Abstract
Background
Disturbed sleep timing is common in bipolar disorder (BD). However, most research is based upon self-reports. We examined relationships between subjective versus objective assessments of sleep timing in BD patients versus controls.
Methods
We studied 61 individuals with bipolar I or II disorder and 61 healthy controls. Structured clinical interviews assessed psychiatric diagnoses, and clinician-administered scales assessed current mood symptom severity. For subjective chronotype, we used the Composite Scale of Morningness (CSM) questionnaire, using original and modified (1, ¾ , ⅔ , and ½ SD below mean CSM score) thresholds to define evening chronotype. Objective chronotype was calculated as the percentage of nights (50%, 66.7%, 75%, or 90% of all nights) with sleep interval midpoints at or before (non-evening chronotype) vs. after (evening chronotype) 04:15:00 (4:15:00 AM), based on 25–50 days of continuous actigraph data.
Results
BD participants and controls differed significantly with respect to CSM mean scores and CSM evening chronotypes using modified, but not original, thresholds. Groups also differed significantly with respect to chronotype based on sleep interval midpoint means, and based on the threshold of 75% of sleep intervals with midpoints after 04:15:00. Subjective and objective chronotypes correlated significantly with one another. Twenty-one consecutive intervals were needed to yield an evening chronotype classification match of ≥ 95% with that made using the 75% of sleep intervals threshold.
Limitations
Limited sample size/generalizability.
Conclusions
Subjective and objective chronotype measurements were correlated with one another in participants with BD. Using population-specific thresholds, participants with BD had a later chronotype than controls.
Introduction
Bipolar disorder (BD) is a severe and highly recurrent mood disorder that affects approximately 2–4% of the U.S. population, and entails a significant public health burden, including high rates of suicide, disability, hospitalization, unemployment, and social impairment (Merikangas et al., 2007; Merikangas et al., 2011). Given this impact, it is crucial to establish aspects of illness that can be targeted in treatment, or that can be used to identify subgroups of patients for whom specific treatments could be more beneficial.
Studies have consistently suggested an association between BD and delayed circadian phase, that is, a preference for later (eveningness) rather than earlier (morningness) timing of sleep and daily activities (Melo, Abreu, Linhares Neto, de Bruin, & de Bruin, 2016). This preference is often referred to as “chronotype”. In the past, chronotype has been commonly assessed using self-report questionnaires, such as the Horne-Östberg Morningness-Eveningness Questionnaire (MEQ) (Horne & Östberg, 1976), the Composite Scale of Morningness (CSM) (Smith, Reilly, & Midkiff, 1989), and the Munich Chronotype Questionnaire (MCTQ) (Roenneberg et al., 2007). With some variation, these metrics have assessed individual differences in preferred sleep and rise times, tiredness in the morning or evening, and the perceived optimal time to perform daily activities. Although high levels of between-measure reliability had been reported among various questionnaires (Thun et al., 2012), this has not been surprising, as these measures tended to share items. Importantly, such approaches do not speak to the validity/discriminant utility of subjective self-reported chronotypes.
More recently, chronotypes have been assessed using objective markers of circadian rhythms, such as the timing of motor activity or patterns of daily melatonin or cortisol levels. However, a recently published review of 42 chronotype studies in over 3,400 patients with BD noted that most patient samples (81.5%) were assessed using only self-report measures. This review concluded eveningness, rather than morningness, was significantly more common in BD (Melo et al., 2016). This is in contrast to the general adult population, in which morningness is more common than eveningness (Paine, Gander, & Travier, 2006; Taillard, Philip, Chastang, & Bioulac, 2004).
In addition, some data have suggested that the eveningness observed among individuals with BD might be independent of current mood state (Seleem et al., 2015), but could be related to greater illness severity. As assessed using CSM mean scores (Smith et al., 1989), adults with bipolar I disorder (BD-I) and bipolar II disorder (BD-II) (n = 257) had evening preference relative to both a non-BD psychopathology group (n = 105) and to healthy controls (n = 55) (Seleem et al., 2015). Furthermore, evening preference was not associated with current mood state, suggesting that eveningness may be a trait-like characteristic in BD (Seleem et al., 2015). Another study using CSM mean scores similarly showed that relative to unscreened controls (n = 349), adults with BD (n = 75) had significantly more eveningness (Mansour et al., 2005). In that study, an association between rapid mood swings and eveningness was observed, suggesting that eveningness may be related to greater illness severity among BD participants. The classic BD diurnal mood variation has involved more morning and less evening depression (Germain et al., 2007), consistent with individuals with BD preferring evenings.
Eveningness has also been documented in youth with BD. Consistent with adult findings, Kim et al. found that their subsample of 13–17 year old youth with BD (n = 17) reported significantly greater eveningness relative to healthy control youth (n = 24) (Kim et al., 2014). Some adult BD studies found eveningness was associated with younger age (Mansour et al., 2005), while others found no such association (Ahn et al., 2008).
Some issues merit particular consideration when evaluating self-report chronotype studies. First, self-report questionnaires reflect individuals’ perceptions of their sleep timing, and thus may or may not reflect objective behavior. A discrepancy between subjective and objective measures of sleep has been previously found in participants with BD (Gershon et al., 2012; Harvey, Schmidt, Scarna, Semler, & Goodwin, 2005; Millar, Espie, & Scott, 2004). To date, there have been few studies using both subjective and objective chronotype measures (Boudebesse et al., 2014; Roenneberg et al., 2007; Thun et al., 2012). In one such study, seven days of actigraphic data were collected to validate self-reported chronotype in 166 unscreened college students (Thun et al., 2012). The findings showed that self-reported chronotype was significantly correlated with actigraphy measures of bed and rise times, consistent with the notion that self-report and actigraphy chronotypes could be comparable. However, this study’s reliance on college students limited generalizability of the results and does not speak to the validity of such chronotype measures in BD samples. Suggestive of the validity of self-reported chronotype in BD, another study of adults with BD reported substantial significant (r = −0.63 and −0.69, both ps <0.0001, N = 26, respectively) correlations between CSM score and two actigraphy-derived markers of circadian phase using 21 days of actigraphy (Boudebesse et al., 2014). However, the sample size was small (N = 26) and as such, the question of relationships between subjective and objective chronotypes requires further assessment. In yet another validation study, an actigraphy-derived marker of circadian phase, midsleep time, or the halfway point between sleep onset and sleep offset time, was found to have a substantial significant (r = −0.56, p < 0.0001, N = 50) correlation with self-reported chronotype among healthy adult participants without psychiatric or sleep disorders (Kantermann, Juda, Merrow, & Roenneberg, 2007; Roenneberg et al., 2007).
