Abstract
Background
Although alcohol misuse is associated with deleterious outcomes in critically ill patients, its detection by either self-report or examination of biomarkers is difficult to obtain consistently. Phosphatidylethanol (PEth) is a direct alcohol biomarker that can characterize alcohol consumption patterns; however, its diagnostic accuracy in identifying misuse in critically ill patients is unknown.
Methods
PEth values were obtained in a mixed cohort comprised of 122 individuals from medical and burn intensive care units (n=33), alcohol detoxification unit (n=51), and healthy volunteers (n=38). Any alcohol misuse and severe misuse were referenced by AUDIT and AUDIT-C scores separately. Mixed effects logistic regression analysis was performed and the discrimination of PEth was evaluated using the area under the receiver operating characteristic (ROC) curve.
Results
The area under the ROC curve for PEth was 0.927 (95% CI: 0.877, 0.977) for any misuse and 0.906 (95% CI: 0.850, 0.962) for severe misuse defined by AUDIT. By AUDIT-C, the area under the ROC curves were 0.948 (95% CI: 0.910, 0.956) for any misuse and 0.913 (95% CI: 0.856, 0.971) for severe misuse. The PEth cut-points of ≥ 250 ng/mL and ≥ 400 ng/mL provided optimal discrimination for any misuse and severe misuse, respectively. The positive predictive value for ≥ 250 ng/mL was 88.7% (95% CI: 77.5%, 95.0%) and negative predictive value was 86.7% (95% CI: 74.9%, 93.7%). PEth ≥ 400 ng/mL achieved similar values and similar results were shown for AUDIT-C. In a subgroup analysis of critically ill patients only, test characteristics were similar to the mixed cohort.
Conclusions
PEth is a strong predictor and has good discrimination for any and severe alcohol misuse in a mixed cohort that includes critically ill patients. Cut-points at 250 ng/mL for any, and 400 ng/mL for severe, are favorable. External validation will be required to establish these cut-points in critically ill patients.
Keywords: Phosphatidylethanol, biomarker, alcohol misuse, critical illness
INTRODUCTION
According to the Centers for Disease Control (CDC), alcohol misuse is a leading cause of premature mortality in the United States and is attributable to 1 in 10 deaths in working-age adults (Stahre et al., 2014). CDC criteria for alcohol misuse are met when alcohol consumed exceeds the lower risk limits of daily and/or weekly alcohol use (Bouchery et al., 2011). Patients with alcohol misuse arriving to the hospital in critical condition develop more complications, have longer recovery times, develop organ dysfunction more frequently, and are at an increased risk for death (Griffin et al., 2009, Silver et al., 2008, Clark et al., 2013, O’Brien et al., 2007, Saitz R, 1997, Afshar et al., 2015, Afshar et al., 2014, Mokdad et al., 2000). Therefore, methods to facilitate prompt identification of alcohol misuse would be helpful in prognostication of outcomes among hospitalized patients.
Current methods to identify alcohol misuse in critically ill patients are fraught with problems. Methods relying on measurement of alcohol biomarkers, such as blood alcohol concentration (BAC) have poor accuracy in detection of alcohol misuse. For example, although 30% of trauma patients present with an elevated BAC, this may reflect multiple patterns of alcohol consumption, ranging from heavy daily use to intermittent binge (Afshar et al., 2015). Not surprisingly, clinical studies relying on BAC in health outcomes have reported mixed results (Friedman, 2012, Jurkovich Gregory J, 1993, Shandro et al., 2009).
Indirect alcohol biomarkers such as gamma-glutamyl-transpeptidase (GGT), carbohydrate deficient transferrin (CDT), and mean corpuscular volume (MCV) are confounded by sex, age, non-alcohol comorbidities, and acute organ dysfunction (Wurst et al., 2015b) that are commonly present in critically ill patients. Ethyl glucuronide (EtG) and ethyl sulfate (EtS) are promising direct alcohol biomarkers, but their values may be confounded by acute kidney injury, or diminished after large volume resuscitation (Hoiseth et al., 2012, Wurst et al., 1999) that is commonly employed in critically ill patients. Self-report methods to quantify alcohol consumption, such as the Alcohol Use Disorder Identification Test (AUDIT), are the current standard for identifying alcohol misuse at many medical centers with Screening, Brief Intervention, and Referral to Treatment (SBIRT) programs. However, self-report AUDIT in critically ill patients is often difficult to obtain routinely due to altered mental status and lack of capacity common to critically ill patients when they are initially admitted to intensive care.
In contrast, Phosphatidylethanol (PEth) homologues are direct alcohol biomarkers with longer half-lives than BAC and are not altered by organ dysfunction (Wurst et al., 2015b). PEth homologues are a group of phospholipids formed primarily in the red blood cell membrane from phosphatidylcholine in the presence of alcohol by the enzyme phospholipase D. They have a half-life of approximately 4 to 10 days with detection windows up to 3 weeks in active drinkers with alcohol use disorders (AUD) (Winkler et al., 2013). PEth has been validated to identify harmful consumption patterns in a variety of settings including forensic pathology, treatment programs, motor vehicle assessment programs, and prenatal screening (Bakhireva et al., 2014, Helander et al., 2012, Marques et al., 2011, Schrock et al., 2016). Recently, clinical laboratories in Sweden have instituted a cut-point level at 210 ng/mL for excessive alcohol consumption (Helander and Hansson, 2013). However, evaluation of PEth as a marker for alcohol misuse in hospitalized settings is lacking, and sparse data are available regarding critically ill patients.
Despite a growing body of evidence for PEth in outpatient and community settings, no test characteristics exist to identify alcohol misuse in critically ill patients where health outcomes can be acutely and severely impacted. The aim of this study is to examine the utility of PEth to identify an alcohol misuse phenotype that can be inclusive of multiple patterns of harmful alcohol consumption, including moderate and severe alcohol misuse, with a focus on the critically ill. We hypothesize that PEth can discriminate alcohol misuse in critically ill patients.
MATERIALS AND METHODS
Individuals and Settings
This was a multicenter mixed cohort of 122 individuals prospectively recruited between 2014 and 2016 from either (1) hospital burn or medical intensive care units (ICU), (2) an inpatient alcohol detoxification unit, along with (3) healthy control subjects. The medical and burn ICUs included sites at University of Colorado Denver and Loyola University Chicago. Burn patients included were those with >15% burn injury or suspected inhalation injury admitted to a burn ICU at either the University of Colorado Hospital in Denver, CO (UCD), or Loyola University Medical Center in Chicago, IL (LUC). Exclusion criteria included any of the following: age less than 18 years of age; expected to die within 48 hours of admission; admitted greater than 24 hours after injury; and pregnancy. Patients admitted to the medical ICU at UCD who were included were those with respiratory failure requiring invasive mechanical ventilation. Exclusion criteria for these patients were: age less than 18 years or greater than 90 years of age; pregnancy; organ transplant; chronic immunosuppression; prior respiratory disease; patients at-risk for increase intracranial pressure; or expected survival of less than six months. Blood was obtained from patients with critical illness within 36 hours of their ICU admission under a waiver of consent. Ambulatory individuals (AUD individuals and controls) completed the AUDIT survey with the oversight of trained clinical research coordinators who routinely perform AUDIT surveys in the outpatient setting, including in an alcohol detoxification facility. In the critical care setting, the capacity of patients to undergo the informed consent process was assessed daily by trained research personnel who are experienced in clinical research in the intensive care unit. Once a patient regained capacity in the opinion of the research personnel, this was additionally confirmed by the patient’s care provider (usually a registered nurse). Patients determined to have capacity were then approached to complete the informed consent process for research. Once informed consent was obtained, the AUDIT survey was completed with the support of trained research personnel.
