples was performed by an established standard operating protocol. In brief, a suspension of TP53 antigen-microspheres was prepared by diluting the coupled microsphere stocks (1×106 beads/mL) to a final concentration of 50 beads/μL in PBS buffer. Aliquots of microsphere suspension were placed in each well of a 96-well polystyrene microplate. Standard curves for quantitating TP53 autoantibody were plotted from triplicate assays of half-log dilution series of the TP53 autoantibody calibrator with concentrations ranging from 0.031 U/mL to 0.5 U/mL. Positive and negative controls for TP53 autoantibody were prepared in triplicate according to the manufacturer's instructions. Serum samples for assay (2 μL) were diluted with serum matrix to mimic the native analyte environment. Serum samples were added to 96-well polystyrene microplates and incubated with TP53-coupled microspheres for 1 hour at room temperature with gentle shaking. To wash the beads, each plate was clipped onto a magnetic plate separator for 1 min and the liquid was discarded by inverting the plate. Following washing with PBS buffer, microspheres were incubated with detection antibody biotinylated goat anti-human IgG. The plates were covered to protect them from light for 30 min at room temperature with gentle shaking. Plates were washed two times with PBS buffer and microspheres were incubated with fluorescence reporter Streptavidin-R-phycoerythrin (SAPE). The plates were covered to protect them from light and incubated for 30 min at room temperature with gentle shaking. After a final wash, the beads were re-suspended in PBS buffer and fluorescence measured on the MAGPIX system (Luminex Corp., Austin, TX) with at least 50 beads per well. The data were acquired and analyzed by xPONENT software version 4.2 (Luminex Corp., Austin, TX).
Patient serum sample sets (details available in the Supplementary Materials and Methods)
The MDACC-NROSS samples included 50 preoperative sera from patients with stage III-IV invasive epithelial ovarian/tubal/peritoneal cancer in the MD Anderson Cancer Center Gynecologic Cancer Tissue Bank and 216 sera from healthy controls who did not develop ovarian cancer on the NROSS coordinated by the MDACC Ovarian SPORE (7). The AOCS sample set included preoperative sera from 108 clinically diagnosed patients with invasive epithelial ovarian/tubal/peritoneal cancer, preoperative sera from 109 patients with benign ovarian tumors and age-matched sera from 464 healthy Australian controls. The UKCTOCS sample set was obtained from participants of this population-based, multi-center randomized controlled trial of ovarian cancer screening in the United Kingdom (10,37). The sample set included 164 screen-detected and 56 screen-negative women with 1,053 preclinical serial samples predating diagnosis of invasive epithelial ovarian/tubal/peritoneal cancer up to 5 years and 619 age-matched controls (3,069 serial samples) who did not develop any type of cancer during follow up. The combined sample sets are generally representative of the performance of multimodal screening in UKCTOCS, but have been enriched for screen negative cases. Ethical approval was obtained from these studies from the appropriate IRB/ethical committees. All participants had provided consent for use of samples in ethically approved secondary studies.
Statistical analysis
Setting a threshold for detecting TP53 autoantibody positivity in ovarian cancer samples
We chose our cutoff to achieve high specificity in multiple datasets as described below. We applied bootstrapping to construct 95% confidence intervals for the TP53 autoantibody cut-off values associated with 98% specificity in the MDACC, AOCS and UKCTOCS cohorts. A cut-off value of 78 U/mL, which falls within all three of the above 95% confidence intervals and provides an overall specificity of 97.4%, was chosen as the common threshold for detecting ovarian cancer.
Comparing early detection times for CA125 and TP53 autoantibody
Line plots and dot plots were drawn to compare differences in early detection times between TP53 autoantibody, CA125, and ROCA in the longitudinal samples of UKCTOCS cohort. Wilcoxon signed rank tests were performed to assess whether the additional early detection time provided by TP53 autoantibody improved upon CA125 or ROCA when both tests indicated a case. In addition, Fisher exact tests were used to assess the independence of TP53 autoantibody and ROCA in the three sample cohorts. Bootstrapping was applied to determine whether TP53 autoantibody could detect ROCA negative cases in the UCKTOCS cohort.
Comparing single and combination marker panels
Receiver Operating Characteristic (ROC) analysis adapted for early-detection-times was performed to compare performance between single and combination biomarker panels in the UKCTOCS cohort. The R package partial ROC (pROC) was used for ROC computations. Longitudinal sets of values were reduced to single values as follows. First, 35 U/mL and 78 U/mL were specified as cut-off values for CA125 and TP53 autoantibody, respectively. If a participant, either a case or a control, was never detected by one of the biomarkers, the maximum value of that marker was assigned to that participant. If a marker ever exceeded its cut-off, the value seen at the first time that the marker exceeded its cut-off was recorded as the summary value. If a case was detected by both CA125 and TP53 autoantibody assays, and the difference in detection times was larger than a certain threshold, such as 3, 6, or 12 months, a zero for the biomarker that detected the cancer later was recorded, as an indication that the latter marker had “failed” by not catching the tumor as early as it could have been caught. After this adjustment for early-detection-time, ROC analyses were performed both for single biomarkers and biomarker combinations. For the biomarker combination, a logistic regression model was built with the summary values as inputs and case/control status as targets, and the predicted logistic regression values were applied as the biomarker combination “score”. ROC curves were plotted both for the single biomarker and for the biomarker combination. Area under the curve (AUC), partial AUC (pAUC), sensitivity and accuracy were computed at 98% specificity. Next, bootstrapping was used to compute 95% confidence intervals for the AUC, as well as p values for the differences in AUC and pAUC values between single biomarkers and the biomarker combination.
Results
A sensitive and robust xMAP bead-based immunoassay has been developed to quantify TP53 autoantibody
We have developed a sensitive, rapid and high-throughput MagPlex microsphere-based immunoassay for quantitating anti-TP53-specific autoantibody in small volumes (2 μL) of serum. The procedure for immunoassay development, validation and assessment of performance in nested case-control studies has been outlined in Supplementary Figure 1. After establishing and optimizing of the assay method, a validation study was performed to demonstrate the accuracy, reproducibility and reliability of the TP53 autoantibody immunoassay. Serum samples with three different TP53 autoantibody titers (low, medium and high range of each sample) were measured in triplicate. The intra-assay coefficient of variation was 3.5-4.6% and the inter-assay coefficient of variation was 6.6% on three consecutive days. The linearity (R-square) was 0.9936 and the linear range was between 5.83 and 250 U/mL. The Limit of Detection (LoD) value was 5.83 U/mL and the maximum detectable TP53 autoantibody titer was 250 U/mL for this immunoassay.
A common cut-off value for the TP53 autoantibody immunoassay was chosen at 97.4% specificity across three clinical datasets
To determine a common cut-off value for the TP53 autoantibody immunoassay, we first used a bootstrap technique to construct 95% confidence intervals (CIs) at the 98th percentile of TP53 autoantibody values for healthy controls across three datasets (Supplementary Figure 2). For the MDACC-NROSS dataset, the 98th percentile for controls was 100.9 (95% CIs, 32.4-148.3) U/mL. For the AOCS dataset, the 98th percentile for healthy controls was 48.5 (95% CIs, 35.0-84.4) U/mL. For the UKCTOCS dataset, where serial samples were available from each healthy control, we chose the single highest value from each participant to calculate the 98th percentile of 94.6 (95% Cis, 65.6-128.0). A common cut-off value of 78 U/mL was chosen for TP53 autoantibody levels. This value was included within the 95% CIs for the 98th percentile across all datasets (Supplementary Figure 2). Using all controls, this provided a specificity of 97.4%. TP53 autoantibody levels above 78 U/mL were considered positive.
