Abstract
Background
An early age of drinking initiation (ADI) has been associated with increased risk for alcohol use disorders (AUDs), but the consistency of this risk across diverse samples has not been well studied. The purpose of this study was to examine whether the pathway from ADI to AUD symptoms by early adulthood is moderated by ethnicity and possessing an alcohol metabolizing gene ALDH2*2 variant allele.
Methods
We used multigroup structural equation modeling, including five groups split by ethnicity and ALDH2*2, to examine the consistency of the path from ADI to AUD symptoms in 604 Chinese-, Korean-, and White-American college students. We further examined the effects of ALDH2*2, ethnicity, and their interaction in Asians to better understand their unique contributions to the moderation.
Results
The association between ADI and AUD symptoms was moderated, with ADI negatively associated with AUD symptoms among Koreans without ALDH2*2 and Whites, but not among Koreans with ALDH2*2 or Chinese regardless of ALDH2*2. Both ALDH2*2 and ethnicity within Asians contributed unique variability in the effect.
Conclusions
Ethnicity and ALDH2*2 altered the relationship of ADI as a risk factor for AUD symptoms. Being Chinese and possessing an ALDH2*2 allele within Koreans both buffered against the risk for AUD symptoms associated with earlier ADI, indicating that this relationship can be attenuated by protective factors.
Keywords: ALDH2, ethnicity, conduct disorder, age at first drink, alcohol use disorders
An earlier age of drinking initiation (ADI) has been found to be a risk factor for the development of alcohol use disorders (AUDs) in both cross-sectional (Chou and Pickering, 1992; DeWit et al., 2000; Grant and Dawson, 1997; Hingson et al., 2006) and longitudinal studies (Fergusson et al., 1994; Hawkins et al., 1997; Prescott and Kendler, 1999a). Twin studies have found approximately one third of the variance in alcohol dependence is shared with ADI (Fowler et al., 2007; Prescott and Kendler, 1999a). Behavioral genetics studies, however, also indicate environmental factors are more strongly implicated in ADI, whereas genetic factors are more strongly associated with AUDs (Agrawal and Lynskey, 2008; Fowler et al., 2007; Maes et al., 1999; Pagan et al., 2006; Prescott and Kendler, 1999b).
Several explanations for mechanisms underlying the relationship between early ADI and the development of AUDs have been hypothesized (see Kuntsche et al., 2013; Maimaris and McCambridge, 2014). The causative model purports that early ADI causes disruption of the normal course of both intellectual and social development, which increases risk for a number of pathologies including AUDs (Brown et al., 2008; DeWit et al., 2000; Goudriaan et al., 2007; York, 1999). Alternatively, the marker hypothesis proposes ADI is a marker of other causal factors, such as the broader construct of behavioral undercontrol, which is expressed as a constellation of early emerging problem behaviors including conduct disorder (CD), poor academic achievement, and early drinking (Babor et al., 1992; Cloninger, 1987; Donovan and Jessor, 1985; McGue et al., 2001; Sher et al., 1991). Support for the causal model comes from studies that find ADI continues to predict AUDs even after covarying for additional risk factors (Dawson et al., 2008; Grant and Dawson, 1997; Hingson et al., 2006; Pederson and Skrondal, 1998; Sartor et al., 2006; 2008). For example, in a study of Australian twins, Agrawal et al (2009) concluded that after adjusting for the genetic confounding between ADI and AUD risk, ADI remained a risk factor for AUD. The marker hypothesis is supported by prospective research findings that a variety of behavioral problems assessed in middle childhood predict early ADI (McGue et al., 2001), and the association between ADI and alcohol dependence becomes non-significant after covarying for other risk factors (King and Chassin, 2007).
Limited research has examined ADI as a risk factor for AUDs in ethnically diverse samples, although some support has been found (Grant and Dawson, 1997; Hawkins et al., 1997). For example, in a longitudinal study ADI was found to mediate the effects of parental, peer, and school factors and ethnicity on risk for alcohol misuse seven years later, and the effects of other risk factors became non-significant after controlling for ADI (Hawkins et al., 1997). However, a more recent study found ADI was a predictor of alcohol problems by early adulthood in White- but not African-American females (Sartor et al., 2013). No previous studies have examined whether this relationship is found in individuals of Asian descent.
ADI has been found to differ across ethnic groups. In the National Longitudinal Study of Adolescent Health (1994–2008), Asian youth reported later ADI on average (16.1 years with 67.9% having initiated) than White youth (14.9 years with 84.1% having initiated; Clark et al., 2013). Two recent studies also examined alcohol initiation rates within Asian American subgroups (Shih et al., 2015, Kane et al., 2017). A study of middle school students in Southern California (Shih et al., 2015) did not find significant differences in alcohol initiation rates across seven subgroups of Asian American adolescents, although the authors noted a few trends by subgroup including that initiation of alcohol use was higher for Filipino (14.9%) and Korean (12.7%) adolescents and lower for Indian (4.4%) and Chinese (6.7%) adolescents. Results from the National Survey on Drug Use and Health (2002–2013) found a different pattern for early alcohol initiation (defined as alcohol use prior to age 14 years) among Asian American subgroups (Kane et al., 2017). Vietnamese American youth had the highest rate of early alcohol initiation (13.5%) and Indian American youth had the lowest rate (4.9%), with Chinese (9.4%) and Korean (9.5%) American youth having intermediate rates.