The control and alcohol detoxification cohorts had previously been enrolled in a protocol to examine the impact of harmful alcohol use on organ dysfunction. Recruitment for these studies focused on determining differences referable to AUDs in individuals who were otherwise healthy in comparison to healthy controls, matched on age, gender, and smoking habits. The exclusion criteria for the control and alcohol detoxification cohorts were intended to provide case-control groupings as part of a NIAAA-funded project to support clinical studies in alcohol research. Individuals with an AUD were recruited from the Denver Comprehensive Addictions Rehabilitation and Evaluation Services Center, affiliated with the Denver Health and Hospital System in Denver, CO. Inclusion criteria included any of the following: AUDIT score of ≥8 for men or ≥5 for women (Reinert and Allen, 2007); age ≥21 years. Control individuals without AUDs were recruited via approved flyers posted on the university campus and by print and electronic mail advertisements in the Denver metropolitan area. The control individuals underwent an informed consent process that first began with telephone screening regarding health history. On the telephone, they were told that the study was designed to examine the health of their lungs; they were not told that alcohol consumption (or not) was an additional qualification. After answering questions related to lung and general health via phone, an AUDIT score was completed by the subjects. Elevated AUDIT scores resulted in exclusion from the study. Both AUD and control individuals who were found to have any the following were excluded: liver disease, gastrointestinal bleeding, left ventricular ejection fraction <50%, myocardial infarction, severe valvular dysfunction, end-stage renal disease requiring dialysis, serum creatinine ≥2 mg/dl, diabetes mellitus, HIV, or lung disease defined as an abnormal chest radiograph; concurrent illicit drug use; pregnancy; or abnormal nutrition status.
The Institutional Review Boards of UCD and LUC approved this study. Ambulatory individuals provided written informed consent before participating, while critically ill individuals completed the informed consent process after regaining capacity to consent.
Definitions and Reference Standards for Alcohol Assessment
In this pragmatic design, we incorporated the AUDIT as the current reference standard from hospitals that care for acutely ill patients and as such have SBIRT programs. The term alcohol misuse using the AUDIT is applied in this study for the following reasons: (a) it represents excessive consumption that exceeds the lower risk limits, (b) the term is used by national health organizations (Coombes, 2009, Moyer, 2013), (c) it is associated with deleterious outcomes (Reisinger et al., 2015), and (d) it has good construct validity (Reinert and Allen, 2007). The 10-item AUDIT is a screening questionnaire developed by the World Health Organization (WHO) to discriminate alcohol consumption above the lower risk limits (Saunders et al., 1993), and it has been validated in both inpatient and outpatient settings (Bradley et al., 2007; MacKenzie et al., 1996). The AUDIT score ranges from 0–40 and correlates clinically with different categories of alcohol misuse (Saunders et al., 1993, Reinert and Allen, 2007) with sex-specific cut-points having better validation (Neumann et al., 2004). An AUDIT score above the sex-specific cut-points (≥5 and ≥8 for females and males) covers a wide spectrum of alcohol problems, ranging from hazardous alcohol use, abuse or harmful consumption, to dependence and AUD (Saunders et al., 1993, Reinert and Allen, 2007). In accordance, we defined the lower risk limit of any alcohol misuse as an AUDIT score ≥5 and ≥8 for females and males, respectively. Severe alcohol misuse is defined as an AUDIT score ≥13 and ≥16 for females and males, respectively (Saunders et al., 1993, Clark et al., 2013, Donovan et al., 2006). The AUDIT-C, an abbreviated tool, consists of the first three full AUDIT questions quantifying alcohol consumption, and has been shown to have good test characteristics for assessing alcohol misuse in acutely ill patients (Reisinger et al., 2015). The AUDIT-C was used as another reference in this investigation to address only the questions around quantification of alcohol consumption (Hahn et al., 2016). AUDIT-C scores of ≥3 and ≥4 were used for females and males, respectively, for the lower risk limit of any misuse, while scores of ≥6 and ≥8 were used to define severe misuse (Reisinger et al., 2015, Rubinsky et al., 2013). Blood alcohol concentration (mg/dL) was also collected as part of routine clinical assessment in the critically ill cohort.
Dried Blood Spot (DBS) Collection and PEth Laboratory Analysis
DBS collection was obtained from whole blood venipuncture samples within 36 hours of hospital presentation. DBS was stored at room temperature in a bloodspot drying box prior to shipment. Specimens were analyzed at United States Drug Testing Laboratories using a previously published method (Jones et al., 2012). The method monitored a single isomer of PEth (palmitoyl/oleoyl), which is a phospholipid containing 16:0 and 18:1 fatty acids and is the most prevalent PEth homologue in human blood (Faller et al., 2013).
Analysis Plan
In univariable analysis, continuous variables were evaluated as medians with interquartile ranges and analyzed using either Wilcoxon rank sum or Kruskal-Wallis nonparametric tests. Categorical variables were analyzed using Chi-square or Fisher’s Exact tests. These tests were performed as descriptive statistics between study cohorts. In addition, the Kruskal-Wallis test was applied to examine PEth levels between alcohol groups. Bonferroni adjustment was applied for multiple comparisons between groups of alcohol misuse. The Pearson product-moment correlation coefficient was used to test the correlation between AUDIT and PEth. Odds ratios and 95% confidence intervals (CI) for alcohol misuse associated with PEth and adjusted by age, sex, and race were evaluated in logistic regression model.
Odds ratios and 95% confidence intervals (CI) for alcohol misuse associated with each predictor were also evaluated in separate mixed effects logistic regression models with random intercepts to account for study location and site (ICU versus non-ICU; UCD vs. LUC). Discrimination of PEth was evaluated using the area under the receiver operating characteristic (ROC) curve in mixed effects logistic regression with both the AUDIT and the AUDIT-C as the reference. ROC curves were also shown for the critically ill subgroup (the cohort of interest for this study) in fixed logistic regression.
Optimal cut-point levels were derived using the highest Youden indices (J) to maximize accuracy and minimize error, particularly false positives. This method has previously been shown to retain the intended, unbiased meaning for diagnostic accuracy whereas other methods in choosing cut-points may differ due to investigator bias. (Perkins and Schisterman, 2006). The area under the ROC curves was computed for the derived cut-points and 5,000 bootstrap samples were used (Steyerberg et al., 2001) to estimate the 95% confidence intervals (CI) for the cut-points. Analysis was performed on the overall sample and for subgroups by age, sex, and race. Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated for the derived cut-points in the discrimination any alcohol misuse and severe misuse as defined by the AUDIT and the AUDIT-C references. Test characteristics for the PEth cut-points were also examined in the critically ill cohort only. Analysis was performed using SAS Version 9.4 (SAS Institute, Cary, NC).