Elevated TP53 autoantibody levels were detected in 30% of pre-treatment sera from predominantly late stage (III/IV) ovarian cancer patients in the MDACC/NROSS dataset
We first analyzed the MDACC-NROSS set of specimens to validate the newly developed immunoassay for detecting TP53 autoantibody in human serum samples. Patient characteristics and TP53 autoantibody positivity by stage or histology are described in Supplementary Table 1. Results are summarized in Figure 1. With a 78 U/mL cut-off value for TP53 autoantibody, 15 of 50 sera (30%) from cancer cases were positive, whereas 5 of 216 sera (2.3%) of healthy controls exhibited TP53 autoantibody (Figure 1, A).
Figure 1.
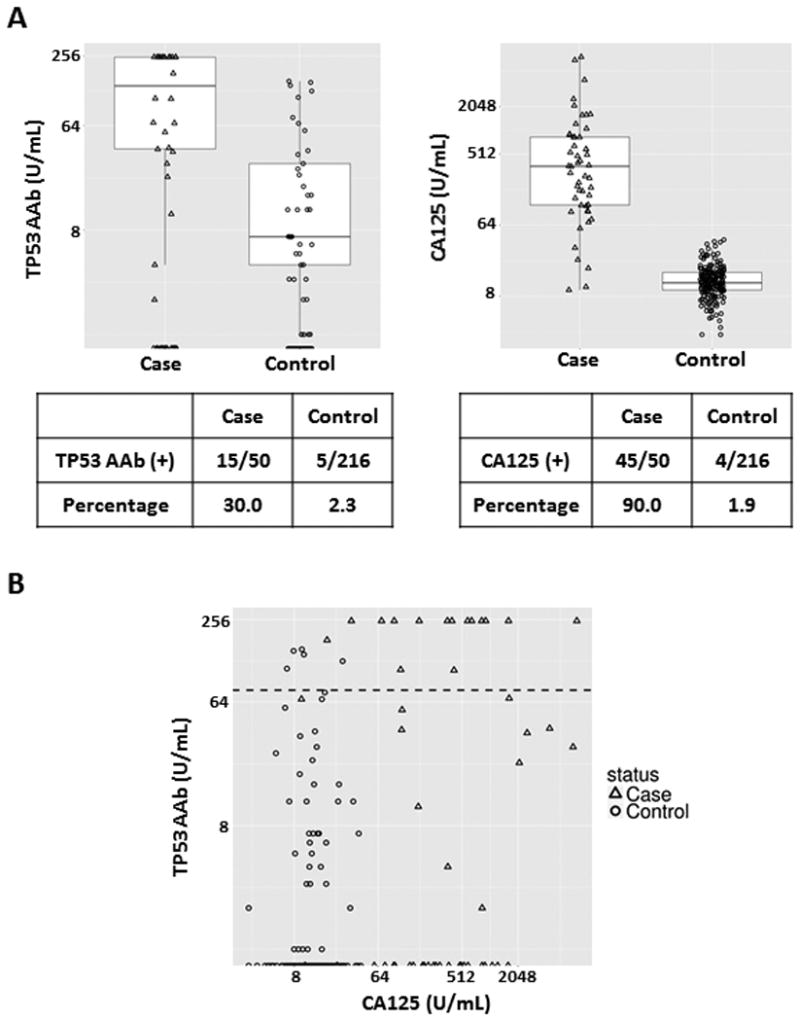
TP53 autoantibody titers and CA125 values in serum samples from the MDACC-NROSS study. A, Box plots for TP53 autoantibody and CA125. Each box exhibits maximum, upper quartile, median, lower quartile and minimum values. Triangles represent cases and circles represent controls. The lower tables display TP53 autoantibody and CA125 positive numbers and percentages in the case and control groups. B, Scatter plot of TP53 autoantibody titers and CA125 values. Triangles represent ovarian cancer cases and circles represent controls. The black dashed line represents the common cut-off value (78 U/mL) of TP53 autoantibody.
We also compared CA125 values (the cut-off = 35 U/mL) with TP53 autoantibody levels in each cancer case (Figure 1, A). CA125 was positive in 45 of 50 cases (90%) and 4 of 216 controls (1.9%). The box plot showed that both TP53 autoantibody and CA125 values were significantly elevated in cases when compared to controls. Remarkably, 12 of 15 (80%) ovarian cancer patients had extremely high titers of TP53 autoantibody (>250 U/mL) (Figure 1, B). Elevation of both CA125 and TP53 autoantibody was observed in 13 of 50 (26%). We further evaluated whether TP53 autoantibody correlated with ovarian cancer histology. All TP53 autoantibody positive cases were the serous or serous mixed with endometrioid histotype (Supplementary Table 1).
Elevated TP53 autoantibody levels were detected in 21% of pre-treatment sera from invasive epithelial ovarian/tubal/peritoneal cancer patients in the AOCS dataset
Next we analyzed sera from the AOCS study. Patient characteristics and TP53 autoantibody positivity by stage or histology are described in Supplementary Table 2. TP53 autoantibody values were significantly elevated in ovarian cancer cases when compared to patients with benign ovarian neoplasms or healthy controls. With a 78 U/mL threshold, sera from 23 of 108 invasive epithelial cancer cases (21.3%) had elevated TP53 autoantibody levels (Figure 2). By contrast, 3 of 109 sera (2.8%) from patients with benign ovarian neoplasms and 6 of 464 sera (1.3%) from healthy controls had elevated TP53 autoantibody levels (Figure 2). We further evaluated whether TP53 autoantibody correlated with ovarian cancer stage or histology. Elevated TP53 autoantibody levels were found in 2 of the 12 early stage (I/II) (16.7%) and 19 of 90 late stage (III/IV) (21.1%) patients. All TP53 autoantibody positive cases were the serous histotype (Supplementary Table 2).
Figure 2.
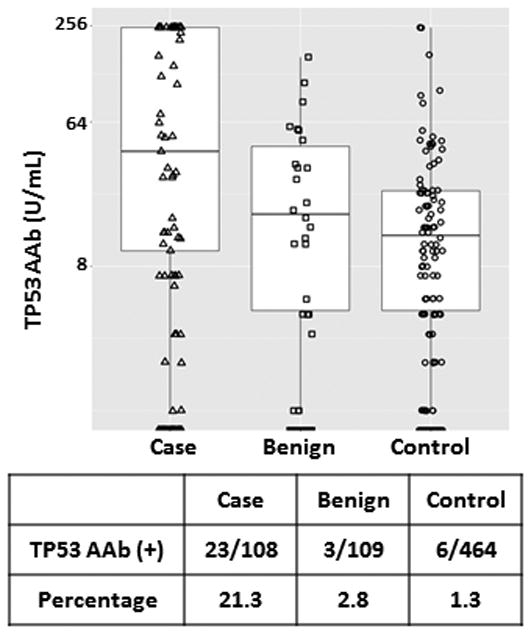
TP53 autoantibody titers in serum samples from the AOCS biobank data set. Box plots for TP53 autoantibody titers. Each box exhibits maximum, upper quartile, median, lower quartile and minimum values. Triangle symbols represent cases, square symbols represent benign ovarian neoplasms, and circle symbols represent controls. The lower table displays TP53 autoantibody positive (above common cut-off 78 U/mL) numbers and percentages in case, benign, and control groups.
