Abstract
Objective
The hippocampus is a key structure implicated in food motivation and intake. Research has shown that the hippocampus is vulnerable to the consumption of a western diet (i.e., high saturated fat and simple carbohydates). Studies of patients with obesity (OB), compared to healthy weight (HW), show changes in hippocampal volume and response to food cues. Moreover, evidence suggests that OB children, relative to HW, have greater hippocampal response to taste. However, no study has examined the association of hippocampal volume with taste functioning in children. We hypothesized that OB children, relative to HW, would show a significant reduction in hippocampal volume and that decreased volume would be significantly associated with greater activation to taste. Finally, we explored whether hippocampal activation would be associated with measures on eating and eating habits.
Subjects
Twenty-five 8–12 year old children (i.e., 13 HW, 12 OB) completed a Magnetic Resonance Imaging scan while participating in a taste paradigm (i.e., 1mL of 10% sucrose or ionic water delivered pseudorandomly every 20 seconds).
Results
Children with OB, relative to HW, showed reduced left hippocampal volume (t=1.994, p=0.03, 95%CI=−40.23,755.42), and greater response to taste in three clusters within the left hippocampus (z=3.3, p=0.001, 95%CI=−0.241, −0.041; z=3.3, p=0.001, 95%CI=−0.2711, −0.0469; z=2.7, p=0.007, 95%CI= −0.6032, −0.0268). Activation within the hippocampus was associated with eating in the absence of hunger (EAH%; t = 2.408, p=0.025, 95%CI= 1.751708, 23.94109) and two subscales on a measure of eating behaviors (Food responsiveness, t = 2.572, p=0.017, 95%CI= 0.9565195, 9.043440; Food enjoyment, t = 2.298, p= 0.032, 95%CI= 0.2256749, 4.531298).
Conclusion
As hypothesized, OB children, relative to HW, had significantly reduced hippocampal volume, and greater hippocampal activation to taste. Moreover, hippocampal activation was associated with measures of eating. These results contribute to research on the relationship between obesity, overeating and cognitive impairment.
Keywords: obesity, MRI, brain imaging, Hippocampus
Introduction
Approximately one-third of children in the United States are either overweight or obese (1). Childhood obesity (OB) is associated with poorer health outcomes and is highly correlated with adult obesity (2–4). Research suggests that obesity is associated with brain changes which may lead to hyperphagia and cognitive impairment (5). In particular, increased weight is associated with damage in memory regions (i.e., hippocampus) and faster rates of cerebral atrophy, as well as increased dementia risk in elderly adults (6–9). Due to the high prevalence of obesity in the United States, further research is needed to establish whether these observed changes can be seen in early childhood, to understand mechanisms which may impact critical neural developmental periods.
Overeating is a major contributor to obesity (10). Although the hypothalamus and hindbrain are identified as key neural structures in eating (11–13), other less studied higher order brain regions, such as the hippocampus, are also considered crucial to the initiation and termination of eating (11, 14). The hippocampus plays an important role in food intake regulation by detecting learned signals which are paired with eating and the consequences of eating (11, 15–17). The hippocampus receives input from brain regions involved in the perception of internal cues, taste, reward (e.g., hypothalamus, thalamus, amygdala), as well as metabolic and neurochemical signals known to be associated with energy intake and weight regulation (e.g., Ghrelin, CCK, Insulin) (15, 18). Animal research shows that damage or inactivation of this area impairs perception and processing of interoceptive signals of energy states, increases food-seeking behaviors and food intake, and decreases the postprandial intermeal interval (i.e., amount of time from the end of one meal to the beginning of the next) (16, 18, 19). Moreover, in humans, the hippocampus is implicated in eating behaviors and body weight regulation. In amnestic patients with bilateral hippocampal damage, difficulties in identifying hunger states and consumption of several meals consecutively are reported (15, 20, 21).
Due to its role in eating, the hippocampus has become a region of interest in the study of obesity. Obesity is associated with inflammation, gliosis and reductions in grey matter in the hippocampus (5, 9, 22, 23), which have been associated with memory and hippocampal-dependent cognitive impairments (24–26). These changes in hippocampal grey matter volume are hypothesized to be related to the consumption of western diets (i.e., High saturated fat foods) (14, 18, 27, 28). In animals, signs of hippocampal pathology (e.g., damage to the blood brain barrier (BBB) and mRNA expression) were detected after only ten days on a western diet (29). Moreover, in adults, a western diet, independent of normal aging, was associated with a smaller left hippocampal volume (30), while in children ages eight to ten years, OB children showed reduced hippocampal volume, relative to healthy-weight (HW) (9). A “vicious cycle of obesity and cognitive decline” was proposed such that a high-fat, high-sugar diet impacts hippocampal structure and function through a break down of the blood brain barrier(14). Damage in this area can then result in an inability to inhibit the activation of memories related to food and the rewarding consequences of eating, and increased food intake due to difficulties in detecting hunger and satiety signals (14, 15, 17, 18).
An emerging body of research in humans is beginning to demonstrate functional activation differences in the hippocampus among individuals who are OB and HW. Changes in hippocampal functioning have been detected early in the lifespan as higher waist circumference was associated with greater hippocampal activation in adolescents in response to high calorie food images(31). Additionally, abnormal activity in the hippocampus in response to a satiating meal does not appear to return to its previous functionality after weight reduction (32). Interestingly, no study has examined both functional and structural differences between OB and HW individuals. Considering that structural changes to the hippocampus are associated with changes in function, more research is needed on whether both structure and functional changes are detected. This is especially important in youth, as the hippocampus develops through mid-adolescence (33).