RESULTS
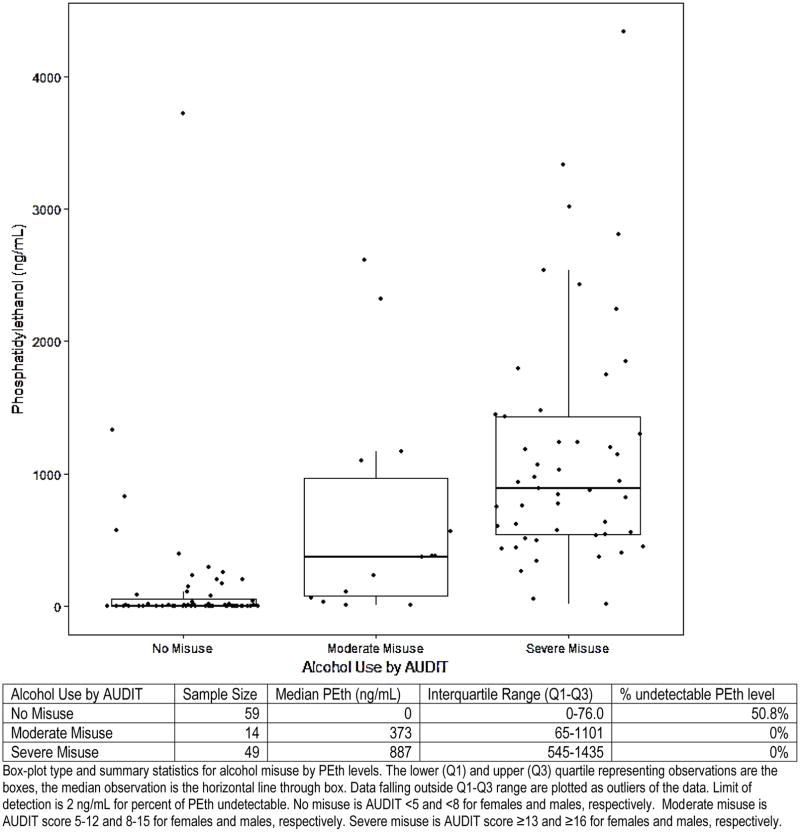
The mixed cohort consisted of 27.1% (n=33) critically ill patients from the medical and burn intensive care units, 41.8% (n=51) patients from an inpatient alcohol detoxification unit, and 31.1% (n=38) healthy controls. The median age of the cohort was 43.5 (IQR 36–50) and 76.2% (n=93) were male (Table 1). Alcohol misuse, defined by AUDIT scores ≥5 for females and ≥8 for males, was present in 51.6% (n=63) of the cohort. The median PEth level of the mixed cohort was 216 ng/mL (IQR 8–887). Among the critically ill cohort, 27.0% met criteria for any level of alcohol misuse. PEth levels by category of alcohol misuse are displayed in Figure 1. Median PEth values were significantly different among groups (p<0.001). In analysis with adjustment for multiple comparisons, intergroup differences in median PEth levels (no misuse vs. moderate misuse, moderate misuse vs. severe misuse, and no misuse vs. severe misuse) were significant as well (p<0.01 for all comparisons).
Table 1.
Demographics and alcohol information
| Characteristic | Total (n=122) | ICU (n=33) | AUD (n=51) | Control (n=38) | p-value |
|---|---|---|---|---|---|
| Age years, median (IQR) | 43.5 (36–51) | 54 (41–64) | 42 (36–48) | 39 (36–48) | 0.10 |
| Sex (male), n (%) | 93 (76.2) | 21 (63.6) | 43 (84.3) | 29 (76.3) | 0.14 |
| Race (white), n (%) | 89 (72.9) | 25 (75.8) | 32 (62.8) | 29 (76.3) | 0.13 |
| Any level of alcohol misuse* | 63 (51.6) | 9 (27.3) | 51 (100) | 0 (0) | NA |
| Severe alcohol misuse† | 49 (40.1) | 6 (18.2) | 42 (82.4) | 0 (0) | NA |
| AUDIT, median (IQR) | 6.5 (1–28) | 3 (0–12) | 30 (18–35) | 2 (0–4) | <0.01 |
| AUDIT-C, median (IQR) | 5 (1–10) | 6 (2–9) | 11 (9–12) | 1.5 (1–3) | <0.01 |
| PEth (ng/mL), median (IQR) | 261 (8–887) | 12 (0–344) | 887 (535–1479) | 10 (0–109) | <0.01 |
| Alcoholic drink in Past 7 days, y (%)(n=107) | 61 (57.0) | 9 (45.0) | 49 (96.1) | 3 (8.3) | <0.01 |
Characteristics are represented as medians with interquartile ranges for continuous variables by cohort, and analyzed using Wilcoxon rank sum tests. The associations between study cohort and categorical variables were assessed using chi-square or Fisher’s Exact tests.
AUDIT ≥5 in females and ≥8 in males
AUDIT ≥13 in females and ≥16 in males
Figure 1.