A second consideration for evaluating chronotype is that questionnaire-established cutoff scores commonly used to classify participants into chronotypes may over-identify morning over evening chronotypes. Indeed, the validation studies noted above used questionnaire means rather than original questionnaire thresholds when comparing participants with morning versus evening chronotypes. Thus, chronotype questionnaires have generally been either used as continuous measures (Boudebesse et al., 2014; Roenneberg et al., 2007), or the original questionnaire thresholds have been modified to determine chronotype classifications (Thun et al., 2012). Thun and associates rationalized that using quartile scores, instead of the original questionnaire thresholds, allowed them to balance the group sizes. This appeared to also increase statistical power. The ability to classify chronotypes in larger numbers of patients could have substantial merit; potentially improving the selection of appropriate treatments and aiding in the development of chronobiologically-focused treatments for subgroups of BD patients.
The aims of the present study were to characterize chronotype in participants with BD relative to healthy controls with no history of psychiatric/sleep disorders, using both self-report and actigraphy-based assessments of chronotype, and to examine relationships between these two measures of chronotype. We use both continuous and categorical approaches. The continuous approach allows for a dimensional assessment of chronotype, whereas the categorical approach provides more immediate clinical utility with regard to treatment considerations. We hypothesized that relative to controls, participants with BD would exhibit significantly greater eveningness using a continuous approach, and that, using a categorical approach, a significantly greater proportion of participants with BD relative to controls would be classified with evening chronotypes. We further hypothesized that self-report and actigraphy-based measurements of evening chronotype would correlate significantly with one-another.
Method
Participants and Procedure
Participants were pooled from two studies to enhance sample size and thus statistical power. The first sub-sample consisted of 76 adults (age 18–64 years): 36 adults with inter-episode (for a least 1 month) BD-I or BD-II and 40 adult healthy controls with no history of sleep or psychiatric disorders. The second sub-sample consisted of 46 youth (age 14–21 years): 25 with inter-episode (for a least 1 month) BD-I or BD-II and 21 youth healthy controls with no history of psychiatric or sleep disorder. Both sub-samples were recruited from the community. These studies were approved by University of California’s Committee for the Protection of Human Subjects and Stanford University’s Administrative Panel on Human Subjects Research, respectively. Participants who contacted the lab were initially screened for their likelihood of meeting the study’s eligibility criteria (described below) with a phone interview. This screening interview included an abbreviated version of the Structured Clinical Interview for DSM (SCID) (First, Spitzer, Gibbon, & Williams, 2007) with additional probes from the mood module. Written informed consent was obtained prior to participation in protocol activities. Consented participants completed a demographics/medication questionnaire, and were administered diagnostic and symptom interviews to assess for lifetime psychiatric disorders and past-month mood symptom levels. Eligible participants were also given an actigraph, a watch-like activity-monitoring device, to be worn continuously on the non-dominant wrist. In sub-samples 1 and 2, participants wore these devices for approximately 50 and 25 days, respectively.
Study exclusion criteria included presence of serious medical or neurological conditions (e.g., Alzheimer’s disease, significant head trauma), alcohol or substance abuse or dependence in the past six months, shift work, an unstable living arrangement, or a primary sleep disorder not attributable to another medical or psychiatric condition (i.e. primary insomnia, sleep apnea, circadian rhythm disorder, or moderate to severe parasomnia). BD participants were additionally required to be inter-episode at study entry. Inter-episode status was defined by absence of a current depressive, manic, hypomanic, or mixed episode in the preceding month, as assessed by the Structured Clinical Interview for DSM-IV (SCID; First, Spitzer, Gibbon, & Williams, 2007) for adult participants (18 years or older) and by the Kiddie-Schedule for Affective Disorders and Schizophrenia - Present and Lifetime Version (K-SADS-PL; Kaufman et al., 2001) for youth under the age of 18.
Lifetime Psychiatric Disorders
Lifetime and current Axis-I disorders were assessed using the Structured Clinical Interview for DSM-IV (SCID) (First et al., 2007) for adult participants (18 years or older) and the Kiddie-Schedule for Affective Disorders and Schizophrenia - Present and Lifetime Version (K-SADS-PL; Kaufman et al., 2001) for youth under the age 18 years. For youth, both parent- and child-reports were used. Interviews were administered in-person by trained master’s- or doctoral-level interviewers.
Mood Symptom Severity
Current symptom severity was measured using the Young Mania Rating Scale (YMRS) (Young, Biggs, Ziegler, & Meyer, 1978) and the Clinician-Rated Inventory of Depressive Symptomatology (IDS-C) (Rush, Gullion, Basco, Jarrett, & Trivedi, 1996) for adults or the Children’s Depression Rating Scale-Revised (CDRS-R) (Poznanski, Freeman, & Mokros, 1985) for youth under age 18 years. Interviews were administered in-person by trained master’s or doctoral-level interviewers.
Subjective (Self-Reported) Chronotypes
The Composite Scale of Morningness (CSM) (Smith et al., 1989) was used to measure subjective self-reported chronotype. This questionnaire consisted of 13 items evaluating preference in timing of daily activities. The CSM has been shown to have good psychometric properties for use in adults (Smith et al., 1989) and adolescents (Tonetti, Adan, Di Milia, Randler, & Natale, 2015). Cross-national studies with adolescents (9–20 years old) have reported adequate internal consistency (Cronbach's alpha values ranging from 0.61 to 0.86) (Tonetti et al., 2015), and have been validated against the subjective MEQ (Horne & Östberg, 1976) and objective cortisol awakening responses (Randler & Schaal, 2010). Total scores ranged from 13 to 55. The original CSM thresholds delineated total scores ≤ 22 as evening chronotype (a preference for evening activities), scores between 23 and 43 as an intermediate chronotype, and scores ≥ 44 as morning chronotype (a preference for morning activities). We categorized each of our participants as an “evening” or “non-evening” chronotype (inclusive of morning and intermediate chronotypes), based on studies showing associations between BD and eveningness (Melo et al., 2016). We calculated the mean and standard deviation (SD) CSM total score for our entire (BD and healthy control) sample, and modified the original CSM total score maximum threshold for eveningness (≤ 22) to increase sensitivity to evening chronotype using modified (numerically higher) CSM total score maximum thresholds of at least 1, ¾, ⅔ , ½ and ¼ SD below our observed CSM full sample total score mean.
