Abstract
Development of non-infectious subunit vaccines is hampered by a slow pipeline of new adjuvants to replace or enhance alum in part because expectations of safety are high. Transient vaccine side effects are not clinical priorities because they cause no lasting harm and vaccine development has appropriately been focused on avoidance of serious adverse events. As a result, surprisingly little is known about the extent to which side effects caused by a vaccines reactogencicity are predictive of successful immunization outcomes. Recent clinical studies of pertussis and human papillomavirus vaccines adjuvanted with alum or the TLR4 agonist monophosphoryl lipid A can be used to advance understanding of the relationship between vaccine side effects and immunization outcomes.
Introduction
Vaccines saved an estimated 730 000 lives and prevented 21 million hospitalizations in the United States from 1994 to 2013 [1] but they remain underutilized. The highly effective HPV vaccine, Gardasil, was a break-through advance in cancer prevention due to the fact that HPV causes almost 30 000 cancers of the cervix, anus, vulva/vagina, penis or oropharynx per year in the US and over 500 000 annual cases worldwide. Nevertheless, HPV vaccine usage is low. The most recent CDC report shows that fewer than half of adolescent girls in the U.S. were adequately immunized in 2015 [2,3]. Although multiple overlapping factors affect uptake of a new vaccine, concern about side effects is frequently cited by parents who decline HPV immunizations for their teen-aged children [4–7,8•,9] (Table 1). In these surveys, 16–55% of parents cited vaccine side effects and concern about short-term health problems as one of the reasons for their refusal (Table 1). A multi-year study of the ‘main reason’ for vaccine refusals found that ‘vaccine safety/side effects’ was cited by an increasing proportion of parents, nearly quadrupling over three years from 4.4% to 16.4% to become one of the top two reasons HPV immunizations were declined (Figure 1). If vaccine reactogenicity has even a small effect on vaccine uptake, it multiplies into a much larger problem given the enormous numbers of people involved in prophylactic immunization programs. In this short review we will consider recent developments in pertussis immunization, one of the first examples of public health being indirectly damaged by vaccine reactogenicity, as well as lessons gleaned from head-to-head comparisons of HPV vaccines containing different adjuvants.
Table 1.
| Study | Survey period, location | Participants | % Refusal HPV immunization | Parental reasons for refusal |
|---|---|---|---|---|
| Darden et al. (2013) [6] | 2008–2010, US | N = 98 000 parents of boys or girls age 13–17 | 40–44% ‘had ever refused’ | ‘Safety concerns/side effects’ tripled as the main reason cited for refusal, from 4% to 16% |
| Kester et al. (2013) [7] | 2010, US | N = 501 parents of girls age 14–17 | 51% had not vaccinated | 36% cited ‘Concern for vaccine side effect’ as one of the reasons |
| Dorell et al. (2014) [5] | 2010, US | N = 4103 parents of girls age 13–17 | 20% refused | 55% cited ‘Concern about shortterm problems like fever or discomfort’ as one of the reasons |
| Gilbert et al. (2016) [4] | 2013, Canada | N = 5720 parents of girls age 12–14 | 14% refused | 36% ‘concerned about the potential side effects of vaccines’ as one of the reasons |
| Gilkey et al. (2017) [8*] | 2014–2015, US | N = 1484 parents of boys or girls age 11–17 | 29% refused | 18% cited ‘Concern for short-term health problems’ as one of the reasons |
| Dayal et al. (2017) [9] | 2015, US (Texas) | N = 60 parents of girls age 9–18 | 23% refused | ‘Perceived HPV vaccine harm’ was the most predictive of parental refusal |
Figure 1.
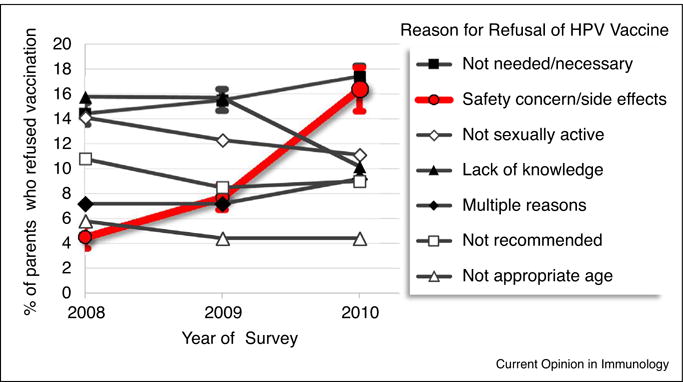
The main reasons parents refused HPV immunization for their teen-aged children. About 20% of parents who responded to the National Immunization Survey – Teens 2008–2010 (N ~ 33 000/year) had refused HPV immunizations for their teen-age daughters and cited one of the indicated statements as ‘the main reason’ for their decision. Error bars denoting 95% confidence intervals are shown only for ‘safety concern/side effects’ and ‘not needed/not necessary’ for clarity. Data from Darden et al. (2013) [6].
The fall and rise of pertussis
Vaccine side effects are not generally perceived as a problem when infectious diseases are prevalent as was the case with the initial immunization campaign to prevent whooping cough, a highly transmissible respiratory disease in which pertussis toxin-induced coughing lasts weeks and can become so forceful that it results in cracked ribs, collapsed lungs, hernias and bleeding in the brain [10]. Pertussis vaccines containing inactivated Bordetella pertussis bacteria plus diphtheria and tetanus toxoid proteins (DTP) began to be used widely in the 1940s and were a success despite high reactogenicity. In the United States, for example, cases of whooping cough were reduced from the hundreds of thousands per year to a low of ~1000 cases in 1976 [11,12•]. However, whooping cough incidence has recently ticked up again with the 2–5 year periodicity that is typical of B. pertussis outbreaks. At first the resurgence involved unimmunized infants who have always been at risk, but beginning in 2004–2005 it included an alarming number of infections of vaccinated school-age who should have been protected, reaching a recent peak of 48 000 annual cases in 2012 [12•,13]. Why have so many cases, the most since 1955, occurred in a country with comparatively high levels of pertussis vaccine coverage? The answer involves a fascinating mix of human perceptions of risk, public policy responses, and the complex immunology of alum-adjuvanted vaccines.