Box plots of PEth levels by AUDIT categories of alcohol misuse
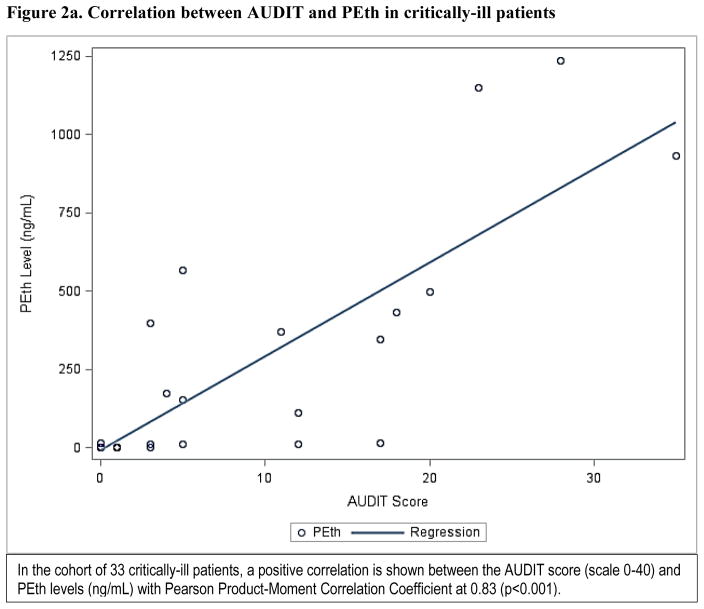
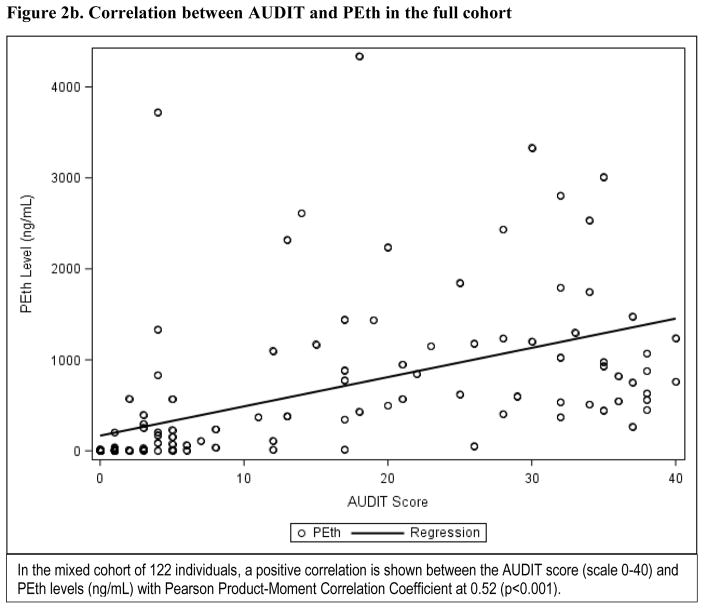
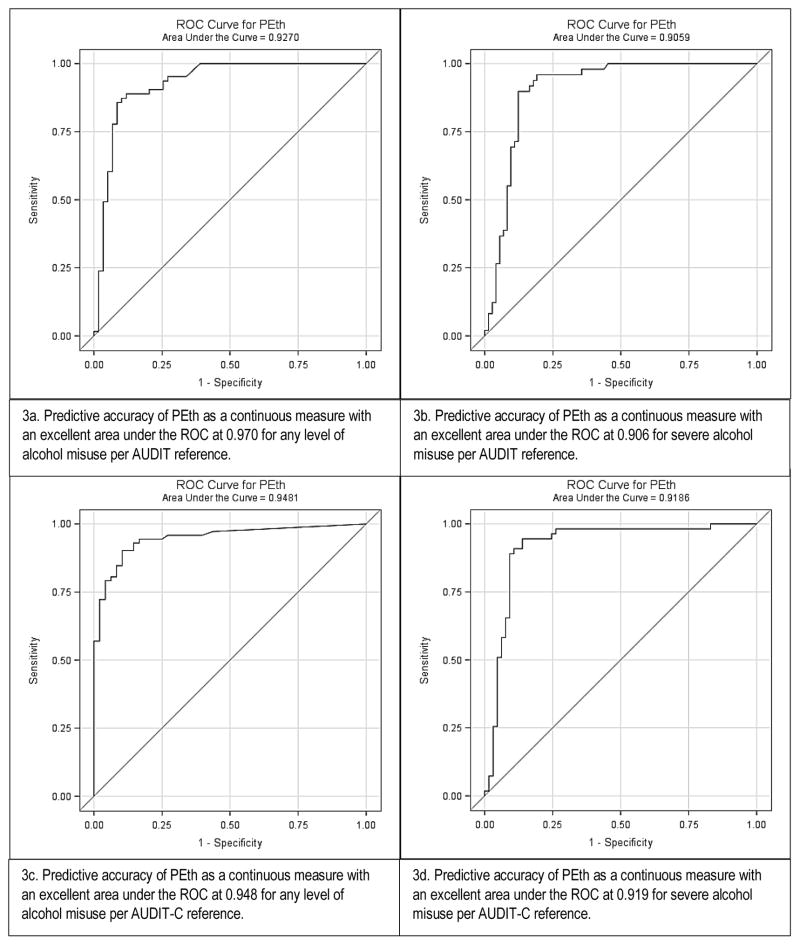
PEth levels as a continuous measure had a positive correlation with the AUDIT score (Pearson product-moment correlation coefficient at 0.52, p<0.001) in the mixed cohort (Figure2a), and an even stronger correlation in the critically ill cohort only (Pearson product-moment correlation coefficient at 0.83, p<0.001) (Figure 2b). In the critically ill cohort, 27.3% (n=9) had a detectable PEth level but undetectable BAC. Of those patients with undetectable BAC but detectable PEth level, approximately half (n=4) screened positive for any level of alcohol misuse. No associations between race, sex, or age and any measure of alcohol misuse were found in logistic regression analyses. The area under the ROC curve for PEth as a continuous measure was 0.927 (95% CI: 0.877, 0.977) for any level of alcohol misuse, and 0.906 (95% CI: 0.850, 0.962) for severe alcohol misuse, defined by the AUDIT reference in the analysis for the mixed cohort. Similarly, using the AUDIT-C as a reference, the area under the ROC curve for PEth was 0.948 (95% CI: 0.910, 0.956) for any level of alcohol misuse and 0.913 (95% CI: 0.856, 0.971) for severe misuse (Figures 3a–3d). In the critically ill cohort only, the area under ROC curve was similar to the mixed cohort for alcohol misuse (0.929; 95% CI: 0.847, 1.000). The area under the ROC curves for PEth by both AUDIT and AUDIT-C reference and across the subgroups of age, sex, and race are shown in Table 2.
Figure 2.
Correlation between AUDIT and PEth in critically-ill patients
Figures 3a–d.
ROC Curves for PEth predicting alcohol misuse by AUDIT and AUDIT-C
Table 2.
Area under the Receiver Operative Characteristic (ROC) curve for PEth in the mixed cohort and by patient characteristics
| PEth for Any Level of Alcohol Misuse (AUDIT)1 | PEth for Severe Alcohol Misuse (AUDIT)2 | Peth Any Level of Alcohol Misuse (AUDIT-C)3 | PEth for Severe Alcohol Misuse (AUDIT-C)4 | |
|---|---|---|---|---|
| Area under the ROC (95% Confidence Interval) | ||||
|
| ||||
| Overall | 0.927 (0.870, 0.962) | 0.906 (0.845, 0.958) | 0.948 (0.906, 0.981) | 0.919 (0.856, 0.965) |
|
| ||||
| Age | ||||
| < 40 | 0.944 (0.863, 0.990) | 0.952 (0.873, 1.000) | 0.931 (0.843, 0.992) | 0.961 (0.893, 1.000) |
| ≥ 40 | 0.917 (0.835, 0.968) | 0.878 (0.788, 0.954) | 0.959 (0.909, 0.995) | 0.892 (0.807, 0.963) |
|
| ||||
| Sex | ||||
| Male | 0.914 (0.841, 0.962) | 0.873 (0.790, 0.945) | 0.945 (0.895, 0.982) | 0.908 (0.833, 0.972) |
| Female | 0.980 (0.926, 1.000) | 0.994 (0.964, 1.000) | 0.945 (0.823, 1.000) | 0.938 (0.820, 1.000) |
|
| ||||
| Race | ||||
| White | 0.906 (0.833, 0.953) | 0.935 (0.822, 0.960) | 0.952 (0.903, 0.989) | 0.907 (0.834, 0.968) |
| Non-white | 0.988 (0.946, 1.000) | 0.896 (0.815, 1.000) | 0.952 (0.861, 1.000) | 0.929 (0.788, 1.000) |
PEth for predicting any level of alcohol misuse per AUDIT reference
PEth for predicting severe alcohol misuse per AUDIT reference
The optimal PEth cut-points to discriminate any level of alcohol misuse and severe alcohol misuse were derived from the mixed cohort. The PEth cut-point of ≥250 ng/mL provided optimal discrimination for any level of alcohol misuse, while a cut-point ≥400 ng/mL provided optimal discrimination for severe alcohol misuse. The positive predictive value for PEth ≥ 250 ng/mL to identify any level of alcohol misuse via AUDIT was 88.7% (95% CI: 77.5%, 95.0%) and the negative predictive value was 86.7% (95% CI: 74.9%, 93.7%) (Table 3). PEth ≥ 400 ng/mL achieved a positive predictive value at 83.0% (95% CI: 69.7%, 91.1%) and a negative predictive value at 92.8% (95% CI: 83.2%, 97.3%) for severe alcohol misuse by AUDIT. Areas under the ROC curves were similar when these cut-points were applied to the AUDIT-C definitions for any level of alcohol misuse and for severe alcohol misuse as well. A range of PEth cut-points with their respective test characteristics are shown in the Table 4.