Objective (Actigraphy-Based) Chronotypes
Objective chronotypes were computed based on movement data obtained from Actiwatches (Philips Inc., Bend, OR) worn continuously on the non-dominant wrist (Sadeh, Hauri, Kripke, & Lavie, 1995). Embedded within each Actiwatch is a piezoelectric sensor that detects movement acceleration, a processor to convert this information to an activity count over a specified time interval (one minute intervals were used for this study), and non-volatile memory to store data. Movement data stored as the integrated movement in one-minute intervals were downloaded and pre-processed using Actiware software (Philips Inc., Bend OR). These data were then scored by research assistants trained to identify sleep intervals (i.e., times from sleep onset to offset) based on activity levels, and data obtained from daily sleep diaries provided by participants (Morin & Espie, 2003). Midpoints of all sleep intervals were calculated. Naps or sleep intervals occurring between 10:00 AM (10:00:00) and 10:00 PM (22:00:00) with a duration of less than 4 hours, were omitted from analyses to avoid over-representation of evening chronotypes.
In order to make meaningful comparisons of sleep onset, offset, and midpoint times, we standardized sleep interval times as the number of seconds from midnight the previous day (e.g., a midpoint occuring at 4:38 AM (04:38:00) would be assigned a value of 103,080 seconds). For interpretation purposes, we translated these raw time values into their respective 24-hour clock hours:minutes:seconds format in our reporting of data in this manuscript. Midpoints after 4:15 AM (04:15:00) were considered to reflect sleep intervals of evening preference, while those before or that time were assigned non-evening preference (Roenneberg et al., 2007).
Statistical Analyses
Statistical analyses were performed using Statistical Package for the Social Science (SPSS v22.0, IBM Corporation, Armonk, New York) software and Stata 13 SE (StataCorp, College Station, Texas) on a MacBook Pro computer (Apple Computers; Cupertino, California). Frequency distributions of the two study sub-samples were found to be normal. Combining the two study samples, the BD and control groups were compared on chronotypes using subjective questionnaire-based and objective actigraphy-based assessments. First, between-group (BD versus healthy controls) differences were examined with respect to subjective CSM mean scores, and with respect to proportions of evening chronotypes according to the original as well as our modified (SD-based) CSM thresholds. Next, using all available objective (actigraphy-derived) sleep interval midpoints per person, within-person sleep interval midpoint means were calculated and between-group (BD versus healthy controls) differences in objective sleep interval midpoint means were compared. Finally, we classified each objective sleep interval as evening vs. non-evening chronotype based on sleep interval midpoints occurring after versus before/at 04:15:00, respectively (Roenneberg et al., 2007), calculated percent of sleep interval midpoints qualifying as evening vs. non-evening chronotype, and tested for between-group differences in proportion of evening chronotypes.
Bivariate correlations were used to examine associations between subjective CSM total scores and objective actigraphy-derived sleep interval midpoint means, and to examine associations between subjective CSM total scores or actigraphy-derived sleep interval midpoint means and medication use. In an effort to account for effects of medications on chronotype, analyses were repeated using partial correlations, examining the association between CSM total scores and actigraphy-derived sleep interval midpoint means while controlling for prescription psychotropic medication use (i.e., any mood stabilizer, any antipsychotic, any antidepressant, any anxiolytic/hypnotic, and any other prescription psychotropic medication use). Finally, a sensitivity analysis was conducted to determine the minimum number of actigraphy-derived sleep intervals needed to accurately classify participants with evening chronotype. Accuracy was based on the cumulative actigraphy-derived sleep intervals needed to match evening chronotype classification based upon the 75% threshold of all actigraphy-derived sleep intervals available. Student’s t-tests were used to compare continuous parameters, and Fisher’s exact Chi-Square tests were used to compare catgeorical parameters. We used a two-tailed significance threshold with p < 0.05, and no correction for multiple comparisons.
Results
Demographic and clinical variables
Table 1 provides demographic and clinical sample characteristics. Distributions of age, gender, race, education, and current employment status were not significantly different between the BD and healthy control groups (all p’s > 0.10). As expected, our inter-episode BD group participants compared to healthy controls had significantly higher mean IDS-C depressive symptom scores in sub-sample 1 (t(73) = 7.8, p < 0.001) and CDRS depressive symptom scores in sub-sample 2 (t(44) = 5.4, p < 0.001), as well as significantly higher entire sample mean YMRS manic symptom scores (t(118) = 6.2, p < 0.001).
Table 1.
Sample Demographic and Clinical Characteristics
| Bipolar Disorder (n=61) | Healthy Control (n=61) | χ2 or t value | p value | |
|---|---|---|---|---|
| Age, in years, Mean (SD) | 28.2 (11.5) | 27.9 (13.6) | 0.14 | 0.89 |
| Female, n (%) | 41 (67.2) | 31 (50.8) | 3.39 | 0.10 |
| White, n (%) | 41 (67.2) | 32 (52.5) | 2.76 | 0.14 |
| Full-Time Employment/Student, n (%) | 39 (63.9) | 43 (70.5) | 0.56 | 0.56 |
| Bipolar disorder type I/II, n (%) | 53 (86.9)/8 (13.1) | -- | -- | -- |
| Illness duration, in years, Mean (SD) | 12.7 (10.6) | -- | -- | -- |
| Age of onset, in years, Mean (SD) | 15.4 (7.8) | -- | -- | -- |
| IDS-C/CDRS-R score, Mean (SD) | 8.61 (4.63)/27.4 (7.71) | 2.28 (2.00)/18.2 (1.21) | 7.79/5.41 | both < 0.001 |
| YMRS score, Mean (SD) | 3.80 (3.43) | 0.86 (1.21) | 6.22 | < 0.001 |
| Number of psychotropics, Mean (SD) | 2.2 (1.5) | -- | -- | -- |
| Any mood stabilizer, n (%) | 42 (68.9) | -- | -- | -- |
| Any antipsychotic, n (%) | 27 (44.3) | -- | -- | -- |
| Any antidepressant, n (%) | 20 (32.8) | -- | -- | -- |
| Any anxiolytic/hypnotic, n (%) | 14 (23.0) | -- | -- | -- |
| Any other psychotropic, n | 8 (13.1) | -- | -- | -- |
Note. SD = standard deviation. IDS-C = Inventory of Depressive Symptomatology – Clinician Rating; CDRS-R = Children’s Depressive Rating Scale – Revised; YMRS = Young Mania Rating Scale. In sub-sample 1, one healthy control lacked IDSC and YMRS score and one healthy control lacked YMRS score. Other psychotropics included stimulants, guanfacine, and other anticonvulsants (i.e., oxcarbazepine, topiramate). Underlines indicate t- (rather than χ2) values.