In the 1970s the pronounced inflammatory reactogenicity of DTP vaccination became associated [14,15], erroneously [16–19], with neurological damage. Because whooping cough had become rare, side effects of immunization began to be perceived as the greater threat causing vaccination rates to drop in several industrialized countries. Parent refusals to allow pertussis immunization for their children began in Japan and spread to Sweden, the United Kingdom, the Russian Federation and others [20]. All of these countries then suffered outbreaks of pediatric whooping cough while countries with more rigid immunization compliance avoided outbreaks, including the U. S., the former East Germany, Poland and Hungary [20]. In 1981, an acellular version of pertussis vaccine (aP) with less reactogenicity was developed in Japan after pains-taking identification of endotoxin-minimized protein fractions that conferred protective immunity [15]. These were adsorbed on alum along with diphtheria and tetanus toxoid antigens and the resulting subunit vaccine, DTaP, was immediately adopted as a replacement for DTP in Japan. The new formulation worked exactly as intended: reactogenicity was reduced, public alarm abated, vaccine rates rose and cases of whooping cough returned to minimal levels by 1985 [20].
And yet, whooping cough has returned. Several non-exclusive explanations have been proposed, including pockets of vaccine refusals despite strong evidence of safety, appearance of vaccine-resistant strains of B. pertussis or parapertussis, improved detection of milder cases of whooping cough, and loss of primary efficacy or durability of immune memory or both. Recent analyses of whooping cough rates show that protective immunity wanes rapidly after completion of the recommended childhood series of five immunizations with DTaP [21], or after a sixth booster at age 10 with Tdap, a formulation of DTaP with lower doses of diphtheria and pertussis antigens Tdap approved for use in adolescents and adults [22•]. These findings are surprising given that the primary efficacy of DTaP had been confirmed in several early vaccine trials [23,24] and that DTaP is effective when deployed to contain outbreaks [20,25]. However, epidemiological studies strongly support the conclusion that immunity was more durable when whole cell DTP vaccines were in use as compared to the currently approved DTaP formulations [26,27]. In addition, recent computational modeling shows that waning immunity explains recent increases in whooping cough occurrence [28•]. Hence, a consensus is emerging that the re-occurrence of whooping cough is due to a failure of subunit pertussis vaccines to establish durable immune memory [12•].
Identifying and fixing the problem with DTaP subunit vaccines
A critical first step to restoring long-term vaccine efficacy is deciphering the mechanism(s) responsible for waning immunity after DTaP vaccination. Several groups are making advances in this regard despite a paucity of head-to-head comparisons to DTP, which is no longer approved for use. In one such study, children were immunized with DTaP or a whole cell DTP available at the time in the Netherlands. Humoral responses to three B. pertussis virulence factors (pertussis toxin, filamentous hemagglutinin and pertactin) were measured 4–6 weeks and 2 years after immunization and compared to those of children who had cleared natural infections over matching time frames [29]. Consistent with DTaPs primary efficacy [20,23–25], peak serum antibody titers were actually somewhat higher after immunization with DTaP than DTP [29]; unfortunately, statistical variability was too great for antibody half-lives to be calculated with precision. Other studies of humoral responses to DTaP, without comparisons to DTP, show a striking pattern of selective durability: the half-lives of humoral responses to diphtheria and tetanus toxoid proteins are high, a decade or more, while those specific for pertussis antigens are just 6–12 months despite being components of the same vaccine [12•,29–32]. This selectivity indicates non-durable memory is not a simple or uniform failure of alums adjuvant function.
A second critical step is identifying which of the many factors present in DTP but not DTaP were responsible for its superior durability. Whole fixed B. pertussis cells contain many more protein antigens than the 3–5 purified pertussis proteins present in various manufacturer’ formulations of DTaP. Some investigators have proposed that additional pertussis antigens are needed [33] such as the B. pertussis virulence factor adenylate cyclase toxin (ACT) which is protective in mice and immunogenic in baboons [34,35•]. DTP also contained ligands for several Toll-like receptors, TLR1, 2, 4, 5, 6 and 9 [36] whereas subunit DTaP has only the conventional vaccine adjuvant alum, often faulted for its Th2-bias. Th2-skewing by alum has long been recognized [37] and is evident in two comparison studies that showed whole cell B. pertussis generated Th1 or Th1/Th17-type responses in humans and baboons, respectively, whereas DTaP generated a mixture of Th1 and Th2 outcomes [12•,38,39]. In the baboon study, Th1/Th2 mixed differentiation was correlated with partial immunity in that aP vaccinated animals were protected from whooping cough disease but not from colonization and transmission of B. pertussis. Another important finding is successive DTaP immunizations result in progressively lower IgG1:IgG4 ratios of pertussis antigen-specific antibodies [40]. This shift from Th1-associated to Th2-associated antibody isotypes is consistent with partial immunity because IgG4 can neutralize toxins and microbes but cannot fix complement nor can it bind FcRγIIIβ and FcRγIIIα (in some people) [41] to mobilize neutrophils and NK cells. Given these findings Th2-bias after alum-adjuvanted immunization is indeed likely to be a contributing factor, although it does not explain the strikingly shorter half-lives of serum titers of pertussis antigen-specific antibodies as compared to those for diphtheria and tetanus toxoid proteins.
The absence of TLR ligands from DTaP seems certain to diminish its immunogenicity relative to whole cell DTP but public and regulatory concern about vaccine side effects makes a return to use of reactogenic DTP unlikely. Selective restoration of adjuvant functions to alum-adjuvanted DTaP is worth considering, although there is both promise and peril in this approach. The adjuvant system AS04 (GSK), a combination adjuvant consisting of alum adsorbed with monophosphoryl lipid A (MPL), has several adjuvant properties that make it promising as a DTaP additive. However, AS04 (hereafter alum + MPL) exacerbates some of the inflammatory effects of alum which may have been pronounced enough to dissuade some parents from accepting HPV immunizations for their children (Table 1 and Figure 1). In the next section we will introduce and consider these promising adjuvant properties of alum + MPL, as well as the need to advance our detailed understanding of vaccine side effects so that immune protection can be boosted without proportional increases in reactogenicity.
