Abstract
Vectors based on cytomegalovirus (CMV) represent a novel vaccine platform that maintains high frequencies of non-exhausted effector memory T cells in both CMV sero-positive and sero-negative individuals. In non-human primate models, CMV vectored vaccines provide unprecedented protection against simian immunodeficiency virus (SIV). Moreover, CMV vectors can be genetically altered to program highly diverse CD8+ T cell responses that differ in their epitope targeting including conventional, MHC-I restricted CD8+ T cells as well as unconventional CD8+ T cells restricted by MHC class II or non-polymorphic MHC-E. By modifying cytomegaloviral determinants that control unconventional T cell priming it is possible to uniquely tailor the CD8+ T cell response for each individual disease target in order to maximize prophylactic or therapeutic protection.
Traditional vaccination seeks to recapitulate the protective immune memory elicited by natural infection by administration of a vaccine containing a nonpathogenic alternative to the pathogen, such as an attenuated variant or a subunit vaccine. Generally, this approach works well for infectious diseases that elicit a strong and long-lasting protective immune response upon primary infection, e.g. smallpox, polio, yellow fever, and all of the typical childhood infectious diseases. However, some infectious diseases do not elicit protective immune responses or have the capacity to evade natural immunity, resulting in repeated infection (Malaria) or chronic infection (TB, HIV). In these instances, classical vaccines have either resulted in less than optimal protection or have failed altogether [1–4]. Thus, simply amplifying natural immune responses with empiric vaccination has not worked well for these immune evasive pathogens, and have led to the hypothesis that vaccine-mediated protection against such infections might require that vaccine-elicited immunity be “unnatural”, that is, qualitatively different from that induced by natural infection and designed to exploit specific immunologic vulnerabilities of the pathogen in question. Even more difficult is the eradication of an established chronic infection by therapeutic vaccination since such an immunotherapy would not only need to counter established pathogen immune evasion, but would also have to work in a setting of exhausted T cells, and pathogen exploitation of immunological sanctuaries [5,6].
The failure of HIV vaccine development for the past 3 decades well illustrates these points. Based on the ability of CD8+ T cells in some individuals with protective MHC-Ia alleles to exert stringent (elite) control viral replication, there was initial hope that powerful prime-boost vaccines that greatly increase CD8+ T cell response magnitude would provide meaningful efficacy. Experiments in nonhuman primates have not, however, supported this hope as vaccine-induced, anamnestic, HIV/SIV-specific CD8+ T cell responses still arrive at sites of infection too late to allow for most recipients to attain elite controller status, and if viral replication is not stringently controlled, mutational escape eventually allows the virus to evade the immune response [2]. Indeed, current thinking is that HIV vaccines must either prevent infection altogether or shut-down infection very early, prior to seeding of long-term viral reservoirs. Antibody (Ab) responses, which can potentially prevent establishment of infection, would seem to be the logical solution to this problem, but most natural virus- or vaccine-induced anti-envelope Abs are not able to neutralize HIV or to prevent infection by other mechanisms [3,7]. There are exceptions to this general rule, though, and much of current HIV vaccine development is focused on these exceptions, in particular on the development of vaccines that specifically elicit broadly neutralizing Abs. These Abs, identified in a subset of subjects with chronic infection, are natural, but highly unusual, both in their etiology and their ability to neutralize the majority of HIV strains. The reverse engineering of immunogens capable of “unnaturally” eliciting one or more of these unique Abs in all vaccinated people remains an important, but as yet unmet, goal [8].
Our approach to HIV vaccine development differs from all others as we hope to elicit and maintain a HIV-targeting T cell response profile that is qualitatively different both with respect to T cell phenotype as well as T cell specificity to that either elicited by HIV or by other vaccine approaches. A key aspect of this approach is the concept of early interception of cells infected by incoming viruses by continuously maintaining a circulating and tissue-resident population of effector-differentiated, HIV-specific T cells. Such responses would continuously monitor the sites of HIV entry and early dissemination and thus potentially could intercept infection at its earliest stage. The induction and maintenance of such effector-memory T cells is accomplished by using vaccine vectors derived from cytomegalovirus (CMV), a widely prevalent herpesvirus that is unusual in its capacity to elicit and maintain high frequencies of effector memory T cells in both lymphoid and extra-lymphoid sites. This effector memory concept has been reviewed in the past [2,9]. Indeed, the immunization of rhesus macaques (RM) with rhesus CMV (RhCMV) carrying inserts derived from simian immunodeficiency virus (SIV) protects more than 50% of the RM against challenge with a highly virulent strain of SIV against which traditional vaccine approaches have failed in the past [10,11]. Moreover, in those monkeys that controlled infection a unique pattern of protection emerged: while all of these monkeys manifested virologic and/or immunologic evidence of SIV infection early after SIV challenge, this evidence of viral infection waned over time until by about 18 months after infection, the initially infected, protected monkeys could no longer be differentiated from vaccinated monkeys not exposed to SIV by highly sensitive virologic and immunologic methods, including at necropsy, the first apparent clearance of a lentivirus by an immunologic mechanism [12].