Among BD participants, 53 of 61 (86.9%) had BD-I, and 41 of 61 (65.6%) had at least one lifetime comorbid Axis-I psychiatric diagnosis. Comorbid psychiatric diagnoses included panic disorder (n = 19), social phobia (n = 12), generalized anxiety disorder (n = 11), alcohol/substance abuse (n = 10), specific phobia (n = 9), post-traumatic stress disorder (n = 7), eating disorder (n = 5), separation anxiety disorder (n = 2), agoraphobia (n = 2), obsessive-compulsive disorder (n = 2), and attention deficit and hyperactivity disorder (n = 1) (not tabulated). Fifty-four of the 61 BD group participants (88.5%) were currently taking prescription psychotropic medications. Current prescription psychotropic medications included mood stabilizers (lithium, valproate, lamotrigine, or carbamazepine, n = 42), antipsychotics (n = 27), antidepressants (n = 20), anxiolytics/hypnotics (n = 14), and others (e.g., stimulants, other anticonvulsants, n = 8). Four participants were using levothyroxine (in addition to taking psychotropic medications). This medication was excluded from analysis of current prescription psychotropics.
Group Differences in Subjective CSM Scores and Evening Chronotype Classification
Frequency distributions of CSM scores for the BD and healthy control groups were normally distributed. Table 2 depicts BD versus healthy control group comparisons of CSM mean scores and the subjective categorical classifications of evening chronotypes, with the latter based upon CSM total scores using original and modified (SD-based) thresholds. The BD versus healthy control group had significantly more eveningness considered continuously (i.e., a significantly lower CSM mean score, 33.1±8.3 versus 36.1±6.9, t(109) = 2.1, p = 0.04). CSM scores were significantly correlated with anxiolytic/hypnotic use (r = −.37, p = 0.006, n = 54) but not with any other prescription psychotropic medication use (all p’s ranged between 0.2 and 0.8).
Table 2.
Group Differences in Composite Scale of Morningness (CSM) Mean Total Scores and in Proportions of Participants Classified with Evening Chronotype, Based Upon Original and Modified CSM Scale Thresholds.
| Bipolar Disorder (n=54) | Healthy Control (n=57) | t or χ2 | p-value | |
|---|---|---|---|---|
|
|
||||
| Total CSM Score, Mean (SD) | 33.1 (8.3) | 36.1 (6.9) | 2.1 | 0.04 |
| Evening chronotype classification | ||||
| Original threshold, n (%) | 3 (5.6) | 1 (1.8) | 1.2 | 0.36 |
| ≤ 1 SD threshold, n (%) | 13 (24.1) | 5 (8.8) | 4.8 | 0.04 |
| ≤ ¾ SD threshold, n (%) | 16 (29.6) | 7 (12.3) | 5.1 | 0.03 |
| ≤ ⅔ SD threshold, n (%) | 19 (35.2) | 7 (12.3) | 8.1 | 0.007 |
| ≤ ½ SD threshold, n (%) | 24 (44.4) | 9 (15.8) | 10.9 | 0.002 |
| ≤ ¼ SD threshold, n (%) | 27 (50.0) | 20 (35.1) | 2.5 | 0.13 |
Note. SD = standard deviation. CSM = Composite Scale of Morningness. The original CSM threshold entailed total score of ≤ 22 for evening chronotype. CSM total scores ≤ 1 SD (total ≤ 26.8), ≤ ¾ SD (total ≤ 28.8), ≤ ⅔ SD (total ≤ 29.4), and ≤ ½ SD (total ≤ 30.7) and ≤ ¼ SD (total ≤ 32.7) below our sample mean (34.6) were our modified thresholds for evening chronotypes. Seven bipolar disorder group participants and 4 control group participants were missing CSM total scores. Underlines indicate t- (rather than χ2) values.
Using the original CSM threshold (total score ≤ 22) for chronotype classification, did not yield a significant between-group difference (p = 0.36) in proportion of evening chronotypes, as only three (of 54) BD group participants and one (of 57) healthy control group participant met the original CSM threshold for classification as evening chronotype. The CSM mean score and SD for the full sample were 34.6 and 7.8, respectively. Using our modified CSM evening chronotype thresholds of ≤ 1 SD below mean (total score ≤ 26.8, p = 0.04), ≤ ¾ SD below mean (total score ≤ 28.8, p = 0.03), ≤ ⅔ SD below mean (total score ≤ 29.4, p = 0.007), and ≤ ½ SD below mean (total score ≤ 30.7, p = 0.002) but not ≤ ¼ SD below mean (total score ≤ 32.7, p = 0.13) yielded significant between-group (BD versus healthy control) differences in rates of evening chronotypes.
Group Differences in Objective Actigraphy-Derived Sleep Interval Midpoints and Evening Chronotype Classification
Table 3 depicts BD versus healthy control group comparisons of mean values of objective actigraphy-derived sleep interval midpoints and objective categorical classifications of evening chronotypes, with the latter based upon percentages of sleep interval midpoints meeting threshold for eveningness. Among the 5,230 observed sleep intervals, 192 naps were omitted as described in the Methods section, leaving 5,038 sleep intervals included in our analyses. Relative to healthy controls, participants with BD had significantly more eveningness considered continuously (i.e., a significantly later sleep interval midpoint means, 04:48:00±01:23:00 versus 04:10:00±01:03:00, t(120) = 2.8, p = 0.005). Female participants with BD had a significantly later sleep interval midpoint mean than did female controls, t(70) = 2.81, p = 0.007. Adolescent participants (age < 18 years) with BD had a significantly later sleep interval midpoint mean than did adolescent control participants, t(28) = 2.90, p = 0.007. A linear regression analysis with diagnostic group, age, gender, and employment/student status entered as predictors and sleep interval midpoint entered as the outcome variable yielded a significant overall model, F(4,117) = 4.74, p = 0.001. Both diagnostic group (β = 0.27, t = 3.04, p = 0.003) and age (β = −0.27, t = 2.87, p = 0.005) were significant predictors of sleep interval midpoint.