This study aimed to fill the gap in the literature by evaluating hippocampal differences among 8–12 year old OB and HW children. To our knowledge, this is the first study to compare both hippocampal volume with its functional response to pleasant tastes in youth. We expect that OB children will show reduced hippocampal volume compared to HW children. Additionally, we expect that OB children, relative to HW, will display significantly greater activation in response to taste in the hippocampus. Novel to this study, we predict that hippocampal volume will be associated with activation in the hippocampus in response to taste. We will also exam whether responsivity in the hippocampus was associated with eating behaviors. These findings could contribute to the current knowledge base regarding the neural underpinnings of obesity and food cue reactivity, and resulting cognitive impairments.
Methods
Subjects
Twelve OB children and 13 age and gender matched HW children (8–12 years old) were recruited from the community and participated in this study. A subset of this sample was analyzed in a prior publication which focused on responses to appetitive tastes in the insula and amygdala in OB compared to HW children following a satiating meal (34). The functional analyses for this manuscript included the same sample as in a prior publication (34), but specifically focused on hippocampal functioning, while the structural analyses included two additional children that were not in the original study. Children were recruited who were either OB (>95%BMI) or HW (<85% BMI)), right-handed, fluent in English, and liked cheese pizza (needed for the Eating in the Absence of Hunger (EAH) task). Exclusion criteria included any diagnosis of an eating disorder (diagnosed by Child Eating Disorder Examination [chEDE])(35) or other significant psychiatric disorder (Mini International Neuropsychiatric Interview for Children and Adolescents [MINI-KID])(36). In addition, children could not have any other medical/neurologic concerns or conditions contraindicative to MRI (e.g., traumatic brain injuries, surgical metallic implants and claustrophobia). Child height in centimeters and weight in kilograms were measured twice using a portable Schorr height board (Schorr Inc, Olney, MD) and Tanita Digital Scale (model WB-110A). Using the average of the 2 values for height and weight, Body Mass Index (BMI;kg/m2) was calculated and translated to BMI for age percentile score using the CDC growth charts (37). This study conformed to the Institutional Review Board regulations of the University of California, San Diego. Written informed assent and consent was acquired from the children and their parents respectively. This study represents a secondary data analyses of primary aims previously published (34).
Experimental Design
During the first study visit, the MINI-KID (36) and chEDE-C(35) were used to rule out any significant psychiatric or eating disorders in children. Parents completed the Child Eating Behavior Questionnaire (CEBQ-PR) (38, 39) and children participated in the EAH (40) paradigm, which measures the percent of daily caloric needs consumed of snack foods when sated in a free access session (EAH%) (40, 41). Prior to the scan, children participated in a mock-scan to acclimate to the noises and experience of the MRI scanner. During a second visit, following a standardized breakfast (i.e., one bagel with cream cheese, one banana, orange juice), children completed a 1-hour functional magnetic resonance imaging (fMRI) scan during which structural scans, in addition to a taste paradigm previously described elsewhere (42), were performed.
MRI/fMRI
Imaging data were collected with a 3T Signa Excite scanner (GE Medical Systems). FMRI was collected with gradient-recalled echoplanar imaging (EPI) (TR=2000 ms, TE=30 ms, flip angle=80°, 64 × 64 matrix, ASSET factor=2, 40 2.6-mm ascending interleaved axial slices with a 0.4-mm gap, 200 volumes) (34). The first four volumes of each run were discarded so as to discount T1 saturation. EPI-based field maps were acquired for correcting susceptibility-induced geometric distortions (34). A high resolution T1-weighted image (SPGR, TI=600 ms, TE=min full, flip angle=8°, 256 × 192 matrix, 170 1.2 mm contiguous slices) was obtained for subsequent spatial normalization and later used for structural analyses.
Definition of Anatomical Regions of Interest
Our region of interest (ROI) (i.e., bilateral hippocampus) was chosen based on literature showing the importance of this region in feeding behaviors and body weight regulation. A single bilateral ROI for the hippocampus was derived from the Harvard-Oxford Atlas (43).
Statistical Analysis
All children (i.e., 13 HW, 12 OB) were included in the structural analyses. The Freesurfer version 5.3.0 image analysis suite (http://surfer.nmr.mgh.harvard.edu/) was used for the volumetric segmentation of subcortical gray and white matter regions. Individual cortical and subcortical region volumes for each subject were normalized to the subject’s total brain volume estimated by the Freesurfer segmentation process. The Freesurfer segmentation files for all subjects were visually inspected for quality assurance. No segmentation file required hand-editing. This method has been previously described in detail (44–46). The individual brain volume segments for each group (i.e., HW and OB) were then averaged over all subjects for each group and compared using linear regression models in R.
Analysis of Functional NeuroImages (AFNI) software (47) and R statistical packages (http://www.r-project.org) (48) were used to preprocess and analyze functional images, as described in our publication (34). Briefly, EPI images were motion-corrected and aligned to high-resolution anatomical images. AFNI’s 3dToutcount was used to generate outliers and volumes with 10% of voxels marked as outliers were not used in subsequent analyses. Based on motion exclusion criteria (e.i., greater than 20% of the volumes being censored and/or over 3mm of movement), two children in the OB group were excluded from the fMRI analyses. Ten OB children (50% female; BMI>95th% for age; age 10.09 ± 1.00 years) and 13 HW children (38.4% female; BMI<85th%; age 10.38 ± 1.26 years) were included in functional analyses.
A linear mixed effects (LMEs) analysis in R was performed for each voxel within the left and right hippocampus. Two separate models were constructed in which subject was treated as a random effect. In one model, diagnosis (OB, HW) was treated as the between subjects factor and condition (sucrose, water) as the within subjects factor. As no group by condition interaction was observed, we reduced the model to include group (OB, HW) and condition (i.e., combining the sucrose and water conditions) as the between subjects factors. Small volume correction was determined with Monte-Carlo simulations (via AFNI’s 3dClustSim) to guard against false positives. To correct for multiple comparisons, a thirteen-voxel cluster-size threshold and a twelve-voxel cluster-size threshold were used in the left and right hippocampus respectively.