Unexpectedly, our work in the RM model revealed another remarkable and unique characteristic of RhCMV vectors - an unprecedented ability of RhCMV to elicit CD8+ T cells that were “unconventional” in their MHC- restriction and epitope targeting. In the vast majority of adaptive CD8+ T cell responses, the elicited CD8+ T cells recognize peptides presented by “classical”, i.e. polymorphic, MHC class Ia molecules. Peptide loading of MHC-Ia may occur in the endoplasmic reticulum (ER) upon TAP-dependent import from the cytoplasm of infected cells (direct presentation), or alternatively, MHC-Ia molecules can be processed in the endolysosomal compartment or phagosomes of professional antigen presenting cells (APC) (cross-presentation) [13]. In striking contrast, we observed that CD8+ T cells elicited by RhCMV vectors recognized peptides either in the context of MHC-II or the non-classical, highly conserved MHC-E molecule [14,15]. MHC-II molecules are generally loaded exogenously in the endolysosomal MHC-II loading compartment of APC and recognized by CD4+ T cells [16]. In contrast, MHC-E (HLA-E in humans) mostly serves an immunological purpose not involving antigen presentation to T cells at all. Instead, MHC-E is loaded in a TAP-dependent manner with the highly conserved peptide VMAPRTL(V/L/I)L (VL9) encoded in the leader sequence of classical MHC-I molecules [17]. Upon display at the cell surface, the MHC-E/VL9 complex serves as a ligand for inhibitory NK cells receptors and as such acts as an inhibitory “self”-signal to NK cells [18]. While there have been occasional reports of CD8+ T cells recognizing peptides in the context of MHC-II [19] or MHC-E [20], the broad induction of such CD8+ T cells against any RhCMV vector-expressed protein was unprecedented. The discovery that CMV vectors are capable of inducing unconventional CD8+ T cells thus clearly falls into the qualitatively different category of vaccination approaches and represents a new paradigm in vaccine development. This finding has potentially far-reaching implications since this strategy enables targeting pathogens with novel and “unexpected” immune responses that a given pathogen has not yet “learned” how to escape. Importantly, unconventional CD8+ T cells recognize SIV-infected cells demonstrating that MHC-II and MHC-E can be loaded with peptides in SIV-infected CD4+ T cells [15]. Moreover, some HIV-derived peptides are known to be loaded into HLA-E [21], rendering it highly likely that HLA-E restricted CD8+ T cells would be able to recognize HIV-infected targets. Thus, despite their inability to elicit unconventional CD8+ T cells, SIV/HIV can nevertheless be targeted by such T cells. In contrast to priming of naive T cells, it takes very few MHC/peptide complexes to stimulate memory T cells. It is therefore likely that many pathogens can be recognized by unconventional CD8+ T cells elicited by CMV vectors even if these pathogens are unable to elicit such responses.