Table 3.
Group Differences in Objective Actigraphy-Derived Sleep Interval Midpoint Means and in Proportions of Participants Classified with Evening Chronotype, Based Upon Percent of Sleep Intervals with Midpoints After 04:15:00.
| Bipolar Disorder (n=61) | Healthy Control (n=61) | t or χ2 | p-value | |
|---|---|---|---|---|
|
|
||||
| Number of sleep intervals assessed, Mean (SD) | 40.6 (15.2) | 42.0 (16.1) | 0.5 | 0.64 |
| Sleep interval midpoint time (hours:minutes:seconds), Mean (SD) | 04:48:00 (01:23:00) | 04:10:00 (01:03:00) | 2.8 | 0.005 |
| Evening chronotype classification | ||||
| ≥ 50.0% of midpoints after 04:15:00, n (%) | 33 (54.1) | 25 (41.0) | 2.1 | 0.20 |
| ≥ 66.7% of midpoints after 04:15:00, n (%) | 27 (44.3) | 17 (27.9) | 3.6 | 0.09 |
| ≥ 75.0% of midpoints after 04:15:00, n (%) | 24 (39.3) | 11 (18.0) | 6.8 | 0.016 |
| ≥ 90.0% of midpoints after 04:15:00, n (%) | 11 (18.0) | 6 (9.8) | 1.7 | 0.30 |
Note. Sleep interval midpoint means calculated across all sleep intervals per-person, then per group. Underlines indicate t- (rather than χ2) values.
Using evening chronotype thresholds of percentages of sleep interval midpoints occurring past 04:15:00, the ≥ 75% (p = 0.016) threshold but not the ≥ 50% (p = 0.20), ≥ 66.7% (p = 0.09), or ≥ 90% (p = 0.30) thresholds yielded a significant between-group (BD versus healthy control) difference in evening chronotypes. Mean values of actigraphy-derived sleep interval midpoints were significantly correlated with antipsychotic use (r = −0.25, p = 0.50, n = 61) but not with any other prescription psychotropic medication use (all p’s ranged between 0.2 and 0.8).
Association Between Subjective Composite Scale Scores and Objective Actigraphy-Derived Sleep Interval Midpoints
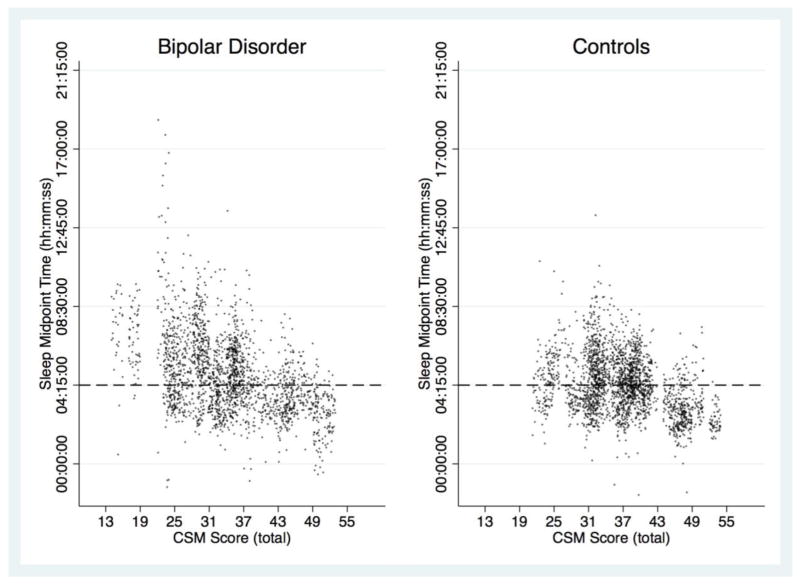
Bivariate correlations between CSM total scores and within-person actigraphy-derived sleep interval midpoint means were statistically significant within each group (BD group: r = −0.64, p < 0.0001, n = 54; control group: r = −0.42, p = 0.001, n = 57), as well as across the full sample (r = −0.57, p < 0.0001, N = 111). Among BD participants, the correlation between CSM total scores and sleep interval midpoint means remained statistically significant after controlling for mood stabilizer, antipsychotic, antidepressant, anxiolytic/hypnotic use, and other prescription psychotropic medication use (partial r = −0.61, p < 0.0001, n = 47). For illustrative purposes, Figure 1 depicts a scatterplot of the correlation between continuous subjective self-report (CSM total score) and all available actigraphy-derived sleep interval midpoints, stratified by participant group (BD and healthy controls).
Figure 1.
Correlations Between Subjective Composite Scale of Morningness (CSM) Total Score and Actigraphy-Derived Sleep Interval Midpoints, Stratified by Participant Group (BD and healthy controls).
Correlations between categorically identified evening chronotypes using the subjective CSM threshold of ≤ ½ SD below mean (total score ≤ 30.7) and the objectively identified evening chronotypes using the ≥ 75% threshold of actigraphy-derived sleep interval midpoints showed that the two methods were significantly correlated in the whole sample, r = 0.36, p < 0.0001, n = 111, and within the BD group , r = 0.43, p = 0.001, n = 54, but not within the healthy control group, r = 0.08, p = 0.57, n = 57 (not illustrated).
Associations Between Symptom Severity and Subjective Composite Scale Total Scores or Objective Actigraphy-Derived Sleep Interval Midpoints
Among BD participants and healthy controls, CSM total scores were not significantly correlated with YMRS manic symptom scores, IDS-C depression symptom scores (among sub-sample 1 participants), nor with CDRS depression scores (among sub-sample 2 participants) (all p’s > 0.66, not illustrated). Similarly, among BD group participants and healthy controls, actigraphy-derived sleep interval midpoint was not significantly associated with YMRS manic symptom scores, IDS-C depression symptom scores (among sub-sample 1 participants), nor with CDRS depression scores (among sub-sample 2 participants) (all p’s > 0.26, not illustrated).