Correlational analysis
Huber robust regression models were performed in R to examine potential correlations between the mean percent signal change in each significant cluster within the left hippocampus and left hippocampal grey matter volume in all children (i.e. N=23). Potential correlations between measures of eating variables and significant clusters in the functional analyses were also examined.
Results
Participant Demographics
There were no significant differences in age (t = 0.68, p = 0.51), gender (χ2 = 0.03, p = 0.87) or race (χ2 = 0.12, p = 0.73) between OB or HW children (Table 1). OB children had significantly higher BMIs relative to HW (BMI, t = 7.77, p < 0.0001; BMI %, t = 5.13, p < 0.0001). OB children, relative to HW, also scored significantly higher on six measures of the CEBQ-PR (Table 1), and tended to have higher EAH (p < 0.07). No differences in age, gender or race were found in the functional MRI subsample (i.e., 13 HW, 10 OB).
Table 1.
Values are means and standard deviations from the mean. No significant differences were found between healthy weight and obese children on age, gender, or race. As expected, obese children, relative to healthy weight, had a significantly higher BMI (p < 0.001). Obese children, relative to healthy weight, also had significantly greater scores on six measures of the Child Eating Behavior Questionnaire Parent-Report (CEBQ-PR) (i.e., Food Responsiveness, p < 0.001; Emotional Over Eating, p < 0.05; Enjoyment of Food, p < 0.01; Satiety Responsiveness, p < 0.01; Slowness of Eating, p < 0.05; Food Fussiness, p = 0.05), and tended to score higher on EAH (p < 0.07). P values derived from T-test.
| Subject Demographics | Healthy Weight (N=13) | Obese (N=12) |
|---|---|---|
| N, female | 5 (38.4%) | 5 (42%) |
| Age, years | 10.38 ± 1.26 | 10.08 ± 1.00 |
| BMI, kg/m2 | 17.71 ± 1.90 | 26.10 ± 3.23*** |
| Ethnicity | ||
| N, White | 8 (62%) | 6 (50%) |
| N, Black | 5 (38%) | 5 (42%) |
| N, Asian | 0 | 1 (8%) |
| EAH | 9.63 ± 2.96 | 17.10 ± 2.50 |
| Child Eating Behavior Questionnaire (Parent-Report) | ||
| Food Responsiveness | 2.20 ± 0.23 | 3.92 ± 0.34*** |
| Emotional Over Eating | 1.60 ± 0.17 | 2.60 ± 0.41* |
| Enjoyment of Food | 3.79 ± 0.13 | 4.58 ± 0.20** |
| Desire to Drink | 2.36 ± 0.14 | 2.90 ± 0.39 |
| Satiety Responsiveness | 2.74 ± 0.15 | 1.94 ± 0.19** |
| Slowness of Eating | 2.62 ± 0.13 | 1.86 ± 0.24* |
| Emotional Under Eating | 2.83 ± 0.26 | 2.93 ± 0.28 |
| Food Fussiness | 2.40 ± 0.18 | 3.22 ± 0.33* |
p < 0.05,
p<0.01,
p < 0.001
Structural analyses
In OB children, linear regression models showed a significant reduction in left hippocampal volume relative to HW children (t = 1.994, p = 0.03) (Figure 1). No significant group differences were found in right hippocampal volume (p = 0.34).
Figure 1.
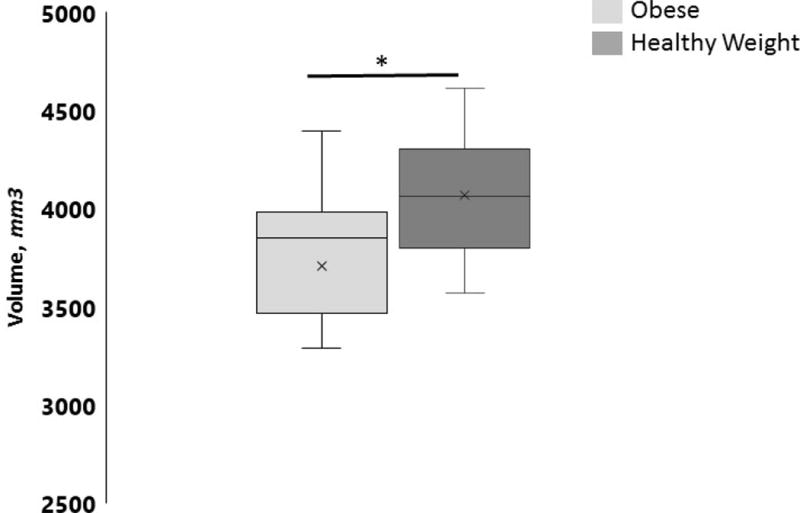
Comparison of left hippocampal volume between obese (OB) and healthy weight (HW) children. OB children had significantly lower left hippocampal volume compared to HW children (t = 1.994, p = 0.03). Data are means of group left hippocampal volume. * p < 0.05.
Functional imaging results
LME models revealed a significant main effect of group in the left hippocampus in three clusters. Post hoc analyses showed that OB children, compared to HW, had significantly greater response to taste (sucrose and water) within the tail (dorsal) (Cluster 1: 24, −33, −8, z = 3.3, p = 0.001), body (Cluster 2: −31, −21, −15, z = 3.3, p = 0.001) and head (ventral) (Cluster 3: −20, −8, −24, z = 3.3, p = 0.007) of the left hippocampus (Figure 2). No interaction of group by condition or main effect of condition was found. No significant effects were seen in the right hippocampus.
Figure 2.