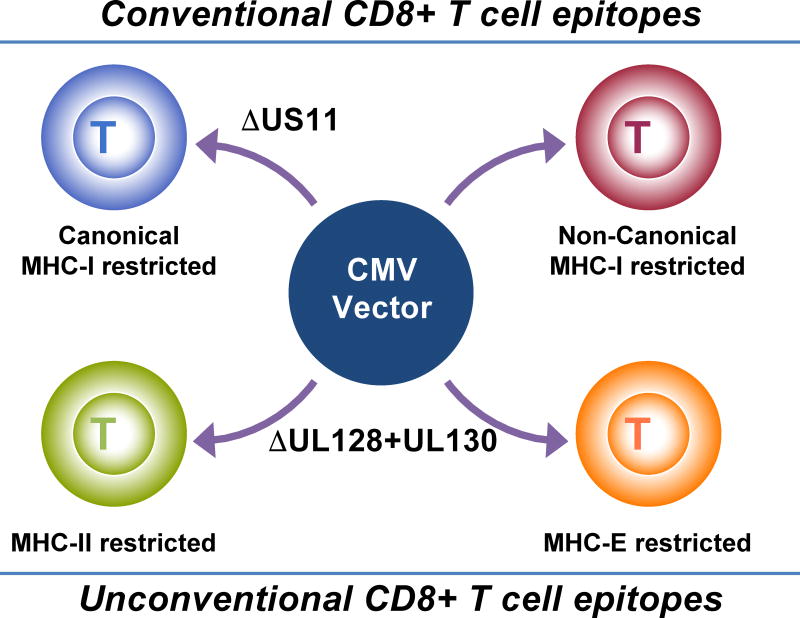
Interestingly, unconventional CD8+ T cells were not found in RM naturally infected with RhCMV. Instead, all CD8+ T cells were restricted by classical MHC-Ia molecules [15]. Similarly, CD8+ T cells of HCMV-infected humans generally recognize peptides in the context of MHC-Ia. A notable exception is CD8+ T cells recognizing a VL9 mimic encoded by the HCMV gene UL40 if the UL40 VL9 peptide is not identical to the endogenous VL9 peptide [22]. (This finding demonstrates that, in principle, HCMV is capable of eliciting HLA-E restricted CD8+ T cells in humans). As it turns out, unconventional CD8+ T cells were only induced in animals immunized with recombinant RhCMV that had been cloned from the fibroblast adapted strain 68-1. As is observed for both HCMV and RhCMV, passaging in fibroblasts routinely results in loss of the so-called pentameric glycoprotein complex [23]. This complex facilitates infection of non-fibroblast cells such as endothelial, epithelial and myeloid cells, but limits viral secretion from fibroblasts, hence the selection or mutants in vitro. Two proteins of this complex, UL128 and UL130, had been deleted spontaneously from the RhCMV 68-1 [24]. Remarkably, re-insertion of these genes into the 68-1 genome resulted in a recombinant virus (68-1.2) that only elicited MHC-I restricted CD8+ T cells, similar to wildtype RhCMV [15]. Thus, depending on the presence/absence of these proteins we can elicit a completely different set of CD8+ T cells (Fig. 1).
Figure 1. Genetically distinct RhCMV vectors elicit four different CD8+ T cell responses each recognizing non-overlapping sets of peptides.
Conventional CD8+ T cell epitopes are peptides presented by polymorphic MHC-Ia molecules. Wildtype RhCMV elicits a “non-canonical” subset of conventional CD8+ T cell epitopes that are rarely observed in the context of other vector platforms or viral infections since US11 prevents the induction of the “canonical” subset of conventional CD8+ T cell epitopes, i.e. epitopes that are frequently observed in a non-CMV context. Deletion of US11 from wildtype RhCMV thus results in the induction of conventional CD8+ T cells that recognize both canonical and non-canonical epitopes. Unconventional CD8+ T cell epitopes are peptides that are either presented by the non-polymorphic MHC-I molecule MHC-E, or by MHC-II molecules. Unconventional responses are observed upon deletion of UL128 and UL130. Additional deletion of US11 results in CD8+ T cells that recognize both conventional canonical and unconventional epitopes, but not conventional non-canonical epitopes.