These few studies indicate differences in ADI across Asian and White American youth, but whether ADI is a consistent predictor of AUD across these ethnic groups remains to be determined. Studies that examine diverse samples and also take into account additional risk factors that vary across ethnic groups can provide insight into the stability of the relationship between ADI and the development of AUDs. The purpose of this study is to examine ADI as a risk factor for AUD symptoms after taking into account two additional risk factors for AUD that also have been found to differ in their prevalence rates across Asian and White American youth, CD symptoms and the alcohol metabolizing gene ALDH2*2 variant allele (rs671, ALDH2*Lys487), to better understand underlying pathways for the development of AUDs and the mechanistic role of ADI in this process.
This study builds upon our prior work that examined CD, ALDH2*2, ethnicity, and alcohol problems by incorporating ADI. In a previous study using path models with the current sample, we examined whether ethnic group differences in alcohol dependence could be explained by differences in CD and ALDH2*2 (Luczak et al., 2004). We found ALDH2*2 and CD partially accounted for ethnic group differences in rates of alcohol dependence in an additive, not an interactive, manner. The additive effect for CD and ALDH2*2 is consistent with these two variables being part of separate mechanistic pathways (i.e. behavioral undercontrol and pharmacological vulnerability pathways). In addition, we found ethnic group differences that were not accounted for by these models, suggesting the importance of other variables.
The current study extends this work to examine ethnicity and ALDH2*2 as moderators of the pathway from ADI to AUD after covarying for CD. Importantly, the variant ALDH2*2 allele, which produces an inactive enzyme that results in increased levels of acetaldehyde during alcohol metabolism that is associated with more intense responses to alcohol and decreased risk for AUDs (see Li, 2000; Luczak et al., 2006; Wall et al., 2016), is expected to exert an influence on AUDs once an individual begins to drink. ALDH2*2 is extremely rare in Whites, but the allele is present in about one third of Korean and one half of Chinese individuals (see Eng et al., 2007), so studying these ethnic groups provides a unique opportunity to incorporate this genetic risk factor into ADI-AUD developmental pathway models. Examining the relationships between ADI and AUD in individuals with variant ALDH2*2 alleles can help elucidate the stability and strength of the relationship between ADI and AUD across individuals whose physical reactions to alcohol are expected to vary once drinking is initiated.
Our primary hypothesis was that the relationship between ADI and AUD symptoms would differ by ALDH2*2 status, with ALDH2*2 buffering the risk associated with earlier ADI. We did not make a priori predictions about the role of ethnicity as a moderator of this relationship, but we tested for differences in this relationships across ethnic groups given our prior finding of variation among Chinese-, Korean-, and White-Americans on alcohol dependence that was not accounted for by either CD or ALDH2*2. Covarying for CD, which we previously reported was highest in the Koreans and lowest in the Chinese in our sample, enables us to examine the ADI-AUD relationship above and beyond the already well-established relationship of CD with AUD while also removing any confound with ADI. Understanding the stability of the ADI-AUD relationship in a diverse sample with variations in the prevalence of multiple risk factors will help to advance our knowledge of how genetic and ethnic group variables combine and interact with ADI to contribute to the development of AUD.
Materials and Methods
Participants
Participants were 604 college students recruited from the University of California, San Diego ranging in age from 21 to 26 years (M = 21.95, SD = 1.26). All participants reported that both biological parents were entirely of Chinese (n = 190; 52% female), Korean (n = 214; 50% female), or White (n = 200; 47% female) heritage.
Procedure and Measures
Participants were recruited via advertisements on campus and in the school newspaper that did not state the purpose of the study. Participants were informed about our procedures for protecting confidentiality, including obtaining a Certificate of Confidentiality from the U.S. Department of Health and Human Services that restricts access to individual data by subpoena. Informed written consent for participation was obtained.
Each participant completed an individual assessment with a trained research interviewer. The interview included the Semi-Structured Assessment for the Genetics of Alcoholism (SSAGA; Bucholz et al., 1994), which obtains drinking milestones and DSM-IV CD and AUD symptoms. Participants also provided a blood sample via fingerprick onto filter paper, which was sent to the Alcohol Research Center at Indiana University for genotyping at the ALDH2 loci using enzymatic amplification of genomic DNA and allele-specific oligonucleotides as previously described (see Luczak et al., 2001).
Variables in the Models
Groups Based on ALDH2*2 and Ethnicity
ALDH2 genotyping indicated that 91 (48%) Chinese were ALDH2*1/*1, 84 (44%) were ALDH2*1/*2, and 15 (8%) were ALDH2*2/*2, and that 141 (66%) Koreans were ALDH2*1/*1, 67 (31%) were ALDH2*1/*2, and 6 (3%) were ALDH2*2/*2. No Whites had an ALDH2*2 allele. Given that the effects of these alleles are not linear (see Li, 2000; Luczak et al., 2006) and to increase power, the groups were dichotomized for statistical comparisons on the basis of the presence (+) or absence (−) of ALDH2*2 allele, which is consistent with prior studies (e.g., Hendershot et al., 2005; Luczak et al., 2004). We then crossed Ethnicity (Chinese, Korean, White) and ALDH2*2 (+/−) to create five groups for the multiple-group path model: Chinese(−), Chinese(+), Korean(−), Korean (+), and White. Using ethnicity/ALDH2*2 groups takes into account the issue of population stratification in our analyses and allows for the effects of a genetic variant that differs in prevalence across ethnic groups and the effects of ethnicity to be examined in a single model.