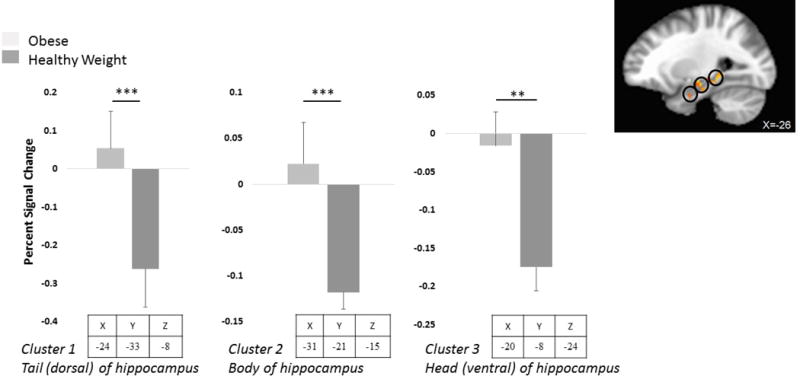
Brain activation by taste (i.e., water and sucrose combined) in obese (OB) and healthy weight (HW) children. In OB children, relative to HW, brain activation by taste was significantly higher in the tail (dorsal), body and head (ventral) portions of the left hippocampus. p values derived from Huber robust regressions and r values derived from Pearson product-moment correlations. *p < 0.01, **p < 0.001
Correlations between functional activation and grey matter volume
Huber robust regressions in R, including all children, assessed associations between hippocampal volume and strength of left hippocampal activation in response to taste in each of the three significant clusters. A trend for significance was found in the dorsal left hippocampus (37 voxels) such that stronger activation to taste tended to be associated with reduced left hippocampal volume (t = −1.56, p = 0.07). A significant association was found in the body of the left hippocampus (24 voxels) such that stronger activation to taste was significantly associated with reduced left hippocampal volume (t = −2.22, p = 0.02). No association was found between activation in the ventral left hippocampus (23 voxels) and left hippocampal volume (t = −1.40, p = 0.18) (Figure 3).
Figure 3.
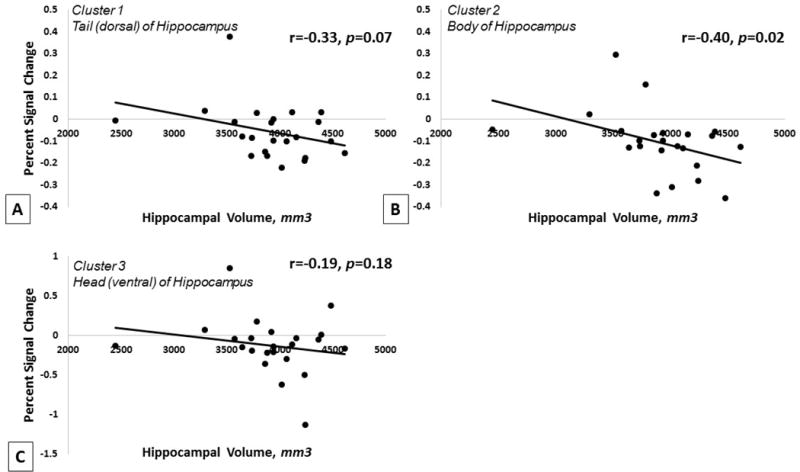
Association between A) left hippocampal activation in the tail (dorsal) of the hippocampus and left hippocampal grey matter volume, B) left hippocampal activation in the body of the hippocampus and left hippocampal grey matter volume, and C) left hippocampal activation in the head (ventral) of the hippocampus and left hippocampal grey matter volume, across all participants included in fMRI analyses (OB and HW). Results show a negative trend with lower activation in the dorsal hippocampus associated with greater left hippocampal volume, and a significant association with lower activation in the body of the hippocampus associated with significantly greater left hippocampal volume. No association was found in the ventral hippocampus. p values derived from Huber robust regressions and r values derived from Pearson product-moment correlation.
Correlations between functional activation and eating variables
Huber robust regressions were performed in R to assess the association between total EAH% and strength of left hippocampal activation in response to taste in all significant clusters. Results showed a significant association in the ventral left hippocampus (23 voxels) such that stronger activation to taste was significantly associated with greater total calories consumed during EAH (t = 2.408, p = 0.025) (Figure 4). No significant associations were found in the two other clusters within the left hippocampus.
Figure 4.
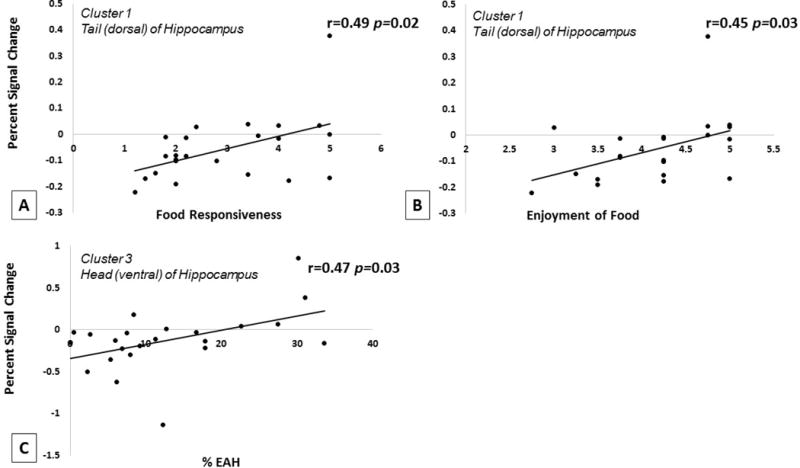
Association between A) left hippocampal activation in the tail (dorsal) of the hippocampus and food responsiveness, B) left hippocampal activation in the dorsal hippocampus and food enjoyment, and C) left hippocampal activation in the head (ventral) of the hippocampus and % EAH, across all children included in fMRI analyses (OB and HW). Results show significant positive associations with greater activation in the dorsal left hippocampus associated with food responsiveness and enjoyment, and greater activation in the ventral left hippocampus associated greater % EAH. p values derived from Huber robust regressions and r values derived from Pearson product-moment correlations.
