Abstract
Purpose
Carcinosarcomas (CS) are highly aggressive gynecologic malignancies containing both carcinomatous and sarcomatous elements with heterogeneous HER2/neu expression. We compared the efficacy of SYD985, (Synthon Biopharmaceuticals BV), a novel HER2-targeting antibody-drug conjugate (ADC), to Trastuzumab emtansine (T-DM1, Genentech-Roche) against primary uterine and ovarian CS.
Experimental Design
Eight primary CS cell lines were evaluated for HER2/neu surface expression by IHC and gene amplification by FISH assays. The in vitro experiments included cytotoxicity, antibody-dependent cellular cytotoxicity (ADCC), proliferation, viability and bystander killing. In vivo activity was studied in mouse xenograft and patient-derived-xenograft (PDX) models.
Results
SYD985 and T-DM1 induced similar levels of ADCC against CS cell lines with low and high HER2/neu expression when challanged in the presence of effector cells. In contrast, SYD985 was 7 to 54 fold more potent than T-DM1 in the absence of effector cells. SYD985, unlike T-DM1, was active against CS demonstrating low or heterogeneous HER2/neu expression. Specifically, the mean IC50’s were 0.060 µg/mL and 3.221 µg/mL (p<0.0001) against HER2/neu 0/1+ cell lines and 0.013 µg/mL and 0.096 µg/mL (p<0.0001) against HER2/neu 3+ cell lines for SYD985 vs T-DM1, respectively. Importantly, unlike T-DM1, SYD985 induced efficient bystander killing of HER2/neu 0/1+ tumor cells admixed with HER2/neu 3+ cells. In vivo studies confirmed that SYD985 is more active than T-DM1 in CS and highly effective against HER2/neu expressing xenografts and PDX.
Conclusions
SYD985 may represent a novel and highly effective ADC against HER2-expressing CS. Clinical studies with SYD985 in patients harboring chemotherapy-resistant CS with low/moderate and high HER2 expression are warranted.
Keywords: SYD985, T-DM1, Uterine Carcinosarcoma, Ovarian Carcinosarcoma, HER2
INTRODUCTION
Carcinosarcomas also known as mixed malignant Müllerian tumors (MMMT) are rare but highly aggressive gynecologic malignancies accounting for less than 5% of all gynecologic cancers (1–3). Carcinosarcomas can arise in the ovary, cervix or uterus. Previously published data from the SEER database suggests that age adjusted rate of carcinosarcoma development in the uterus is 0.6/100,000 and in the ovary 0.19/100,000 (4). Despite aggressive surgical and adjuvant therapy, 5 year survival rates for uterine carcinosarcoma are approximately 60% for early (stage I/II) disease and 9–22% for more advanced disease (5). Similarly, ovarian carcinosarcomas account for less than 4% of all newly diagnosed ovarian carcinomas and carry a significantly worse prognosis than their epithelial ovarian carcinoma counterparts (6). Standard treatment for carcinosarcoma is aggressive surgical debulking followed by chemotherapy with or without radiation. The rarity of these tumors has made determination of the best adjuvant therapy difficult and completing large prospective randomized trials remains challenging. As a result, a considerable amount of effort has been made by our group as well as others to better understand the biology of the disease, its developmental origins as well as common genetic alterations and activated molecular pathways in an attempt to improve treatments and ultimately survival (7).
Carcinosarcomas are composed of an epithelial component as well as a sarcomatous component. Many theories regarding the mechanism of development of this tumor, composed of two dissimilar cell populations, have been proposed (7). Importantly, recent comphrensive whole exome sequencing genetic studies from our research group have provided strong experimental evidence to suggest that ovarian and uterine carcinosarcoma originate as epithelial tumors and only subsequently may undergo mesenchymal transformation (8–11). These genetic landscape results are consistent with previous clinical/pathological studies showing that the epithelial component of the carcinosarcoma drives the growth and proliferation of these rare and biologically aggressive gynecologic cancers. Because of the reported overexpression and/or gene amplification of HER2 in over one third of uterine serous carcinoma (12, 13) which represents the high grade epithelial component of a large number of gynecologic CS (10), HER2 may represent an attractive target for anticancer therapy (4).
HER2-directed antibody antitumor activity is mainly due to inhibition of intracellular signaling via the phosphatidylinositol 3-kinase (PI3K) and the mitogen-activated protein kinase (MAPK) pathways, and activation of immune response through antibody-dependent cellular cytotoxicity (ADCC), and complement-dependent cytotoxicity (CDC) (14, 15). Currently the three monoclonal HER2-directed antibody drugs approved by the Food and Drug Administration (FDA) as clinical therapies in tumors overexpressing HER2 include Trastuzumab (approved in 1998) (16), Pertuzumab (approved in 2012), and Trastuzumab emtansine (T-DM1) (approved in 2013) (17, 18). Antibody drug conjugates (ADCs) such as T-DM1 are considered to be sophisticated drug delivery systems that provide one of the most promising approaches to improve the therapeutic window of existing cytotoxic agents such as tubulin-targeting agents (19–21). Trastuzumab emtansine (T-DM1), approved as a second-line monotherapy for the treatment of relapsed HER2 positive metastatic breast cancer, includes trastuzumab covalently linked with a non-cleavable linker to the antimitotic agent emtansine (DM1) (22, 23). Once the ADC is internalized into tumor cells by receptor-mediated endocytosis and processed, DM1 is released and binds to tubulin and inhibits microtubule assembly and causes apoptosis in dividing tumor cells (24–27). Importantly, T-DM1, although highly active against tumor cells overexpressing HER2, does not induce significant bystander killing since the linker between trastuzumab and DM1 is stable in structure. SYD985 (Synthon Biopharmaceuticals BV, Nijmegen, the Netherlands) is a novel HER2-targeting ADC with a ‘cleavable’ linker that couples a potent duocarmycin payload, valine-citrulline-seco DUocarmycin hydroxyBenzamide Azaindole (vc-seco-DUBA), to trastuzumab (28–30). SYD985 has shown impressive preclinical results in breast cancer and endometrial cancer (31) and encouraging clinical activity in Phase I clinical trials (25–27). The toxic payload in SYD985 (i.e., DUBA) alkylates DNA resulting in DNA damage, mitochondrial stress, impaired DNA transcription, apoptosis, and ultimately cell death in both dividing and non-dividing cells (28–30). Importantly, once the linker is cleaved by enzymes in endosomes and lysosomes, membrane-permeable active toxin from SYD985 is released. This linker cleavage property has been shown to cause cell killing of not only HER2/neu positive cells but also of neighboring HER2 negative tumor cells (i.e., bystander killing effect)(31). HER2/neu protein over-expression (ie, 3 + by IHC) in CSs has been reported in 22–56% of carcinomatous component compared to low to negligible HER2/neu expression in the sarcomatous component (32–34). Taken together, these data suggest that while a significant subset of CS patients may potentially benefit from current HER2/neu targeted therapies such as Trastzumab and T-DM1 (ie, 3+ patients), a novel HER2/neu-targeting ADC active against CS with low/moderate or heterogeneous HER2/neu expression might significantly increase the number of patients benefitting from this therapeutic approach.
Accordingly, the objective of this paper was to compare the anti-tumor activity of SYD985 to the FDA-approved ADC T-DM1 against primary uterine and ovarian CS cell lines with different HER2/neu expression status. We demonstrate for the first time that SYD985 is significantly more potent than T-DM1 in CS in comparative in vitro and in vivo experiments. More importantly, our results show that SYD985, unlike T-DM1, is able to induce a significant bystander effect against tumor cells with low/negligible HER2/neu expression when admixed with HER2/neu 3+ cells, suggesting potential clinical activity against not only the HER2/neu positive epithelial but also the HER2/neu negative sarcomatous component of CS.