The rise and fall of MPL in the United States
MPL is a low toxicity agonist of TLR4 derived from the lipopolysaccharide (LPS) component of gram-negative bacterial cell wall. It was created in the 1970s by Edgar Ribi, a scientist at the US Rocky Mountain Laboratories, through systematic manipulation of the structure of LPS with acid and base hydrolysis in an effort to develop a detoxified form of ‘Coleys toxin’ [42,43] for cancer therapy [44]. In early tests, the anti-tumor activity of MPL appeared to be unimpaired relative to its parental LPS but with as little as 0.1% as much inflammatory toxicity. MPL is now a component in several adjuvant systems developed by GSK including AS04, alum + MPL, used in Cervarix and Fendrix [45,46]. It is the first — and so far only — refined TLR ligand to achieve clinical and regulatory success in a prophylactic vaccine intended for healthy individuals, where expectations of safety are extraordinarily high. MPL therefore provides an important model for those seeking to understand how vaccine reactogenicity can be uncoupled from adjuvanticity at the level of adaptive priming.
Beneficial adjuvant functions of MPL
Three clinical trials performed as head-to-head comparisons of first-generation and second-generation versions of vaccines, adjuvanted with alum alone or alum + MPL, provide strong evidence of the ‘value added’ by MPL. Two of these were large trials of HPV vaccines that differ primarily in adjuvant composition, Gardasil (Merck) adjuvanted with alum and its competitor Cervarix (GSK) adjuvanted with alum + MPL. Both vaccines contain self-assembling L1 capsid proteins from the two most prevalent oncogenic serotypes, HPV-16 and HPV-18 adsorbed to alum, although Gardasil has a different alum salt and contains L1 from two additional, non-oncogenic HPV serotypes [47]. The HPV-010 Study Group compared the immunogenicity and safety of Cervarix and Gardasil in a head-to-head, randomized and double-blinded clinical trial of a 3-dose immunization schedule initiated with 1100 study participants and reported their findings in a remarkable series of publications [48–53]. A separate head-to-head comparison of Gardasil and Cervarix by the HPV-071 study group was conducted with 700 adolescent girls, age 9–14, using 2-dose or 3-dose immunization schedules [54,55]. In each trial, both vaccines were highly effective as defined by seroconversion of ~ 100% of the study participants. Neither vaccine was associated with increased risk of serious AEs (SAE), new onset autoimmune diseases, chronic diseases or other medically significant conditions in these and many other studies [47].
Relative to Gardasil (alum), Cervarix (alum + MPL) generated markedly higher peak titers of HPV-neutralizing antibodies, a difference that persisted for at least 60 months in women aged 18–45 and 36 months year in girls aged 9–14 (Figure 2). Durability of protective immunity was also likely to be enhanced by the addition of MPL because fewer Cervarix-immunized women fell back into seronegative status after 5 years [52]. It is important to note that no loss of seropositivity was observed in girls age 9–14 given either Gardasil or Cervarix vaccine, underscoring the current recommendations for HPV vaccines to be routinely administered at age 11–12 [47]. Although MPL is the most prominent difference in composition between Gardasil and Cervarix, caution is warranted in drawing the conclusion that Cervarixs enhanced humoral responses are attributable only to MPL, and not the other differences between the vaccines. For the comparison of Fendrix to Engerix-B, however, MPL is the sole difference in vaccine composition as both vaccines are manufactured by GSK using the identical alum salts and HBsAg antigen preparations. When tested in about 100 adult men and women (Figure 2) the vaccine containing alum + MPL again generated markedly higher titers of antigen-specific antibodies than its alum-only counterpart [56•,57], strongly supporting the conclusion that improved immunization outcomes are attributable to the addition of MPL.
Figure 2.
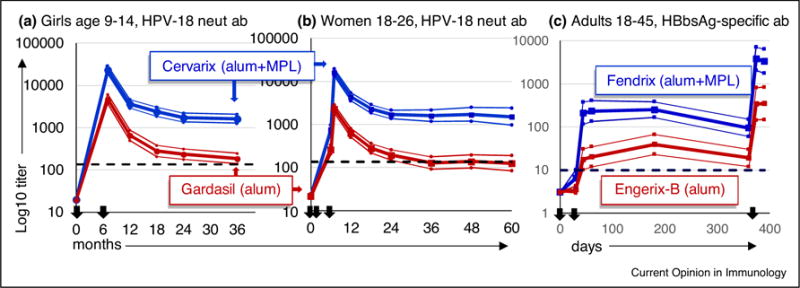
Humoral responses to vaccines adjuvanted with alum + MPL versus alum alone. Serum titers from three clinical studies performed as double-blind, randomized head-to-head comparisons of vaccines are shown; N is for according-to-protocol. (a) Cervarix versus Gardasil in seronegative girls age 9–14, N = 187 according-to-protocol (ATP). Study participants were immunized twice at 0 and 6 months and serum titers of HPV-18 neutralizing antibody were measured from 7 through 36 months; data from [54,55]. (b) Cervarix versus Gardasil in seronegative women age 18–26, N = 248 ATP. Participants were immunized thrice at 0, 1–2 and 6 months and serum titers of HPV-18 neutralizing antibody were measured from 6 through 60 months; data from one of three age-stratified groups reported in [48–53]. (c) Fendrix versus Engerix-B in seronegative women and men age 18–45, N = 104 ATP. Participants were immunized on days 0, 30 and 360 and serum titers of HBsAg-specific antibody were measured from 30 to 390 days; data from [56•,57]. N values are for participants who completed each study according-to-protocol. (All) Black arrows: vaccine immunizations in each study. Bold lines: geometric mean titers of serum antibodies. Thin lines: upper and lower confidence intervals (95%). Dotted lines: neutralizing Ab titer of women who had cleared natural HPV infection (a, b) or HBsAg-specific titer associated with immunity to HBV (c).
In all three of these vaccine trials, MPL exemplifies the classically beneficial functions of an adjuvant: immune responses that are faster, stronger and longer lasting. In the context of pertussis immunization, these adjuvant properties would seem likely to improve DTaP vaccines as well, given they generate tepid antibody responses relative to natural infection that are not durable. MPL in DTaP vaccine would probably favor Th1 responses to pertussis antigens [58,59].