Table 3.
Test Characteristics for PEth to detect any alcohol misuse and severe alcohol misuse (AUDIT and AUDIT-C)
| Estimate, % (95% Confidence Interval) | Sensitivity | Specificity | Positive predictive value | Negative predictive value |
|---|---|---|---|---|
| PEth ≥ 250 ng/mL | AUDIT any level of alcohol misuse (≥8 for men, ≥5 for women) | |||
| All Groups* (n=122) | 87.3 (76.0, 94.0) | 88.1 (76.5, 94.7) | 88.7 (77.5, 95.0) | 86.7 (74.9, 93.7) |
| ICU group (n=33) | 66.7 (35.4, 88.7) | 95.2 (74.1, 99.8) | 88.9 (50.7, 99.4) | 83.3 (61.8, 94.5) |
| PEth ≥ 400 ng/mL | AUDIT severe alcohol misuse (≥16 for men, ≥13 for women) | |||
| All Groups* (n=122) | 89.8 (77.0, 96.2) | 87.7 (77.4, 93.9) | 83.0 (69.7, 91.5) | 92.8 (83.2, 97.3) |
| ICU group (n=33) | 71.4 (30.3, 94.9) | 96.2 (78.4, 99.7) | 83.3 (36.5, 99.1) | 92.6 (74.2, 98.7) |
| PEth ≥ 250 ng/mL | AUDIT-C any level of alcohol misuse (≥4 for men, ≥3 for women) | |||
| All Groups* (n=122) | 80.6 (69.2, 88.6) | 91.7 (79.1, 97.3) | 93.5 (83.5, 97.9) | 75.9 (62.5, 85.7) |
| ICU group (n=33) | 66.7 (35.4, 88.7) | 94.7 (71.9, 99.7) | 88.9 (50.7, 99.4) | 81.8 (59.0, 94.0) |
| PEth ≥ 400 ng/mL | AUDIT-C severe alcohol misuse (≥8 for men, ≥6 for women) | |||
| All Groups* (n=122) | 83.6 (70.7, 91.8) | 89.2 (78.5, 95.2) | 86.8 (74.0, 94.1) | 86.6 (75.5, 93.3) |
| ICU group (n=33) | 66.7 (24.1, 94.0) | 92.0 (72.5, 98.6) | 66.7 (24.1, 94.0) | 92.0 (72.5, 98.6) |
Burn and medical intensive care unit patients, inpatient alcohol detoxification patients, and control group.
Table 4.
Range of sensitivities and specificities for PEth
| PEth | Alcohol Misuse | |
|---|---|---|
| Sensitivity | Specificity | |
| 202 | 88.9% | 82.8% |
| 204 | 88.9% | 84.5% |
| 230 | 88.9% | 86.2% |
| 236 | 88.9% | 87.9% |
| 255 | 87.3% | 87.9% |
| 267 | 87.3% | 89.7% |
| 294 | 85.7% | 89.7% |
| 344 | 85.7% | 91.4% |
| 370 | 84.1% | 91.4% |
| 371 | 82.5% | 91.4% |
| 377 | 81.0% | 91.4% |
| 382 | 79.4% | 91.4% |
| 404 | 77.8% | 93.1% |
In the critically ill cohort, the positive predictive values for PEth ≥ 250 ng/mL for any level of alcohol misuse and PEth ≥ 400 ng/mL for severe alcohol misuse were similar to the mixed cohort at 88.9% (95% CI: 50.7, 99.4) and 83.3% (95% CI: 61.8%, 94.5%), respectively. The negative predictive values were also similar at 83.3% (95% CI: 36.5%, 99.1%) and 92.6 (74.2%, 98.7%). The complete test characteristics are displayed in Table 3.
DISCUSSION
Using the AUDIT and AUDIT-C as reference standards, PEth is a strong predictor and carries good discrimination for identifying any level of alcohol misuse in a mixed cohort that includes critically ill patients. The derivation of cut-points at 250 ng/mL for any level of alcohol misuse, and 400 ng/mL for severe alcohol misuse carried good test characteristics with NPV and PPV at or above 83%. We also demonstrated that the overall cut-points performed similarly in the cohort of critically ill patients only, although with greater variability in precision and a lower sensitivity and PPV. The results we observed provide information regarding optimal cut-points to discriminate alcohol misuse in critically ill patients and may help to better identify misuse as a comorbid condition.
Alcohol misuse is frequently present in critically ill patients with reports ranging between 12% and 35% depending on the hospital setting. These patients have higher rates of sepsis, organ failure, and hospital mortality (O’Brien et al., 2007, Rivara et al., 1993). Deriving and validating cut-points of PEth allows providers to identify patients at-risk for poor outcomes associated with alcohol misuse (Clark et al., 2013, Afshar et al., 2014, Afshar et al., 2015, Mokdad et al., 2000, Rivara et al., 1993). Studies in critically ill patients have used the AUDIT to identify alcohol misuse and it remains a commonly accepted method implemented in SBIRT programs to target resources towards patients with misuse. A small cohort study performed in an emergency department using the AUDIT as a reference produced an area under the ROC curve for PEth at 0.672 but did not report cut-point levels (Kip et al., 2008). These results may have differed from the results in our critically ill cohort because the authors examined patients in the emergency department and excluded those with any illicit drug use, an elevated BAC, presence of liver disease, and self-report may be less reliable in the emergency department.