MATERIALS AND METHODS
Establishment of Cell Lines
Study approval was obtained from the Institutional Review Board at Yale University, and all patients signed consent prior to tissue collection according to the institutional guidelines. Eight primary carcinosarcoma (CS) cell lines (cell lines characteristics, epithelial/stromal components of each cell line and tissue source are described in Table 1) were established from chemotherapy-naïve patients at the time of primary staging surgery after sterile processing of fresh tumor biopsy samples, as described previously and evaluated in our study (10,12). All revived cells were used within 50 passages, and cultured for less than 6 months. Tumors were staged according to the International Federation of Gynecology and Obstetrics staging system. Patient characteristics are noted in Table 1. Primary uterine and ovarian cell lines with limited passages were used in the experiments listed below and corresponding cell blocks were analyzed for HER2 surface expression by immunohistochemistry (IHC) and fluorescent in situ hybridization (FISH).
Table 1.
Characteristics and demographic data of carcinosarcoma cell lines used
| Cell line | Age | Race | FIGO stage |
Primary site |
Histology | IHC | FISH | Flow cytometry (MFI) |
|
|---|---|---|---|---|---|---|---|---|---|
| EC | SC | ||||||||
|
| |||||||||
| SARARK-1* | 70 | A | IC | Uterus | Homologous | 0 | NA | 17.2 | |
| END+CC | ESS | ||||||||
|
| |||||||||
| SARARK-3 | 74 | W | IIIC | Ovary | Heterologous | 0 | NT | 22.8 | |
| SER | CDRS | ||||||||
|
| |||||||||
| SARARK-4 | 77 | W | IIIC | Ovary | Heterologous | 0 | NA | 32.36 | |
| SER | CDRS | ||||||||
|
| |||||||||
| SARARK-6* | 78 | W | IV | Ovary | Homologous | 3+ | A | 508.91 | |
| SER | CDR | ||||||||
|
| |||||||||
| SARARK-7* | 55 | W | IV | Ovary | Heterologous | 1+ | NA | 38.35 | |
| CC+ SER | CDRS | ||||||||
|
| |||||||||
| SARARK-8 | 46 | W | IIB | Uterus | Homologous | 0 | NA | 12.06 | |
| UND | ESS | ||||||||
|
| |||||||||
| SARARK-9* | 66 | W | IIIC2 | Uterus | Homologous | 3+ | A | 300.74 | |
| SER | ESS | ||||||||
|
| |||||||||
| SARARK-10 | 63 | W | IVB | Uterus | Homologous | 1+ | NT | 23.05 | |
| SER | ESS | ||||||||
FIGO: International Federation of Gynecology and Obstetrics; EC: epithelial component; SC: sarcomatous component; END: endometrioid; CC: clear cell; ESS: endometrial stromal sarcoma; SER: serous; CDRS: chondrosarcoma; CDR: chondroid; UND: undifferentiated; IHC: immunohistochemistry; FISH: fluorescent in situ hybridization; MFI: mean fluorescence intensity; NT: not tested; NA: not amplified; A: amplified.
Cell lines used in in vitro and in vivo experiments.
SYD985 and T-DM1
T-DM1 (batch N0001B02; Roche, Basel, Switzerland) was purchased by Synthon Biopharmaceuticals BV, Nijmegen, the Netherlands. SYD985 was prepared as previously described (29). Briefly, vc-seco-DUBA was coupled to a cysteine residue of trastuzumab after partial reduction of the inter-chain disulfides. SYD985 was further purified to deliver a well-defined ADC predominantly consisting of species with a drug to antibody ratio (DAR) of 2 and 4, yielding a mean DAR of 2.8 (28, 29).
Immunostaining of Cell Blocks of Primary CS
Cell blocks from all eight CS cell lines described in Table 1 and tumor blocks from OM(M)98 (ie, a freshly established CS-PDX1 used in the in vivo experiments described below) were reviewed by a gynecologic surgical pathologist to confirm the presence of carcinosarcoma cells. Briefly, HER2 immunohistochemical staining was performed on paraffin-embedded 5 µm sections of cell blocks after deparaffinisation and rehydration, using the c-erbB-2 antibody (Thermo Fisher Scientific, Fremont, CA) at 1:800 dilution. HER2 staining intensity was graded per the American Society of Clinical Oncology and the College of American Pathologists (ASCO/CAP) 2013 breast scoring criteria (20). Appropriate positive and negative controls were used with each case.
Fluorescent In Situ Hybridization (FISH) of Cell Blocks From Primary CS
Fluorescent in situ hybridization (FISH) analysis was performed using the PathVysion HER2 DNA FISH Kit (Abbott Molecular Inc., Abbott Park, IL, USA) according to the manufacturer’s instructions. Cell block sections of 5 µm were deparaffinised and rehydrated, followed by acid pretreatment and proteinase K digestion. A probe mix containing an orange probe directed against the HER2 gene (Vysis, Inc., Downers Grove, IL, USA, LSI HER2) and a green probe directed against the pericentromeric region of chromosome 17 (Vysis CEP 17) were added and specimens were denatured for 5 minutes at 73°C. Slides were then incubated overnight in a humidity chamber at 37°C and washed the day after when a fluorescence mounting medium, containing 4, 6-diamidino-2-phenylindole (DAPI), was applied. Fluorescent signals in at least 30 non-overlapping interphase nuclei with intact morphology were scored using a Zeiss Axioplan 2 microscope (Carl Zeiss Meditec, Inc., Dublin, CA, USA) with a 100× planar objective, using a triple band-pass filter that permits simultaneous blue, green, and red colors. Tumor cells were scored for the number of orange (HER2) and green (chromosome 17) signals. A case was scored as amplified when the ratio of the number of fluorescent signals of HER2 to chromosome 17 was ≥2.
Tests for ADCC
Standard 4-hour chromium (51Cr) release assay was performed to measure the cytotoxic reactivity of Ficoll-Hypaque-separated PBLs from several healthy donors in combination with trastuzumab, T-DM1 and SYD985 against primary CS target cell lines at effector to target ratios (E:T) of 20:1 and 40:1. The release of 51Cr from target cells was measured as evidence of tumor cell lysis after exposure of the tumor cells to 2.5 µg/ml of trastuzumab or 2.5 µg/ml of T-DM1 and SYD985. Dose-response experiments were performed in order to determine the optimal antibody dosing for ADCC experiments. The negative control condition was the incubation of target cells alone. As a positive control condition, 0.1% sodium dodecyl sulfate (SDS) was used to achieve complete lysis of target cells. Chimeric anti-CD20 mAb rituximab 2.5 µg/ml was used as the negative control for trastuzumab, T-DM1 and SYD985 in all bioassays. The percentage cytotoxicity of trastuzumab or T-DM1 was calculated by the following formula: % cytotoxicity = 100 × (E-S)/(T-S), where E is the experimental release, S is the spontaneous release by target cells, T is the maximum release by target cells lysed with 0.1% SDS.
Cell Viability Assay
CS cell lines were plated at log phase of growth in 6-well tissue culture plates at a density of 20,000–40,000 cells in RPMI 1640 media (Life Technologies, Carlsbad, CA) supplemented with 10% fetal bovine serum, 1% penicillin/streptomycin (Mediatech, Manassas, VA), and 0.3% fungizone (Life Technologies, Carlsbad, CA). Cells were incubated at 37°C, 5% CO2. After 24 hours of incubation, cells were treated with SYD985, T-DM1, trastuzumab and isotype control ADC (i.e., rituximab conjugated to vc-seco-DUBA: SYD989). SYD985, T-DM1, and SYD989 were used at scalar concentrations of 0.005µg/ml, 0.05µg/ml, 0.5µg/ml, 2µg/ml and 8µg/ml. Trastuzumab was used at concentrations of 1µg/ml, 5µg/ml, 10µg/ml, 40µg/ml, and 100µg/ml. Three days after drug treatment, cells were harvested in their entirety, centrifuged and stained with propidium iodide (2µl of 500 µg/ml stock solution in PBS). Analysis was performed using a flow cytometry based assay to quantify percent viable cells as a mean ± SEM relative to untreated cells as 100% viable controls. A minimum of three independent experiments per cell line were performed.