ADI
Participants reported their age (in years) when they first had a full drink, not a sip, of alcohol. A drink was defined as 12 oz of beer, 4 oz of wine, or a single shot (1–1.5 oz) of 80 proof of alcohol. Five Chinese, five Koreans, and four Whites had not consumed a full drink. Five individuals reported ages of 5 and 7 years, which was >3 SD below the mean, and thus were collapsed (i.e., winsorized) into the score of 8 years (coinciding with a z score of −3.04 in the original distribution). This resulted in an 8-year-old age category that contained 1.7% of the data.
AUD Symptoms
The dependent variable in our model was lifetime DSM-IV (APA, 1994) AUD symptoms. AUD symptom counts in the current sample ranged from 0 to 11. We used a square-root transformation to account for the bottom-heavy distribution, improving the skew statistic from 2.09 to −0.84.
CD Symptoms
We included CD symptoms prior to age 15 years as a covariate in the model. A unique feature of the SSAGA is that it assesses psychiatric symptoms independent of substance abuse. Thus, no symptom of CD in our data was the result of substance use. CD symptom counts in the current sample ranged from 0 to 8. We used a square-root transformation to account for the bottom-heavy distribution, improving the skew statistic from 1.85 to 0.62.
Gender
We included Gender (0 = female, 1 = male) as a covariate in the model.
Analyses
Analyses were conducted in SPSS version 24 (IBM corp, 2016) and Mplus version 8 (Muthén and Muthén, 1998–2017). Our primary analyses tested a single multigroup path model using a series of constraints and releases across the five ALDH2*2/ethnic groups. Means were free to vary across groups, and gender and CD Symptoms were included as covariates. Model constraints were compared using loglikelihood (−2LL), Akaike information criterion (AIC), and Δχ2 statistics. We first compared the model fit with the ADI-AUD Symptoms path fixed versus freed across the five groups. When we found the model fit significantly improved by freeing this path, we then tested whether a series of 2 and 3 groups could be constrained without significantly reducing model fit to determine which groups were statistically equivalent and which were not. In a final step, we tested whether the relationship between ADI and AUD Symptoms was significantly different from 0.
Lastly, in a follow-up analysis of Asians only, we further examined whether ethnicity (Chinese, Korean) and ALDH2*2 were each significant moderators of the relationship ADI and AUD Symptoms using Model 3 in the PROCESS macro version 2.16.3 (Hayes, 2012–2016; 2013). ALDH2*2 was included as a moderator of ethnicity, which itself was a direct moderator of the relationship ADI and AUD Symptoms, and gender and CD Symptoms were entered as covariates (see Figure 1). Because there is no ALDH2*2 variation in Whites, only Asians were included in this analysis. This analysis allowed us to more fully explicate the extent to which ALDH2*2, Asian ethnic subgroup, and their interaction each accounted for the significant group effects we found in the multigroup models using groups created from the combination of ALDH2*2 and ethnic group.
Figure 1.
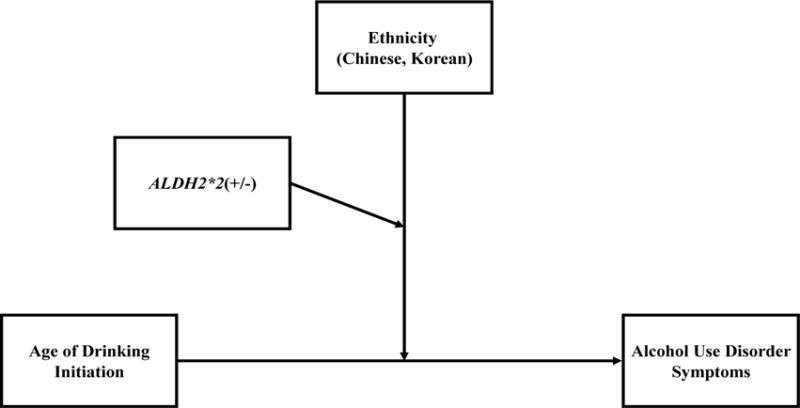
Moderated Moderation Model of Ethnicity and ALDH2*2 as Moderators of the Path from Age of Drinking Initiation (ADI) to Alcohol Use Disorder (AUD) Symptoms in Asians Only
Results
Descriptive Statistics
Table 1 shows the means and intercorrelations for the model variables stratified into the five ALDH2*2/ethnic groups. CD Symptoms were positively associated with AUD Symptoms among all groups and were negatively associated with ADI among all groups except Chinese(−). ADI was negatively associated with AUD Symptoms among all groups except Chinese(−). ANOVAs indicated ADI, F (4, 599) = 5.21, p < .001, CD Symptoms, F (4, 599) = 4.30, p = .002, and AUD Symptoms, F (4, 599) = 16.35, p < .001, levels differed across the five groups. Tukey HSD posthoc comparisons found significant mean differences in ADI for Whites with Korean(−) and Chinese(+); in CD Symptoms for Korean(−) with Chinese(−) and Chinese(+); and in AUD Symptoms for Whites with Chinese(−), Chinese(+), and Korean(+), and for Korean(−) with Chinese(−) and Chinese(+).
Table 1.
