Abstract
Aberrations in telomere biology are among the earliest events in prostate cancer tumorigenesis and continue during tumour progression. Substantial telomere shortening occurs in prostate cancer cells and high-grade prostatic intraepithelial neoplasia. Not all mechanisms of telomere shortening are understood, but oxidative stress from local inflammation might accelerate prostatic telomere loss. Critically short telomeres can drive the accumulation of tumour-promoting genomic alterations; however, continued telomere erosion is unsustainable and must be mitigated to ensure cancer cell survival and unlimited replication potential. Prostate cancers predominantly maintain telomeres by activating telomerase, but alternative mechanisms of telomere extension can occur in metastatic disease. Telomerase activity and telomere length assessment might be useful in prostate cancer diagnosis and prognosis. Telomere shortening in normal stromal cells has been associated with prostate cancer, whereas variable telomere lengths in prostate cancer cells and telomere shortening in cancer-associated stromal cells correlated with lethal disease. Single-agent telomerase-targeted treatments for solid cancers were ineffective in clinical trials but have not been investigated in prostate cancer and might be useful in combination with established regimens. Telomere-directed strategies have not been explored as extensively. Telomere deprotection strategies have the advantage of being effective in both telomerase-dependent and telomerase-independent cancers. Disruption of androgen receptor function in prostate cancer cells results in telomere dysfunction, indicating telomeres and telomerase as potential therapeutic targets in prostate cancer.
Early studies of linear yeast artificial chromosomes identified three essential elements required to ensure correct duplication and segregation of linear eukaryotic chromosomes1: an origin of replication2,3, a centromere4,5, and a pair of telomeres at the extreme ends of the chromosome6. Human telomeres consist of a highly conserved, G-rich, repetitive hexanucleotide sequence (TTAGGG)n7,8, approximately 5–15 kb in length9. A complex of six proteins — collectively termed shelterin — is associated with the telomeric DNA repeats (telomeric repeat-binding factor 1 and 2 (TERF1 and TERF2, also known as TRF1 and TRF2), TRF2-interacting protein 1 (TERF2IP, also known as RAP1), TRF1-interacting nuclear factor 2 (TINF2, also known as TIN2), protection of telomeres protein 1 (POT1), and tripeptidyl-peptidase 1 (TPP1))10–20. The linear nature of human chromosomes poses several biological dilemmas that these telomeric nucleoproteins help mitigate. The extreme ends of chromosomes are potential substrates for exonucleolytic degradation and can also be recognized as a DNA double-strand break by the DNA damage response (DDR) pathway. Telomeres of sufficient length safeguard against exonuclease activity and DDR recognition by forming specialized T-loop structures21 and serve as a scaffold for shelterin proteins that inhibit potentially deleterious DNA repair mechanisms at the telomeres20.
Telomeres are integral to cellular proliferation barriers that ensure finite replicative capacity in cells, serving as a potent anticancer mechanism22,23. During DNA replication, synthesis on the lagging DNA strand of linear templates is incomplete (end replication problem)24,25, resulting in the loss of ~50 terminal nucleotides in each round of cellular division26. For a limited number of population doublings, telomeres buffer against the loss of information- carrying DNA sequences. However, when telomeres become substantially shortened, the DDR pathway is activated on one or more telomeres, and cell cycle progression is arrested via the tumour suppressor p53 pathway27–29. This state of cell cycle arrest is termed replicative senescence and occurs after ~50 cell divisions (the Hayflick limit), depending on cell type. Failure to block cell cycling via the p53 pathway can have devastating genomic consequences, manifested as end-to-end chromosomal fusions, anaphase bridges, nonreciprocal translocations and aneuploidy. All these processes help to promote cellular transformation via the stochastic inactivation of tumour suppressor genes and activation of oncogenes30–34.
To achieve unlimited replicative capacity, cancer cells must eventually resolve the end replication problem. Predominately, cancers maintain their telomeres by activating telomerase, a telomere-specific enzyme that extends telomeres35, therefore obviating the end replication problem. Approximately 5–10% of cancers employ a telomerase-independent mechanism to maintain and extend telomeres called the alternative lengthening of telomeres (ALT), which is thought to rely on homology-directed DNA recombination36,37. ALT is frequently observed in nonepithelial cancers, but ALT has been reported in a subset of advanced, lethal metastatic prostate tumours38 (FIG. 1). By contrast, ALT has not been observed in primary prostate cancers, of which the vast majority, if not all, employ telomerase for telomere maintenance39.