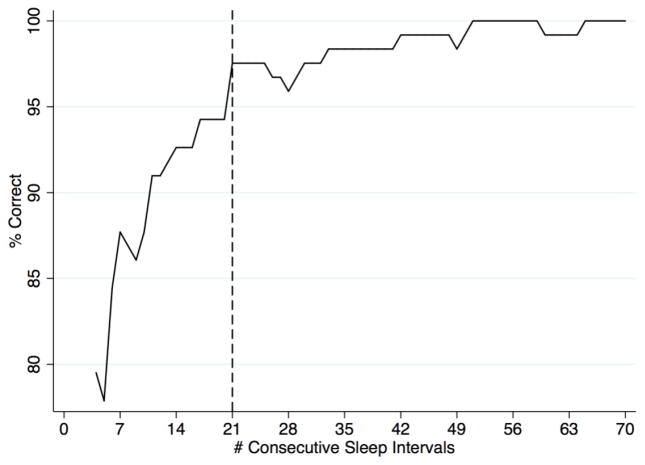
Number of Actigraphy-Derived Sleep Intervals Needed to Accurately Classify Participants with Evening Chronotype
The number of consecutive sleep intervals needed to correctly classify ≥ 95% of participants with objective evening chronotype based upon the 75% threshold classification was 21 (Figure 2). Given that participants varied in number of sleep intervals, we repeated this analysis limiting our sample to participants with ≥ 30 consecutive sleep intervals. The resulting re-analysis showed a similar pattern.
Figure 2.
Number of Consecutive Actigraphy-Derived Sleep Intervals Needed for Accurate Classification of Evening Chronotype
Note. Percent Correct is defined by whether an evening chronotype classification based on cumulative actigraphy-derived sleep intervals matched the classification based upon all actigraphy-derived sleep intervals available.
Discussion
Prior studies have highlighted associations between BD and a preference for later (eveningness) rather than earlier (morningness) timing of sleep and daily activities (Melo et al., 2016). However, much of this work has been carried out using subjective self-reports. Given the potential inaccuracies of subjective self-reports and the density and ease of objective data collection using wearable sensors, we sought to examine relationships between subjective self-reported and objective actigraphy-based chronotype metrics in BD participants versus healthy controls. Using objective actigraphy-derived sleep interval data for both continuous (mean values of sleep midpoints) and categorical (≥ 75% of sleep midpoints after 04:15:00) approaches, BD participants had significantly more eveningness than healthy controls. Similarly, using the subjective self-report (CSM total score) derived continuous metric, BD participants had significantly more eveningness (lower mean CSM total score) than controls. In contrast, the CSM’s original threshold for classifying evening chronotype yielded no significant between-group (BD versus healthy control) difference in proportion of evening chronotype, likely due to limited statistical power as the original threshold identified only 3 BD participants and 1 healthy control with evening chronotype. However, modifying CSM thresholds to ≤ 1, ¾, ⅔, ½ (but not ¼) SD below our observed CSM mean yielded significantly greater proportions of subjective evening chronotypes in BD than in healthy controls.
The ability to accurately assess chronotype in patients with BD has both clinical and research implications. Clinically, identification of BD patients for whom chronotherapies, or circadian rhythm-oriented pharmacological and behavioral treatments that could beneficially modify sleep and activity timing, may enhance quality of life and functioning, aid in treatment compliance, and improve long-term illness outcomes. Moreover, given that the circadian system is involved in basic regulatory functions such as hormone release, inflammatory processes, and metabolism (Hagenauer & Lee, 2012; T. W. Kim, Jeong, & Hong, 2015; McGinnis & Young, 2016; Sancar & Brunner, 2014; Tsang, Astiz, Friedrichs, & Oster, 2016), detecting and treating dysregulated circadian rhythms could have far reaching effects on clinical outcomes in patients with BD. From a research perspective, the ability to classify participants into groups according to chronotype may facilitate understanding of roles of circadian systems in BD. Additionally, consistent with recent advances in precision medicine initiatives, chronotype identification has the potential to lead to development of highly targeted new treatments to address circadian rhythm dysregulation in individual BD patients.
Chronotype has commonly been measured using subjective self-report questionnaires, which are relatively inexpensive and easy to administer. However, self-reports may only reflect individuals’ perceptions of their sleep timing, rather than actual behavior. Increasingly, the ubiquity of wearable sensors makes it possible to objectively measure sleep timing unobtrusively and on a large scale. However, the actigraphy method requires participants to wear devices continuously. Our data suggest that despite the potential theoretical limitations of subjective self-reported chronotypes, this approach yields data that generally overlap those from objective chronotypes. Our study also highlights the utility of examining chronotype as both a summary measure and on a night-to-night basis. We found that even among those who were categorized with overall evening chronotypes, there was still considerable variability in night-to-night chronotype. Nevertheless, we found that 21 consecutive sleep intervals are sufficient for accurately determining evening chronotype. These considerations highlight the possibilities of using night-to-night chronotype data as a way to provide “just-in-time” interventions to regulate sleep patterns in people with BD.
Our study is among the first to investigate relationships between subjective self-report-based versus objective actigraphy-based chronotypes in a substantial sample of well-characterized individuals with BD and healthy controls not only during adulthood, but also during adolescence. However, several limitations need to be considered. First, our BD participants were all inter-episode, and mostly white and female, limiting the generalizability of our findings to more diverse samples. Second, although we obtained over 5,000 actigraphy-measured sleep intervals, our relatively small participant sample size (61 BD and 61 healthy control participants) may have limited statistical power. Replication with larger and more diverse samples is warranted. Third, most of the BD participants were taking prescription psychotropic medications, relative to none of the healthy control group. However, partial correlations revealed that subjective CSM mean total scores and objective actigraphy-derived mean sleep interval midpoints remained significantly correlated among BD participants after controlling for most prescription psychotropic medication use, with the important exceptions of antipsychotics, which were significantly correlated with actigraphy-derived sleep interval midpoint means, and anxiolytic/hypnotics, which were significantly correlated with CSM total scores. The use of antipsychotics and anxiolytics/hypnotics, in particular, should be carefully assessed in studies of chronotype, as these medications influence sleep quality and timing. Similarly, some types of non-pharmacological treatments can include sleep regularization as one of the therapeutic targets (e.g., Interpersonal and Social Rhythm Therapy). Because we did not assess for the presence of non-pharmacological treatments in the current study, we cannot test for the extent to which these treatments may have influenced chronotype in our sample. Future research should assess the influence of psychotherapy with specific sleep-related indications on chronotype. Fourth, although all BD participants were inter-episode at study entry, BD participants reported significantly higher depressive (assessed with IDSC or CDRS) and manic (assessed with YMRS) symptom severity at baseline compared to healthy controls. However, neither depressive nor manic symptoms at baseline were significantly associated with CSM total scores or sleep interval midpoints among BD participants. The absence of a significant association between symptom severity and chronotype is consistent with previous findings in which evening preference was not associated with current mood symptoms among BD participants (Seleem et al., 2015) and is suggestive of the idea that evening chronotype may be a trait characteristic of BD that is unaffected by current mood state. Fifth, our data do not address the environmental or life circumstances (e.g. resulting in having to retire later or arise earlier) that may have influenced the observed group differences in chronotype.