Bystander killing
Briefly, a 1:1 ratio of HER2/neu 3+ positive CS cells (i.e., SARARK-6) and HER2/neu negative USC cells (i.e., USC ARK-4) stably transfected with a Green Fluorescence Protein (GFP) plasmid (pCDH-CMV-MCSEF1-copGFP, a kind gift from Dr. Simona Colla, MDACC), were mixed (20,000 cells/well of each cell type) and plated in 6-well plates (3mL/well). After an overnight incubation, SYD985, T-DM1 or isotype control ADC at a concentration of 1µg/ml or vehicle were added. After a 72-hour incubation, cells were harvested in their entirety, centrifuged and stained with propidium iodide (2µl of 500 µg/ml stock solution in PBS) to facilitate the identification of dead CS cells. Analysis was performed using flow cytometry based assay to recognize to quantify percent viable cells as a mean ± SEM relative to untreated cells as 100% viable controls. A minimum of three independent experiments were performed.
In vivo treatment
The in vivo antitumor activity of SYD985 versus T-DM1 was tested in xenograft models with 3+ and 1+ HER2/neu expression established from primary CS cell lines and in freshly explanted PDXs with 3+ HER2/neu expression. Specimen collection and all animal experiments were approved by the institutional ethical committee (HIC) and Institutional Animal Care and Use Committee (IACUC) of Yale University. Briefly, six to eight week old CB-17/SCID mice were given a single subcutaneous injection of 7 × 106 CS SARARK-6 cells (HER2/neu 3+) in approximately 200 µL of a 1:1 solution of sterile PBS containing cells and Matrigel (BD Biosciences) while for the PDX experiments, the 3+ HER2/neu CS OM(M)98 (ie, CS-PDX1) was obtained from a surgical specimen at the time of a staging procedure of a CS patient (stage IVB) and placed into a sterile Petri dish containing phosphate-buffered saline (PBS), then sliced into 5 × 5 × 2 mm fragments. Typically, one fragment was implanted into a subcutaneous area in the right or left flanks in combination with matrigel. The size of the implanted tumour was checked 1 – 3 times per week using Vernier calipers when the implanted tissue was palpable, and the volume was calculated as (length × width2)/2. Once the tumor volume was approximately 0.2 cm3 for the xenografted cell lines and about 0.4 cm3 for the PDX, the mice were randomized into treatment groups (n=5); those treated with SYD985 (3mg/mg and 10mg/kg), T-DM1 (10 mg/kg), isotype control ADC (SYD989) (3mg/kg and 10mg/kg), and phosphate-buffered saline (PBS). Drug dosages were chosen according to previous studies conducted on different xenograft models (28, 29). All treatment drugs were given as a single intravenous (IV) injection based on prior literature (28, 29). Mice were observed for overall survival as the primary outcome measure. Tumor measurements were recorded twice weekly. Mice were sacrificed if tumor volume reached 1.5 cm3 using the formula (width)2 × height/2. Animal care and euthanasia were carried out according to the rules and regulations as set forth by the Institutional Animal Care and Use Committee (IACUC).
Statistical analysis
Statistical analysis was performed using GraphPad Prism version 6 (GraphPad Software, Inc. San Diego, CA). The differences in ADCC levels by 4-hr chromium release assays as well as the inhibition of proliferation in the CS cell lines after exposure to SYD985 were evaluated by the two-tailed unpaired student t-test. Overall survival data were analyzed and plotted using the Kaplan-Meier method. Survival curves were compared using the log-rank test. Differences in all comparisons were considered statistically significant at p-values < 0.05.
RESULTS
HER2/neu expression in primary CS cell lines
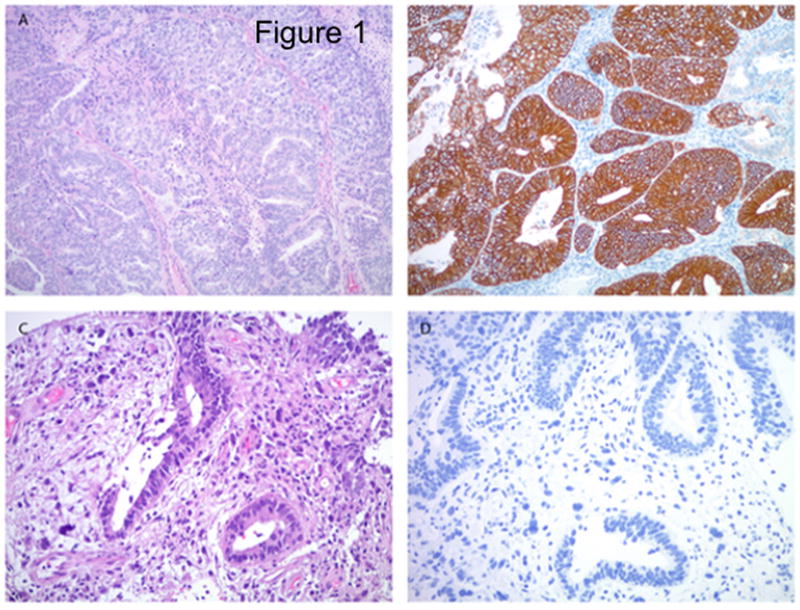
We evaluated erbB-2 gene amplification by FISH and surface HER2/neu protein expression by IHC in eight primary CS culture cell lines (Table 1) as well as the tumor blocks obtained from OM(M)98 (ie CS-PDX1). Gene amplification and high levels (3+ staining) of HER2 protein expression by IHC were detected in 25% of the CS cell lines (i.e. 2 out of 8) and in OM(M)98. Of interest, as described in Figure 1, OM(M)98 CS was found to harbor a 3+ HER2/neu serous carcinoma component while the homologous sarcomatous component was found Her2 negative by IHC. Two cell lines had low (1+) and four had negligible (0) HER2 expression on IHC, while there were no cell lines with 2+ expression (Table 1). On the basis of the HER2/neu results, we selected a total of 4 primary CS cell lines with similar growth rate and different HER2 expression and OM(M)98 (ie CS-PDX1) for the additional in vitro and in vivo experiments described below.
Figure 1.
Her2 expression by immunohistochemistry in uterine carcinosarcoma. The serous carcinoma component (a) shows strong membranous Her2 immunoreactivity (b), while the homologous sarcomatous component (c) - diffusely infiltrating around the malignant glands – is Her2 negative (d). Original magnification 100× (panels a and b) and 200× (panels c and d).
SYD985, T-DM1, and trastuzumab mediated ADCC against HER2-positive primary CS
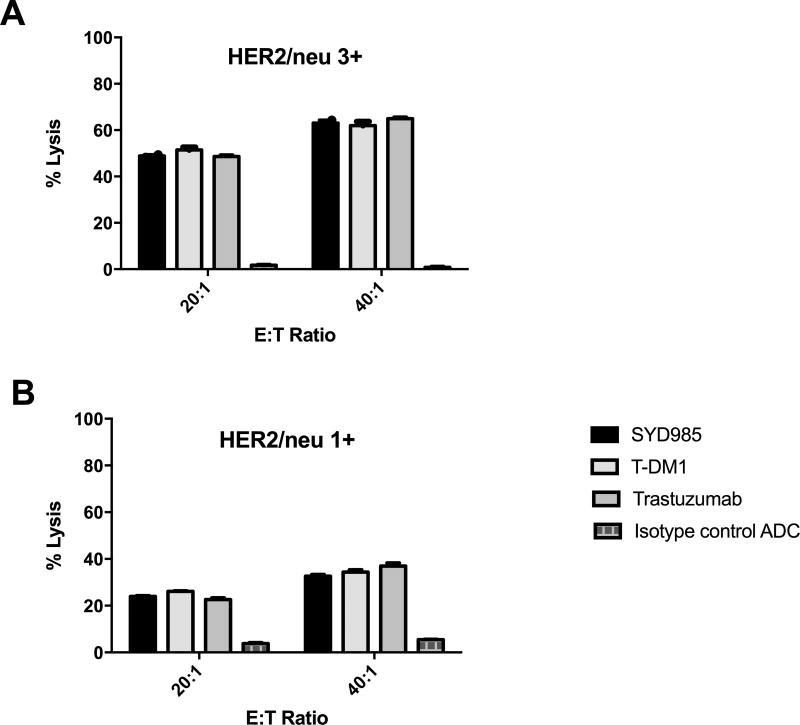
Three representative primary CS cell lines were tested for their sensitivity to PBL-mediated cytotoxicity when challenged with heterologous PBLs collected from several healthy donors in standard 4-h 51Cr release assays. CS cell lines were consistently found to be resistant to PBL-mediated cytotoxicity when combined with PBLs and isotype control ADC (2.5 µg/mL) at E:T ratios of 20:1 and 40:1 (mean ± SEM cytotoxicity of 3.8± 0.40% with PBL alone and mean ± SEM cytotoxicity of 5.45 ± 0.15% in the presence of isotype control ADC + PBL, respectively)(Figure 2). We then investigated the sensitivity of CS cell lines to heterologous PBLs in the presence of trastuzumab, SYD985, and T-DM1 at 2.5 µg/mL (Figure 2). SYD985, T-DM1 and trastuzumab (T) were similarly effective in inducing strong ADCC against primary CS cell lines expressing HER2/neu at high levels (i.e., SARARK-6, SARARK-9) with mean cytotoxicity ± SEM = 55.9 ± 7.08% for SYD985 vs. 72.9 ± 10.55% for T-DM1 vs. 56.7 ± 8.16% for Trastuzumab, P = 0.744. Similarly, no significant differences between SYD985, T-DM1, or trastuzumab in the induction of ADCC were detected against CS cell lines with low (1+) HER2 expression (p=0.2455, Figure 2).