The association between parent report of the child’s food responsiveness, enjoyment of food and satiety responsiveness and strength of left hippocampal activation in response to taste in each of the three significant clusters was also assessed. Results showed a significant association in the dorsal left hippocampus (37 voxels) with the food responsiveness (t = 2.572, p= 0.017) and enjoyment of food (t = 2.298, p= 0.032) (Figure 4). No other associations were found between the other two clusters and the subscales on the CEBQ-PR.
Discussion
This is the first study to demonstrate differences in hippocampal structure and function between OB and HW children. Prior studies have focused on regions associated with food reward and motivation such as the amygdala, insula, and ventral striatum (5). The hippocampus has been shown to be important in feeding behaviors and weight regulation through its role in the perception and processing of hunger and satiety signals, as well as in its role in initiating or terminating eating using learned interoceptive satiety cues (11, 14, 15, 17). In this study, OB children, relative to HW, showed significantly reduced left hippocampal volume as well as a hypersensitivity to taste cues in three clusters within in left hippocampus. Activation to taste in the left hippocampus was negatively associated with left hippocampal volume. This could suggest a hypersensitivity to food taste in OB children, relative to HW, as evidenced by increased scores on behavioral measures of food sensitivity (i.e., CEBQ-PR food responsiveness subscale) or an over-compensation in this region due to a reduction in volume. Importantly, this study demonstrated that functional activation to taste in the hippocampus was associated with eating in the lab, food responsiveness and enjoyment of food in children. In sum, our data show that OB children, relative to HW, had lower hippocampal volume and greater activation in the left hippocampus, and that activation in this region is associated with eating when satiated, responsiveness to food, and greater enjoyment of food.
Our study adds to a small body of literature showing a left hippocampal volume reduction in OB children, compared to HW. This finding is consistent with one other study which examined the association between obesity in children and reductions in hippocampal volume (9). These observed changes in the hippocampus could be due to the impact of sustained consumption of a western diet on the blood brain barrier (BBB) (14). The maintenance of a western diet may lead to damage of the BBB including a reduction in expression of the proteins that make up the BBB and an increase in BBB permeability (49). The BBB in the hippocampal formation area is especially prone to damage associated with the western diet (49, 50). This increased vulnerability is thought to be a result of the high nutrient demands and pronounced cellular plasticity of the hippocampus (14, 51). As a result, hippocampal functioning is particularly susceptible to damage by western diets. It should be noted that other processes (i.e., inflammation and hormonal imbalance) have also been implicated in the relationship between obesity and hippocampal damage (5, 22). Moreover, our data are cross-sectional, and it is impossible to determine whether changes in structure preceed or are a result of children becoming obese from a sustained western diet. Future studies should include a dietary recall variable to assess the effect of the consumption of a western diet. In addition, prior research has shown that the left hippocampus may be more prone to neurodegeneration (30). Greater vulnerability of the left hippocampus, relative to the right, is consistent with our results and may be explained by the functional lateralization of this region. The left hippocampus is more involved in context-dependent episodic/autobiographical memory (52). Since eating is a social experience, involving more contextual cues, the left hippocampus may be more implicated in food cue signaling and satiety, making it more vulnerable to the effects of diet.
Our results also showed that OB children had higher responsivity to taste in the dorsal, body, and ventral portions of the left hippocampus compared to HW children. This is in line with a prior study in adolescents which showed that waist circumference was significantly positively associated with activation in the left hippocampus in response to high-calorie food pictures (31). Although the dorsal and ventral portions of the hippocampus differ in terms of functioning(53), no significant difference in activation were found among our three clusters. Yet, this study demonstrates that altered response to food tastes in the hippocampus using a taste paradigm can be seen as early as 8 years old. Considering that the hippocampus is involved in memory, place preference and hunger and satiety detection, these results could help explain why OB children tend to overeat as they may be less able to perceive and process satiety signals.
Hippocampal activation in response to food taste was also associated with reduced hippocampal volume in OB and HW children. In particular, our study showed associations between left hippocampal volume and activation to taste, which is consistent with the “vicious cycle of obesity and cognitive decline” (14). Damage to the integrity of the BBB, and resulting increased BBB impermeability, due to consumption of the Western diet (14, 49) could lead to a heightened vulnerability of the hippocampus to toxins or illnesses and thus to alterations in hippocampal functioning. In addition, animal research has shown that long term consumption of a western diet may lead to neuroinflammation in the hippocampus (54), reduced hippocampal levels of brain-derived neurotrophic factor (55) (BDNF; i.e., protein promoting neurogenesis, synaptic transmission, and memory performance), and impairements in long-term potentiation in the hippocampus (56). Therefore, it is possible that a greater consumption of the western diet could lead to damage to the hippocampus and heightened response in the hippocampus to food taste as a compensatory mechanism. Future longitudinal studies should explore this further using dietary recall.
Moreover, this study found associations between structure and activation in the hippocampus in one out of the three significant clusters in the left hippocampus. It is possible that more clusters would show significance in older individuals who have been obese for longer (5). It is also possible that certain individuals at risk for obesity could have a predisposition for hippocampal dysfunction, making them susceptible to overeating and further dysfunction in this brain region.
Importantly, this study demonstrates that response to taste in the hippocampus is associated with eating behavior in OB and HW children. Greater response to taste within the ventral left hippocampus was positively associated with the total amount of calories consumed when sated. In addition, greater response to taste within the dorsal left hippocampus was positively associated with parent report of child’s food responsiveness and enjoyment of food. Taken together, these results demonstrate that activation to taste in the hippocampus is associated with eating, reward and responsiveness to food in this sample.