PEth has repeatedly demonstrated both good sensitivity and specificity for multiple patterns of alcohol consumption, outperforming indirect biomarkers (i.e. CDT, GGT, MCV, AST) (Hartmann et al., 2007, Walther et al., 2015) without confounding by comorbidities or demographics (Stewart et al., 2014, Stewart et al., 2009). We examined the area under the ROC curve and test statistics for PEth and found similar discrimination across age, sex, and race subgroups. Good diagnostic accuracy remained even after restricting analyses to the critically ill group alone. Area under the ROCs above 90% for PEth in this study are among the highest reported in the literature but will require further validation in a larger sample size of critically ill patients. Few patients without a quantifiable PEth level were labelled as alcohol misusers, reflective of the near perfect specificity that has been described previously (Wurst et al., 2015a).
The large majority of the evidence around PEth has involved drinking experiments and target specific patterns of alcohol consumption. Our derived cut-point at 250 ng/mL for any level of alcohol misuse was designed to represent a myriad of alcohol consumption patterns that are harmful. The case-mix of alcohol use in hospitalized patients is complex with crossover between both acute and chronic alcohol use. The PEth cut-points derived in this study are not intended to differentiate between specific patterns of alcohol consumption such as binge or daily heavy use, but rather they identify patients with alcohol behaviors indicating misuse and likely to be associated with a deleterious impact on outcomes. Drinking experiments with heavy daily consumption have been associated with lower PEth levels than what we reported. In one study, binge consumption to 100 mg/dL daily for five consecutive days yielded PEth levels that range between 74ng/mL to 237 ng/mL between days 3 and 6 (Gnann et al., 2012). Another study of inpatients and outpatients with liver disease demonstrated 91% sensitivity and 77% specificity for a PEth cut-point at 80 ng/mL approximating four drinks daily (Stewart et al., 2014) that may constitute alcohol misuse. Other studies around repeated, moderate to heavy alcohol consumption have identified cut-points between 210 ng/mL and 700 ng/mL with differences due to consumption patterns (Schrock et al., 2016, Helander and Hansson, 2013). Prolonged alcohol exposures in community settings outside of drinking experiments have generated PEth levels around 500 ng/mL (Isaksson et al., 2011, Wurst et al., 2015b). The levels of PEth in alcohol dependent individuals admitted for detoxification vary between clinical studies. A recent meta-analysis reported a mean level around 2,400 ng/mL, much higher than our cohort’s median level around 900 ng/mL (Viel et al., 2012). Other factors regulating the synthesis and elimination of PEth may further contribute to variance among individuals (Hahn 2016). Our application is unique among these published investigations in that values were measured in critically ill patients, and were reflective of alcohol misuse according to AUDIT scores obtained in parallel.
Many aspects of testing are attractive for clinical application. The DBS method is straightforward and samples can be preserved at room temperature without altering their integrity. Research staff in our study were trained by a short instructional video on collection. PEth levels from one study demonstrated consistency in measurements between capillary-DBS, whole blood, or venous-DBS (Kummer et al., 2016) allowing for multiple routes of blood collection, and PEth can be assayed as soon as 60 minutes after alcohol consumption (Gnann et al., 2012). Finally, unlike whole blood venipuncture, PEth does not continue to increase after DBS collection in individuals that had an elevated BAC at time of blood draw (Schrock et al., 2014).
Several limitations apply to this study. Our use of the AUDIT as the reference standard was intended to follow its application in SBIRT programs across the United States, but the AUDIT as a reference poses its own potential problems since it is subject to recall bias and self-report may be flawed. Spectrum bias may be present in our mixed study cohort that included healthy volunteers and individuals with known heavy alcohol consumption. This is supported by a much weaker correlation between PEth and AUDIT in the mixed cohort than in the critically ill cohort only. However, we demonstrated similar discrimination for alcohol misuse in the cohort of critically ill patients compared to the mixed cohort, but the precision (95% CI) was lower likely due to the small sample size. Therefore, our results should be interpreted with caution since the sample does not fully represent all drinking patterns with robust sample size. Additionally, other direct biomarkers may also be useful in this setting that include Ethyl Glucuronide and Ethyl Sulfate.
CONCLUSION
This is the first study to address the role of PEth in a cohort including critically ill patients. We demonstrate good diagnostic accuracy for PEth in discriminating alcohol misuse with useful cut-points to risk stratify patients. Further validation in a more representative sample of critically ill patients is needed prior to clinical and research application.
Acknowledgments
FUNDING INFORMATION: This research was supported by the National Institute of General Medical Sciences R01GM115257 (EJK), and the National Institute of Alcoholism and Alcohol Abuse, R24AA019661 (EB), K23AA024503 (MA), K23AA021814 (BJC), K23AA022126 (EML).
Footnotes
CONFLICT OF INTEREST: No conflicts of interest to disclose amongst the authors.
References
- AFSHAR M, NETZER G, MURTHI S, SMITH GS. Alcohol exposure, injury, and death in trauma patients. J Trauma Acute Care Surg. 2015;79:643–8. doi: 10.1097/TA.0000000000000825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AFSHAR M, SMITH GS, TERRIN ML, BARRETT M, LISSAUER ME, MANSOOR S, JEUDY J, NETZER G. Blood alcohol content, injury severity, and adult respiratory distress syndrome. The Journal of Trauma and Acute Care Surgery. 2014;76:1447–55. doi: 10.1097/TA.0000000000000238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAKHIREVA LN, LEEMAN L, SAVICH RD, CANO S, GUTIERREZ H, SAVAGE DD, RAYBURN WF. The validity of phosphatidylethanol in dried blood spots of newborns for the identification of prenatal alcohol exposure. Alcohol Clin Exp Res. 2014;38:1078–85. doi: 10.1111/acer.12349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOUCHERY EE, HARWOOD HJ, SACKS JJ, SIMON CJ, BREWER RD. Economic costs of excessive alcohol consumption in the U.S., 2006. American journal of preventive medicine. 2011;41:516–24. doi: 10.1016/j.amepre.2011.06.045. [DOI] [PubMed] [Google Scholar]