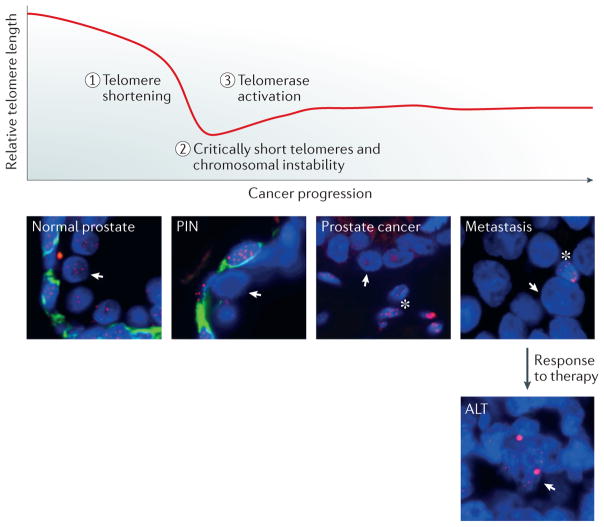
Figure 1. Telomere shortening during prostate tumorigenesis and cancer progression.
Telomeres gradually shorten in each round of cell division owing to incomplete replication of the lagging DNA strand during DNA synthesis. To sustain unlimited replicative capacity, prostate cancer cells activate telomerase; however, they maintain short telomeres during prostate tumorigenesis and cancer progression, which can be seen in biopsy samples following fluorescence in situ hybridization staining (telomeres: red, Cy3-labelled anti-telomeric probe; basal cells: green, anti-cytokeratin antibody 34bE12; DNA: blue, DAPI). In the normal prostate, telomere lengths are relatively similar between luminal (arrows) and basal cells. However, in prostatic intraepithelial neoplasia (PIN), telomere staining in the luminal cell is substantially less than in the basal cells, indicative of telomere shortening in the luminal compartment. In prostate cancer, which lacks basal cells, the telomere staining is substantially less than in neighbouring stromal cells (asterisks). Likewise, in cancer metastases, telomere staining is less than in infiltrating lymphocytes (asterisks, reduced nucleus size). In a subset of metastatic prostate cancer cells, alternative lengthening of telomeres (ALT) mechanisms are activated, possibly as a response to treatment or environmental factors. Bright telomeric DNA foci are characteristic of ALT.
In this Review, we highlight the function of telomeres that specifically relate to prostate cancer. We describe the short-telomeres phenotype observed in the majority of precancerous prostate lesions and prostate tumours and the potential sources of telomere shortening in prostate cancer. Furthermore, we discuss how telomere shortening and telomerase activation result in genomic instability, consequently contributing to tumour promoting mutations. Finally, we delineate the clinical implications of telomere dysfunction and the therapeutic potential of treatments targeting telomerase and telomeres in patients with prostate cancer.
Role in prostate pathophysiology
Telomerase, a reverse transcriptase, extends chromosome ends with a concatemer of the telomeric TTAGGG repeat sequence40. The creation of the enzyme is a complex orchestration of several proteins and nucleic acids participating in the biogenesis and localization of telomerase41. The core components for DNA extension activity consist primarily of the catalytic telomerase reverse transcriptase protein (TERT) and the telomerase RNA component (TERC, also known as hTR), which contains the repetitive telomeric sequence that functions as a template for the reverse transcriptase42,43. Telomerase expression is repressed in most human somatic cells, but detectable levels of activity exist in germline and somatic stem cells44.
Prostate cancers activate telomerase to maintain telomeres, but the telomeres in prostate cancer cells, as directly assessed in situ, are abnormally short in the vast majority of clinical samples compared with matched adjacent normal prostate tissue39. Southern blot analysis of terminal restriction fragments (TRFs) shows that the average telomere length of normal prostate tissue is 6.6 kb, whereas prostate cancer tissue has a considerably shorter average telomere length of 5.4 kb39. This finding is perhaps not surprising, considering that even adult stem cells in epithelial tissues with activated telomerase are subject to telomere shortening over time, presumably because the amount of telomerase present is unable to fully offset the end replication problem. In the same study, the average telomere length of BPH tissue was 6.4 kb, which is comparable to that of the normal prostate. These reported telomere lengths are probably an overestimation of the actual telomere length, as the TRF Southern blot technique detects not only pure TTAGGG telomeric repeats but also degenerate and variant subtelomeric sequences, resulting in estimates that are 4 kb higher than the actual telomere region consisting exclusively of telomere repeats26,45. In addition, contaminating normal cells artificially increase measured average telomere lengths in bulk tissue analyses.
Similar to prostate cancer, prostatic intraepithelial neoplasia (PIN) and BPH are characterized by an abnormal increase in cell proliferation. Localized prostate cancer has an ~7-fold increased cell proliferation rate compared with normal prostatic epithelial tissue46. High-grade PIN (HGPIN) tissue proliferates ≥6-fold faster than normal prostate46, and has abnormally short telomeres47. BPH tissue proliferates at a 2–3-fold higher rate than normal prostate48 but, surprisingly, does not display the telomere shortening expected to accompany abnormally high cell proliferation in a telomerase-negative setting39.