Elevated TP53 autoantibody levels were detected in 21% of sera from invasive epithelial ovarian cancer patients in the longitudinal UKCTOCS preclinical dataset
Availability of sera from the UKCTOCS trial permitted detection of TP53 autoantibody in preclinical sera from a large number of women who subsequently developed invasive epithelial ovarian cancer. Patient characteristics and TP53 autoantibody positivity by stage or histology are shown in Table 1. Elevated TP53 autoantibody levels were found in sera from 43 of 220 patients (19.5%) with invasive epithelial ovarian cancer and in sera from 17 of 619 subjects (2.7%) without cancer detected during the study (Figure 3, A). The TP53 autoantibody levels were also compared to ROCA/CA125 values in each of the ovarian cancer cases. In the UKCTOCS dataset, ROCA could detect 164 of 220 (74.5%) cases and 34 cases were detected by both ROCA and anti-TP53 autoantibody assays. In box plots, both values were significantly elevated in cases compared to controls (Figure 3, A). In addition, scatter plot analysis suggested that CA125 is, as anticipated, a good biomarker to separate cases from controls (Figure 3, B). We further evaluated whether TP53 autoantibody correlated with ovarian cancer stage or histology. Elevated TP53 autoantibody levels were found in 11 of the 81 early stage (I/II) (13.6%) and 32 of 139 late stage (III/IV) (23.0%) patients (Supplementary Table 3). Some 76.7% of cancer cases with elevated TP53 autoantibody levels were of serous histotype, but 1-2 cases with endometrioid, clear cell and mucinous histotype were also associated with elevated TP53 autoantibody (Table 1).
Table 1.
Characteristics of patients with invasive epithelial ovarian/tubal/peritoneal cancer by ROCA/CA125 status, histotype and TP53 autoantibody positivity in the UKCTOCS study. *TP53 AAb (+): TP53 autoantibody screen positive; N/A: Not available
| ROCA/CA125 (+) N= 164 |
ROCA/CA125 (-) N = 56 |
||||
|---|---|---|---|---|---|
|
| |||||
| Histology | TP53 AAb (+)* No. (%) | Histology | TP53 AAb (+)* No. (%) | ||
|
|
|
||||
| Type | No. | Type | No. | ||
| Serous | 119 | 27 (22.7%) | Serous | 28 | 6(21.4%) |
| Mucinous | 1 | 0 (0.0%) | Mucinous | 1 | 1 (100.0%) |
| Endometrioid | 19 | 2 (10.5%) | Endometrioid | 6 | 0 (0.0%) |
| Clear cell | 7 | 0 (0.0%) | Clear cell | 7 | 1 (14.3%) |
| Poorly differentiated | 17 | 4 (23.5%) | Poorly differentiated | 11 | 0 (0.0%) |
| Mixed | 1 | 1 (100.0%) | N/A | 3 | 1 (33.3%) |
| Total | 164 | 34 (20.7%) | Total | 56 | 9(16.1%) |
Figure 3.
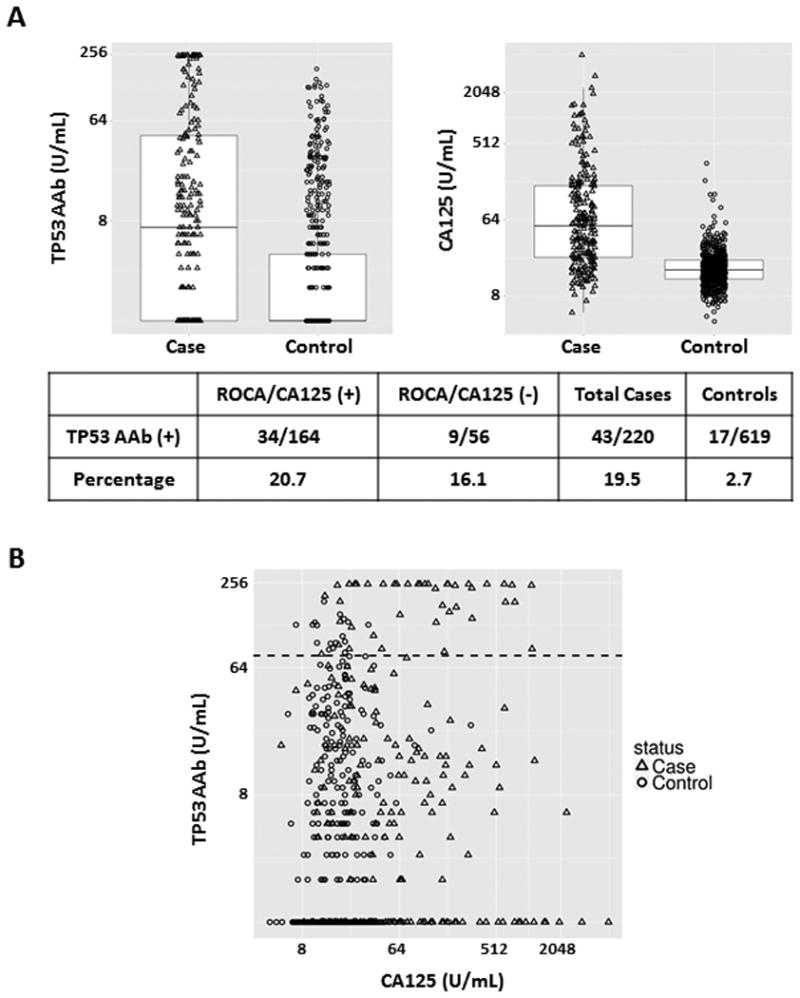
TP53 autoantibody titers and CA125 values in serum samples from the UKCTOCS screening trial. A, Box plots for TP53 autoantibody and CA125. Each box exhibits maximum, upper quartile, median, lower quartile and minimum values. Triangles represent cases and circles represent controls. The lower table displays TP53 autoantibody positive numbers and percentages in ROCA/CA125 (+) case, ROCA/CA125 (-) case and control groups. B, Scatter plots of TP53 autoantibody levels and CA125 values. Triangles represent ovarian cancer cases and circles represent controls. The black dashed line represents the common cut-off value (78 U/mL) for TP53 autoantibody.
Elevated TP53 autoantibody levels were found in sera from 16% of ovarian cancer patients whose disease was not detected with increasing CA125 and the ROCA
In the UKCTOCS dataset, among the 220 women who went on to develop ovarian cancer, 164 cases (74.5%) were detected with rising CA125 using the ROCA and TVS (screen positives) and 56 cases (25.5%) were not detected by ROCA (screen negative). Thirty-four of 164 (20.7%) screen positive cases and 9 of 56 (16.1%) screen negative cases had elevated levels of TP53 autoantibody (Figure 3, A). Thus, in retrospect, TP53 autoantibody could identify 16% of ovarian cancer cases missed by the ROCA.