- BRADLEY KA, DEBENEDETTI AF, VOLK RJ, WILLIAMS EC, FRANK D, KIVLAHAN DR. AUDIT-C as a brief screen for alcohol misuse in primary care. Alcohol Clin Exp Res. 2007;31:1208–17. doi: 10.1111/j.1530-0277.2007.00403.x. [DOI] [PubMed] [Google Scholar]
- CLARK BJ, WILLIAMS A, FEEMSTER LM, BRADLEY KA, MACHT M, MOSS M, BURNHAM EL. Alcohol screening scores and 90-day outcomes in patients with acute lung injury. Critical Care Medicine. 2013;41:1518–25. doi: 10.1097/CCM.0b013e318287f1bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COOMBES R. NHS still failing to tackle alcohol misuse despite rise in admissions. BMJ. 2009;339:b3125. doi: 10.1136/bmj.b3125. [DOI] [PubMed] [Google Scholar]
- DONOVAN DM, KIVLAHAN DR, DOYLE SR, LONGABAUGH R, GREENFIELD SF. Concurrent validity of the Alcohol Use Disorders Identification Test (AUDIT) and AUDIT zones in defining levels of severity among out-patients with alcohol dependence in the COMBINE study. Addiction. 2006;101:1696–704. doi: 10.1111/j.1360-0443.2006.01606.x. [DOI] [PubMed] [Google Scholar]
- FALLER A, RICHTER B, KLUGE M, KOENIG P, SEITZ HK, SKOPP G. Stability of phosphatidylethanol species in spiked and authentic whole blood and matching dried blood spots. Int J Legal Med. 2013;127:603–10. doi: 10.1007/s00414-012-0799-y. [DOI] [PubMed] [Google Scholar]
- FRIEDMAN LS. Dose–response relationship between in-hospital mortality and alcohol following acute injury. Alcohol. 2012;46:769–775. doi: 10.1016/j.alcohol.2012.08.006. [DOI] [PubMed] [Google Scholar]
- GNANN H, WEINMANN W, THIERAUF A. Formation of phosphatidylethanol and its subsequent elimination during an extensive drinking experiment over 5 days. Alcohol Clin Exp Res. 2012;36:1507–11. doi: 10.1111/j.1530-0277.2012.01768.x. [DOI] [PubMed] [Google Scholar]
- GRIFFIN R, POE AM, CROSS JM, RUE LW, 3RD, MCGWIN G., JR The association between blood alcohol level and infectious complications among burn patients. J Burn Care Res. 2009;30:395–9. doi: 10.1097/BCR.0b013e3181a28966. [DOI] [PubMed] [Google Scholar]
- HAHN JA, ANTON RF, JAVORS MA. The Formation, Elimination, Interpretation, and Future Research Needs of Phosphatidylethanol for Research Studies and Clinical Practice. Alcohol Clin Exp Res. 2016;40:2292–2295. doi: 10.1111/acer.13213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARTMANN S, ARADOTTIR S, GRAF M, WIESBECK G, LESCH O, RAMSKOGLER K, WOLFERSDORF M, ALLING C, WURST FM. Phosphatidylethanol as a sensitive and specific biomarker: comparison with gamma-glutamyl transpeptidase, mean corpuscular volume and carbohydrate-deficient transferrin. Addict Biol. 2007;12:81–4. doi: 10.1111/j.1369-1600.2006.00040.x. [DOI] [PubMed] [Google Scholar]
- HELANDER A, HANSSON T. National harmonization of the alcohol biomarker PEth. Lakartidningen. 2013;110:1747–8. [PubMed] [Google Scholar]
- HELANDER A, PETER O, ZHENG Y. Monitoring of the alcohol biomarkers PEth, CDT and EtG/EtS in an outpatient treatment setting. Alcohol Alcohol. 2012;47:552–7. doi: 10.1093/alcalc/ags065. [DOI] [PubMed] [Google Scholar]
- HOISETH G, NORDAL K, PETTERSEN E, MORLAND J. Prolonged urinary detection times of EtG and EtS in patients with decreased renal function. Alcohol Clin Exp Res. 2012;36:1148–51. doi: 10.1111/j.1530-0277.2011.01713.x. [DOI] [PubMed] [Google Scholar]
- ISAKSSON A, WALTHER L, HANSSON T, ANDERSSON A, ALLING C. Phosphatidylethanol in blood (B-PEth): a marker for alcohol use and abuse. Drug Test Anal. 2011;3:195–200. doi: 10.1002/dta.278. [DOI] [PubMed] [Google Scholar]
- JONES J, JONES M, PLATE C, LEWIS D. The Detection of 1-Palmitoyl-2-oleoyl-<i>sn</i>-glycero-3-phosphoethanol and Ethyl Glucuronide in Human Umbilical Cord. American Journal of Analytical Chemistry. 2012;03:800–810. doi: 10.4236/ajac.2012.31012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JURKOVICH GREGORY JRFP, COPASS MICHAEL. The Effect of Acute Alcohol Intoxication and Chronic Alcohol Abuse on Outcome from Trauma. JAMA. 1993;270:51–56. [PubMed] [Google Scholar]
- KIP MJ, SPIES CD, NEUMANN T, NACHBAR Y, ALLING C, ARADOTTIR S, WEINMANN W, WURST FM. The usefulness of direct ethanol metabolites in assessing alcohol intake in nonintoxicated male patients in an emergency room setting. Alcohol Clin Exp Res. 2008;32:1284–91. doi: 10.1111/j.1530-0277.2008.00696.x. [DOI] [PubMed] [Google Scholar]
- KUMMER N, INGELS AS, WILLE SM, HANAK C, VERBANCK P, LAMBERT WE, SAMYN N, STOVE CP. Quantification of phosphatidylethanol 16:0/18:1, 18:1/18:1, and 16:0/16:0 in venous blood and venous and capillary dried blood spots from patients in alcohol withdrawal and control volunteers. Anal Bioanal Chem. 2016;408:825–38. doi: 10.1007/s00216-015-9169-1. [DOI] [PubMed] [Google Scholar]
- MACKENZIE D, LANGA A, BROWN TM. Identifying hazardous or harmful alcohol use in medical admissions: a comparison of audit, cage and brief mast. Alcohol Alcohol. 1996;31:591–9. doi: 10.1093/oxfordjournals.alcalc.a008195. [DOI] [PubMed] [Google Scholar]
- MARQUES P, HANSSON T, ISAKSSON A, WALTHER L, JONES J, LEWIS D, JONES M. Detection of phosphatidylethanol (PEth) in the blood of drivers in an alcohol ignition interlock program. Traffic Inj Prev. 2011;12:136–41. doi: 10.1080/15389588.2010.544048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOKDAD AH, MARKS JS, STROUP DF, GERBERDING JL. Actual causes of death in the United States, 2000. JAMA. 2004;291:1238–45. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- MOYER VA. Screening and behavioral counseling interventions in primary care to reduce alcohol misuse: U.S. preventive services task force recommendation statement. Annals of Internal Medicine. 2013;159:210–8. doi: 10.7326/0003-4819-159-3-201308060-00652. [DOI] [PubMed] [Google Scholar]
- NEUMANN T, NEUNER B, GENTILELLO LM, WEISS-GERLACH E, MENTZ H, RETTIG JS, SCHRODER T, WAUER H, MULLER C, SCHUTZ M, MANN K, SIEBERT G, DETTLING M, MULLER JM, KOX WJ, SPIES CD. Gender differences in the performance of a computerized version of the alcohol use disorders identification test in subcritically injured patients who are admitted to the emergency department. Alcohol Clin Exp Res. 2004;28:1693–701. doi: 10.1097/01.alc.0000145696.58084.08. [DOI] [PubMed] [Google Scholar]