Figure 2.
A) ADCC results (mean ± SD) of SYD985, T-DM1 (ie, SYD995), Trastuzumab (ie, SYD 997) and ADC isotype control (ie, SYD 989) in two representative HER2 3+ expressing cell lines (ie, SARARK-6: ovary as the primary site and SARARK-9: uterus as the primary site). B) A representative 1+ HER2 expressing cell line (SARARK-7: ovary as the primary site). No significant differences in ADCC between SYD985, T-DM1, or Trastuzumab were detected in CS cell lines with different HER2 expression.
Cytotoxicity of SYD985 versus T-DM1 in vitro
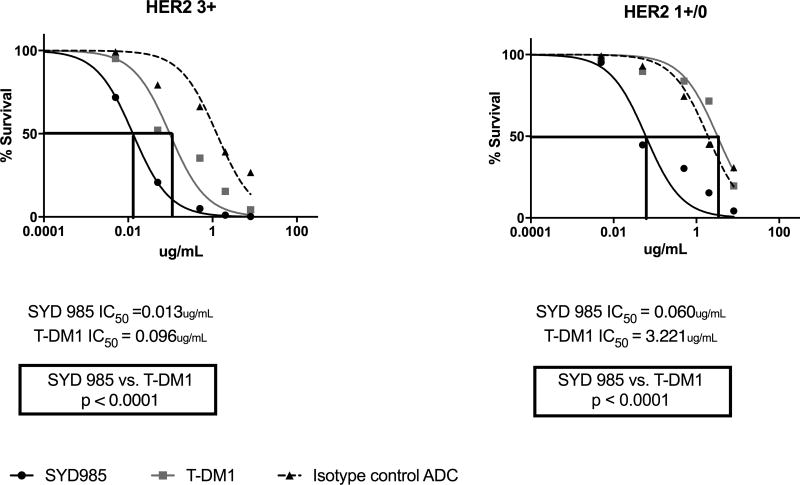
Next, we exposed four cell lines with different HER2/neu expression (Table 1) to scalar concentrations of ADC in the absence of PBL for a total of 3 days. As representatively demonstrated in Figure 3, SYD985 was significantly more potent in inducing cell death than T-DM1 in all CS cell lines tested regardless of the level of HER2/neu expression (Figure 3). In the HER2/neu 3+ cell lines, SYD985 exhibited a mean IC50 of 0.013 µg/mL while T-DM1 exhibited a mean IC50 = 0.096 µg/mL (p<0.0001). In the HER2/neu 1+ cell lines, SYD985 exhibited a mean IC50 of 0.060 µg/mL while T-DM1 exhibited a mean IC50 = 3.221 µg/mL (p<0.0001).
Figure 3.
IC50 dose response curves of SYD985, T-DM1 and ADC isotype control in all CS cell lines tested in vitro [i.e., HER2 3+ cell lines (SARARK-6 and SARARK-9), p = <0.0001 and HER2 1+/0 cell lines (SARARK-1 and SARARK-7), p = <0.0001] at 3 days. SARARK-6 and SARARK-7 are ovarian in origin. SARARK-1 and SARARK-9 are uterine in origin.
Bystander killing in vitro
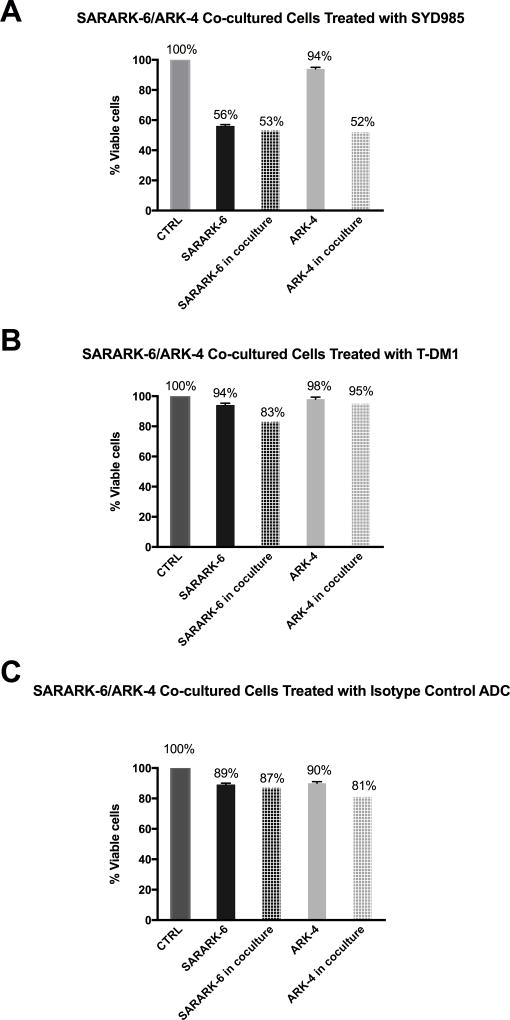
Next, we evaluated the ability of SYD985 and T-DM1 to induce bystander cytotoxicity of CS cells with low/negligible HER2 expression (i.e., GFP-ARK-4) when admixed with SARARK-6 (HER2/neu 3+) cells for 96 hours. As shown in the Figure 4a for SYD985, while no increase in SARARK-6 killing was noted when SARARK-6 cells were co-cultured with ARK-4 (p= 0.17), SARARK-6/ARK-4 co-cultures yielded a significant increase (ie, 42%, p= 0.01) in the amount of bystander killing of HER2 low/negligible ARK-4 cells when compared to controls. In contrast, minimal bystander cytotoxicity was detected when SARARK-6/ARK-4 co-cultures were challenged with T-DM1 (ie, 3%, p = 0.16) or isotype control ADC for 96 hours (Figure 4b and 4c, respectively).
Figure 4.
A) Cytotoxicity induced on HER2 3+ CS (SARARK-6), HER2 0/1+ USC (ARK-4), and SARARK-6/ARK-4 co-cultures with 1µg/ml of SYD985, T-DM1 and ADC isotype control. A significant increase in the amount of killing of HER2 0/1+ USC cells was detected when compared to control (p= 0.01) after treatment with SYD985. Cell viability of ARK-4 decreased from 94% to 52% after co-incubation of ARK-4 with SARARK-6, showing bystander killing effect of SYD985 in the presence of HER2 3+ cell line SARARK-6. B) Cytotoxicity induced on HER2 3+ CS (SARARK-6), HER2 0/1+ USC (ARK-4), and SARARK-6/ARK-4 co-cultures with 1µg/ml of SYD995 (T-DM1). No significant increase in the amount of killing of HER2 0/1+ USC cells was detected when compared to control (p= 0.29) after treatment with T-DM1. Cell viability of ARK-4 decreased from 98% to 95% after co-incubation of ARK-4 with SARARK-6, which failed to show any bystander killing effect of T-DM1 in the presence of HER2 3+ cell line SARARK-6. C) Cytotoxicity induced on HER2 3+ CS (SARARK-6), HER2 0/1+ USC (ARK-4), and SARARK-6/ARK-4 co-cultures with 1µg/ml of SYD989 (isotype control ADC). No significant increase in the amount of killing of HER2 0/1+ USC cells was detected when compared to control (p= 0.51) after treatment with isotype control ADC.