Vaccine reactogenicity associated with alum +MPL
Each of the vaccine trials also recorded the percentages of study participants who experienced any of several anticipated side effects indicative of vaccine reactogenicity. We graphed the percentages measured after the first vaccine injection in all three studies side-by-side (Figure 3). Comparison of the graphs reveals patterns that may be useful in understanding the functional relevance of vaccine side effects. First, the frequencies of participants who experienced a particular side effect were surprisingly consistent given the range of cohort demographics, which included girls, adult women and adult men of several nationalities. This consistency indicates the underlying biological mechanisms are less likely to be obscured by social behaviors or other complicating factors. Second, vaccines adjuvanted with alum alone (Gardasil and Engerix-B) are fairly reactogenic with broad effects on general and local symptoms. Vaccines adjuvanted with alum + MPL are even more reactogenic, which raises the troubling prospect that enhancement of immunity is not separable from proportional increases in undesirable side effects. As noted by the study authors [48,54,56•], however, the increases associated with MPL were largely restricted to local side effects, lasting injection site pain, redness and swelling, and not general side effects other than myalgia. Further analysis is needed, but this pattern suggests separation of beneficial immunization outcomes from systemic side effects is possible. Injection site pain was common with alum-adjuvanted vaccines and markedly exacerbated by the addition of MPL. This correlation is intriguing given that sensory neurons express TLR4 and CD14 [60] and LPS sensitizes or activates nociceptive ion channels involved in pain sensation [61,62,63•].
Figure 3.
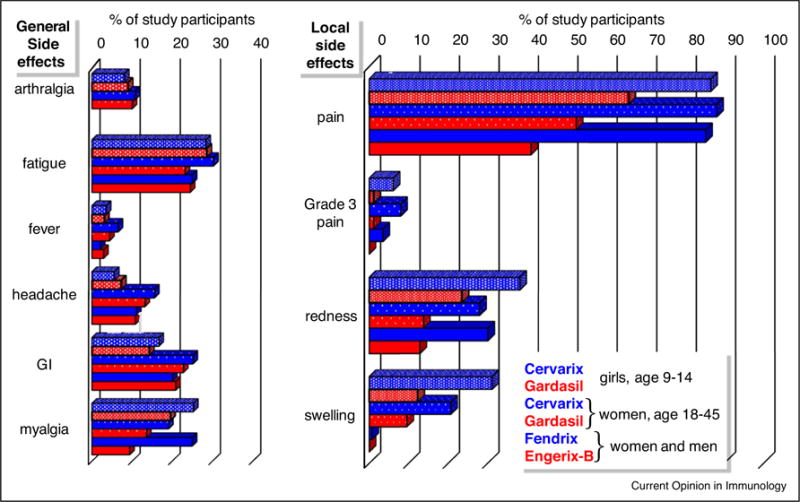
Inflammatory side effects of vaccines adjuvanted with alum + MPL or alum. The percentages of participants who experienced general or local adverse events after vaccine dose 1 in the clinical studies described in Figure 2 are shown. Blue and red bars: instances of adverse events after intramuscular injection of vaccines containing alum + MPL (Cervarix and Fendrix) or alum alone (Gardasil and Engerix-B), respectively. Instances of arthralgia were not recorded after administration of Fendrix or Engerix-B. For each cluster shown, bars from top-to-bottom correspond to adverse events after injection of Cervarix versus Gardasil in N = 716 girls age 9–14 from France, Hong Kong, Singapore and Sweden; Cervarix versus Gardasil in N = 249 women age 18–26 from the US; and Fendrix versus Engerix-B in N = 282 adult women and men age 18–45 from Belgium and Germany. Data from [53,55,57]; N indicates all participants enrolled in each study who received vaccine dose 1 regardless of pre-immune status or completion of the immunization series according-to-protocol.
No pain no gain?
As noted earlier, vaccine side effects appears to be a contributing factor in parental refusals to immunize their children, yet one more factor weighing down on vaccination rates that remain below public health goals. Public health experts are needed to address the social causes of low vaccine coverage, but immunologists can and should help by learning how to minimize even mild forms of vaccine reactogenicity. Achieving this goal begins by asking some basic questions usually overlooked in clinical studies. Are inflammatory side effects predictive of more effective, longer-lasting immunization outcomes? Are side effects even necessary, or is it conceivable that future vaccinations could be entirely forgettable events? Several studies of pain relief medications for vaccination side effects have been conducted that might help provide answers, but surprisingly few included measurement of immunization outcomes. In this handful of studies, prophylactic paracetamol (acetaminophen) treatment had either no or modest effects on secondary antibody titers in vaccinated groups of adults, toddlers and children [64–66]. More such studies are needed, but the implication is that immunization outcomes may not be critically dependent on inflammatory processes responsible for vaccine side effects, at least those responsive to paracetamol. Another approach to learn more about side effects is to analyze safety data from vaccine trials to identify which are predictive of improved immunization outcomes, and which are not.
Given the superior function of Cervarix relative to Gardasil in these studies, it is disheartening that Cervarix was withdrawn from the US market in 2016 due to low sales. The reasons for this market failure are not clear, although AS04 and MPL may return as a component of a next-generation shingles vaccine if a recent submission to the FDA is approved. Cervarix is widely available outside of the US, with licensure in the EU, Australia, China and elsewhere, but the U.S. is effectively returned to its pre-TLR adjuvant stage of adjuvant development as a result of the withdrawal of Cervarix (Fendrix was never introduced). Other than alum, only one other vaccine adjuvant is currently in use in the US: the squalene oil MF59 in a seasonal influenza vaccine, Fluad, (Novartis) recently received expedited approval for use in a restricted population of elderly individuals.
Pertussis vaccines may perform better with alum + MPL as adjuvant
Ironically, Gardasil is highly effective with alum as its sole adjuvant suggesting that MPL may have been deployed where it was not needed, in Cervarix, and not where it might be most beneficial: in DTaP subunit vaccines. B. pertussis is a Gram-negative bacterium whose TLR4 stimulatory function is one of the attributes lost in the conversion from whole cell to endotoxin-minimized acellular vaccines and Tlr4 is required for immunity, at least in mouse models of B. pertussis infection. MPL has adjuvant properties that are likely to be needed in an improved pertussis vaccine, properties such as restrained reactogenicity, more durable immunity, less Th2-bias, and higher peak serum titers that approach those of a natural Bordetella infection. GSK Vaccines has two pertussis subunit vaccines in its portfolio, Infanrix (a DTaP) and Boostrix (Tdap), it has AS04, and it has unrivaled experience in bringing vaccines with next-generation adjuvants into clinical use. An AS04-adjuvanted pertussis vaccine is not listed in the GSK Vaccines product pipeline, but one hopes that it is being considered.