- O’BRIEN JM, LU B, ALI NA, MARTIN GS, ABEREGG SK, MARSH CB, LEMESHOW S, DOUGLAS IS. Alcohol dependence is independently associated with sepsis, septic shock, and hospital mortality among adult intensive care unit patients*. Critical Care Medicine. 2007;35:345–350. doi: 10.1097/01.CCM.0000254340.91644.B2. [DOI] [PubMed] [Google Scholar]
- PERKINS NJ, SCHISTERMAN EF. The inconsistency of “optimal” cutpoints obtained using two criteria based on the receiver operating characteristic curve. Am J Epidemiol. 2006;163:670–5. doi: 10.1093/aje/kwj063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REINERT DF, ALLEN JP. The alcohol use disorders identification test: an update of research findings. Alcohol Clin Exp Res. 2007;31:185–99. doi: 10.1111/j.1530-0277.2006.00295.x. [DOI] [PubMed] [Google Scholar]
- REISINGER MW, MOSS M, CLARK BJ NATIONAL HEART L, BLOOD INSTITUTE ACUTE RESPIRATORY DISTRESS SYNDROME NETWORK I. Brief Versus Full Alcohol Use Disorders Identification Test in National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome Network Clinical Trials. Crit Care Med. 2015;43:e382–5. doi: 10.1097/CCM.0000000000001181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RIVARA FP, JURKOVICH GJ, GURNEY JG, SEGUIN D, FLIGNER CL, RIES R, RAISYS VA, COPASS M. The magnitude of acute and chronic alcohol abuse in trauma patients. Arch Surg. 1993;128:907–12. doi: 10.1001/archsurg.1993.01420200081015. discussion 912–3. [DOI] [PubMed] [Google Scholar]
- RUBINSKY AD, DAWSON DA, WILLIAMS EC, KIVLAHAN DR, BRADLEY KA. AUDIT-C scores as a scaled marker of mean daily drinking, alcohol use disorder severity, and probability of alcohol dependence in a U.S. general population sample of drinkers. Alcohol Clin Exp Res. 2013;37:1380–90. doi: 10.1111/acer.12092. [DOI] [PubMed] [Google Scholar]
- SAITZ RGW, MOSKOWITZ MA. The impact of alcohol-related diagnoses on pneumonia outcomes. Archives of Internal Medicine. 1997;157:1446–1452. [PubMed] [Google Scholar]
- SAUNDERS JB, AASLAND OG, BABOR TF, DE LA FUENTE JR, GRANT M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption--II. Addiction. 1993;88:791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- SCHROCK A, HERNANDEZ REDONDO A, MARTIN FABRITIUS M, KONIG S, WEINMANN W. Phosphatidylethanol (PEth) in blood samples from “driving under the influence” cases as indicator for prolonged excessive alcohol consumption. Int J Legal Med. 2016;130:393–400. doi: 10.1007/s00414-015-1300-5. [DOI] [PubMed] [Google Scholar]
- SCHROCK A, THIERAUF A, WURST FM, THON N, WEINMANN W. Progress in monitoring alcohol consumption and alcohol abuse by phosphatidylethanol. Bioanalysis. 2014;6:2285–94. doi: 10.4155/bio.14.195. [DOI] [PubMed] [Google Scholar]
- SHANDRO JR, RIVARA FP, WANG J, JURKOVICH GJ, NATHENS AB, MACKENZIE EJ. Alcohol and Risk of Mortality in Patients With Traumatic Brain Injury. The Journal of Trauma: Injury, Infection, and Critical Care. 2009;66:1584–1590. doi: 10.1097/TA.0b013e318182af96. [DOI] [PubMed] [Google Scholar]
- SILVER GM, ALBRIGHT JM, SCHERMER CR, HALERZ M, CONRAD P, ACKERMAN PD, LAU L, EMANUELE MA, KOVACS EJ, GAMELLI RL. Adverse Clinical Outcomes Associated With Elevated Blood Alcohol Levels at the Time of Burn Injury. Journal of Burn Care & Research. 2008;29:784–789. doi: 10.1097/BCR.0b013e31818481bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STAHRE M, ROEBER J, KANNY D, BREWER RD, ZHANG X. Contribution of excessive alcohol consumption to deaths and years of potential life lost in the United States. Preventing chronic disease. 2014;11:E109. doi: 10.5888/pcd11.130293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEWART SH, KOCH DG, WILLNER IR, ANTON RF, REUBEN A. Validation of blood phosphatidylethanol as an alcohol consumption biomarker in patients with chronic liver disease. Alcoholism, clinical and experimental research. 2014;38:1706–11. doi: 10.1111/acer.12442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEWART SH, REUBEN A, BRZEZINSKI WA, KOCH DG, BASILE J, RANDALL PK, MILLER PM. Preliminary evaluation of phosphatidylethanol and alcohol consumption in patients with liver disease and hypertension. Alcohol Alcohol. 2009;44:464–7. doi: 10.1093/alcalc/agp039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEYERBER EW, HARELL FE, BORSBOOM GJ, EIJKEMANS MJC, VERGOUWE Y, HABBEMA DF. Internal validation of preditive models: Efficiency of some procedures for logistic regression analysis. Journal of Clinical Epidemiology. 2001;54:774–81. doi: 10.1016/s0895-4356(01)00341-9. [DOI] [PubMed] [Google Scholar]
- VIEL G, BROSCOLO-BERTO R, CECCHETTO G, FAIS P, NALESS A, FERRARA SD. Phosphatidylethanol in blood as a marker of chronic alcohol use: a systematic review and meta-analysis. International Journal of Molecular Science. 2012;13:14788–812. doi: 10.3390/ijms131114788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALTHER L, DE BEJCZY A, LOF E, HANSSON T, ANDERSSON A, GUTERSTAM J, HAMMARBERG A, ASANOVSKA G, FRANCK J, SODERPALM B, ISAKSSON A. Phosphatidylethanol is Superior to Carbohydrate-Deficient Transferrin and gamma-Glutamyltransferase as an Alcohol Marker and is a Reliable Estimate of Alcohol Consumption Level. Alcohol Clin Exp Res. 2015;39:2200–8. doi: 10.1111/acer.12883. [DOI] [PubMed] [Google Scholar]
- WINKLER M, SKOPP G, ALT A, MILTNER E, JOCHUM T, DAENHARDT C, SPORKERT F, GNANN H, WEINMANN W, THIERAUF A. Comparison of direct and indirect alcohol markers with PEth in blood and urine in alcohol dependent inpatients during detoxication. Int J Legal Med. 2013;127:761–8. doi: 10.1007/s00414-012-0812-5. [DOI] [PubMed] [Google Scholar]
- WURST FM, SCHUTTLER R, KEMPTER C, SEIDL S, GILG T, JACHAU K, ALT A. Can ethyl glucuronide be determined in post-mortem body fluids and tissues? Alcohol Alcohol. 1999;34:262–3. doi: 10.1093/alcalc/34.2.262. [DOI] [PubMed] [Google Scholar]
- WURST FM, THON N, YEGLES M, SCHRUCK A, PREUSS UW, WEINMANN W. Ethanol metabolites: their role in the assessment of alcohol intake. Alcohol Clin Exp Res. 2015a;39:2060–72. doi: 10.1111/acer.12851. [DOI] [PubMed] [Google Scholar]
- WURST FM, THON N, YEGLES M, SCHRUCK A, PREUSS UW, WEINMANN W. Ethanol Metabolites: Their Role in the Assessment of Alcohol Intake. Alcohol Clin Exp Res. 2015b doi: 10.1111/acer.12851. [DOI] [PubMed] [Google Scholar]