TP53 autoantibody levels can increase prior to elevation of CA125
To determine whether TP53 autoantibody titers might increase prior to elevation of CA125 above the 35 U/mL threshold or to an indication of elevated risk determined by the ROCA, we measured TP53 autoantibody in serial serum specimens from ovarian cancer cases in the UKCTOCS dataset. Serial values for CA125 and TP53 autoantibody from two representative early-stage cancer cases are plotted in Supplementary Figure 3, A and B. TP53 autoantibody titers were markedly elevated (>250 U/mL) 8 and 7 months before cancer diagnosis, while CA125 levels remained within the normal range (<35 U/mL) during the entire period. In addition, the TP53 autoantibody titers were elevated in cancer cases at late stage (Supplementary Figure 3, C and D). While CA125 remained within normal range, autoantibody titers increased dramatically (190∼250 U/mL) at 4 years 4 months and 1 year 6 months prior to cancer diagnosis.
Among 43 cases with autoantibody elevation, TP53 autoantibody was detected 11.8 months (mean) prior to an elevation of the ROCA and 11.0 months prior to CA125 elevation (≥35 U/mL) (Figure 4, A). In 34 cases with both autoantibody elevation and ROCA positivity, TP53 autoantibody were detected 9.2 months prior to ROCA positivity, and 8.1 months prior to CA125 elevation (Figure 4, B). In 9 cases with autoantibody positivity that were not detected by the ROCA, TP53 autoantibody titers rose 22.9 months (mean) prior to cancer diagnosis (Figure 4, C). Thus TP53 autoantibody levels provide a promising biomarker with clinically significant lead time over either elevation of CA125 levels alone or elevation of risk using the ROCA algorithm to analyze CA125 levels.
Figure 4.
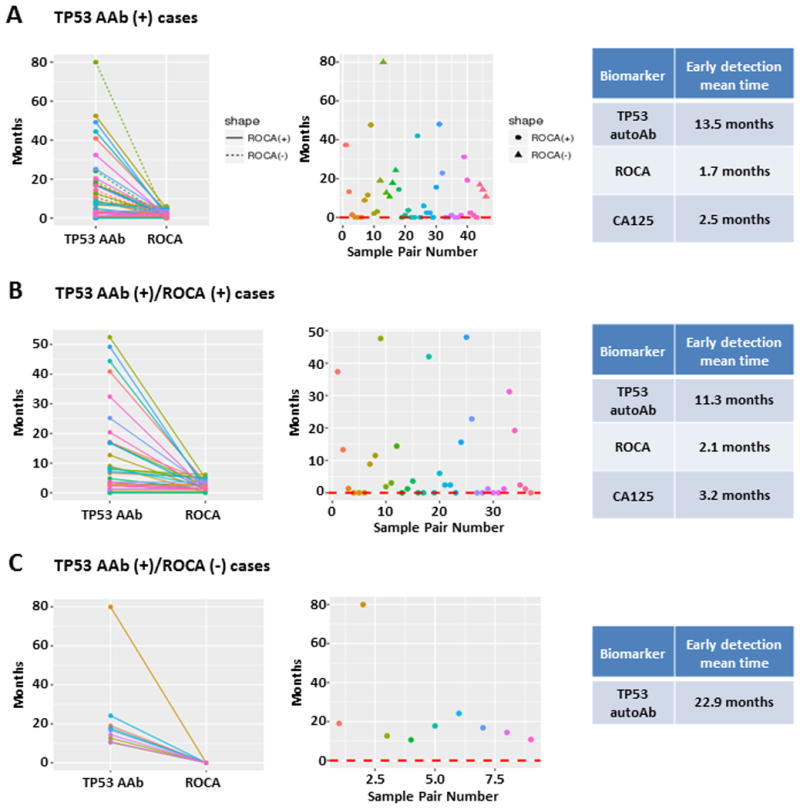
Longitudinal analysis of CA125 values and TP53 autoantibody titers in pre-diagnostic serial serum samples from ovarian cancer patients in the UKCTOCS study. A, Lead time prior to diagnosis for elevated TP53 autoantibody, ROCA and CA125 (>35 U/mL) in 43 cases with elevated TP53 autoantibody. In the left panel solid lines were detected with the ROCA and the dashed lines were not. In the middle panel, circles were detected with the ROCA and the triangles were not. B, Lead time prior to diagnosis for elevated TP53 autoantibody, ROCA and CA125 (>35 U/mL) in 34 cases with elevated TP53 autoantibody who were diagnosed with the ROCA. C, Lead time prior to diagnosis for elevated TP53 autoantibody, ROCA and CA125 (>35 U/mL) in 9 cases with elevated TP53 autoantibody who were not diagnosed with the ROCA. Sample pair number indicates the index of a given sample pairing within each set, ranging from 1 to 46 in A, 1 to 37 in B, and 1 to 9 in C. Colors match between the two plots in each panel, so the dramatic downward segment in C is seen to correspond to sample pair 2, with a difference in detection times of around 80 months.
A combination of TP53 autoantibody and CA125 enhanced detection of ovarian cancer in the UKCTOCS trial
To determine whether TP53 autoantibody levels enhanced the ability of CA125 to detect ovarian cancer, we generated ROC curves to discriminate cancer cases from controls for the UKCTOCS. The values first reaching the TP53 autoantibody cut-off (78 U/mL) and CA125 cut-off (35 U/mL) were chosen for ROC analysis. The AUC for TP53 autoantibody, CA125 and the combination of these two markers have been calculated. In the UKCTOCS dataset (Figure 5, A), the AUC of TP53 autoantibody was 0.699. Adding TP53 autoantibody values to the panel can significantly increase the AUC (CA125, 0.838 vs. TP53 AAb + CA125, 0.867, p= 0.007).
Figure 5.
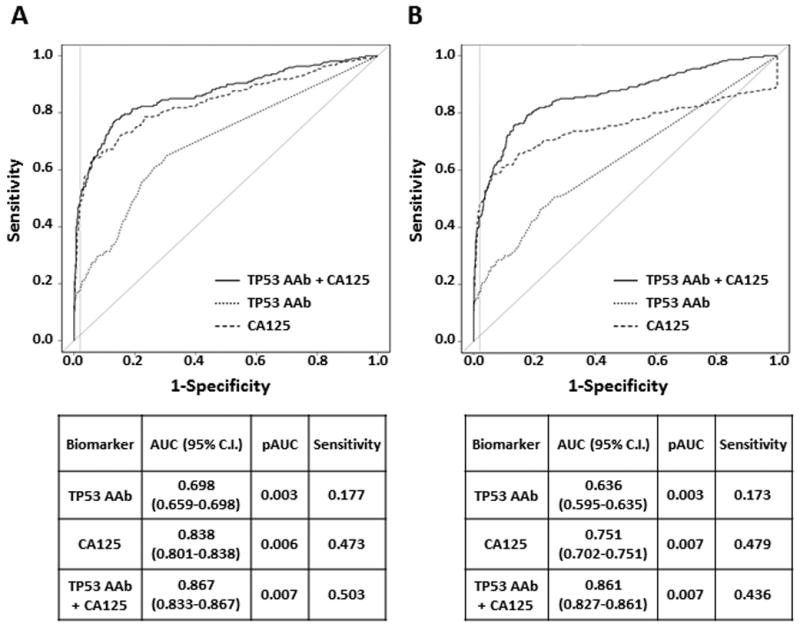
ROC curve analysis for TP53 autoantibody and CA125 biomarkers in the UKCTOCS study. A, The AUC, partial AUC (pAUC) and sensitivity for TP53 autoantibody, CA125 and the combination of these two markers are shown in the table (p value = 0.001 for AUC of CA125 vs CA125+TP53 AAb; p value = 0.097 for pAUC of CA125 vs CA125+TP53 AAb). Dotted line, ROC curve for TP53 autoantibody; dashed line, ROC curve for CA125; solid line, ROC curve for the combination of two biomarkers. The gray vertical lines are corresponding to 0.98 specificity. B, ROC curves for TP53 autoantibody and CA125 in the UKCTOCS study were calculated when 3 months was chosen as the cut-off to define the “significant early detection”. The AUC, partial AUC (pAUC) and sensitivity for TP53 autoantibody, CA125 and the combination of these two markers are shown in the table (p value = 0.000 for AUC of CA125 vs CA125+TP53 AAb; p value = 0.983 for pAUC of CA125 vs CA125+TP53 AAb).