Concluding remarks
In this era of increased public concern of adverse effects of vaccination, understanding how to minimize local and systemic effects of vaccination while maintaining efficacy should be a goal for future vaccines. After all, vaccines can only achieve full benefit if they have widespread acceptance and use. The misperception that vaccine reactogenicity causes unrelated health problems persists today, as in Robert F Kennedy Jrs pronouncement in 2015 that children immunized for MMR are at risk because, ‘They get the shot. That night they have a fever of 103. They go to sleep, and three months later their brain is gone’ [67]. We propose three areas that need be addressed, in the following order of urgency. First, test the addition of MPL to DTaP and Tdap vaccines. Just one group appears to have tested a generic form of MPL as an additive for DTaP, reporting in 2007 that it improved efficacy, but only in mice and durability was not assessed [68]. Next, advance our understanding of the extent to which transient vaccine side effects are associated with desired immunization outcomes in vaccine trials. And finally, apply what is learned about side effects to discover how they can be uncoupled from adjuvanticity so that future vaccines can be effective, and forgettable.
Acknowledgments
Funding
Preparation and submission of this review was supported by the Barnstable-Brown Foundation, the Commonwealth of Kentucky Research Challenge Trust Fund and the National Institute Of Allergy And Infectious Diseases of the National Institutes of Health under Award Number R01AI127970. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The authors thank all investigators and study participants who contributed to development of vaccines to prevent whooping cough and cervical cancer, and regret that not all publications that deserve recognition could be listed as references.
Footnotes
Conflict of interest
None declared.
Edited by Ross Kedl and Robert Seder
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
• of outstanding interest
- 1.Whitney CG, Zhou F, Singleton J, Schuchat A. Benefits from immunization during the vaccines for children program era — United States, 1994–2013. MMWR Morb Mortal Wkly Rep. 2014;63:352–355. [PMC free article] [PubMed] [Google Scholar]
- 2.Reagan-Steiner SYD, Jeyarajah J, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years — United States, 2015. MMWR Morb Mortal Wkly Rep. 2016;65:850–858. doi: 10.15585/mmwr.mm6533a4. [DOI] [PubMed] [Google Scholar]
- 3.Meites EKA, Markowitz LE. Use of a 2-dose schedule for human papillomavirus vaccination — updated recommendations of the advisory committee on immunization practices. MMWR Morb Mortal Wkly Rep. 2016;65:1405–1408. doi: 10.15585/mmwr.mm6549a5. [DOI] [PubMed] [Google Scholar]
- 4.Gilbert NL, Gilmour H, Dube E, Wilson SE, Laroche J. Estimates and determinants of HPV non-vaccination and vaccine refusal in girls 12 to 14 y of age in Canada: results from the Childhood National Immunization Coverage Survey, 2013. Hum Vaccine Immunother. 2016;12:1484–1490. doi: 10.1080/21645515.2016.1153207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dorell C, Yankey D, Jeyarajah J, Stokley S, Fisher A, Markowitz L, Smith PJ. Delay and refusal of human papillomavirus vaccine for girls, National Immunization Survey — teen, 2010. Clin Pediatr. 2014;53:261–269. doi: 10.1177/0009922813520070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Darden PM, Thompson DM, Roberts JR, Hale JJ, Pope C, Naifeh M, Jacobson RM. Reasons for not vaccinating adolescents: National Immunization Survey of Teens, 2008– 2010. Pediatrics. 2013;131:645–651. doi: 10.1542/peds.2012-2384. [DOI] [PubMed] [Google Scholar]
- 7.Kester LM, Zimet GD, Fortenberry JD, Kahn JA, Shew ML. A national study of HPV vaccination of adolescent girls: rates, predictors, and reasons for non-vaccination. Matern Child Health J. 2013;17:879–885. doi: 10.1007/s10995-012-1066-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8•.Gilkey MB, Calo WA, Marciniak MW, Brewer NT. Parents who refuse or delay HPV vaccine: differences in vaccination behavior, beliefs, and clinical communication preferences. Hum Vaccin Immunother. 2017;13:680–686. doi: 10.1080/21645515.2016.1247134. In this recent survey, 25% of US parents reported they had refused or decided not to get HPV immunization for their children. Of these, 18% were concerned about short-term health problems and 50% about lasting health problems. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dayal K, Robinson S, Schoening J, Smith MC, Kim SC. Predictors of human papillomavirus vaccine uptake or intent among parents of preadolescents and adolescents. J Nurs Educ Pract. 2017;7:35. [Google Scholar]
- 10.Kroger A, Hamborsky J. Epidemiology and Prevention of Vaccine-Preventable Diseases. 13. The Pink Book: Public Health Foundation; 2015. [Google Scholar]
- 11.Cherry JD. Historical review of pertussis and the classical vaccine. J Infect Dis. 1996;174(Suppl 3):S259–S263. doi: 10.1093/infdis/174.supplement_3.s259. [DOI] [PubMed] [Google Scholar]
- 12•.Warfel JM, Edwards KM. Pertussis vaccines and the challenge of inducing durable immunity. Curr Opin Immunol. 2015;35:48–54. doi: 10.1016/j.coi.2015.05.008. The authors provide a detailed review of the many moving parts involved in pertussis immunization and the complexity of the problem posed by waning immunity to whooping cough. [DOI] [PubMed] [Google Scholar]