However, although pentameric glycoprotein-intact RhCMV vectors carrying SIV antigens elicited T cells broadly recognizing many MHC-Ia-restricted epitopes within a given antigen, “canonical” MHC-I restricted epitopes, i.e. epitopes known to be immunodominant in the context of SIV or other vector systems, were notably absent [10,15]. This lack of canonical MHC-I restricted CD8+ T cells was shown to be due to the RhCMV homologue of HCMV US11. In the absence of US11, RhCMV elicits canonical MHC-I restricted CD8+ T cells on the background of either 68-1 [15] or 68-1.2 vectors (unpublished). Thus US11-deleted 68-1-based vectors elicit not only MHC-II and MHC-E restricted CD8+ T cells, but also canonical MHC-I restricted CD8+ T cells, whereas 68-1.2-based vectors elicit both canonical and non-canonical MHC-I restricted CD8+ T cells upon deletion of US11 (Fig. 1). The US11 protein has been studied extensively and shown to eliminate nascent MHC-I proteins by ER-associated degradation [25]. Thus, it is likely that the elimination of MHC-I by US11 in infected cells results in specific inhibition of “normal” i.e. otherwise immunodominant peptide loading. US11 belongs to the US6 family of glycoproteins of which several prevent antigen presentation by interfering with biosynthesis, intracellular transport or peptide loading of MHC-I molecules [26,27]. Why US11 uniquely prevents the induction of canonical CD8+ T cells is presently unknown. However, evasion of CD8+ T cell recognition by US6 family genes enabled CMV vectors to be used repeatedly and in individuals that are chronically infected with CMV [28]. This is yet another unique feature of CMV vectors that enables the use of CMV vectors regardless of the sero-status of the vaccine recipient, an important aspect of the clinical translating CMV vectors since the vast majority of the world’s population is chronically infected with CMV.
In ongoing work we are identifying viral and host determinants of unconventional CD8+ T cell priming which will enable us to further fine-tune the CD8+ T cell responses including vectors that exclusively elicit MHC-II or MHC-E-restricted CD8+ T cells. By using different vector backbones it will thus be possible to interrogate the protective function of each CD8+ T cell population against different pathogens in suitable non-human primate models. Ultimately, we can thus tailor vector backbones to generate optimal responses for individual pathogens. For instance in the case of SIV, using vector backbones eliciting increased frequencies of protective CD8+ T cells while reducing non-protective or neutral CD8+ T cells might increase the percent of animals protected upon SIV challenge.
Going forward, the major goal will be to determine whether such unconventional CD8+ T cells can be elicited in humans. CMVs are highly species-specific and results obtained in RM using RhCMV need to be recapitulated in humans with HCMV. However, the genomes of RhCMV and HCMV are similar enough to permit the generation of chimeric viruses to examine whether, in the context of RhCMV, HCMV-derived genes perform similar functions than their RhCMV homologs. So far, HCMV genes were consistently found to be able to substitute for RhCMV, e.g. HCMV UL128, UL130 and US11 prevent the induction of unconventional or canonical CD8+ T cells by RhCMV lacking the corresponding homologues (unpublished results). By designing human HCMV vectors with genetic modifications similar to the respective RhCMV vectors it is expected that similar CD8+ T cell response profiles will be elicited in humans.
The clinical testing of HCMV-based vaccines requires the development of attenuated vectors with reduced pathogenic potential while maintaining the unique immunological characteristics described above. Studies in murine CMV (MCMV) suggest that effector memory T cell responses are maintained by spread-deficient viruses, i.e. recombinant, gene-deleted viruses that are still DNA replication competent, but have lost their ability to assemble functional virions or that generate non-infectious virus particles [29–31]. In fact, such single-cycle viruses elicit higher T cell responses, at least initially, due to reduced dendritic cell depletion [32]. Unpublished work in the RM model supports the conclusion that immunogenicity can be uncoupled from pathogenicity. However, not all attenuation strategies seem to maintain the full spectrum of immune responses in the RM model and attenuating mutations therefore need to be carefully vetted in the RM model prior to designing corresponding attenuated HCMV vectors.
Only a very limited number of attenuated HCMV strains or recombinants have been tested in humans so far and all of them in the context of developing a vaccine against CMV [33]. (The goal of such vaccine development is to generate immune responses that recapitulate or potentially improve on the limited natural immunity that, while unable to efficiently prevent reinfection, clearly controls viremia and thus HCMV-associated diseases.) Unfortunately, published results suggest that none of the previously tested attenuated HCMV strains and recombinants are suitable as vector backbones. For example, HCMV recombinants rendered replication-deficient by expressing unstable viral transcriptional activators and replication enzymes are not expected to maintain antigen presentation and thus effector memory T cells over time [34]. Moreover, chimeric HCMV recombinants of two fibroblast-adapted strains (Towne and Toledo) were unable to re-infect seropositive individuals [35] and did not elicit effector memory T cell responses in sero-negative individuals [36]. Despite truncation of UL128, Towne/Toledo chimeras were unable to elicit unconventional T cell responses indicating that loss of the pentameric complex alone is insufficient for T cell reprogramming [37]. These results are consistent with recent observations in the RhCMV model revealing that not only loss of the pentamer but additional requirements for cell tropism as well as the presence and absence of viral gene products modulating T cell priming in a positive or negative manner, respectively, are required for unconventional T cell priming (unpublished results). Different vector backbones and attenuation strategies will thus be required to recapitulate the unique immunological characteristics of RhCMV-derived vaccines with life-attenuated HCMV vectors. If successful however, the clinical translation of results obtained with CMV vectors in animal models will have a lasting impact on vaccine development since it allows vaccine developers to unleash new types of immune responses optimized to prevent and cure pathogens that have resisted traditional vaccine approaches.