Since the regular ROC analysis may not reflect the real situation that TP53 autoantibody has lead time prior to CA125, we performed new ROC analyses incorporating lead time. ROC analysis adapted for early-detection-times was performed to compare performance between single and combination marker panels in the UKCTOCS cohort. Partial ROC curves for TP53 autoantibody and CA125 in the UKCTOCS study were calculated when 3, 6 and 12 months were chosen as the cut-off to define the “significant early detection”. In ROC result with 3 months cut-off (Figure 5, B), the AUC of TP53 autoantibody was 0.636. Adding TP53 autoantibody to the panel can significantly increase the AUC (CA125, 0.751 vs TP53 AAb + CA125, 0.861, P= 0.000). Using 6 and 12 months cut-off did not obviously change AUCs of single and combination biomarker panels (Supplementary Figure 4, A and B). Thus, a combination of CA125 and TP53 autoantibody significantly enhance the ROC curve relative to either biomarker alone.
Similar sensitivity for detecting TP53 autoantibody was obtained with patient specific mutant TP53 and wild-type TP53
At a set cut-off value (78 U/mL) for TP53 autoantibody, our immunoassay using wild-type TP53 detected autoantibody in 21∼30% of invasive epithelial ovarian cancer patients in three data sets. As virtually all high grade serous cancers, which comprise approximately 60% of all ovarian cancers, have TP53 mutations at a variety of sites, we may have missed autoantibody against epitopes expressed by these mutant TP53 proteins, but not by wild-type TP53. Indeed, our previous screening result in the AOCS trial showed that cancer cases who harbored TP53 mutant gene have higher TP53 autoantibody immune responses compared with TP53 wild-type cancer cases (Supplementary Figure 5). This result is consistent with previous reports (34). Therefore, we asked whether assays incorporating the specific mutant TP53 proteins might detect antibody in a larger fraction of patients or at higher titer.
At present, 95 different TP53 mutations have been identified in high grade serous ovarian cancers (18). Since TP53 gene status has been sequenced for each case in the AOCS trial, we expressed the specific mutant proteins from the ovarian cancers of these patients using a mammalian protein expression system. We then established immunoassays and compared autoantibody titers to those obtained with wild-type TP53 with every mutant TP53 assay's reference level set at 98% specificity in AOCS controls. We selected 15 TP53 point mutation candidates for multiplex immunoassay development based on previous screening result in the AOCS trial (Supplementary Figure 6). Candidates were chosen where mutations at the same locus were associated with anti-TP53 antibody or no antibodies in different patients or mutations that were associated with intermediate TP53 wild-type autoantibody titers.
When sera from 29 patients in the AOCS study were assayed against 15 corresponding mutant TP53 proteins and the results compared to those from assays against wild-type TP53 protein, increased sensitivity was not observed (Supplementary Table 4). Patients with undetectable TP53 wild-type autoantibody had undetectable TP53 mutant autoantibody titers. Differences in titers, when present, did not exceed thresholds for either TP53 wild-type or TP53 mutant for positivity at 98% specificity. Subsequently, we analyzed sera from the AOCS trial against TP53 wild-type and 9 mutant protein candidates. When compared to titers against TP53 wild-type protein, most of the TP53 mutant proteins detected the same number or fewer cases (19.4-22.2%) with the thresholds set at 98% specificity (Supplementary Table 5). In general, they detected exactly the same cases as TP53 wild-type protein. Only TP53 R248W, R273H and R273L mutant proteins detected an additional 2-3 more cases (24.0-25.0%) compared with TP53 wild-type protein (Supplementary Table 5). Consequently, it appears that the predominant epitope(s) recognized by the autologous humoral response is likely to be expressed on the wild-type TP53 protein.
Discussion
In this study, we have developed a new, high-throughput xMAP bead-based immunoassay for quantitating TP53-specific autoantibody in ultra-low volumes of human serum. This assay was used to analyze sera from patients with invasive ovarian, tubal and primary peritoneal cancer from three large data sets to test whether TP53 autoantibody might serve as a biomarker for early detection of ovarian cancer. We set a cut-off value of TP53 autoantibody titers (78 U/mL) for each study, reflecting a specificity of 97.4%. Our assay detected elevated levels of TP53 autoantibody in 21% to 30% of cases at the time of clinical diagnosis, whereas previous studies had found TP53 autoantibody in around 15% of cases (20-36). In the UKCTOCS trial, TP53 autoantibody could detect 16% of ovarian cancer patients who were not detected using the CA125 based multimodal strategy. Remarkably, in cases in the UKCTOCS set in screen detected cases that were TP53 autoantibody positive, levels rose a mean of 11.0 months earlier than did CA125 (>35 U/mL) and 11.8 months earlier than the ROCA. In cases that were not screen detected using the CA125-based ROCA screening strategy, TP53 autoantibody titers rose an average of 22.9 months before cancer diagnosis. Among more than 110 ovarian cancer biomarkers identified to date, this is the first promising candidate that is able to detect invasive epithelial ovarian cancer at an earlier time than CA125 (38). Recently, Russell et. al. identified new biomarkers Protein Z, Fibronectin and C-reactive protein by the proteomic platform and those biomarkers showed a lead time on CA125 in small UKCTOCS sample sets (39,40).
The TP53 gene is frequently mutated in a variety of human cancers and plays a crucial role in their development, continued growth and genetic instability. Our immunoassay for detecting TP53 autoantibody may have potential applications for early detection of other types of cancer. Indeed, detection of TP53 autoantibody has also been reported in other cancers such as esophageal cancer (41,42), head and neck cancer (43,44), colorectal cancer (45-47), hepatocellular carcinoma (48,49), lung cancer (50-52) and breast cancer (53,54), etc. If TP53 autoantibody is used for screening in a general population, more prevalent cancers may also be detected. This could be an asset rather than a limitation, if a cost-effective diagnostic algorithm were developed to differentiate other types of cancer in addition to ovarian cancer in women with an elevated anti-TP53 autoantibody titer.