In vivo antitumor activity of SYD985 versus T-DM1
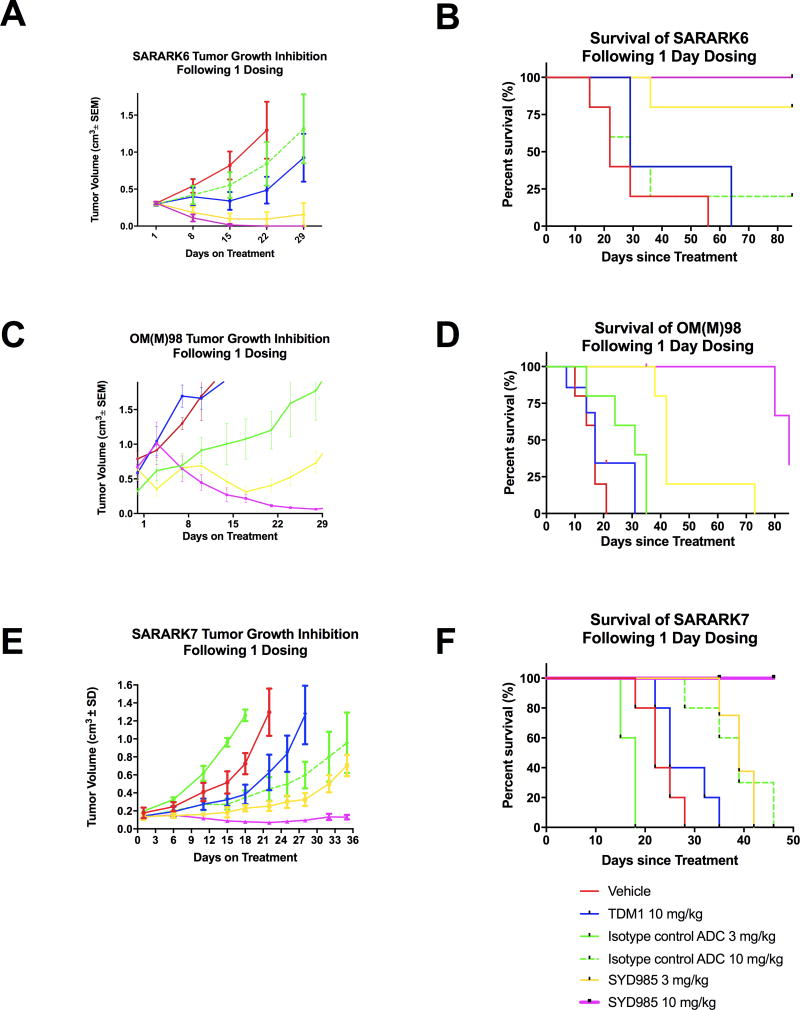
The in vivo effects of SYD985 and T-DM1 were determined by establishing xenografts from primary CS cell lines with 3+ and 1+ HER2/neu expression (i.e., SARARK-6 and SARARK-7, respectively) and PDXs with 3+ HER2/neu expression (i.e., OM(M)98). As described in Methods, after the tumors had reached the goal size, animals were randomized into treatment groups and treated as described previously (31). Tumors were assessed once weekly and mice were sacrificed if tumors became necrotic, reached a volume of 1.5cm3, or mice appeared to be in poor health. Treatment with a single injection of SYD985 showed remarkable inhibition of tumor growth in mice harboring both xenografts and PDX with 3+ HER2/neu expression as well as against xenografts with 1+ HER2/neu expression (Figure 5a, 5c and 5e). Specifically, in SARARK-6 (HER2/neu 3+) xenografts, we detected a significant difference in growth inhibition between animals treated with SYD985 3 mg/kg (p=0.0006 at 29 days) and 10 mg/kg (p=0.00001 at 29 days) when compared to T-DM1 at 10 mg/kg (Figure 5a). Accordingly, a significant survival advantage was seen in SYD985 treated xenografts (Figure 5b). Specifically, when comparing SYD985 10mg/kg single injection to T-DM1 10mg/kg single injection, there was a significant difference in mean overall survival of 85 days versus 43 days, respectively, (p=0.0079). The significant survival difference was maintained when SYD985 3 mg/kg single injection was compared to T-DM1 10 mg/kg, with mean overall survival of 75 days versus 43 days, respectively (p=0.0238). Notably, 5 out of 5 (100%) and 3 out of 5 (60%) of the mice were alive and disease free at 90 days after a single injection of SYD985 at a dose of 10mg/kg and 3 mg/kg, respectively. In contrast, there was no statistically significant difference in mean overall survival when comparing SYD989 10 mg/kg single injection to T-DM1 10 mg/kg single injection (mean overall survival of 37 days versus 43 days, respectively)(p=0.5873). Survival benefit in mice treated with 10 mg/kg of isotype control ADC was inferior to SYD985 dosed at both 3mg/kg and 10 mg/kg (Figure 5b), indicating that SYD985-induced anti-tumor activity in these models is at least partly mediated through HER2. Importantly, SYD985 was also highly active against OM(M)98, a HER2/neu 3+ PDX recently established in our laboratory. Specifically, we detected a significant difference in growth inhibition between animals treated with SYD985 3 mg/kg (p<0.005 at 7 days) and 10 mg/kg (p<0.005 at 7 days) when compared to T-DM1 at 10 mg/kg (Figure 5c). Accordingly, a significant survival advantage was seen in SYD985 treated PDX when compared to control treatments (Figure 5d). Specifically, when comparing SYD985 10mg/kg single injection to T-DM1 10mg/kg single injection, there was a significant difference in median overall survival of 108 days versus 17 days, respectively (p=0.02). When comparing SYD985 3mg/kg single injection to T-DM1 10mg/kg single injection there was also a significant difference in median overall survival- 42 days versus 17 days, respectively (p<0.005).
Figure 5.
A) Antitumor activity of SYD985 compared to T-DM1 and ADC isotype control in CS xenograft tumor model with SARARK-6 (HER2/neu 3+). B) Overall survival in mice inoculated with CS xenografts with SARARK-6 (HER2/neu 3+), after treatment with vehicle, single injection SYD985 (3mg/kg and 10mg/kg), and single injection T-DM1 (10mg/kg) and ADC isotype control (10 mg/kg). C) Antitumor activity of SYD985 compared to T-DM1 and ADC isotype control in CS PDX model with OM(M)98 (HER2/neu 3+). D) Overall survival in mice inoculated with CS PDX OM(M)98, after treatment with vehicle, single injection SYD985 (3mg/kg and 10mg/kg), and single injection T-DM1 (10mg/kg) and ADC isotype control (3 mg/kg). E) Antitumor activity of SYD985 compared to T-DM1 and ADC isotype control in CS xenograft model with SARARK-7 (HER2/neu 1+). F) Overall survival in mice inoculated with CS xenografts with SARARK-7 (HER2/neu 1+), after treatment with vehicle, single injection SYD985 (3 mg/kg and 10 mg/kg), and single injection T-DM1 (10 mg/kg) and ADC isotype control (10 mg/kg). Mice were treated with a single dose administered intravenously as described in Methods. A significant difference in growth inhibition was detected in SYD985-treated groups at the dose of 3 mg/kg and 10 mg/kg when compared to the other treatment groups in 3+ HER2/neu expressing SARARK-6 and 1+ HER2/neu expressing SARARK-7 xenogratfs as well as in 3+ HER2/neu expressing OM(M)98 PDXs. Significantly prolonged overall survival across SYD985-treated groups was detected when compared to the other treatment groups in both SARARK-6 and SARARK-7 xenogratfs as well as in OM(M)98 PDXs.