Highlights.
Effector memory T cell (TEM) inducing vaccines represent a novel paradigm in vaccine development that enables the early intercept of incoming or reactivating pathogens
Cytomegalovirus (CMV)-based vectors elicit and maintain high frequency TEM to inserted antigens
Rhesus CMV-based vaccines control and clear highly pathogenic simian immunodeficiency virus (SIV)
Specific deletions in the RhCMV genome permit the programming of CD8+ T cells to four different, non-overlapping sets of epitopes restricted by MHC-I, MHC-II or MHC-E molecules
CMV-based vaccines can be designed to elicit CD8+ T cell responses that exploit any given pathogen’s immunologic vulnerability and thereby provide optimal protection
Acknowledgments
We gratefully acknowledge the large teams of investigators involved in the design, construction and immunological analysis of CMV-based vaccine vectors. Their tireless efforts enable the paradigm-shifting results reviewed here. This work was supported by the National Institutes of Health [grant numbers AI094417, AI054292, DE021291, AI095113, AI117802, AI059457, OD010850, OD011092, GM065794, HHSN272201100013C, AI100645] and the Bill & Melinda Gates Foundation, Seattle, WA [grant numbers OPP1108533 and OPP1133649].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement: Drs. Picker and Fruh have a significant financial interest in Vir Biotechnology, Inc., a company that may have a commercial interest in the results of this research and technology. The potential individual and institutional conflicts of interest have been reviewed and managed by OHSU.
References
- 1.Hawn TR, Day TA, Scriba TJ, Hatherill M, Hanekom WA, Evans TG, Churchyard GJ, Kublin JG, Bekker LG, Self SG. Tuberculosis vaccines and prevention of infection. Microbiol Mol Biol Rev. 2014;78:650–671. doi: 10.1128/MMBR.00021-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Picker LJ, Hansen SG, Lifson JD. New paradigms for HIV/AIDS vaccine development. Annu Rev Med. 2012;63:95–111. doi: 10.1146/annurev-med-042010-085643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stephenson KE, D'Couto HT, Barouch DH. New concepts in HIV-1 vaccine development. Curr Opin Immunol. 2016;41:39–46. doi: 10.1016/j.coi.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cowman AF, Healer J, Marapana D, Marsh K. Malaria: Biology and Disease. Cell. 2016;167:610–624. doi: 10.1016/j.cell.2016.07.055. [DOI] [PubMed] [Google Scholar]
- 5.Bui JK, Mellors JW. Reversal of T-cell exhaustion as a strategy to improve immune control of HIV-1. AIDS. 2015;29:1911–1915. doi: 10.1097/QAD.0000000000000788. [DOI] [PubMed] [Google Scholar]
- 6.Fukazawa Y, Lum R, Okoye AA, Park H, Matsuda K, Bae JY, Hagen SI, Shoemaker R, Deleage C, Lucero C, et al. B cell follicle sanctuary permits persistent productive simian immunodeficiency virus infection in elite controllers. Nat Med. 2015;21:132–139. doi: 10.1038/nm.3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burton DR, Mascola JR. Antibody responses to envelope glycoproteins in HIV-1 infection. Nat Immunol. 2015;16:571–576. doi: 10.1038/ni.3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moore PL, Williamson C. Approaches to the induction of HIV broadly neutralizing antibodies. Curr Opin HIV AIDS. 2016;11:569–575. doi: 10.1097/COH.0000000000000317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Masopust D, Picker LJ. Hidden memories: frontline memory T cells and early pathogen interception. J Immunol. 2012;188:5811–5817. doi: 10.4049/jimmunol.1102695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hansen SG, Ford JC, Lewis MS, Ventura AB, Hughes CM, Coyne-Johnson L, Whizin N, Oswald K, Shoemaker R, Swanson T, et al. Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine. Nature. 2011;473:523–527. doi: 10.1038/nature10003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hansen SG, Vieville C, Whizin N, Coyne-Johnson L, Siess DC, Drummond DD, Legasse AW, Axthelm MK, Oswald K, Trubey CM, et al. Effector memory T cell responses are associated with protection of rhesus monkeys from mucosal simian immunodeficiency virus challenge. Nat Med. 2009;15:293–299. doi: 10.1038/nm.