We also evaluated the potential benefit in combining CA125 with TP53 autoantibody for detecting ovarian cancer. ROC curve analysis for the UKCTOCS dataset showed that combination of CA125 with TP53 autoantibody can significantly increase the AUC (Figure 5, A). To further confirm those results, we performed new ROC analyses incorporating 3, 6 and 12 months lead time of CA125 and TP53 autoantibody (Figure 5, B and Supplementary Figure 4, A and B). Combination of CA125 with TP53 autoantibody can significantly increase the AUC (Figure 5, B; CA125, 0.751 v.s. TP53 AAb + CA125, 0.861, P= 0.000). Our data suggest that the addition of TP53 autoantibody may improve upon CA125 alone or the ROCA for identifying patients with early stage ovarian cancer. Further large-scale clinical studies will be required to evaluate the clinical efficacy of a combination of CA125 with TP53 autoantibody assays for detecting ovarian cancer.
We tested whether using assays that incorporated the mutant TP53 protein corresponding to that in the ovarian cancer from each patient might provide a more sensitive assay than wild-type TP53. Similar sensitivity was obtained with specific mutant and wild-type TP53. Given current technology, mutant proteins and peptides could be incorporated in assays for early detection, if there were an advantage to this approach. However, we did not find such an advantage in this study. Consequently, this was an important issue to resolve. This also suggests that the autoantibody might be directed against wild-typeTP53 and the amount of non-degraded TP53 in ovarian cancer cells might be an important factor in developing autoantibody.
To our knowledge, this study is the first to assay TP53 autoantibody in large numbers of preclinical sera. As only 20-25% of patients with invasive epithelial ovarian cancer have elevated levels of autoantibody against TP53, this biomarker may have only a limited, but potentially significant, impact on the ability to detect early stage disease. Our study does demonstrate that TP53 autoantibody levels: (1) can complement CA125 at the time of diagnosis; (2) provide substantial lead time over CA125 in a fraction of cases; and (3) could contribute one important member of a panel of biomarkers that would improve substantially upon the performance of CA125. Given the proof of concept that TP53 autoantibody can be elevated in advance of CA125, we are identifying other autoantibodies that would complement TP53 autoantibody levels and create a panel of tests that would detect CA125 negative cases and provide lead time in a greater fraction of cases.
Supplementary Material
TRANSLATIONAL RELEVANCE.
The biomarker CA125 can be detected in 80% of patients with advanced stage invasive epithelial ovarian cancer and in 50-60% of patients with early stage disease. Whereas small volumes of ovarian cancer may not shed sufficient CA125 to be detected, small amounts of tumor associated antigen(s) could stimulate production of detectable autoantibody. We have detected TP53 autoantibody in approximately 20% of ovarian cancers and in 16% that could not be detected with CA125 alone. We have observed elevated titers of TP53 autoantibody 11 months prior to elevation of CA125 and 23 months prior to diagnosis in patients not detected with CA125. Our observations provide proof of concept that autoantibodies can detect ovarian cancer in advance of shed tumor associated antigens such as CA125. Measurement of CA125 in combination with TP53 autoantibody and other autoantibodies could provide an effective strategy for earlier detection of patients with invasive epithelial ovarian cancer.
Acknowledgments
We are grateful to the UKCTOCS participants who donated their samples for use in secondary studies. The authors are solely responsible for the design of the study, the analysis and interpretation of the data, the writing of the article, and the decision to submit the article for publication. We also acknowledge the contribution of the AOCS nurses and research assistants and would like to thank all of the women who participated in the study.
Financial support: This work was supported by funds from the Early Detection Research Network (5 U01 CA200462-02) and the MD Anderson Ovarian SPORE (P50 CA83639), National Cancer Institute, Department of Health and Human Services; the Cancer Prevention Research Institute of Texas (RP160145); Golfer's Against Cancer, the Mossy Foundation, the Roberson Endowment, National Foundation for Cancer Research; UT MD Anderson Women's Moon Shot and generous donations from Stuart and Gaye Lynn Zarrow The UT MD Anderson Cancer Center Odyssey Program provided support to WLY, the Theodore N. Law Endowment for Scientific Achievement to WLY; the Clyde H. Wright Memorial Fund to WLY and Bristol-Myers Squibb Award in Clinical Research to WLY. SJS received additional support from the NCI Early Detection Research Network (CA152990). UKCTOCS was core funded by the Medical Research Council, Cancer Research UK, and the Department of Health with additional support from the Eve Appeal, Special Trustees of Bart's and the London, and Special Trustees of UCLH and supported by researchers at the National Institute for Health Research University College London Hospitals Biomedical Research Centre. AOCS was supported by the U.S. Army Medical Research and Materiel Command under DAMD17-01-1-0729, The Cancer Council Victoria, Queensland Cancer Fund, The Cancer Council New South Wales, The Cancer Council South Australia, The Cancer Foundation of Western Australia, The Cancer Council Tasmania and the National Health and Medical Research Council of Australia (NHMRC; ID400413, ID400281). The AOCS gratefully acknowledges additional support from S. Boldeman, the Agar family, the Peter MacCallum Cancer Centre Foundation, Ovarian Cancer Australia and Ovarian Cancer Action (UK).
Footnotes
Disclosure of potential conflicts of interest: Dr. Wei-Wu He is the former chief executive officer of OriGene Technologies, Inc. Dr. Robert Bast receives royalties from Fujirebio Diagnostics Inc. for discovery of CA125. Other authors have no conflicts of interest.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Espey DK, Wu XC, Swan J, Wiggins C, Jim MA, Ward E, et al. Annual report to the nation on the status of cancer, 1975-2004, featuring cancer in American Indians and Alaska Natives. Cancer. 2007;110(10):2119–52. doi: 10.1002/cncr.23044. [DOI] [PubMed] [Google Scholar]
- 3.Aletti GD, Dowdy SC, Podratz KC, Cliby WA. Relationship among surgical complexity, short-term morbidity, and overall survival in primary surgery for advanced ovarian cancer. Am J Obstet Gynecol. 2007;197(6):676 e1-7. doi: 10.1016/j.ajog.2007.10.495. [DOI] [PubMed] [Google Scholar]