To confirm the activity of SYD985 in vivo in low HER2/neu expressing tumors, additional experiments were performed by establishing xenografts from a primary CS cell line with 1+ HER2/neu expression (i.e., SARARK-7). Similarly to the in vivo results with the HER2/neu 3+ CS models, in SARARK-7 xenografts the difference in growth inhibition between animals treated with SYD985 3 mg/kg and 10 mg/kg when compared to T-DM1 at 10 mg/kg was highly significant (ie, p=0.002 at 25 days and p=0.00009 at 22 days, respectively) (Figure 5e). Accordingly, a significant survival advantage was seen in SYD985 treated xenografts (Figure 5f). Specifically, when comparing SYD985 10mg/kg single injection to T-DM1 10mg/kg single injection, there was a significant difference in mean overall survival of undefined days versus 25 days, respectively, (p=0.0018).
DISCUSSION
Carcinosarcomas are rare but highly aggressive gynecologic cancers composed of both an epithelial and sarcomatous component. The rarity, aggressive biologic nature, and rapid development of chemotherapeutic resistance make these tumors difficult to cure and extremely difficult to study within clinical trials.
Our group has recently used whole exome sequencing (WES) to analyze the genetic landscape of a large number of uterine and ovarian CS as well as multi region WES to resolve the evolutionary histories of the carcinomatous and sarcomatous elements (10). In this comprehensive report, in agreement with previously published clinical data (35), we demonstrated at molecular level that carcinomatous and sarcomatous elements derive from a common precursor having mutations typical of carcinomas (10). These results raised the possibility that treatments targeting genes and pathways within the carcinomatous elements such as HER2 may prove efficacious in treating both carcinomatous and sarcomatous elements (10).
As of April 2015, the U.S. Food and Drug Administration (FDA) has approved five therapies for HER2-positive breast, gastric, and non-small cell lung cancer patients. The first class of drugs includes monoclonal antibodies against the extracellular domain of the HER2 receptor, including trastuzumab, ado-trastuzumab emtansine (T-DM1), and pertuzumab (27, 36, 37). In addition to these antibodies, two FDA-approved small-molecule tyrosine kinase inhibitors (TKIs), lapatinib and afatinib, inhibit the intracellular kinase domain of the HER receptor to prevent signaling. In contrast to breast cancer, therapy with trastuzumab alone (i.e.GOG 181B)(38) and lapatinib alone (i.e.GOG 229D)(39) revealed no responses in women with recurrent HER2 overexpressing endometrial cancer. While there is disagreement regarding the reasons why trastuzumab and lapatinib failed to demonstrate any significant durable clinical benefit (40), these clinical trials do suggest, as recently demonstrated by our research group (41, 42) that the limited benefit in endometrial cancer patients may be at least partially due to an innate or rapidly-acquired resistance to drugs targeting the HER2/neu pathways.
In an effort to optimize the use of HER2 blockade in the next phase of CS treatment and meaningfully alter patient outcomes, multiple groups including our own, have investigated the frequency of HER2 expression in uterine and ovarian CS with a reported range of overexpression of 25%–56% (32–34, 43, 44). Along these lines, Nicoletti et al., recently compared the activity of T-DM1 and Trastuzumab (T) against HER2 positive and negative primary CS cell lines in vitro followed by developing a supportive CS in vivo model. In Nicoletti’s study, T-DM1 and T were similarly effective in inducing strong ADCC against CS overexpressing HER2 at 3+ levels. In contrast, T-DM1 was dramatically more effective than T in inhibiting cell proliferation and, more importantly, in reducing tumor formation in CS xenografts overexpressing HER2 with a significantly longer survival when compared to T (44). Unfortunately, the activity of TDM-1 was evlauted in this study only against HER2/neu 3+ CS by IHC and c-erbB gene amplified endometrial cancer cell lines.
Expanding the patient population who would benefit from HER2 targeted therapies to patients harboring tumors with low (1+) or moderate (2+) HER2/neu expression might significantly expand the number of treatment-eligible patients. Consistent with this view, Black et al., investigated the efficacy of SYD985 in USC and compared the anti-tumor activity of SYD985 to T- DM1 against multiple primary USC cell lines expressing different levels of HER2/neu both in vitro and in vivo (31). The data from Black et al’s study demonstrated a remarkable antitumor activity of SYD985 against USC with not only strong (3+) but also with low to moderate (i.e., 1+/2+) HER2/neu expression (31). In agreement with Black’s results in USC, in the current study, we were able to demonstrate potent antitumor activity of SYD985 against both CSs with high (ie, 3+) and low (ie, 1+) HER2 expression. Importantly, our experiments demonstrated that SYD985 has consistently stronger cytotoxicity, when compared to T-DM1, against both high and low HER2/neu expressing CS cell lines in vitro and in vivo in SCID mice harboring CS xenografts with 3+ and 1+ HER2/neu expression. In this regard, while SYD985 and T-DM1 evoked similar levels of ADCC against HER2/neu expressing CS cell lines in the presence of effector peripheral blood lymphocytes (PBLs), SYD985 was significantly more cytotoxic against primary CS cell lines with HER2/neu expression of 1+ and 3+ in absence of PBLs. Specifically, in HER2/neu 3+ cell lines, we found SYD985 to be more than 7 folds more potent than T-DM1 (p <0.0001) in inducing cell death, while in HER2/neu 1+ cell lines, SYD985 was 54 folds more potent than T-DM1. In vivo data in multiple animal models harbouring CS xenografts with 3+ and 1+ HER2/neu expression and PDXs with 3+ HER2/neu expression were confirmatory of the in vitro results demonstrating high efficacy of SYD985. Indeed, one injection of SYD985 was enough to cure 100% and 60% of the mice harboring 3+ HER2/neu cells without any recurrence when treated with 10 mg/kg and 3mg/kg, respectively (45).
The cleavage of duocarmycine from its linker, unlike the cleavage of DM1, may take place in CS not only within HER2/neu overexpressing tumor cells after antibody internalization (28, 29) but also extracellularly within the tumor microenvironment, inducing a potent bystander effect. Consistent with this view, we found SYD985 to be signifcantly more potent than T-DM1 in its ability to induce bystander killing of low/negative HER2/neu expressing tumor cells admixed with HER2/neu positive tumor cells (31). Taken together, these results strongly suggest that SYD985 may represent a significantly more effective therapeutic tool when compared to T-DM1 or trastuzumab in HER2/neu expressing CS. Indeed, SYD985 may be effective not only against CS with low to moderate HER2/neu expression in the epithelial component but also against the mesenchymal components of CS, which is commonly reported to have negligible HER2/neu expression. Consistent with this view, encouraging clinical results have recently been reported with SYD985 in Phase I clinical trials in patients with locally advanced or metastatic solid tumors (NCT02277717) (30).
In conclusion, we have demonstrated that SYD985 is a novel ADC with remarkable activity against CS with strong (3+) as well as low (i.e., 1+) HER2/neu expression. SYD985 is significantly more potent than T-DM1 in comparative experiments and unlike T-DM1, is significantly active against the epithelial CS component overexpressing HER2/neu but also, potentially, against sarcomatous CS components showing low to negligible HER2/neu expression (ie, bystander killing). Clinical studies with SYD985 in CS patients harboring disease resistant to standard salvage chemotherapy are warranted.
TRANSLATIONAL RELEVANCE.
Carcinosarcomas (CSs) of the uterus and ovaries are uncommon but highly aggressive neoplasms characterized by a biphasic histology of carcinomatous and sarcomatous elements. In an effort to develop novel, active agents for these deadly gynecologic malignancies, we evaluated the efficacy of SYD985, (Synthon Biopharmaceuticals BV), a novel HER2-targeting antibody-drug conjugate (ADC) composed of the monoclonal antibody (mAb) trastuzumab linked to a highly potent DNA-alkylating agent (i.e., duocarmycin). Our results demonstrated SYD985 is a novel ADC with remarkable and significantly more potent activity than T-DM1 against uterine and ovarian CS with strong (3+) as well as low (i.e., 1+) HER2/neu expression. Clinical studies with SYD985 in patients harboring chemotherapy-resistant uterine and ovarian CS with HER2 expression are warranted.