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12**.Hansen SG, Piatak M, Jr, Ventura AB, Hughes CM, Gilbride RM, Ford JC, Oswald K, Shoemaker R, Li Y, Lewis MS, et al. Immune clearance of highly pathogenic SIV infection. Nature. 2013;502:100–104. doi: 10.1038/nature12519. Using multiple highly sensitive methods it is demonstrated that monkeys immunized with RhCMV expressing SIV antigens eliminate virulent SIV over time despite being infected initially. This is the first demonstration of immune-mediated clearance of a lentivirus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blander JM. The comings and goings of MHC class I molecules herald a new dawn in cross-presentation. Immunol Rev. 2016;272:65–79. doi: 10.1111/imr.12428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14**.Hansen SG, Wu HL, Burwitz BJ, Hughes CM, Hammond KB, Ventura AB, Reed JS, Gilbride RM, Ainslie E, Morrow DW, et al. Broadly targeted CD8(+) T cell responses restricted by major histocompatibility complex E. Science. 2016;351:714–720. doi: 10.1126/science.aac9475. This paper shows that RhCMV strain 68-1 elicits CD8+ T cells recognizing peptides presented by the non-polymorphic MHC-E molecule. Together with ref. 15, this work shows that 68-1-based vectors exclusively elicit CD8+ T cells that are unconventional in their epitope restriction, either MHC-II or MHC-E. Interestingly, the peptide repertoire presented by MHC-E is highly diverse with very limited structural constraints suggesting that a monomorphic MHC-I molecule can elicit CD8+ T cell responses to almost as many different epitopes than multiple polymorphic MHC-I alleles that each have a more limited peptide repertoire. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15**.Hansen SG, Sacha JB, Hughes CM, Ford JC, Burwitz BJ, Scholz I, Gilbride RM, Lewis MS, Gilliam AN, Ventura AB, et al. Cytomegalovirus vectors violate CD8+ T cell epitope recognition paradigms. Science. 2013;340:1237874. doi: 10.1126/science.1237874. It is shown that RhCMV vectors based on strain 68-1 elicit unconventional CD8+ T cell responses recognizing approximately three times as many epitopes within a given antigen compared to conventional vaccine vectors, with the majority of these epitopes being recognized in the context of MHC-II. Repair of the RhCMV orthologs of UL128 and UL130 results in conventional, MHC-I-restricted CD8+ T cell responses to subdominant, non-canonical epitopes, whereas vectors lacking the US11-ortholog elicit canonical, MHC-I restricted epitopes. This is the first demonstration of a vector platform that can be programmed to elicit CD8+ T cell responses that differ in their epitope targeting and MHC-restriction. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garstka MA, Neefjes J. How to target MHC class II into the MIIC compartment. Mol Immunol. 2013;55:162–165. doi: 10.1016/j.molimm.2012.10.022. [DOI] [PubMed] [Google Scholar]
- 17.Braud V, Jones EY, McMichael A. The human major histocompatibility complex class Ib molecule HLA-E binds signal sequence-derived peptides with primary anchor residues at positions 2 and 9. Eur J Immunol. 1997;27:1164–1169. doi: 10.1002/eji.1830270517. [DOI] [PubMed] [Google Scholar]
- 18.Braud VM, Allan DS, O'Callaghan CA, Soderstrom K, D'Andrea A, Ogg GS, Lazetic S, Young NT, Bell JI, Phillips JH, et al. HLA-E binds to natural killer cell receptors CD94/NKG2A, B and C. Nature. 1998;391:795–799. doi: 10.1038/35869. [DOI] [PubMed] [Google Scholar]