Despite these limitations, our study is among the first to assess chronotype in a substantial sample of well-characterized BD participants relative to healthy controls using both subjective self-report and an objective actigraphy-derived marker of circadian phase (sleep interval midpoint) based on several weeks of actigraphy data per participant. Our findings confirm prior associations between BD and evening chronotype and highlight that both subjective questionnaire-based (albeit with some chronotype threshold modification) and objective actigraphy-based chronotype assessments can identify between group-differences in the proportion of evening chronotype using categorical approaches. Moreover, our findings show that subjective questionnaire-based and objective actigraphy-based continuous and categorical chronotype assessments significantly correlate with one-another and have considerable overlap, respectively. Given that subjective chronotypes yielded data that generally overlap with those from actigraphy-based measurement, our study confirms the utility of self-report questionnaires for chronotype assessment in BD samples. Nevertheless, actigraphy-based measurement yields more fine-grained information regarding night-to-night variability in chronotype, which may be valuable for tracking circadian variation over time as it relates to treatment compliance and long-term illness outcomes.
Highlights.
Chronotype measured in 61 BD subjects and 61 controls via self-report and actigraphy
BD subjects showed more evening chronotype than controls using both measures
Self-report and actigraphy chronotype correlated within groups and across full sample
21 nights of actigraphy correctly identified evening chronotype in ≥ 95% of subjects
Acknowledgments
This research was supported by the National Institute of Mental Health (NIMH) Research Scientist Development Award K01MH100433 and an NIMH Ruth L. Kirschstein National Research Service Award Postdoctoral Fellowship F32MH76339 to Dr. Gershon.
Footnotes
Contributors
Dr. Gershon contributed to the research, data analysis, and writing of the manuscript. Drs. Kaufmann, Depp, Miller, Zeitzer, and Ketter, and Dennis Do, contributed to the data analysis and writing of the manuscript. All authors have approved the final article.
Disclosure of Financial Relationships
Drs. Gershon, Kaufmann, Depp, Do, and Zeitzer report no financial relationships with commercial interests. Dr. Miller has received Grant/Research Support from Merck & Co., Inc. and Sunovion Pharmaceuticals. Dr. Ketter has received Grant/Research Support from the Agency for Healthcare Research and Quality, AstraZeneca Pharmaceuticals LP, Cephalon Inc., Eli Lilly and Company, National Institute of Mental Health, Pfizer Inc., and Sunovion Pharmaceuticals; Consultant Fees from Allergan, Inc., Avanir Pharmaceuticals, Bristol-Myers Squibb Company, Cephalon Inc., Forest Pharmaceuticals, Janssen Pharmaceutica Products, LP, Merck & Co., Inc., Sunovion Pharmaceuticals, and Teva Pharmaceuticals; Lecture Honoraria from Abbott Laboratories, Inc., AstraZeneca Pharmaceuticals LP, GlaxoSmithKline, and Otsuka Pharmaceuticals; and Publication Royalties from American Psychiatric Publishing, Inc. In addition, Dr. Ketter’s spouse is an employee of and holds stock in Janssen Pharmaceuticals.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahn YM, Chang J, Joo YH, Kim SC, Lee KY, Kim YS. Chronotype distribution in bipolar I disorder and schizophrenia in a Korean sample. Bipolar Disorders. 2008;10(2):271–275. doi: 10.1111/j.1399-5618.2007.00573.x. [DOI] [PubMed] [Google Scholar]
- Boudebesse C, Geoffroy PA, Bellivier F, Henry C, Folkard S, Leboyer M, Etain B. Correlations between objective and subjective sleep and circadian markers in remitted patients with bipolar disorder. Chronobiology International. 2014;31(5):698–704. doi: 10.3109/07420528.2014.895742. [DOI] [PubMed] [Google Scholar]
- Carskadon MA, Vieira C, Acebo C. Association between puberty and delayed phase preference. Sleep. 1993;16(3):258–262. doi: 10.1093/sleep/16.3.258. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Structured clinical interview for DSM-IV-TR axis I disorders Patient edition (SCID-I/P, 1/2007 revision) Biometrics Research Department, New York, NY: New York State Psychiatric Institute; 2007. [Google Scholar]
- Germain A, Nofzinger EA, Meltzer CC, Wood A, Kupfer DJ, Moore RY, Buysse DJ. Diurnal variation in regional brain glucose metabolism in depression. Biological Psychiatry. 2007;62(5):438–445. doi: 10.1016/j.biopsych.2006.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon A, Thompson WK, Eidelman P, McGlinchey EL, Kaplan KA, Harvey AG. Restless pillow, ruffled mind: sleep and affect coupling in interepisode bipolar disorder. Journal of Abnormal Psychology. 2012;121(4):863–873. doi: 10.1037/a0028233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagenauer MH, Lee TM. The neuroendocrine control of the circadian system: adolescent chronotype. Frontiers in Neuroendocrinology. 2012;33(3):211–229. doi: 10.1016/j.yfrne.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey AG, Schmidt DA, Scarna A, Semler CN, Goodwin GM. Sleep-related functioning in euthymic patients with bipolar disorder, patients with insomnia, and subjects without sleep problems. American Journal of Psychiatry. 2005;162(1):50–57. doi: 10.1176/appi.ajp.162.1.50. [DOI] [PubMed] [Google Scholar]
- Horne JA, Östberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. International Journal of Chronobiology. 1976;4(2):97–110. [PubMed] [Google Scholar]