As far as we are aware, this is the first study to specifically examine the response to taste in the hippocampus in children as young as 8 years old. It is crucial to study the impact of obesity on the brain as childhood is an important stage in neural development. The hippocampus has been shown to continue developing into mid adolescence (33). Therefore, damage done during developmental years could have long lasting effects and could predispose individuals to a lifetime of overeating. Our own review found that OB children, compared to HW, have poorer cognitive functioning exhibited by deficits in the areas of executive functioning, attention, visuo-spatial and motor skills, learning and memory, language and academic achievement (57). These observed deficits in executive dysfunction, motor skill, and academic achievement have been related to obesity-related behaviors (e.g., increased disinhibited eating and sedentary activity). It is possible that these changes perpetuate overeating or worsen if these children remain in a state of obesity. The hippocampus is one of two regions known for neurogenesis, which further supports the need for early intervention to promote potential recovery of function in this region (33). Thus, these findings show the importance to develop interventions to promote healthy eating and reduce food cue reactivity, and highlights the need to intervene earlier than 8 years of age.
A strength of our study is the use of a sample of young children which provides a better understanding of the development of underlying mechanisms and of the early neural changes associated with obesity. Our study adds to this growing body of literature by showing that these changes can be detected earlier than previously reported (58). In addition, this study examined both structural and functional changes in the hippocampus, and the relationship between the two, in a pediatric sample. Although one study demonstrated similar differences among OB and HW children in hippocampal structure (9), none to date has examined differences among OB and HW children’s hippocampal functioning and none have demonstrated the relationship between activation in the hippocampus and eating behavior, food responsiveness and enjoyment of food. As in all studies, there are weaknesses that need to be considered. Our sample size was relatively small and had high inter-subject variability. Additionally, this study is cross-sectional, limiting causal implications. Another limitation was the lack of a neurocognitive measure to assess for overall cognitive ability, which prevented the exploration of the association between hippocampal volume and function, and general cognitive impairment.
However, these results raise questions for further research regarding the relationship between obesity and hippocampal functioning in youth. It is unclear whether changes in hippocampal volume and structure preceed weight gain in youth, or are a result of obese status. It is also unclear if changes in hippocampal volume and structure would persist over time or whether these observed changes are reversible. Future studies should implement a longitudinal design to examine whether a state of childhood obesity leads to alterations in hippocampal response and overall volume, and whether these changes then perpetuate into adulthood. In addition, interventions to promote a healthy diet (i.e., minimizing western diets) in children across the weight spectrum could potentially prevent hippocampal damage and dysfunction.
In conclusion, this study suggests that OB children, compared to HW, have reduced left hippocampal volume, alterations in activation to food taste in this region, and a relationship between structure and function in the hippocampus. Importantly, alterations in hippocampal activation are associated with overeating when sated, as well as food responsiveness and enjoyment of food in children, identifying the hippocampus as a key structure involved in obesity and eating behavior in youth.
Acknowledgments
Supported by grants to KB (R01DK094475; R01 DK075861; K02HL112042; R21HD074987; R01DK103554; Department of Pediatrics, UCSD) and WK (MH046001, MH042984, MH066122; MH001894; MH092793), the Price Foundation, and the Davis/Wismer Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclaimer statement: Authors have no conflicts to declare
References
- 1.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311(8):806–14. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Biro FM, Wien M. Childhood obesity and adult morbidities. Am J Clin Nutr. 2010;91(5):1499S–505S. doi: 10.3945/ajcn.2010.28701B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Franks PW, Hanson RL, Knowler WC, Sievers ML, Bennett PH, Looker HC. Childhood obesity, other cardiovascular risk factors, and premature death. N Engl J Med. 2010;362(6):485–93. doi: 10.1056/NEJMoa0904130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reilly JJ, Kelly J. Long-term impact of overweight and obesity in childhood and adolescence on morbidity and premature mortality in adulthood: systematic review. Int J Obes (Lond) 2011;35(7):891–8. doi: 10.1038/ijo.2010.222. [DOI] [PubMed] [Google Scholar]
- 5.Carnell S, Gibson C, Benson L, Ochner CN, Geliebter A. Neuroimaging and obesity: current knowledge and future directions. Obes Rev. 2012;13(1):43–56. doi: 10.1111/j.1467-789X.2011.00927.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gustafson D, Lissner L, Bengtsson C, Bjorkelund C, Skoog I. A 24-year follow-up of body mass index and cerebral atrophy. Neurology. 2004;63(10):1876–81. doi: 10.1212/01.wnl.0000141850.47773.5f. [DOI] [PubMed] [Google Scholar]
- 7.Whitmer RA, Gustafson DR, Barrett-Connor E, Haan MN, Gunderson EP, Yaffe K. Central obesity and increased risk of dementia more than three decades later. Neurology. 2008;71(14):1057–64. doi: 10.1212/01.wnl.0000306313.89165.ef. [DOI] [PubMed] [Google Scholar]