- 19*.Ranasinghe S, Lamothe PA, Soghoian DZ, Kazer SW, Cole MB, Shalek AK, Yosef N, Jones RB, Donaghey F, Nwonu C, et al. Antiviral CD8+ T Cells Restricted by Human Leukocyte Antigen Class II Exist during Natural HIV Infection and Exhibit Clonal Expansion. Immunity. 2016;45:917–930. doi: 10.1016/j.immuni.2016.09.015. It is shown for the first time that some HIV-infected individuals who spontaneously control virus in the absence of antiretroviral therapy contain HIV-specific CD8+ T cells restricted by MHC-II. Importantly, these unconventional CD8+ T cells effictively killed autologous, HIV-infected CD4+ T cells thus suggesting that these T cells contribute to HIV control. These observations also demonstrate that while the induction of MHC-II-restricted CD8+ T cells might be rare in the context of HIV, such unconventional CD8+ T cells can nevertheless recognize HIV-infected targets. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joosten SA, Sullivan LC, Ottenhoff TH. Characteristics of HLA-E Restricted T-Cell Responses and Their Role in Infectious Diseases. J Immunol Res. 2016;2016:2695396. doi: 10.1155/2016/2695396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21*.Davis ZB, Cogswell A, Scott H, Mertsching A, Boucau J, Wambua D, Le Gall S, Planelles V, Campbell KS, Barker E. A Conserved HIV-1-Derived Peptide Presented by HLA-E Renders Infected T-cells Highly Susceptible to Attack by NKG2A/CD94-Bearing Natural Killer Cells. PLoS Pathog. 2016;12:e1005421. doi: 10.1371/journal.ppat.1005421. It is demonstrated that the highly conserved peptide AISPRTLNA derived from the HIV-1 capsid protein is presented by HLA-E. While this peptide is not recognized by inhibitory NK cell receptors it will enable the recognition of HIV infected CD44+ T cells by HLA-E-restricted CD8+ T cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pietra G, Romagnani C, Mazzarino P, Falco M, Millo E, Moretta A, Moretta L, Mingari MC. HLA-E-restricted recognition of cytomegalovirus-derived peptides by human CD8+ cytolytic T lymphocytes. Proc Natl Acad Sci U S A. 2003;100:10896–10901. doi: 10.1073/pnas.1834449100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sinzger C, Digel M, Jahn G. Cytomegalovirus cell tropism. Curr Top Microbiol Immunol. 2008;325:63–83. doi: 10.1007/978-3-540-77349-8_4. [DOI] [PubMed] [Google Scholar]
- 24.Gill RB, Jason Bowman J, Krogmann T, Wollenberg K, Asher DM, Cohen JI. Coding potential of UL/b' from the initial source of rhesus cytomegalovirus Strain 68-1. Virology. 2013;447:208–212. doi: 10.1016/j.virol.2013.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van der Wal FJ, Kikkert M, Wiertz E. The HCMV gene products US2 and US11 target MHC class I molecules for degradation in the cytosol. Curr Top Microbiol Immunol. 2002;269:37–55. doi: 10.1007/978-3-642-59421-2_3. [DOI] [PubMed] [Google Scholar]
- 26.Powers C, DeFilippis V, Malouli D, Fruh K. Cytomegalovirus immune evasion. Curr Top Microbiol Immunol. 2008;325:333–359. doi: 10.1007/978-3-540-77349-8_19. [DOI] [PubMed] [Google Scholar]
- 27.Pande NT, Powers C, Ahn K, Fruh K. Rhesus Cytomegalovirus Contains Functional Homologues of US2, US3, US6, and US11. J Virol. 2005;79:5786–5798. doi: 10.1128/JVI.79.9.5786-5798.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hansen SG, Powers CJ, Richards R, Ventura AB, Ford JC, Siess D, Axthelm MK, Nelson JA, Jarvis MA, Picker LJ, et al. Evasion of CD8+ T cells is critical for superinfection by cytomegalovirus. Science. 2010;328:102–106. doi: 10.1126/science.1185350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mohr CA, Arapovic J, Muhlbach H, Panzer M, Weyn A, Dolken L, Krmpotic A, Voehringer D, Ruzsics Z, Koszinowski U, et al. A spread-deficient cytomegalovirus for assessment of first-target cells in vaccination. J Virol. 2010;84:7730–7742. doi: 10.1128/JVI.02696-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Snyder CM, Cho KS, Bonnett EL, Allan JE, Hill AB. Sustained CD8+ T cell memory inflation after infection with a single-cycle cytomegalovirus. PLoS Pathog. 