- Kantermann T, Juda M, Merrow M, Roenneberg T. The human circadian clock's seasonal adjustment is disrupted by daylight saving time. Current Biology. 2007;17(22):1996–2000. doi: 10.1016/j.cub.2007.10.025. [DOI] [PubMed] [Google Scholar]
- Kim KL, Weissman AB, Puzia ME, Cushman GK, Seymour KE, Wegbreit E, … Dickstein DP. Circadian Phase Preference in Pediatric Bipolar Disorder. Journal of Clinical Medicine. 2014;3(1):255–266. doi: 10.3390/jcm3010255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TW, Jeong JH, Hong SC. The impact of sleep and circadian disturbance on hormones and metabolism. International Journal of Endocrinology. 2015;2015:591729. doi: 10.1155/2015/591729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour HA, Wood J, Chowdari KV, Dayal M, Thase ME, Kupfer DJ, … Nimgaonkar VL. Circadian phase variation in bipolar I disorder. Chronobiology International. 2005;22(3):571–584. doi: 10.1081/CBI-200062413. [DOI] [PubMed] [Google Scholar]
- McGinnis GR, Young ME. Circadian regulation of metabolic homeostasis: causes and consequences. Nature and Science of Sleep. 2016;8:163–180. doi: 10.2147/NSS.S78946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo MC, Abreu RL, Linhares Neto VB, de Bruin PF, de Bruin VM. Chronotype and circadian rhythm in bipolar disorder: A systematic review. Sleep Medicine Reviews. 2016 doi: 10.1016/j.smrv.2016.06.007. [DOI] [PubMed] [Google Scholar]
- Merikangas KR, Akiskal HS, Angst J, Greenberg PE, Hirschfeld RM, Petukhova M, Kessler RC. Lifetime and 12-month prevalence of bipolar spectrum disorder in the National Comorbidity Survey replication. Archives of General Psychiatry. 2007;64(5):543–552. doi: 10.1001/archpsyc.64.5.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merikangas KR, Jin R, He JP, Kessler RC, Lee S, Sampson NA, … Zarkov Z. Prevalence and correlates of bipolar spectrum disorder in the world mental health survey initiative. Archives of General Psychiatry. 2011;68(3):241–251. doi: 10.1001/archgenpsychiatry.2011.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar A, Espie CA, Scott J. The sleep of remitted bipolar outpatients: a controlled naturalistic study using actigraphy. Journal of Affective Disorders. 2004;80(2–3):145–153. doi: 10.1016/S0165-0327(03)00055-7. [DOI] [PubMed] [Google Scholar]
- Morin CM, Espie CA. Insomnia: A Clinical Guide to Assessment and Treatment. New York: Kluwer Academic/Plenum Publishers; 2003. [Google Scholar]
- Paine SJ, Gander PH, Travier N. The epidemiology of morningness/eveningness: influence of age, gender, ethnicity, and socioeconomic factors in adults (30–49 years) Journal of Biological Rhythms. 2006;21(1):68–76. doi: 10.1177/0748730405283154. [DOI] [PubMed] [Google Scholar]
- Poznanski EO, Freeman LN, Mokros HB. Children's Depression Rating Scale-Revised. Psychopharmacological Bulletin. 1985;21:979–989. [Google Scholar]
- Randler C, Schaal S. Morningness-eveningness, habitual sleep-wake variables and cortisol level. Biological Psychology. 2010;85(1):14–18. doi: 10.1016/j.biopsycho.2010.04.006. [DOI] [PubMed] [Google Scholar]
- Roenneberg T, Kuehnle T, Juda M, Kantermann T, Allebrandt K, Gordijn M, Merrow M. Epidemiology of the human circadian clock. Sleep Medicine Reviews. 2007;11(6):429–438. doi: 10.1016/j.smrv.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Gullion CM, Basco MR, Jarrett RB, Trivedi MH. The Inventory of Depressive Symptomatology (IDS): psychometric properties. Psychological Medicine. 1996;26(3):477–486. doi: 10.1017/s0033291700035558. [DOI] [PubMed] [Google Scholar]
- Sadeh A, Hauri PJ, Kripke DF, Lavie P. The role of actigraphy in the evaluation of sleep disorders. Sleep. 1995;18(4):288–302. doi: 10.1093/sleep/18.4.288. [DOI] [PubMed] [Google Scholar]
- Sancar G, Brunner M. Circadian clocks and energy metabolism. Cellular and Molecular Life Sciences. 2014;71(14):2667–2680. doi: 10.1007/s00018-014-1574-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seleem MA, Merranko JA, Goldstein TR, Goldstein BI, Axelson DA, Brent DA, … Birmaher B. The longitudinal course of sleep timing and circadian preferences in adults with bipolar disorder. Bipolar Disorders. 2015;17(4):392–402. doi: 10.1111/bdi.12286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CS, Reilly C, Midkiff K. Evaluation of three circadian rhythm questionnaires with suggestions for an improved measure of morningness. Journal of Applied Psychology. 1989;74(5):728–738. doi: 10.1037/0021-9010.74.5.728. [DOI] [PubMed] [Google Scholar]
- Taillard J, Philip P, Chastang JF, Bioulac B. Validation of Horne and Ostberg morningness-eveningness questionnaire in a middle-aged population of French workers. Journal of Biological Rhythms. 2004;19(1):76–86. doi: 10.1177/0748730403259849. [DOI] [PubMed] [Google Scholar]
- Thun E, Bjorvatn B, Osland T, Steen VM, Sivertsen B, Johansen T, … Pallesen S. An Actigraphic Validation Study of Seven Morningness-Eveningness Inventories. European Psychologist. 2012;17(3):222–230. doi: 10.1027/1016-9040/a000097. [DOI] [Google Scholar]
- Tonetti L, Adan A, Di Milia L, Randler C, Natale V. Measures of circadian preference in childhood and adolescence: A review. European Psychiatry. 2015;30(5):576–582. doi: 10.1016/j.eurpsy.2015.01.006. [DOI] [PubMed] [Google Scholar]
- Tsang AH, Astiz M, Friedrichs M, Oster H. Endocrine regulation of circadian physiology. The Journal of Endocrinology. 2016;230(1):R1–R11. doi: 10.1530/JOE-16-0051. [DOI] [PubMed] [Google Scholar]
- Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. British Journal of Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]