Means (Standard Errors) and Intercorrelations among Childhood Conduct Disorder (CD) Symptoms, Age of Drinking Initiation (ADI), and Alcohol Use Disorder (AUD) Symptoms stratified by Ethnicity and ALDH2*2(+/−) Groups
| M | (SD) | Correlations
|
|||
|---|---|---|---|---|---|
| CD Symptoms | ADI | AUD Symptoms | |||
| Chinese(−) (n = 91, 48% female) | |||||
| CD Symptoms | 0.65 | (0.86) | – | −.10 | .25* |
| ADI (age in years) | 16.8 | (3.62) | – | −.04 | |
| AUD Symptoms | 0.53 | (1.39) | – | ||
| Chinese(+) (n = 99, 55% female) | |||||
| CD Symptoms | 0.75 | (1.34) | – | −.27** | .27* |
| ADI (age in years) | 17.2 | (2.71) | – | −.26* | |
| AUD Symptoms | 0.33 | (0.91) | – | ||
| Korean(−) (n = 141, 50% female) | |||||
| CD Symptoms | 1.33 | (1.63) | −.28*** | .46*** | |
| ADI (age in years) | 17.1 | (2.37) | – | −.41*** | |
| AUD Symptoms | 1.40 | (2.30) | |||
| Korean(+) (n = 73, 51% female) | |||||
| CD Symptoms | 1.27 | (1.84) | – | −.51*** | .27* |
| ADI (age in years) | 16.6 | (2.95) | – | −.21† | |
| AUD Symptoms | 0.84 | (1.61) | – | ||
| White (n = 200, 47% female) | |||||
| CD Symptoms | 0.97 | (1.30) | – | −.33*** | .43*** |
| ADI (age in years) | 15.95 | (2.39) | – | −.38*** | |
| AUD Symptoms | 1.83 | (2.43) | – | ||
Note. All M and SD are raw scores. ADI is in years, winsorized at the lower end as described in the text. For correlations, CD Symptoms and AUD Symptoms are square root transformed counts of DSM-IV conduct disorder and alcohol use disorder symptoms, respectively. Correlations are Pearson and reflect pairwise deletion.
p < .10.
p < .05.
p < .01.
p < .001. (two-tailed tests)
Multigroup Model
Table 2 shows the relative fits of our model after imposing various multigroup constraints. The baseline model with the ADI-AUD Symptoms path fixed across all five groups is shown in row 1. Releasing the path in row 2 improved the model fit, indicating this path was moderated by group. We then constrained the path across select groups based on ethnicity and ALDH2*2 to determine which groups had similar model fits and thus could be constrained to be equivalent (shown in rows 3–9). Constraining the Chinese(−) and (+) groups did not significantly affect model fit, but constraining the Korean(−) and (+) groups did. Constraining the Chinese(+) and Korean(+) groups did not affect model fit, whereas constraining the Chinese(−) and Korean(−) groups did. Constraining Koreans(−) and Whites (who also have no ALDH2*2 allele) also did not significantly affect model fit. Finally, constraining Chinese(−), Chinese(+), and Korean(+) groups did not significantly reduce fit. Thus, the groups were parsimoniously constrained into Whites/Korean(−) and Chinese(−/+)/Korean(+). In this model, the ADI-AUD Symptoms path was significant for Whites/Korean(−), b = −.121, p < .001, but not for Chinese(−/+)/Korean(+), b = −.019, p = .140. Finally, given the small and non-significant ADI-AUD Symptoms path loading for Chinese(−/+)/Korean(+), in our final model (row 10) we constrained the ADI-AUD path to zero for Chinese(−/+)/Korean(+), which did not significantly affect model fit. Thus, the ADI-AUD Symptoms path was significantly negative for the White/Korean(−) groups, but was not statistically different from zero for Chinese(−/+)/Korean(−) groups.
Table 2.
Fit Comparisons of a Multigroup (ALDH2*2/Ethnicity) Model of Age of Drinking Initiation (ADI) to Alcohol Use Disorder (AUD) Symptoms
| ADI-AUD Symptom path | Model Fit
|
Comparative Statistics
|
||||
|---|---|---|---|---|---|---|
| χ2 | df | LL | AIC | Row # compared (df) | Δχ2 (p-value) | |
| Fit Tested Across All 5 groups | ||||||
| 1. Constrained across all 5 groups | 20.71 | 9 | −3108.4 | 6338.7 | – | – |
| 2. Released across all 5 groups | 0.0 | 5 | −3098.0 | 6326.0 | 1 (4df) | 20.71 (< .001) |
|
| ||||||
| Constraints Across Specific Groups | ||||||
| 3. Chinese(−) and Chinese(+) | 1.84 | 6 | −3098.9 | 6325.9 | 2 (1df) | 1.84 (.18) |
| 4. Korean(−) and Korean(+) | 4.57 | 6 | −3100.3 | 6328.6 | 2 (1df) | 4.57 (.033) |
| 5. Chinese(+) and Korean(+) | 0.10 | 6 | −3098.1 | 6324.1 | 2 (1df) | 0.10 (.75) |
| 6. Chinese(−) and Korean(−) | 11.61 | 6 | −3103.8 | 6335.6 | 2 (1df) | 11.61 (.001) |
| 7: Korean(−) and Whites | 0.00 | 6 | −3098.0 | 6324.0 | 2 (1df) | 0.00 (–) |
| 8. Chinese(+/−)/Korean(+) | 1.87 | 7 | −3098.9 | 6323.9 | 2 (2df) | 1.87 (.39) |
| 9. Two Groups: 1) Whites/Korean(−) and 2) Chinese(+/−)/Korean(+) | 1.87 | 8 | −3098.9 | 6321.9 | 2 (3df) | 1.87 (.60) |
| 10. Constrained path in Chi(+/−)/Kor(+) to 0 | 4.04 | 9 | −3100.0 | 6322.1 | 9 (1df) | 2.17 (.14) |
Note. Models covary for conduct disorder symptoms and gender.