- 8.Gustafson D, Rothenberg E, Blennow K, Steen B, Skoog I. An 18-year follow-up of overweight and risk of Alzheimer disease. Arch Intern Med. 2003;163(13):1524–8. doi: 10.1001/archinte.163.13.1524. [DOI] [PubMed] [Google Scholar]
- 9.Bauer CC, Moreno B, Gonzalez-Santos L, Concha L, Barquera S, Barrios FA. Child overweight and obesity are associated with reduced executive cognitive performance and brain alterations: a magnetic resonance imaging study in Mexican children. Pediatr Obes. 2015;10(3):196–204. doi: 10.1111/ijpo.241. [DOI] [PubMed] [Google Scholar]
- 10.Mitchell HH. Overnutrition and obesity. J Clin Nutr. 1952;1(1):66–76. doi: 10.1093/ajcn/1.1.66. [DOI] [PubMed] [Google Scholar]
- 11.Davidson TL, Kanoski SE, Schier LA, Clegg DJ, Benoit SC. A potential role for the hippocampus in energy intake and body weight regulation. Curr Opin Pharmacol. 2007;7(6):613–6. doi: 10.1016/j.coph.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moran TH. Gut peptide signaling in the controls of food intake. Obesity (Silver Spring) 2006;14(Suppl 5):250S–3S. doi: 10.1038/oby.2006.318. [DOI] [PubMed] [Google Scholar]
- 13.Woods SC. Gastrointestinal satiety signals I. An overview of gastrointestinal signals that influence food intake. Am J Physiol Gastrointest Liver Physiol. 2004;286(1):G7–13. doi: 10.1152/ajpgi.00448.2003. [DOI] [PubMed] [Google Scholar]
- 14.Hargrave SL, Jones S, Davidson TL. The Outward Spiral: A vicious cycle model of obesity and cognitive dysfunction. Curr Opin Behav Sci. 2016;9:40–6. doi: 10.1016/j.cobeha.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davidson TL, Jarrard LE. A role for hippocampus in the utilization of hunger signals. Behav Neural Biol. 1993;59(2):167–71. doi: 10.1016/0163-1047(93)90925-8. [DOI] [PubMed] [Google Scholar]
- 16.Davidson TL, Kanoski SE, Chan K, Clegg DJ, Benoit SC, Jarrard LE. Hippocampal lesions impair retention of discriminative responding based on energy state cues. Behav Neurosci. 2010;124(1):97–105. doi: 10.1037/a0018402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benoit SC, Davis JF, Davidson TL. Learned and cognitive controls of food intake. Brain Res. 2010;1350:71–6. doi: 10.1016/j.brainres.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davidson TL, Kanoski SE, Walls EK, Jarrard LE. Memory inhibition and energy regulation. Physiol Behav. 2005;86(5):731–46. doi: 10.1016/j.physbeh.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 19.Henderson YO, Smith GP, Parent MB. Hippocampal neurons inhibit meal onset. Hippocampus. 2013;23(1):100–7. doi: 10.1002/hipo.22062. [DOI] [PubMed] [Google Scholar]
- 20.Rozin P, Dow S, Moscovitch M, Rajaram S. WHAT CAUSES HUMANS TO BEGIN AND END A MEAL? A Role for Memory for What Has Been Eaten, as Evidenced by a Study of Multiple Meal Eating in Amnesic Patients. PSYCHOLOGICAL SCIENCE. 1998;9(5):392–6. [Google Scholar]
- 21.Higgs S, Williamson AC, Rotshtein P, Humphreys GW. Sensory-specific satiety is intact in amnesics who eat multiple meals. Psychol Sci. 2008;19(7):623–8. doi: 10.1111/j.1467-9280.2008.02132.x. [DOI] [PubMed] [Google Scholar]
- 22.Raji CA, Ho AJ, Parikshak NN, Becker JT, Lopez OL, Kuller LH, et al. Brain structure and obesity. Hum Brain Mapp. 2010;31(3):353–64. doi: 10.1002/hbm.20870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shefer G, Marcus Y, Stern N. Is obesity a brain disease? Neurosci Biobehav Rev. 2013;37(10 Pt 2):2489–503. doi: 10.1016/j.neubiorev.2013.07.015. [DOI] [PubMed] [Google Scholar]
- 24.Kanoski SE. Cognitive and neuronal systems underlying obesity. Physiol Behav. 2012;106(3):337–44. doi: 10.1016/j.physbeh.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller AA, Spencer SJ. Obesity and neuroinflammation: a pathway to cognitive impairment. Brain Behav Immun. 2014;42:10–21. doi: 10.1016/j.bbi.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 26.Khan NA, Baym CL, Monti JM, Raine LB, Drollette ES, Scudder MR, et al. Central adiposity is negatively associated with hippocampal-dependent relational memory among overweight and obese children. J Pediatr. 2015;166(2):302–8 e1. doi: 10.1016/j.jpeds.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kanoski SE, Davidson TL. Western diet consumption and cognitive impairment: links to hippocampal dysfunction and obesity. Physiol Behav. 2011;103(1):59–68. doi: 10.1016/j.physbeh.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Francis HM, Stevenson RJ. Higher reported saturated fat and refined sugar intake is associated with reduced hippocampal-dependent memory and sensitivity to interoceptive signals. Behav Neurosci. 2011;125(6):943–55. doi: 10.1037/a0025998. [DOI] [PubMed] [Google Scholar]
- 29.Hargrave SL, Davidson TL, Lee TJ, Kinzig KP. Brain and behavioral perturbations in rats following Western diet access. Appetite. 2015;93:35–43. doi: 10.1016/j.appet.2015.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jacka FN, Cherbuin N, Anstey KJ, Sachdev P, Butterworth P. Western diet is associated with a smaller hippocampus: a longitudinal investigation. BMC Med. 2015;13:215. doi: 10.1186/s12916-015-0461-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wallner-Liebmann S, Koschutnig K, Reishofer G, Sorantin E, Blaschitz B, Kruschitz R, et al. Insulin and hippocampus activation in response to images of high-calorie food in normal weight and obese adolescents. Obesity (Silver Spring) 2010;18(8):1552–7. doi: 10.1038/oby.2010.26. [DOI] [PubMed] [Google Scholar]
- 32.DelParigi A, Chen K, Salbe AD, Hill JO, Wing RR, Reiman EM, et al. Persistence of abnormal neural responses to a meal in postobese individuals. Int J Obes Relat Metab Disord. 2004;28(3):370–7. doi: 10.1038/sj.ijo.0802558. [DOI] [PubMed] [Google Scholar]