Acknowledgments
We thank Synthon Biopharmaceuticals for the supply of SYD985 and its isotype control ADC.
Financial support: This work was supported in part by R01 CA154460-01 and U01 CA176067-01A1 grants from NIH, and grants from the Deborah Bunn Alley Foundation, the Tina Brozman Foundation, the Discovery to Cure Foundation and the Guido Berlucchi Foundation to A.D. Santin. This investigation was also supported by NIH Research Grant CA-16359 from the NCI to A.D. Santin.
Footnotes
Disclosure of Potential Conflicts of Interest
The authors declare no conflict of interest or previous publication. All of the authors fulfill the conditions required for authorship.
Authors' Contributions
Conception and design: G. Menderes, E. Bonazzoli, J. Black, G. Altwerger, N. Buza, M. Azodi, A.D. Santin
Development and methodology: G. Menderes, S. Bellone, E. Bonazzoli, A. Masserdotti, F. Pettinella, L. Zammataro, M. Azodi, A.D. Santin
Acquisition of data (provided animals, acquired and managed patients, provided facilities, etc.): G. Menderes, S. Bellone, J. Black, G. Altwerger, E. Bonazzoli, A.Masserdotti, F.Pettinella, N. Buza, P. Hui, S. Wong, S, D.-A. Silasi, M. Azodi, P.E. Schwartz, A.D. Santin
Analysis and interpretation of data (e.g., statistical analysis, biostati- stics, computational analysis): G. Menderes, E. Bonazzoli, J. Black, L.Zammataro, F. Predolini, N. Buza, P. Hui, M. Azodi, A.D. Santin
Writing, review, and/or revision of the manuscript: G. Menderes, S. Bellone, J. Black, G.Altwerger, F. Predolini, L.Zammataro, N. Buza, P. Hui, F.Pettinella, A. Masserdotti, E. Ratner, D.-A. Silasi, M. Azodi, B. Litkouhi, P.E. Schwartz, A.D. Santin
Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): G. Menderes, E.Bonazzoli, M. Azodi
Study supervision: S. Bellone, M. Azodi, A.D. Santin
References
- 1.Arend R, Doneza JA, Wright JD. Uterine carcinosarcoma. Curr Opin Oncol. 2011;23:531–6. doi: 10.1097/CCO.0b013e328349a45b. [DOI] [PubMed] [Google Scholar]
- 2.Jonson AL, Bliss RL, Truskinovsky A, Judson P, Argenta P, Carson L, et al. Clinical features and outcomes of uterine and ovarian carcinosarcoma. Gynecol Oncol. 2006;100:561–4. doi: 10.1016/j.ygyno.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 4.Guzzo F, Bellone S, Buza N, Hui P, Carrara L, Varughese J, et al. HER2/neu as a potential target for immunotherapy in gynecologic carcinosarcomas. Int J Gynecol Pathol. 2012;31:211–21. doi: 10.1097/PGP.0b013e31823bb24d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gonzalez Bosquet J, Terstriep SA, Cliby WA, Brown-Jones M, Kaur JS, Podratz KC, et al. The impact of multi-modal therapy on survival for uterine carcinosarcomas. Gynecol Oncol. 2010;116:419–23. doi: 10.1016/j.ygyno.2009.10.053. [DOI] [PubMed] [Google Scholar]
- 6.Rauh-Hain JA, Diver EJ, Clemmer JT, Bradford LS, Clark RM, Growdon WB, et al. Carcinosarcoma of the ovary compared to papillary serous ovarian carcinoma: a SEER analysis. Gynecol Oncol. 2013;131:46–51. doi: 10.1016/j.ygyno.2013.07.097. [DOI] [PubMed] [Google Scholar]
- 7.Voutsadakis IA. Epithelial to mesenchymal transition in the pathogenesis of uterine malignant mixed Mullerian tumours: the role of ubiquitin proteasome system and therapeutic opportunities. Clinical & translational oncology : official publication of the Federation of Spanish Oncology Societies and of the National Cancer Institute of Mexico. 2012;14:243–53. doi: 10.1007/s12094-012-0792-4. [DOI] [PubMed] [Google Scholar]
- 8.Castilla MA, Moreno-Bueno G, Romero-Perez L, Van De Vijver K, Biscuola M, Lopez-Garcia MA, et al. Micro-RNA signature of the epithelial-mesenchymal transition in endometrial carcinosarcoma. J Pathol. 2011;223:72–80. doi: 10.1002/path.2802. [DOI] [PubMed] [Google Scholar]
- 9.Zeisberg M, Neilson EG. Biomarkers for epithelial-mesenchymal transitions. J Clin Invest. 2009;119:1429–37. doi: 10.1172/JCI36183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao S, Bellone S, Lopez S, Thakral D, Schwab C, English DP, et al. Mutational landscape of uterine and ovarian carcinosarcomas implicates histone genes in epithelial–mesenchymal transition. Proceedings of the National Academy of Sciences. 2016 doi: 10.1073/pnas.1614120113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mirantes C, Espinosa I, Ferrer I, Dolcet X, Prat J, Matias-Guiu X. Epithelial-to-mesenchymal transition and stem cells in endometrial cancer. Hum Pathol. 2013;44:1973–81. doi: 10.1016/j.humpath.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 12.Zhao S, Choi M, Overton JD, Bellone S, Roque DM, Cocco E, et al. Landscape of somatic single-nucleotide and copy-number mutations in uterine serous carcinoma. Proc Natl Acad Sci U S A. 2013;110:2916–21. doi: 10.1073/pnas.1222577110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buza N, English DP, Santin AD, Hui P. Toward standard HER2 testing of endometrial serous carcinoma: 4-year experience at a large academic center and recommendations for clinical practice. Mod Pathol. 2013;26:1605–12. doi: 10.1038/modpathol.2013.113. [DOI] [PubMed] [Google Scholar]
- 14.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–74. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sukawa Y, Yamamoto H, Nosho K, Ito M, Igarashi H, Naito T, et al. HER2 expression and PI3K-Akt pathway alterations in gastric cancer. Digestion. 2014;89:12–7. doi: 10.1159/000356201. [DOI] [PubMed] [Google Scholar]
- 16.Molina MA, Codony-Servat J, Albanell J, Rojo F, Arribas J, Baselga J. Trastuzumab (herceptin), a humanized anti-Her2 receptor monoclonal antibody, inhibits basal and activated Her2 ectodomain cleavage in breast cancer cells. Cancer Res. 2001;61:4744–9. [PubMed] [Google Scholar]
- 17.Lewis Phillips GD, Li G, Dugger DL, Crocker LM, Parsons KL, Mai E, et al. Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody-cytotoxic drug conjugate. Cancer Res. 2008;68:9280–90. doi: 10.1158/0008-5472.CAN-08-1776. [DOI] [PubMed] [Google Scholar]
- 18.Amiri-Kordestani L, Blumenthal GM, Xu QC, Zhang L, Tang SW, Ha L, et al. FDA approval: ado-trastuzumab emtansine for the treatment of patients with HER2-positive metastatic breast cancer. Clin Cancer Res. 2014;20:4436–41. doi: 10.1158/1078-0432.CCR-14-0012. [DOI] [PubMed] [Google Scholar]
- 19.Ducry L, Stump B. Antibody-drug conjugates: linking cytotoxic payloads to monoclonal antibodies. Bioconjugate chemistry. 2010;21:5–13. doi: 10.1021/bc9002019. [DOI] [PubMed] [Google Scholar]
- 20.Klute K, Nackos E, Tasaki S, Nguyen DP, Bander NH, Tagawa ST. Microtubule inhibitor-based antibody-drug conjugates for cancer therapy. Onco Targets Ther. 2014;7:2227–36. doi: 10.2147/OTT.S46887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perez HL, Cardarelli PM, Deshpande S, Gangwar S, Schroeder GM, Vite GD, et al. Antibody-drug conjugates: current status and future directions. Drug discovery today. 2014;19:869–81. doi: 10.1016/j.drudis.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 22.Mathew J, Perez EA. Trastuzumab emtansine in human epidermal growth factor receptor 2-positive breast cancer: a review. Curr Opin Oncol. 2011;23:594–600. doi: 10.1097/CCO.0b013e32834b895c. [DOI] [PubMed] [Google Scholar]