Moderated Moderation Model
In a final analysis of Asians only (n = 394), we examined a moderated moderation model with ALDH2*2 moderating ethnicity, which was a direct moderator of the ADI-AUD Symptoms relationship. The model accounted for an R2 of .239, F(9, 384) = 13.43, p < .001. The three-way interaction of ADI X ALDH2*2 X Ethnicity was significant (ΔR2 = .018, p = .003), all two-way interactions were p < .10 (ranging from p < .001 to p = .052), and all simple effects were significant for Chinese/Korean (b = 1.34, p < .001), ALDH2*2(−/+) (b = 1.88, p = .047), and ADI (b = 0.07, p = .044). When conditional effects were examined, ALDH2*2(−) was driving the moderation of Ethnicity X ADI interaction (b = −0.13, p < .001), and Korean(−) was driving the moderation of the ADI-AUD Symptoms relationship (b = −0.07, p < .001). These results are consistent with the multigroup models and further show that ALDH2*2, Asian ethnic subgroup, and their interaction each contributed to the significant group effects we found in the multigroup models where the five groups were based on both ALDH2*2 and ethnic group.
Discussion
The present study examined whether ethnicity and ALDH2*2 moderate the risk for AUD symptoms associated with earlier ADI in a sample of Chinese−, Korean−, and White-American college students. The prevalence of several risk and protective factors along the development path for AUDs varies between White and Asian American ethnic groups, which provides an opportunity to examine the ADI-AUD relationship among varying levels of risk. The results of this study extend previous research by revealing a pattern of risk for AUD that varies by both ethnicity and ALDH2*2. Our findings indicate that ADI is not associated with AUD symptoms in Koreans with an ALDH2*2 allele or in Chinese regardless of ALDH2*2 status. Thus, both ALDH2*2 and ethnic subgroup are moderators of this relationship, with the risk of ADI being buffered by possessing a genetic variation that heightens responses to alcohol as well as by additional factors among Chinese that remain to be determined.
This novel finding of variations in the ADI-AUD relationship indicates that it is not just an earlier initiation of drinking that predicts subsequent drinking problems, but that factors involved in the process once an individual has begun to drink are influential in determining whether earlier drinking is predictive of later problems. Our finding that ALDH2*2 alters the effects of earlier drinking on the development of AUD symptoms suggests the relationship between ADI-AUD symptoms is modifiable. In a prospective study of Asian Americans over four years of college (beginning at age 18 years), we previously showed that the relationship between binge drinking and alcohol-related problems differed for individuals with and without an ALDH2*2 allele (Luczak et al., 2014). In that study, which examined only alcohol consumption patterns and problems, the path from consumption to problems was moderated by possession of an ALDH2*2 allele, with binge drinking being a less strong predictor of problems in those with ALDH2*2. These findings suggest an individual’s level of response to alcohol contributes to the likelihood of drinking heavily, but also variability in the relationship between heavy drinking and developing problems. Along these lines, the pharmacological vulnerability model predicts not only that a higher response to alcohol is protective against developing AUDs, but also that a lower response is a risk factor for AUDs (see Luczak et al., 2011; Schuckit et al., 2004; Sher, 1991). Thus, research examining the relationship between ADI and AUD in individuals with a low response to alcohol could provide further insight into variation in the progression from drinking initiation to alcohol-related problems. In addition, longitudinal studies that incorporate both risk and protective factors along the ADI-AUD pathway including a detailed assessment of early drinking patterns would provide a more nuanced understanding of mechanisms underlying developmental differences in the path from initiation to problems.
This study cannot speak directly to whether ADI is a causal factor or a marker, but our findings do indicate that the path from ADI to AUD symptoms can be altered by other factors. Earlier ADI does place individuals at risk for developing AUD symptoms, but it is clear that this relationship has variability that involves a greater constellation of factors than these two factors alone. Genetically informative studies have found environmental factors are more involved in ADI whereas genetic factors are more implicated in AUDs (Agrawal and Lynskey, 2008; Fowler et al., 2007; Maes et al., 1999; Pagan et al., 2006; Prescott and Kendler, 1999b). Our results also suggest that specific genetic variations like ALDH2*2 further affect how more environmentally-influenced risk factors like ADI relate to the development of AUDs, thus helping to inform how gene-environment interactions are involved in the mechanistic pathways. It will also be important to examine what other genetic and environmental factors are found in Chinese Americans that offer further protection from AUDs beyond those afforded by ALDH2*2.
The ADI-AUD relationship was significant in some of our groups after covarying for CD symptoms and gender but not in others, suggesting sample differences may help explain why only some studies have found this ADI-AUD relationship after covarying for CD. In this study, CD symptoms were correlated with AUD symptoms in all ALDH2*2/ethnic groups, and with earlier ADI in all groups except Chinese without ALDH2*2. These results are consistent with the behavioral undercontrol model, with childhood CD symptoms being related to both earlier ADI and higher AUD symptoms by early adulthood, regardless of ALDH2*2 or ethnicity. The robust relationship of CD-ADI and CD-AUD across our groups provides additional support for the generalizability of this developmental pathway in diverse samples. However, once accounting for CD symptoms, ADI remained a significant predictor of AUD symptoms only in Whites and Koreans without ALDH2*2. Understanding when and how ADI contributes additional risk for AUD symptoms above and beyond CD symptoms can be examined in future research with other diverse samples.