- 33.Gomez RL, Edgin JO. The extended trajectory of hippocampal development: Implications for early memory development and disorder. Dev Cogn Neurosci. 2016;18:57–69. doi: 10.1016/j.dcn.2015.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boutelle KN, Wierenga CE, Bischoff-Grethe A, Melrose AJ, Grenesko-Stevens E, Paulus MP, et al. Increased brain response to appetitive tastes in the insula and amygdala in obese compared with healthy weight children when sated. Int J Obes (Lond) 2015;39(4):620–8. doi: 10.1038/ijo.2014.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Watkins B, Frampton I, Lask B, Bryant-Waugh R. Reliability and validity of the child version of the Eating Disorder Examination: a preliminary investigation. Int J Eat Disord. 2005;38(2):183–7. doi: 10.1002/eat.20165. [DOI] [PubMed] [Google Scholar]
- 36.Sheehan DV, Sheehan KH, Shytle RD, Janavs J, Bannon Y, Rogers JE, et al. Reliability and validity of the Mini International Neuropsychiatric Interview for Children and Adolescents (MINI-KID) J Clin Psychiatry. 2010;71(3):313–26. doi: 10.4088/JCP.09m05305whi. [DOI] [PubMed] [Google Scholar]
- 37.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, et al. CDC growth charts: United States. Adv Data. 2000;314:1–27. [PubMed] [Google Scholar]
- 38.Wardle J, Guthrie CA, Sanderson S, Rapoport L. Development of the Children’s Eating Behaviour Questionnaire. J Child Psychol Psychiatry. 2001;42(7):963–70. doi: 10.1111/1469-7610.00792. [DOI] [PubMed] [Google Scholar]
- 39.Carnell S, Wardle J. Measuring behavioural susceptibility to obesity: validation of the child eating behaviour questionnaire. Appetite. 2007;48(1):104–13. doi: 10.1016/j.appet.2006.07.075. [DOI] [PubMed] [Google Scholar]
- 40.Fisher JO, Birch LL. Eating in the absence of hunger and overweight in girls from 5 to 7 y of age. Am J Clin Nutr. 2002;76(1):226–31. doi: 10.1093/ajcn/76.1.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boutelle KN, Zucker NL, Peterson CB, Rydell SA, Cafri G, Harnack L. Two novel treatments to reduce overeating in overweight children: a randomized controlled trial. J Consult Clin Psychol. 2011;79(6):759–71. doi: 10.1037/a0025713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wagner A, Aizenstein H, Mazurkewicz L, Fudge J, Frank GK, Putnam K, et al. Altered insula response to taste stimuli in individuals recovered from restricting-type anorexia nervosa. Neuropsychopharmacology. 2008;33(3):513–23. doi: 10.1038/sj.npp.1301443. [DOI] [PubMed] [Google Scholar]
- 43.Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31(3):968–80. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 44.Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9(2):179–94. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- 45.Fischl B, Liu A, Dale AM. Automated manifold surgery: constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Trans Med Imaging. 2001;20(1):70–80. doi: 10.1109/42.906426. [DOI] [PubMed] [Google Scholar]
- 46.Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33(3):341–55. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- 47.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29(3):162–73. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 48.Team RC. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2013. [Available from: http://www.R-project.org/ [Google Scholar]
- 49.Kanoski SE, Zhang Y, Zheng W, Davidson TL. The effects of a high-energy diet on hippocampal function and blood-brain barrier integrity in the rat. J Alzheimers Dis. 2010;21(1):207–19. doi: 10.3233/JAD-2010-091414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Davidson TL, Hargrave SL, Swithers SE, Sample CH, Fu X, Kinzig KP, et al. Interrelationships among diet, obesity and hippocampal-dependent cognitive function. Neuroscience. 2013;253:110–22. doi: 10.1016/j.neuroscience.2013.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Williamson LL, Bilbo SD. Chemokines and the hippocampus: a new perspective on hippocampal plasticity and vulnerability. Brain Behav Immun. 2013;30:186–94. doi: 10.1016/j.bbi.2013.01.077. [DOI] [PubMed] [Google Scholar]
- 52.Igloi K, Doeller CF, Berthoz A, Rondi-Reig L, Burgess N. Lateralized human hippocampal activity predicts navigation based on sequence or place memory. Proc Natl Acad Sci U S A. 2010;107(32):14466–71. doi: 10.1073/pnas.1004243107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fanselow MS, Dong HW. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010;65(1):7–19. doi: 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Boitard C, Cavaroc A, Sauvant J, Aubert A, Castanon N, Laye S, et al. Impairment of hippocampal-dependent memory induced by juvenile high-fat diet intake is associated with enhanced hippocampal inflammation in rats. Brain Behav Immun. 2014;40:9–17. doi: 10.1016/j.bbi.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 55.Kanoski SE, Meisel RL, Mullins AJ, Davidson TL. The effects of energy-rich diets on discrimination reversal learning and on BDNF in the hippocampus and prefrontal cortex of the rat. Behav Brain Res. 2007;182(1):57–66. doi: 10.1016/j.bbr.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Karimi SA, Salehi I, Komaki A, Sarihi A, Zarei M, Shahidi S. Effect of high-fat diet and antioxidants on hippocampal long-term potentiation in rats: an in vivo study. Brain Res. 2013;1539:1–6. doi: 10.1016/j.brainres.2013.09.029. [DOI] [PubMed] [Google Scholar]
- 57.Liang J, Matheson BE, Kaye WH, Boutelle KN. Neurocognitive correlates of obesity and obesity-related behaviors in children and adolescents. Int J Obes (Lond) 2014;38(4):494–506. doi: 10.1038/ijo.2013.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stice E, Spoor S, Bohon C, Veldhuizen MG, Small DM. Relation of reward from food intake and anticipated food intake to obesity: a functional magnetic resonance imaging study. J Abnorm Psychol. 2008;117(4):924–35. doi: 10.1037/a0013600. [DOI] [PMC free article] [PubMed] [Google Scholar]