- 4.Ashworth A, Balkwill F, Bast RC, Berek JS, Kaye A, Boyd JA, et al. Opportunities and challenges in ovarian cancer research, a perspective from the 11th Ovarian cancer action/HHMT Forum, Lake Como, March 2007. Gynecol Oncol. 2008;108(3):652–7. doi: 10.1016/j.ygyno.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 5.Havrilesky LJ, Sanders GD, Kulasingam S, Myers ER. Reducing ovarian cancer mortality through screening: Is it possible, and can we afford it? Gynecol Oncol. 2008;111(2):179–87. doi: 10.1016/j.ygyno.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 6.Jacobs IJ, Menon U, Ryan A, Gentry-Maharaj A, Burnell M, Kalsi JK, et al. Ovarian cancer screening and mortality in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): a randomised controlled trial. Lancet. 2015 doi: 10.1016/S0140-6736(15)01224-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu KH, Skates S, Hernandez MA, Bedi D, Bevers T, Leeds L, et al. A 2-stage ovarian cancer screening strategy using the Risk of Ovarian Cancer Algorithm (ROCA) identifies early-stage incident cancers and demonstrates high positive predictive value. Cancer. 2013;119(19):3454–61. doi: 10.1002/cncr.28183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Skates SJ. Ovarian cancer screening: development of the risk of ovarian cancer algorithm (ROCA) and ROCA screening trials. Int J Gynecol Cancer. 2012;22(Suppl 1):S24–6. doi: 10.1097/IGC.0b013e318256488a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Skates SJ, Pauler DK, Jacobs I. Screening Based on the Risk of Cancer Calculation from Bayesian Hierarchical Changepoint and Mixture Models of Longitudinal Markers. Journal of the American Statistical Association. 2001;96(454):429–39. [Google Scholar]
- 10.Menon U, Gentry-Maharaj A, Hallett R, Ryan A, Burnell M, Sharma A, et al. Sensitivity and specificity of multimodal and ultrasound screening for ovarian cancer, and stage distribution of detected cancers: results of the prevalence screen of the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS) Lancet Oncol. 2009;10(4):327–40. doi: 10.1016/S1470-2045(09)70026-9. [DOI] [PubMed] [Google Scholar]
- 11.Menon U, Ryan A, Kalsi J, Gentry-Maharaj A, Dawnay A, Habib M, et al. Risk Algorithm Using Serial Biomarker Measurements Doubles the Number of Screen-Detected Cancers Compared With a Single-Threshold Rule in the United Kingdom Collaborative Trial of Ovarian Cancer Screening. J Clin Oncol. 2015;33(18):2062–71. doi: 10.1200/JCO.2014.59.4945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bast RC, Jr, Hennessy B, Mills GB. The biology of ovarian cancer: new opportunities for translation. Nat Rev Cancer. 2009;9(6):415–28. doi: 10.1038/nrc2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Das PM, Bast RC., Jr Early detection of ovarian cancer. Biomark Med. 2008;2(3):291–303. doi: 10.2217/17520363.2.3.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simmons AR, Gentry-Maharaj A, Skates S, Baggerly K, Fourkala O, Ryan A, et al. Validation of a multi-marker panel for early detection of ovarian cancer. J Clin Oncol. 2016;34 (Suppl; abstr 5570) [Google Scholar]
- 15.Terry KL, Schock H, Fortner RT, Husing A, Fichorova RN, Yamamoto HS, et al. A Prospective Evaluation of Early Detection Biomarkers for Ovarian Cancer in the European EPIC Cohort. Clin Cancer Res. 2016;22(18):4664–75. doi: 10.1158/1078-0432.CCR-16-0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dudas SP, Chatterjee M, Tainsky MA. Usage of cancer associated autoantibodies in the detection of disease. Cancer Biomark. 2010;6(5-6):257–70. doi: 10.3233/CBM-2009-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chatterjee M, Tainsky MA. Autoantibodies as biomarkers for ovarian cancer. Cancer Biomark. 2010;8(4-5):187–201. doi: 10.3233/CBM-2011-0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahmed AA, Etemadmoghadam D, Temple J, Lynch AG, Riad M, Sharma R, et al. Driver mutations in TP53 are ubiquitous in high grade serous carcinoma of the ovary. J Pathol. 2010;221(1):49–56. doi: 10.1002/path.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blagosklonny MV. p53 from complexity to simplicity: mutant p53 stabilization, gain-of-function, and dominant-negative effect. FASEB J. 2000;14(13):1901–7. doi: 10.1096/fj.99-1078rev. [DOI] [PubMed] [Google Scholar]
- 20.Angelopoulou K, Rosen B, Stratis M, Yu H, Solomou M, Diamandis EP. Circulating antibodies against p53 protein in patients with ovarian carcinoma. Correlation with clinicopathologic features and survival Cancer. 1996;78(10):2146–52. [PubMed] [Google Scholar]
- 21.Gadducci A, Ferdeghini M, Buttitta F, Fanucchi A, Annicchiarico C, Prontera C, et al. Preoperative serum antibodies against the p53 protein in patients with ovarian and endometrial cancer. Anticancer Res. 1996;16(6B):3519–23. [PubMed] [Google Scholar]
- 22.Marx D, Uebel T, Schauer A, Kuhn W, Meden H. Association of serum autoantibodies to tumor-suppressor gene p53 in patients with ovarian cancer according to status of the disease. Oncol Rep. 1997;4(6):1157–60. doi: 10.3892/or.4.6.1157. [DOI] [PubMed] [Google Scholar]
- 23.Gadducci A, Ferdeghini M, Buttitta F, Cosio S, Fanucchi A, Annicchiarico C, et al. Serum anti-p53 antibodies in the follow-up of patients with advanced ovarian carcinoma. Anticancer Res. 1998;18(5B):3763–5. [PubMed] [Google Scholar]
- 24.Gadducci A, Ferdeghini M, Buttitta F, Cosio S, Fanucchi A, Annicchiarico C, et al. Assessment of the prognostic relevance of serum anti-p53 antibodies in epithelial ovarian cancer. Gynecol Oncol. 1999;72(1):76–81. doi: 10.1006/gyno.1998.5101. [DOI] [PubMed] [Google Scholar]
- 25.Mayerhofer K, Tempfer C, Kucera E, Hefler L, Zeisler H, Kainz C, et al. Humoral p53 antibody response is a prognostic parameter in ovarian cancer. Anticancer Res. 1999;19(1B):875–8. [PubMed] [Google Scholar]
- 26.Vogl FD, Stickeler E, Weyermann M, Kohler T, Grill HJ, Negri G, et al. p53 autoantibodies in patients with primary ovarian cancer are associated with higher age, advanced stage and a higher proportion of p53-positive tumor cells. Oncology. 1999;57(4):324–9. doi: 10.1159/000012069. [DOI] [PubMed] [Google Scholar]
- 27.Abendstein B, Marth C, Muller-Holzner E, Widschwendter M, Daxenbichler G, Zeimet AG. Clinical significance of serum and ascitic p53 autoantibodies in epithelial ovarian carcinoma. Cancer. 2000;88(6):1432–7. doi: 10.1002/(sici)1097-0142(20000315)88:6<1432::aid-cncr22>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 28.Vogl FD, Frey M, Kreienberg R, Runnebaum IB. Autoimmunity against p53 predicts invasive cancer with poor survival in patients with an ovarian mass. Br J Cancer. 2000;83(10):1338–43. doi: 10.1054/bjoc.2000.1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marx D, Frey M, Zentgraf H, Adelssen G, Schauer A, Kuhn W, et al. Detection of serum autoantibodies to tumor suppressor gene p53 with a new enzyme-linked immunosorbent assay in patients with ovarian cancer. Cancer Detect Prev. 2001;25(2):117–22. [PubMed] [Google Scholar]