It is important to acknowledge the limitations of the study. The data collected are based on retrospective self-report interviews, which are subject to response bias. This is particularly important to consider given the possibility of differential accuracy of self-report across ethnic groups (e.g., Bauman and Ennett, 1994), although accuracy is not expected to differ based on ALDH2*2. Longitudinal studies with ages of initiation obtained over time will provide further support of these developmental processes. In addition, the use of a college student sample limits the generalizability of the results to different age groups as well as to the population of non-college attending individuals of the same age. Finally, we were unable to take into account additional genetic (e.g., ADH1B), acculturation, and background-culture variables in our analyses. Future research is needed with additional variables such as acculturation, parental respect, permissive attitudes toward alcohol, etc. (Hahm et al., 2004; Iwamoto et al., 2016; Shih et al., 2012) to investigate how these factors are related to ethnic differences in ADI and alcohol problems.
Despite these limitations, this study contributes to the literature by providing an example of how risk and protective factors combine to alter the development of AUD symptoms, particularly the role of ADI. It also indicates a possible point to intervene in this pathway once drinking has occurred, for example based on response to alcohol and heavy consumption patterns. Finally, this study shows that ethnic group differences in AUD symptoms result not only from different prevalence of risk and protective factors, but also are due to variation in the relationships between vulnerability factors and alcohol outcomes. Such moderation models can be used in future studies to examine mechanistic pathways between risk/protective factors and drinking outcomes in prospective designs.
Acknowledgments
This work was supported by National Institutes of Health grants K02AA00269, K08AA14265, P60AA06420, R01AA11257, R01AA18179, and R01DA35804.
We thank Travis A. R. Cook and Lisa M. Yarnell for their contributions to this work.
Footnotes
The authors have no conflict of interest to declare.
References
- Agrawal A, Lynskey M. Are there genetic influences on addiction: evidence from family, adoption and twin studies. Addiction. 2008;103:1069–1078. doi: 10.1111/j.1360-0443.2008.02213.x. [DOI] [PubMed] [Google Scholar]
- Agrawal A, Sartor CE, Lynskey MT, Grant JD, Pergadia ML, Grucza R, Bucholz KK, Nelson EC, Madden PA, Martin NG, Heath AC. Evidence for an interaction between age at first drink and genetic influences on DSM-IV alcohol dependence symptoms. Alcohol Clin Exp Res. 2009;33:2047–2056. doi: 10.1111/j.1530-0277.2009.01044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th. American Psychiatric Press; Washington, DC: 1994. [Google Scholar]
- Babor TF, Hofmann M, Del Boca FK, Hesselbrock VM. Types of alcoholics: I. evidence for an empirically derived typology based on indicators of vulnerability and severity. Arch Gen Psychiatry. 1992;49:599–608. doi: 10.1001/archpsyc.1992.01820080007002. [DOI] [PubMed] [Google Scholar]
- Bauman KE, Ennett SE. Tobacco use by Black and White adolescents: the validity of self-reports. Am J Pub Health. 1994;84:394–398. doi: 10.2105/ajph.84.3.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SA, McGue M, Maggs J, Schulenberg J, Hingson R, Swartzwelder S, Martin C, Chung T, Tapert SF, Sher K, Winters KC, Lowman C, Murphy S. A developmental perspective on alcohol and youths 16 to 20 years of age. Pediatrics. 2008;121:S290–310. doi: 10.1542/peds.2007-2243D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucholz KK, Cadoret R, Cloninger CR, Dinwiddie SH, Hesselbrock VM, Nurnberger JI, Reich T, Schmidt I, Schuckit MA. A new semi-structured psychiatric interview for use in genetic linkage studies: a report on the reliability of the SSAGA. J Stud Alcohol. 1994;55:149–158. doi: 10.15288/jsa.1994.55.149. [DOI] [PubMed] [Google Scholar]
- Chou SP, Pickering RP. Early onset drinking as a risk factor for lifetime alcohol-related problems. Br J Addict. 1992;87:1199–1204. doi: 10.1111/j.1360-0443.1992.tb02008.x. [DOI] [PubMed] [Google Scholar]
- Clark TT, Doyle O, Clincy A. Age of first cigarette, alcohol, and marijuana use among U.S. biracial/ethnic youth: A population-based study. Addict Behav. 2013;38:2450–2454. doi: 10.1016/j.addbeh.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloninger CR. Neurogenetic adaptive mechanisms in alcoholism. Science. 1987;236:410–416. doi: 10.1126/science.2882604. [DOI] [PubMed] [Google Scholar]
- Dawson DA, Goldstein RB, Chou SP, Ruan WJ, Grant BF. Age at first drink and the first incidence of adult-onset DSM-IV alcohol use disorders. Alcohol Clin Exp Res. 2008;32:2149–2160. doi: 10.1111/j.1530-0277.2008.00806.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWit DJ, Adlaf EM, Offord DR, Ogborne AC. Age at first alcohol use: a risk factor for the development of alcohol disorders. Am J Psychiatry. 2000;157:745–750. doi: 10.1176/appi.ajp.157.5.745. [DOI] [PubMed] [Google Scholar]