- 13.Zhang L, Prietsch SO, Axelsson I, Halperin SA. Acellular vaccines for preventing whooping cough in children. Cochrane Database Syst Rev. 2014:CD001478. doi: 10.1002/14651858.CD001478.pub6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kulenkampff M, Schwartzman J, Wilson J. Neurological complications of pertussis inoculation. Arch Dis Childhood. 1974;49:46–49. doi: 10.1136/adc.49.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kanai K. Japans experience in pertussis epidemiology and vaccination in the past thirty years. Jpn J Med Sci Biol. 1980;33:107–143. doi: 10.7883/yoken1952.33.107. [DOI] [PubMed] [Google Scholar]
- 16.Cherry JD. Pertussis vaccine encephalopathy: it is time to recognize it as the myth that it is. JAMA. 1990;263:1679–1680. [PubMed] [Google Scholar]
- 17.Ray P, Hayward J, Michelson D, Lewis E, Schwalbe J, Black S, Shinefield H, Marcy M, Huff K, Ward J, et al. Encephalopathy after whole-cell pertussis or measles vaccination: lack of evidence for a causal association in a retrospective case– control study. Pediatr Infect Dis J. 2006;25:768–773. doi: 10.1097/01.inf.0000234067.84848.e1. [DOI] [PubMed] [Google Scholar]
- 18.Howson CP, Howe CJ, Fineberg HV. Adverse effects of pertussis and rubella vaccines: a report of the Committee to Review the Adverse Consequences of Pertussis and Rubella Vaccines. In: Howson CP, Howe CJ, Fineberg HV, editors. Adverse Effects of Pertussis and Rubella Vaccines: A Report of the Committee to Review the Adverse Consequences of Pertussis and Rubella Vaccines. The National Academies Collection: Reports funded by National Institutes of Health; 1991. [PubMed] [Google Scholar]
- 19.Griffin MR, Ray WA, Mortimer EA, Fenichel GM, Schaffner W. Risk of seizures and encephalopathy after immunization with the diphtheria–tetanus–pertussis vaccine. JAMA. 1990;263:1641–1645. [PubMed] [Google Scholar]
- 20.Gangarosa EJ, Galazka AM, Wolfe CR, Phillips LM, Gangarosa RE, Miller E, Chen RT. Impact of anti-vaccine movements on pertussis control: the untold story. Lancet. 1998;351:356–361. doi: 10.1016/s0140-6736(97)04334-1. [DOI] [PubMed] [Google Scholar]
- 21.Klein NP, Bartlett J, Rowhani-Rahbar A, Fireman B, Baxter R. Waning protection after fifth dose of acellular pertussis vaccine in children. N Engl J Med. 2012;367:1012–1019. doi: 10.1056/NEJMoa1200850. [DOI] [PubMed] [Google Scholar]
- 22•.Klein NP, Bartlett J, Fireman B, Baxter R. Waning Tdap effectiveness in adolescents. Pediatrics. 2016;137:e20153326. doi: 10.1542/peds.2015-3326. Waning immunity is shown to be a problem even after a sixth immunization with subunit pertussis vaccines, additional and troubling evidence of vaccine failure. Booster immunization with Tdap, the pertussis vaccine recommended for adolescents and adults, is estimated be 69, 57, 25 and then just 9% effective in the four years following its administration at age 10. [DOI] [PubMed] [Google Scholar]
- 23.Bisgard KM, Rhodes P, Connelly BL, Bi D, Hahn C, Patrick S, Glode MP, Ehresmann KR. Pertussis vaccine effectiveness among children 6 to 59 months of age in the United States, 1998–2001. Pediatrics. 2005;116:e285–e294. doi: 10.1542/peds.2004-2759. [DOI] [PubMed] [Google Scholar]
- 24.Stehr K, Cherry JD, Heininger U, Schmitt-Grohé S, Überall M, Laussucq S, Eckhardt T, Meyer M, Engelhardt R, Christenson P. A comparative efficacy trial in Germany in infants who received either the Lederle/Takeda acellular pertussis component DTP (DTaP) vaccine, the Lederle whole-cell component DTP Vaccine, or DT vaccine. Pediatrics. 1998;101:1–11. doi: 10.1542/peds.101.1.1. [DOI] [PubMed] [Google Scholar]
- 25.Kirkland KB, Talbot EA, Decker MD, Edwards KM. Kinetics of pertussis immune responses to tetanus-diphtheria-acellular pertussis vaccine in health care personnel: implications for outbreak control. Clin Infect Dis. 2009;49:584–587. doi: 10.1086/603555. [DOI] [PubMed] [Google Scholar]
- 26.Liko J, Robison SG, Cieslak PR. Priming with whole-cell versus acellular pertussis vaccine. N Engl J Med. 2013;368:581–582. doi: 10.1056/NEJMc1212006. [DOI] [PubMed] [Google Scholar]
- 27.Witt MA, Arias L, Katz PH, Truong ET, Witt DJ. Reduced risk of pertussis among persons ever vaccinated with whole cell pertussis vaccine compared to recipients of acellular pertussis vaccines in a large US cohort. Clin Infect Dis. 2013;56:1248–1254. doi: 10.1093/cid/cit046. [DOI] [PubMed] [Google Scholar]
- 28•.Gambhir M, Clark TA, Cauchemez S, Tartof SY, Swerdlow DL, Ferguson NM. A change in vaccine efficacy and duration of protection explains recent rises in pertussis incidence in the United States. PLoS Comput Biol. 2015;11:e1004138. doi: 10.1371/journal.pcbi.1004138. The complex intersection of microbiology, immunology, psychology and public policy makes discovery of the causes of pertussis vaccine failure a daunting task. This computational approach modeled the effects of several of the possible explanations to determine the impact of each, and found that waning immunity explains the increase in cases of whooping cough. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berbers GAM, van de Wetering MSE, van Gageldonk PGM, Schellekens JFP, Versteegh FGA, Teunis PFM. A novel method for evaluating natural and vaccine induced serological responses to Bordetella pertussis antigens. Vaccine. 2013;31:3732–3738. doi: 10.1016/j.vaccine.2013.05.073. [DOI] [PubMed] [Google Scholar]
- 30.Dalby T, Petersen JW, Harboe ZB, Krogfelt KA. Antibody responses to pertussis toxin display different kinetics after clinical Bordetella pertussis infection than after vaccination with an acellular pertussis vaccine. J Med Microbiol. 2010;59:1029–1036. doi: 10.1099/jmm.0.020826-0. [DOI] [PubMed] [Google Scholar]
- 31.Le T, Cherry JD, Chang S-J, Knoll MD, Lee ML, Barenkamp S, Bernstein D, Edelman R, Edwards KM, Greenberg D, et al. Immune responses and antibody decay after immunization of adolescents and adults with an acellular pertussis vaccine: the APERT study. J Infect Dis. 2004;190:535–544. doi: 10.1086/422035. [DOI] [PubMed] [Google Scholar]