- 30.Numa F, Umayahara K, Suehiro Y, Hirakawa H, Nawata S, Suminami Y, et al. Serum anti-p53 antibodies in uterine and ovarian cancer: association with dna sequence copy number abnormalities. Tumour Biol. 2001;22(3):162–8. doi: 10.1159/000050611. [DOI] [PubMed] [Google Scholar]
- 31.Hogdall EV, Hogdall CK, Blaakaer J, Heegaard NH, Glud E, Christensen L, et al. P53 autoantibodies in sera from Danish ovarian cancer patients and their correlation with clinical data and prognosis. APMIS. 2002;110(7-8):545–53. doi: 10.1034/j.1600-0463.2002.11007805.x. [DOI] [PubMed] [Google Scholar]
- 32.Goodell V, Salazar LG, Urban N, Drescher CW, Gray H, Swensen RE, et al. Antibody immunity to the p53 oncogenic protein is a prognostic indicator in ovarian cancer. J Clin Oncol. 2006;24(5):762–8. doi: 10.1200/JCO.2005.03.2813. [DOI] [PubMed] [Google Scholar]
- 33.Leffers N, Lambeck AJ, de Graeff P, Bijlsma AY, Daemen T, van der Zee AG, et al. Survival of ovarian cancer patients overexpressing the tumour antigen p53 is diminished in case of MHC class I down-regulation. Gynecol Oncol. 2008;110(3):365–73. doi: 10.1016/j.ygyno.2008.04.043. [DOI] [PubMed] [Google Scholar]
- 34.Tsai-Turton M, Santillan A, Lu D, Bristow RE, Chan KC, Shih Ie M, et al. p53 autoantibodies, cytokine levels and ovarian carcinogenesis. Gynecol Oncol. 2009;114(1):12–7. doi: 10.1016/j.ygyno.2009.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anderson KS, Wong J, Vitonis A, Crum CP, Sluss PM, Labaer J, et al. p53 autoantibodies as potential detection and prognostic biomarkers in serous ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2010;19(3):859–68. doi: 10.1158/1055-9965.EPI-09-0880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hafner N, Nicolaus K, Weiss S, Frey M, Diebolder H, Rengsberger M, et al. p53-autoantibody may be more sensitive than CA-125 in monitoring microscopic and macroscopic residual disease after primary therapy for epithelial ovarian cancer. J Cancer Res Clin Oncol. 2013;139(7):1207–10. doi: 10.1007/s00432-013-1432-2. [DOI] [PubMed] [Google Scholar]
- 37.Menon U, Gentry-Maharaj A, Ryan A, Sharma A, Burnell M, Hallett R, et al. Recruitment to multicentre trials--lessons from UKCTOCS: descriptive study. BMJ. 2008;337:a2079. doi: 10.1136/bmj.a2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cramer DW, Bast RC, Jr, Berg CD, Diamandis EP, Godwin AK, Hartge P, et al. Ovarian cancer biomarker performance in prostate, lung, colorectal, and ovarian cancer screening trial specimens. Cancer Prev Res (Phila) 2011;4(3):365–74. doi: 10.1158/1940-6207.CAPR-10-0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Russell MR, Walker MJ, Williamson AJ, Gentry-Maharaj A, Ryan A, Kalsi J, et al. Protein Z: A putative novel biomarker for early detection of ovarian cancer. Int J Cancer. 2016;138(12):2984–92. doi: 10.1002/ijc.30020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Russell MR, D'Amato A, Graham C, Crosbie EJ, Gentry-Maharaj A, Ryan A, et al. Novel risk models for early detection and screening of ovarian cancer. Oncotarget. 2016 doi: 10.18632/oncotarget.13648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cai HY, Wang XH, Tian Y, Gao LY, Zhang LJ, Zhang ZY. Changes of serum p53 antibodies and clinical significance of radiotherapy for esophageal squamous cell carcinoma. World J Gastroenterol. 2008;14(25):4082–6. doi: 10.3748/wjg.14.4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kozlowski M, Kovalchuk O, Niklinski J, Chyczewski L, Staroslawska E, Ciechanski A, et al. Circulating anti-p53 antibodies in esophageal cancer patients. Folia Histochem Cytobiol. 2001;39(Suppl 2):173–4. [PubMed] [Google Scholar]
- 43.Shimada H, Ochiai T, Nomura F. Titration of serum p53 antibodies in 1,085 patients with various types of malignant tumors: a multiinstitutional analysis by the Japan p53 Antibody Research Group. Cancer. 2003;97(3):682–9. doi: 10.1002/cncr.11092. [DOI] [PubMed] [Google Scholar]
- 44.Chow V, Yuen AP, Lam KY, Ho WK, Wei WI. Prognostic significance of serum p53 protein and p53 antibody in patients with surgical treatment for head and neck squamous cell carcinoma. Head Neck. 2001;23(4):286–91. doi: 10.1002/hed.1032. [DOI] [PubMed] [Google Scholar]
- 45.Tang R, Ko MC, Wang JY, Changchien CR, Chen HH, Chen JS, et al. Humoral response to p53 in human colorectal tumors: a prospective study of 1,209 patients. Int J Cancer. 2001;94(6):859–63. doi: 10.1002/ijc.1541. [DOI] [PubMed] [Google Scholar]
- 46.Pedersen JW, Gentry-Maharaj A, Fourkala EO, Dawnay A, Burnell M, Zaikin A, et al. Early detection of cancer in the general population: a blinded case-control study of p53 autoantibodies in colorectal cancer. Br J Cancer. 2013;108(1):107–14. doi: 10.1038/bjc.2012.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lechpammer M, Lukac J, Lechpammer S, Kovacevic D, Loda M, Kusic Z. Humoral immune response to p53 correlates with clinical course in colorectal cancer patients during adjuvant chemotherapy. Int J Colorectal Dis. 2004;19(2):114–20. doi: 10.1007/s00384-003-0553-5. [DOI] [PubMed] [Google Scholar]
- 48.Muller M, Meyer M, Schilling T, Ulsperger E, Lehnert T, Zentgraf H, et al. Testing for anti-p53 antibodies increases the diagnostic sensitivity of conventional tumor markers. Int J Oncol. 2006;29(4):973–80. [PubMed] [Google Scholar]
- 49.Sitruk V, Vaysse J, Chevret S, Ganne-Carrie N, Christidis C, Trinchet J, et al. [Prevalence and prognostic value of serum anti-p53 antibodies in hepatocellular carcinoma. A study of 159 patients] Gastroenterol Clin Biol. 2000;24(12):1159–63. [PubMed] [Google Scholar]
- 50.Zalcman G, Tredaniel J, Schlichtholz B, Urban T, Milleron B, Lubin R, et al. Prognostic significance of serum p53 antibodies in patients with limited-stage small cell lung cancer. Int J Cancer. 2000;89(1):81–6. doi: 10.1002/(sici)1097-0215(20000120)89:1<81::aid-ijc13>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 51.Cioffi M, Vietri MT, Gazzerro P, Magnetta R, D'Auria A, Durante A, et al. Serum anti-p53 antibodies in lung cancer: comparison with established tumor markers. Lung Cancer. 2001;33(2-3):163–9. doi: 10.1016/s0169-5002(01)00201-x. [DOI] [PubMed] [Google Scholar]
- 52.Mack U, Ukena D, Montenarh M, Sybrecht GW. Serum anti-p53 antibodies in patients with lung cancer. Oncol Rep. 2000;7(3):669–74. doi: 10.3892/or.7.3.669. [DOI] [PubMed] [Google Scholar]
- 53.Metcalfe S, Wheeler TK, Picken S, Negus S, Jo Milner A. P53 autoantibodies in 1006 patients followed up for breast cancer. Breast Cancer Res. 2000;2(6):438–43. doi: 10.1186/bcr91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gao RJ, Bao HZ, Yang Q, Cong Q, Song JN, Wang L. The presence of serum anti-p53 antibodies from patients with invasive ductal carcinoma of breast: correlation to other clinical and biological parameters. Breast Cancer Res Treat. 2005;93(2):111–5. doi: 10.1007/s10549-005-4321-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