- Donovan JE, Jessor R. Structure of problem behavior in adolescence and young adulthood. J Consult Clin Psychol. 1985;53:890–904. doi: 10.1037//0022-006x.53.6.890. [DOI] [PubMed] [Google Scholar]
- Eng MY, Luczak SE, Wall TL. ALDH2, ADH1B, and ADH1C genotypes in Asians: a literature review. Alcohol Res Health. 2007;30:22–27. [PMC free article] [PubMed] [Google Scholar]
- Fergusson DM, Lynskey MT, Horwood LJ. Childhood exposure to alcohol and adolescent drinking patterns. Addiction. 1994;8:1007–1016. doi: 10.1111/j.1360-0443.1994.tb03360.x. [DOI] [PubMed] [Google Scholar]
- Fowler T, Lifford K, Shelton K, Rice F, Thapar A, Neale MC, McBride A, Van Den Bree M. Exploring the relationship between genetic and environmental influences on initiation and progression of substance use. Addiction. 2007;102:413–422. doi: 10.1111/j.1360-0443.2006.01694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudriaan AE, Grekin ER, Sher KJ. Decision making and binge drinking: a longitudinal study. Alcohol Clin Exp Res. 2007;31:928–938. doi: 10.1111/j.1530-0277.2007.00378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Dawson DA. Age at onset of alcohol use and its association with DSM-IV alcohol abuse and dependence: results from the National Longitudinal Alcohol Epidemiologic Survey. J Subst Abuse. 1997;9:103–110. doi: 10.1016/s0899-3289(97)90009-2. [DOI] [PubMed] [Google Scholar]
- Hahm HC, Lahiff M, Gutterman NB. Asian American adolescents’ acculturation, binge drinking, and alcohol- and tobacco-using peers. J Community Psychology. 2004;32:295–308. [Google Scholar]
- Hawkins JD, Graham JW, Maguin E, Abbott R, Hill KG, Catalano RF. Exploring the effects of age of alcohol use initiation and psychosocial risk factors on subsequent alcohol misuse. J Stud Alcohol. 1997;58:280–290. doi: 10.15288/jsa.1997.58.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AF. The PROCESS macro for SPSS and SAS, version 2.16.3. 2012–2016 Available at: http://www.processmacro.org/index.html.
- Hayes AF. Introduction to mediation, moderation, and conditional process analysis: a regression-based approach. The Guilford Press; New York: 2013. [Google Scholar]
- Hendershot CS, MacPherson L, Myers MG, Carr LG, Wall TL. Psychosocial, cultural and genetic influences on alcohol use in Asian-American youth. J Stud Alcohol. 2005;66:185–195. doi: 10.15288/jsa.2005.66.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hingson RW, Heeren T, Winter MR. Age at drinking onset and alcohol dependence: age at onset, duration, and severity. Arch Pediatr Adolesc Med. 2006;160:739–746. doi: 10.1001/archpedi.160.7.739. [DOI] [PubMed] [Google Scholar]
- Iwamoto DK, Kaya A, Grivel M, Clinton L. Under-researched demographics: Heavy episodic drinking and alcohol-related problems among Asian American adolescents. Alcohol Res. 2016;38:17–25. [PMC free article] [PubMed] [Google Scholar]
- Kane JC, Damian AJ, Fairman B, Bass JK, Iwamoto DK, Johnson RM. Differences in alcohol use patterns between adolescent Asian American ethnic groups: Representative estimates from the National Survey on Drug Use and Health. Addict Behav. 2017;64:154–158. doi: 10.1016/j.addbeh.2016.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King KM, Chassin L. A prospective study of the effects of age of initiation of alcohol and drug use on young adult substance dependence. J Stud Alcohol. 2007;68:256–265. doi: 10.15288/jsad.2007.68.256. [DOI] [PubMed] [Google Scholar]
- Kuntsche E, Rossow I, Simons-Morton B, Bogt TT, Kokkevi A, Godeau E. Not early drinking but early drunkenness is a risk factor for problem behaviors among adolescents from 38 European and North American countries. Alcohol Clin Exp Res. 2013;37:308–314. doi: 10.1111/j.1530-0277.2012.01895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T-K. Pharmacogenetics of responses to alcohol and genes that influence alcohol drinking. J Stud Alcohol. 2000;61:5–12. doi: 10.15288/jsa.2000.61.5. [DOI] [PubMed] [Google Scholar]
- Luczak SE, Glatt SJ, Wall TL. Meta-analyses of ALDH2 and ADH1B with alcohol dependence in Asians. Psychol Bull. 2006;4:607–621. doi: 10.1037/0033-2909.132.4.607. [DOI] [PubMed] [Google Scholar]
- Luczak SE, Pandika D, Shea SH, Eng MY, Liang T, Wall TL. ALDH2 and ADH1B interactions in retrospective reports of low-dose reactions and initial sensitivity to alcohol in Asian Americans. Alcohol Clin Exp Res. 2011;35:1238–1245. doi: 10.1111/j.1530-0277.2011.01458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luczak SE, Wall TL, Cook TAR, Shea SH, Carr LG. ALDH2 status and conduct disorder mediate the relationship between ethnicity and alcohol dependence in Chinese-, Korean-, and White-American college students. J Abnorm Psychol. 2004;113:271–278. doi: 10.1037/0021-843X.113.2.271. [DOI] [PubMed] [Google Scholar]