- 23.Barginear MF, John V, Budman DR. Trastuzumab-DM1: a clinical update of the novel antibody-drug conjugate for HER2-overexpressing breast cancer. Molecular medicine (Cambridge, Mass) 2012;18:1473–9. doi: 10.2119/molmed.2012.00302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barok M, Tanner M, Koninki K, Isola J. Trastuzumab-DM1 is highly effective in preclinical models of HER2-positive gastric cancer. Cancer Lett. 2011;306:171–9. doi: 10.1016/j.canlet.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 25.Krop IE, Beeram M, Modi S, Jones SF, Holden SN, Yu W, et al. Phase I study of trastuzumab-DM1, an HER2 antibody-drug conjugate, given every 3 weeks to patients with HER2-positive metastatic breast cancer. J Clin Oncol. 2010;28:2698–704. doi: 10.1200/JCO.2009.26.2071. [DOI] [PubMed] [Google Scholar]
- 26.Hurvitz SA, Dirix L, Kocsis J, Bianchi GV, Lu J, Vinholes J, et al. Phase II randomized study of trastuzumab emtansine versus trastuzumab plus docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer. J Clin Oncol. 2013;31:1157–63. doi: 10.1200/JCO.2012.44.9694. [DOI] [PubMed] [Google Scholar]
- 27.Verma S, Miles D, Gianni L, Krop IE, Welslau M, Baselga J, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367:1783–91. doi: 10.1056/NEJMoa1209124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van der Lee MM, Groothuis PG, Ubink R, van der Vleuten MA, van Achterberg TA, Loosveld EM, et al. The Preclinical Profile of the Duocarmycin-Based HER2-Targeting ADC SYD985 Predicts for Clinical Benefit in Low HER2-Expressing Breast Cancers. Mol Cancer Ther. 2015;14:692–703. doi: 10.1158/1535-7163.MCT-14-0881-T. [DOI] [PubMed] [Google Scholar]
- 29.Dokter W, Ubink R, van der Lee M, van der Vleuten M, van Achterberg T, Jacobs D, et al. Preclinical profile of the HER2-targeting ADC SYD983/SYD985: introduction of a new duocarmycin-based linker-drug platform. Mol Cancer Ther. 2014;13:2618–29. doi: 10.1158/1535-7163.MCT-14-0040-T. [DOI] [PubMed] [Google Scholar]
- 30.Elgersma RC, Coumans RG, Huijbregts T, Menge WM, Joosten JA, Spijker HJ, et al. Design, Synthesis, and Evaluation of Linker-Duocarmycin Payloads: Toward Selection of HER2-Targeting Antibody-Drug Conjugate SYD985. Mol Pharm. 2015;12:1813–35. doi: 10.1021/mp500781a. [DOI] [PubMed] [Google Scholar]
- 31.Black J, Menderes G, Bellone S, Schwab CL, Bonazzoli E, Ferrari F, et al. SYD985, a Novel Duocarmycin-Based HER2-Targeting Antibody-Drug Conjugate, Shows Antitumor Activity in Uterine Serous Carcinoma with HER2/Neu Expression. Mol Cancer Ther. 2016;15:1900–9. doi: 10.1158/1535-7163.MCT-16-0163. [DOI] [PubMed] [Google Scholar]
- 32.Livasy CA, Reading FC, Moore DT, Boggess JF, Lininger RA. EGFR expression and HER2/neu overexpression/amplification in endometrial carcinosarcoma. Gynecol Oncol. 2006;100:101–6. doi: 10.1016/j.ygyno.2005.07.124. [DOI] [PubMed] [Google Scholar]
- 33.Amant F, Vloeberghs V, Woestenborghs H, Debiec-Rychter M, Verbist L, Moerman P, et al. ERBB-2 gene overexpression and amplification in uterine sarcomas. Gynecol Oncol. 2004;95:583–7. doi: 10.1016/j.ygyno.2004.07.041. [DOI] [PubMed] [Google Scholar]
- 34.Raspollini MR, Susini T, Amunni G, Paglierani M, Taddei A, Marchionni M, et al. COX-2, c-KIT and HER-2/neu expression in uterine carcinosarcomas: prognostic factors or potential markers for targeted therapies? Gynecol Oncol. 2005;96:159–67. doi: 10.1016/j.ygyno.2004.09.050. [DOI] [PubMed] [Google Scholar]
- 35.McCluggage WG. Malignant biphasic uterine tumours: carcinosarcomas or metaplastic carcinomas? J Clin Pathol. 2002;55:321–5. doi: 10.1136/jcp.55.5.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–92. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 37.Baselga J, Cortes J, Kim SB, Im SA, Hegg R, Im YH, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012;366:109–19. doi: 10.1056/NEJMoa1113216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fleming GF, Sill MW, Darcy KM, McMeekin DS, Thigpen JT, Adler LM, et al. Phase II trial of trastuzumab in women with advanced or recurrent, HER2-positive endometrial carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2010;116:15–20. doi: 10.1016/j.ygyno.2009.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leslie KK, Sill MW, Lankes HA, Fischer EG, Godwin AK, Gray H, et al. Lapatinib and potential prognostic value of EGFR mutations in a Gynecologic Oncology Group phase II trial of persistent or recurrent endometrial cancer. Gynecol Oncol. 2012;127:345–50. doi: 10.1016/j.ygyno.2012.07.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Santin AD. Letter to the Editor referring to the manuscript entitled: "Phase II trial of trastuzumab in women with advanced or recurrent HER-positive endometrial carcinoma: a Gynecologic Oncology Group study" recently reported by Fleming et al., (Gynecol Oncol., 116;15-20;2010) Gynecol Oncol. 2010;118:95–6. doi: 10.1016/j.ygyno.2010.01.043. author reply 6–7. [DOI] [PubMed] [Google Scholar]
- 41.Black JD, Lopez S, Cocco E, Bellone S, Altwerger G, Schwab CL, et al. PIK3CA oncogenic mutations represent a major mechanism of resistance to trastuzumab in HER2/neu overexpressing uterine serous carcinomas. Br J Cancer. 2015;113:1641. doi: 10.1038/bjc.2015.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lopez S, Cocco E, Black J, Bellone S, Bonazzoli E, Predolini F, et al. Dual HER2/PIK3CA Targeting Overcomes Single-Agent Acquired Resistance in HER2-Amplified Uterine Serous Carcinoma Cell Lines In Vitro and In Vivo. Mol Cancer Ther. 2015;14:2519–26. doi: 10.1158/1535-7163.MCT-15-0383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sawada M, Tsuda H, Kimura M, Okamoto S, Kita T, Kasamatsu T, et al. Different expression patterns of KIT EGFR, and HER-2 (c-erbB-2) oncoproteins between epithelial and mesenchymal components in uterine carcinosarcoma. Cancer Sci. 2003;94:986–91. doi: 10.1111/j.1349-7006.2003.tb01389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nicoletti R, Lopez S, Bellone S, Cocco E, Schwab CL, Black JD, et al. T-DM1, a novel antibody-drug conjugate, is highly effective against uterine and ovarian carcinosarcomas overexpressing HER2. Clin Exp Metastasis. 2015;32:29–38. doi: 10.1007/s10585-014-9688-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.English DP, Bellone S, Schwab CL, Bortolomai I, Bonazzoli E, Cocco E, et al. T-DM1, a novel antibody-drug conjugate, is highly effective against primary HER2 overexpressing uterine serous carcinoma in vitro and in vivo. Cancer Med. 2014;3:1256–65. doi: 10.1002/cam4.274. [DOI] [PMC free article] [PubMed] [Google Scholar]