- 32.Amanna IJ, Carlson NE, Slifka MK. Duration of humoral immunity to common viral and vaccine antigens. N Engl J Med. 2007;357:1903–1915. doi: 10.1056/NEJMoa066092. [DOI] [PubMed] [Google Scholar]
- 33.Meade BD, Plotkin SA, Locht C. Possible options for new pertussis vaccines. J Infect Dis. 2014;209:S24–S27. doi: 10.1093/infdis/jit531. [DOI] [PubMed] [Google Scholar]
- 34.Sebo P, Osicka R, Masin J. Adenylate cyclase toxin-hemolysin relevance for pertussis vaccines. Expert Rev Vaccines. 2014;13:1215–1227. doi: 10.1586/14760584.2014.944900. [DOI] [PubMed] [Google Scholar]
- 35•.Eby JC, Gray MC, Warfel JM, Merkel TJ, Hewlett EL. Use of a toxin neutralization assay to characterize the serologic response to adenylate cyclase toxin after infection with Bordetella pertussis. Clin Vaccine Immunol. 2017:24. doi: 10.1128/CVI.00370-16. The authors describe an important advance in antigen identification that could improve subunit pertussis vaccines. Humans and baboons infected with B. pertussis are demonstrated to generate neutralizing humoral responses to a known virulence factor that inhibits multiple leukocyte functions needed for immune defense. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reed SG, Orr MT, Fox CB. Key roles of adjuvants in modern vaccines. Nat Med. 2013;19:1597–1608. doi: 10.1038/nm.3409. [DOI] [PubMed] [Google Scholar]
- 37.Grun JL, Maurer PH. Different T helper cell subsets elicited in mice utilizing two different adjuvant vehicles: the role of endogenous interleukin 1 in proliferative responses. Cell Immunol. 1989;121:134–145. doi: 10.1016/0008-8749(89)90011-7. [DOI] [PubMed] [Google Scholar]
- 38.Ausiello CMUF, La Salla A, Lande R, Cassone A. Vaccine- and antigen-dependent type 1 and type 2 cytokine induction after primary vaccination of infants with whole-cell or acellular pertussis vaccines induction after primary vaccination of infants with whole-cell. Infect Immunity. 1997;65:2168–2174. doi: 10.1128/iai.65.6.2168-2174.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Warfel JM, Zimmerman LI, Merkel TJ. Acellular pertussis vaccines protect against disease but fail to prevent infection and transmission in a nonhuman primate model. Proc Natl Acad Sci U S A. 2014;111:787–792. doi: 10.1073/pnas.1314688110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Diavatopoulos DA, Edwards KM. What is wrong with pertussis vaccine immunity? Why immunological memory to pertussis is failing. Cold Spring Harb Perspect Biol. 2017 doi: 10.1101/cshperspect.a029553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vidarsson G, Dekkers G, Rispens T. IgG subclasses and allotypes: from structure to effector functions. Front Immunol. 2014;5 doi: 10.3389/fimmu.2014.00520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Coley WB., II Contribution to the knowledge of sarcoma. Ann Surg. 1891;14:199. doi: 10.1097/00000658-189112000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tsung K, Norton JA. Lessons from Coleys toxin. Surg Oncol. 2006;15:25–28. doi: 10.1016/j.suronc.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 44.Ribi E, Parker R, Strain SM, Mizuno Y, Nowotny A, von Eschen KB, Cantrell JL, McLaughlin CA, Hwang KM, Goren MB. Peptides as requirement for immuno therapy of the guinea-pig line-10 tumor with endotoxins. Cancer Immunol Immunother. 1979;7:43–58. [Google Scholar]
- 45.Di Pasquale A, Preiss S, Tavares Da Silva F, Garcon N. Vaccine adjuvants: from 1920 to 2015 and beyond. Vaccines (Basel) 2015;3:320–343. doi: 10.3390/vaccines3020320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Garcon N, Segal L, Tavares F, Van Mechelen M. The safety evaluation of adjuvants during vaccine development: the AS04 experience. Vaccine. 2011;29:4453–4459. doi: 10.1016/j.vaccine.2011.04.046. [DOI] [PubMed] [Google Scholar]
- 47.Markowitz LE, Dunne EF, Saraiya M, Chesson HW, Curtis CR, Gee J, Bocchini JA, Jr, Unger ER. Human papillomavirus vaccination: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2014;63:1–30. [PubMed] [Google Scholar]
- 48.Einstein MH, Baron M, Levin MJ, Chatterjee A, Edwards RP, Zepp F, Carletti I, Dessy FJ, Trofa AF, Schuind A, et al. Comparison of the immunogenicity and safety of Cervarix™ and Gardasil® human papillomavirus (HPV) cervical cancer vaccines in healthy women aged 18–45 years. Hum Vaccines. 2009;5:705–719. doi: 10.4161/hv.5.10.9518. [DOI] [PubMed] [Google Scholar]
- 49.Einstein MH, Baron M, Levin MJ, Chatterjee A, Fox B, Scholar S, Rosen J, Chakhtoura N, Lebacq M, van der Most R, et al. Comparison of the immunogenicity of the human papillomavirus (HPV)-16/18 vaccine and the HPV-6/11/16/18 vaccine for oncogenic non-vaccine types HPV-31 and HPV-45 in healthy women aged 18–45 years. Hum Vaccines. 2011;7:1359–1373. doi: 10.4161/hv.7.12.18282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Einstein MH, Baron M, Levin MJ, Chatterjee A, Fox B, Scholar S, Rosen J, Chakhtoura N, Meric D, Dessy FJ, et al. Comparative immunogenicity and safety of human papillomavirus (HPV)-16/18 vaccine and HPV-6/11/16/18 vaccine: follow-up from months 12–24 in a Phase III randomized study of healthy women aged 18–45 years. Hum Vaccines. 2011;7:1343–1358. doi: 10.4161/hv.7.12.18281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Einstein MH, Levin MJ, Chatterjee A, Chakhtoura N, Takacs P, Catteau G, Dessy FJ, Moris P, Lin L, Struyf F, et al. Comparative humoral and cellular immunogenicity and safety of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine and HPV-6/11/16/18 vaccine in healthy women aged 18–45 years: follow-up through month 48 in a phase III randomized study. Hum Vaccine Immunother. 2014;10:3455–3465. doi: 10.4161/hv.36117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Einstein MH, Takacs P, Chatterjee A, Sperling RS, Chakhtoura N, Blatter MM, Lalezari J, David MP, Lin L, Struyf F, et al. Comparison of long-term immunogenicity and safety of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine and HPV-6/11/16/18 vaccine in healthy women aged 18–45 years: end-of-study analysis of a phase III randomized trial. Hum Vaccine Immunother. 2014;10:3435–3445. doi: 10.4161/hv.36121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.HPV-010-Study-Group. Immunogenicity of GlaxoSmithKline Biologicals Human Papillomavirus (HPV) Vaccine Versus Mercks Gardasil® in Healthy Females 18–45 Years of Age: Scientific Result Summary in Clinical Study Register. 2014 [Google Scholar]