- Luczak SE, Wall TL, Shea SH, Byun S, Carr LG. Binge drinking in Chinese, Korean, and White college students: genetic and ethnic group differences. Psych Addict Beh. 2001;15:306–309. doi: 10.1037//0893-164x.15.4.306. [DOI] [PubMed] [Google Scholar]
- Luczak SE, Yarnell LM, Prescott CA, Myers MG, Carr LG, Wall TL. Effects of ALDH2*2 on alcohol consumption and problems over four years of college. J Abnorm Psychol. 2014;123:130–140. doi: 10.1037/a0035486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes HH, Woodard CE, Murrelle L, Meyer JM, Silberg JL, Hewitt JK, Rutter M, Simonoff E, Pickles A, Carbonneau R, Neal MC, Eaves LJ. Tobacco, alcohol and drug use in eight- to sixteen-year-old twins: the Virginia Twin Study of Adolescent Behavioral Development. J Stud Alcohol. 1999;60:293–305. doi: 10.15288/jsa.1999.60.293. [DOI] [PubMed] [Google Scholar]
- Maimaris W, McCambridge JJ. Age of first drinking and adult alcohol problems: systematic review of prospective cohort studies. J Epidemiol Community Health. 2014;68:268–274. doi: 10.1136/jech-2013-203402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGue M, Iacono WG, Legrand LN, Malone S, Elkins I. Origins and consequences of age at first drink: I. associations with substance-use disorders, disinhibitory behavior and psychopathology, and P3 amplitude. Alcohol Clin Exp Res. 2001;8:1156–1165. [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus User’s Guide. Los Angeles: Muthén and Muthén; 1998–2017. [Google Scholar]
- Pagan JL, Rose RJ, Viken RJ, Pulkkinen L, Kaprio J, Dick DM. Genetic and environmental influences on stages of alcohol use across adolescence and into young adulthood. Beh Genet. 2006;36:483–497. doi: 10.1007/s10519-006-9062-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pederson W, Skrondal A. Alcohol consumption debut: predictors and consequences. J Stud Alcohol. 1998;59:32–42. doi: 10.15288/jsa.1998.59.32. [DOI] [PubMed] [Google Scholar]
- Prescott C, Kendler KS. Age at first drink and risk for alcoholism: a noncausal association. Alcohol Clin Exp Res. 1999a;1:101–107. [PubMed] [Google Scholar]
- Prescott C, Kendler KS. Genetic and environmental contributions to alcohol abuse and dependence in a population-based sample of male twins. Am J Psychiatry. 1999b;156:34–40. doi: 10.1176/ajp.156.1.34. [DOI] [PubMed] [Google Scholar]
- Sartor CE, Agrawal A, Lynskey MT, Bucholz KK, Heath AC. Genetic and environmental influences on the rate of progression to alcohol dependence in young women. Alcohol Clin Exp Res. 2008;32:632–638. doi: 10.1111/j.1530-0277.2008.00621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartor CE, Lynskey MT, Heath AC, Jacob T, True W. The role of childhood risk factors in initiation of alcohol use and progression to alcohol dependence. Addiction. 2006;102:216–225. doi: 10.1111/j.1360-0443.2006.01661.x. [DOI] [PubMed] [Google Scholar]
- Sartor CE, Nelson EC, Lynskey MT, Madden PAF, Heath AC, Bucholz KK. Are there differences between young African-American and European-American women in the relative influences of genetics versus environment on age at first drink and problem alcohol use? Alcohol Clin Exp Res. 2013;37:1939–1946. doi: 10.1111/acer.12185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Anderson KG, Brown SA. Testing the level of response to alcohol: social information processing model of alcoholism risk – A 20 year prospective study. Alcohol Clin Exp Res. 2004;12:1881–1889. doi: 10.1097/01.alc.0000148111.43332.a5. [DOI] [PubMed] [Google Scholar]
- Sher K. Children of Alcoholics: A Critical Appraisal of Theory and Research. University of Chicago Press; Chicago: 1991. [Google Scholar]
- Sher KJ, Walitzer KS, Wood PK, Brent EE. Characteristics of alcoholics: putative risk factors, substance use and abuse, and psychopathology. J Abnorm Psychol. 1991;100:427–448. doi: 10.1037//0021-843x.100.4.427. [DOI] [PubMed] [Google Scholar]
- Shih RA, Miles JN, Tucker JS, Zhou AJ, D’Amico EJ. Racial/ethnic differences in the influence of cultural values, alcohol resistance self-efficacy, and alcohol expectancies on risk for alcohol initiation. Psychol Addict Behav. 2012;26:460–470. doi: 10.1037/a0029254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih RA, Tucker JS, Miles JN, Ewing BA, Pedersen ER, D’Amico EJ. Differences in substance use and substance use risk factors by Asian subgroups. Asian Am J Psychol. 2015;6:38–46. doi: 10.1037/a0036251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall TL, Luczak SE, Hiller-Sturmhöfel S. Biology, genetics, and environment: underlying factors influencing alcohol metabolism. Alcohol Res. 2016;38:59–68. [PMC free article] [PubMed] [Google Scholar]
- York JL. Clinical significance of alcohol intake parameters at initiation of drinking. Alcohol. 1999;19:97–99. doi: 10.1016/s0741-8329(99)00020-8. [DOI] [PubMed] [Google Scholar]