2011;7:e1002295. doi: 10.1371/journal.ppat.1002295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Snyder CM, Allan JE, Bonnett EL, Doom CM, Hill AB. Cross-presentation of a spread-defective MCMV is sufficient to prime the majority of virus-specific CD8+ T cells. PLoS One. 2010;5:e9681. doi: 10.1371/journal.pone.0009681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32*.Loo CP, Snyder CM, Hill AB. Blocking Virus Replication during Acute Murine Cytomegalovirus Infection Paradoxically Prolongs Antigen Presentation and Increases the CD8+ T Cell Response by Preventing Type I IFN–Dependent Depletion of Dendritic Cells. The Journal of Immunology. 2016 doi: 10.4049/jimmunol.1600478. Following the demonstration that single cycle MCMV elicits effector CD8+ T cell memory inflation (ref. 30) this group now reports the surprising finding that, during the acute phase of infection, CD8+ T cell frequencies are higher upon infection with spread-deficient as compared to wildtype virus due to decreased type I interferon–dependent depletion of conventional dendritic cells that mediate MHC-I cross-presentation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fu TM, An Z, Wang D. Progress on pursuit of human cytomegalovirus vaccines for prevention of congenital infection and disease. Vaccine. 2014;32:2525–2533. doi: 10.1016/j.vaccine.2014.03.057. [DOI] [PubMed] [Google Scholar]
- 34*.Wang D, Freed DC, He X, Li F, Tang A, Cox KS, Dubey SA, Cole S, Medi MB, Liu Y, et al. A replication-defective human cytomegalovirus vaccine for prevention of congenital infection. Sci Transl Med. 2016;8:362ra145. doi: 10.1126/scitranslmed.aaf9387. This paper describes pre-clinical studies of a pentamer-intact, conditionally replicating HCMV as a vaccine against HCMV. Two essential viral proteins were fused to a degradation domain resulting in a virus that depends on a small molecule stabilizer for viral growth. Despite being replication-deficient in vivo, the virus was immunogenic in animal models. [DOI] [PubMed] [Google Scholar]
- 35.Heineman TC, Schleiss M, Bernstein DI, Spaete RR, Yan L, Duke G, Prichard M, Wang Z, Yan Q, Sharp MA, et al. A phase 1 study of 4 live, recombinant human cytomegalovirus Towne/Toledo chimeric vaccines. J Infect Dis. 2006;193:1350–1360. doi: 10.1086/503365. [DOI] [PubMed] [Google Scholar]
- 36*.Adler SP, Manganello AM, Lee R, McVoy MA, Nixon DE, Plotkin S, Mocarski E, Cox JH, Fast PE, Nesterenko PA, et al. A Phase 1 Study of Four Live, Recombinant Human Cytomegalovirus Towne/Toledo Chimera Vaccines in CMV Seronegative Men. J Infect Dis. 2016 doi: 10.1093/infdis/jiw365. The first clinical testing of live-attenuated, recombinant HCMV in seronegative individuals. The immunogenicity of four different UL128-deficient recombinants between the highly passaged strain Towne and the low-passage strain Toledo were evaluated at relatively low doses (≤1000PFU) in human volunteers. (All chimeras contained the ULb' region of Toledo and are thus deficient for UL128.) In contrast to sero-negative individuals (ref 35), several of the volunteers displayed antibody and T cell responses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37*.Murray SE, Nesterenko PA, Vanarsdall AL, Munks MW, Smart SM, Veziroglu EM, Sagario LC, Lee R, Claas FHJ, Doxiadis IIN, et al. Fibroblast-adapted human CMV vaccines elicit predominantly conventional CD8 T cell responses in humans. J Exp Med. 2017;214:1889–1899. doi: 10.1084/jem.20161988. The T cell responses in some of the sero-negative volunteers inoculated with two of the Towne/Toledo chimeras (Ref. 36) were analyzed in more detail. It is shown that the CD8+ T cell responses to individual epitopes were significantly weaker than those induced by natural HCMV infection but similarly restricted by MHC-1 molecules. Thus, random mutations acquired during fibroblast-adaptation did not enable Towne and Toledo to elicit unconventional CD8+ T cells suggesting that genetic modifications of HCMV genomes need to more closely resemble those found in fibroblast-adapted RhCMV 68-1 to recapitulate the unique ability of RhCMV 68-1 to elicit unconventional CD8+ T cell responses. [DOI] [PMC free article] [PubMed] [Google Scholar]