- 54.Leung TF, Liu AP, Lim FS, Thollot F, Oh HM, Lee BW, Rombo L, Tan NC, Rouzier R, Friel D, et al. Comparative immunogenicity and safety of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine and HPV-6/11/16/18 vaccine administered according to 2- and 3-dose schedules in girls aged 9–14 years: results to month 12 from a randomized trial. Hum Vaccine Immunother. 2015;11:1689–1702. doi: 10.1080/21645515.2015.1050570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.HPV-071-PRI. Immunogenicity and safety study of GlaxoSmithKline Biologicals’ HPV-16/18 L1 AS04 vaccine and Mercks Gardasil® vaccine when administered according to alternative 2-dose schedules in 9–14 year old females. Clinical Study Register. 2012 [Google Scholar]
- 56•.Leroux-Roels G, Marchant A, Levy J, Van Damme P, Schwarz TF, Horsmans Y, Jilg W, Kremsner PG, Haelterman E, Clement F, et al. Impact of adjuvants on CD4(+) T cell and B cell responses to a protein antigen vaccine: results from a phase II, randomized, multicenter trial. Clin Immunol. 2016;169:16–27. doi: 10.1016/j.clim.2016.05.007. The first report of an ongoing clinical study to map immune parameters involved in establishment of human immune memory as a function of several new adjuvants in comparison to alum and alum + MPL (AS04). In this extensive and well-crafted study, adaptive immune responses and vaccine side effects are reported; additional studies of transcriptional profiles and pro-inflammatory factors are in progress that will provide invaluable insights into adjuvant effects on the human immune system. [DOI] [PubMed] [Google Scholar]
- 57.EARLY-CLINRES-002. Comparison of the immunogenicity and safety of various investigational and licensed formulations of Hepatitis B surface antigen (HBsAg) vaccines. Clinical Study Register. 2016 [Google Scholar]
- 58.Coffman RL, Sher A, Seder RA. Vaccine adjuvants: putting innate immunity to work. Immunity. 2010;33:492–503. doi: 10.1016/j.immuni.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Casella CR, Mitchell TC. Putting endotoxin to work for us: monophosphoryl lipid A as a safe and effective vaccine adjuvant. Cell Mol Life Sci. 2008;65:3231–3240. doi: 10.1007/s00018-008-8228-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wadachi R, Hargreaves KM. Trigeminal nociceptors express TLR-4 and CD14: a mechanism for pain due to infection. J Dent Res. 2006;85:49–53. doi: 10.1177/154405910608500108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Diogenes A, Ferraz CC, Akopian AN, Henry MA, Hargreaves KM. LPS sensitizes TRPV1 via activation of TLR4 in trigeminal sensory neurons. J Dent Res. 2011;90:759–764. doi: 10.1177/0022034511400225. [DOI] [PubMed] [Google Scholar]
- 62.Meseguer V, Alpizar YA, Luis E, Tajada S, Denlinger B, Fajardo O, Manenschijn JA, Fernandez-Pena C, Talavera A, Kichko T, et al. TRPA1 channels mediate acute neurogenic inflammation and pain produced by bacterial endotoxins. Nat Commun. 2014;5:3125. doi: 10.1038/ncomms4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63•.Pinho-Ribeiro FA, Verri WA, Jr, Chiu IM. Nociceptor sensory neuron-immune interactions in pain and inflammation. Trends Immunol. 2017;38:5–19. doi: 10.1016/j.it.2016.10.001. This review provides an excellent description of cross talk between the immune system and nociceptor neurons that convey the sensation of pain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Doedee AM, Boland GJ, Pennings JL, de Klerk A, Berbers GA, van der Klis FR, de Melker HE, van Loveren H, Janssen R. Effects of prophylactic and therapeutic paracetamol treatment during vaccination on hepatitis B antibody levels in adults: two open-label, randomized controlled trials. PLOS ONE. 2014;9:e98175. doi: 10.1371/journal.pone.0098175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Prymula R, Siegrist CA, Chlibek R, Zemlickova H, Vackova M, Smetana J, Lommel P, Kaliskova E, Borys D, Schuerman L. Effect of prophylactic paracetamol administration at time of vaccination on febrile reactions and antibody responses in children: two open-label, randomised controlled trials. Lancet. 2009;374:1339–1350. doi: 10.1016/S0140-6736(09)61208-3. [DOI] [PubMed] [Google Scholar]
- 66.Prymula R, Habib A, Francois N, Borys D, Schuerman L. Immunological memory and nasopharyngeal carriage in 4-year-old children previously primed and boosted with 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) with or without concomitant prophylactic paracetamol. Vaccine. 2013;31:2080–2088. doi: 10.1016/j.vaccine.2013.01.044. [DOI] [PubMed] [Google Scholar]
- 67.White JB. Robert Kennedy Jr. warns of vaccine-linked ‘holocaust’. The Sacromento Bee. 2015 [Google Scholar]
- 68.Geurtsen J, Banus HA, Gremmer ER, Ferguson H, de la Fonteyne-Blankestijn LJ, Vermeulen JP, Dormans JA, Tommassen J, van der Ley P, Mooi FR, et al. Lipopolysaccharide analogs improve efficacy of acellular pertussis vaccine and reduce type I hypersensitivity in mice. Clin Vaccine Immunol. 2007;14:821–829. doi: 10.1128/CVI.00074-07. [DOI] [PMC free article] [PubMed] [Google Scholar]