Kinetic studies of abnormal lesions in the prostate also show that BPH tissue is indeed less proliferative than PIN tissue49, which might partially account for the lack of telomere shortening observed in BPH. Alternatively, the inconsistency in telomere length might be caused by a difference in the proliferative topology between BPH and HGPIN tissues50. Normally, proliferation in prostatic epithelial tissues is mainly restricted to the basal cell compartment, where stem cells are thought to reside51–53. However, in HGPIN lesions, hyperproliferation occurs in both basal and luminal epithelial cells54,55 and the majority of proliferation occurs in the luminal compartment50,53,56. Correspondingly, telomere shortening in HGPIN tissue is observed in luminal cells only and not the basal cell compartment47. By contrast, hyperproliferation in BPH occurs in both stromal and epithelial cells. Furthermore, proliferation of epithelial cells in BPH mainly occurs in the basal compartment53,56, similar to normal prostate, which implicates stem cells and stem-like cells in the aetiology of BPH. Consistent with this notion, a report from 2016 suggested that the telomerase-negative epithelial cells in BPH with normal telomere length arise from telomerase-positive progenitor cells that differentiated from stem-like cells residing in the basal compartment57. Thus, the lack of short telomeres in BPH could be caused by widespread proliferation of multiple progenitor cells, in contrast to cancer, which is traditionally thought to be the result of clonal expansion originating from a single transformed cell. HGPIN lesions share many features with prostate cancer; hence, HGPIN are believed to be the malignant precursors of prostate cancer58,59, which strongly implicates telomere shortening as an early event in prostate tumorigenesis (FIG. 1). Evidence to define a concrete timeline of telomere shortening and the transition point from HGPIN to cancer is limited, but telomerase activation is likely to be a key event. The precise timing of telomerase activation during prostate tumorigenesis is unknown. However, studies have indicated that at least a subset of HGPIN lesions have detectable telomerase60,61, suggesting that considerable telomere shortening ostensibly occurs before telomerase activation in HGPIN.
Cancer is a disease of ageing, and prostate cancer is not unusual in that regard. The median age for prostate cancer diagnosis is 67 years62. Prostate cancer is an exceptionally slow-growing cancer compared with other solid tumours63. A clonal outgrowth of prostate cancer has been estimated to take ~40 years to reach a size of 1 cm3 (REF. 46). The even lower rate of proliferation in the normal prostate is not consistent with a timeline that would result in substantial telomere shortening before transformation, and such substantial shortening is also not observed. On the basis of an estimated 500-day turnover rate of the prostatic epithelium46, and the Hayflick limit of 50 population doublings23,64, normal prostate cells would take ~68 years to reach replicative senescence. Furthermore, prostate cells would take an additional 27 years of proliferation to achieve critical telomere lengths, when one or more telomeres become dysfunctional. These estimates assume that, during tumorigenesis, prostate cells need to bypass replicative senescence, that 50 bp of telomere content are lost per division26, and that the difference between senescent cells and cells in crisis is 1 kb of telomere content65. On the basis of these conservative numbers, prostate cells would need to proliferate for almost 100 years before telomeres were sufficiently shortened to result in the telomere-driven genomic instability required for normal prostate cells to develop into precursor lesions. Taken together, the combined observations of relatively slow proliferation rates of normal prostate cells and luminal-cell-specific telomere shortening in prostate cancer and precursor HGPIN lesions suggest that additional factors (for example, oxidative stress, perhaps from local inflammatory processes) might work in concert with cell proliferation to accelerate the process of telomere shortening in precancerous HGPIN lesions and perhaps further in prostate cancer.
Telomeres and genomic alterations
Oxidative DNA damage and inflammation
Telomeres are particularly susceptible to telomere shortening in response to DNA damage, which might be aggravated by persistent inflammation and the production of reactive oxygen species (ROS) (FIG. 2). ROS are inherent byproducts of cellular respiration, arising from multiple endogenous sources, including the electron transport chain in mitochondria66. However, additional endogenous and exogenous sources of free radicals can increase ROS levels above baseline with devastating cellular consequences. A particularly well-characterized effect of elevated ROS levels is increased DNA damage67. The predominant species of free radicals that contribute to cellular DNA damage are hydroxyl radicals (·OH) arising from superoxide radicals (·O2−) and hydrogen peroxide (H2O2)68–70. Guanine has the lowest oxidation potential of the DNA bases71. As a result, the oxidation of guanine to form 8-oxoguanine is the most abundant of the many DNA mutations that arise from ROS exposure72.
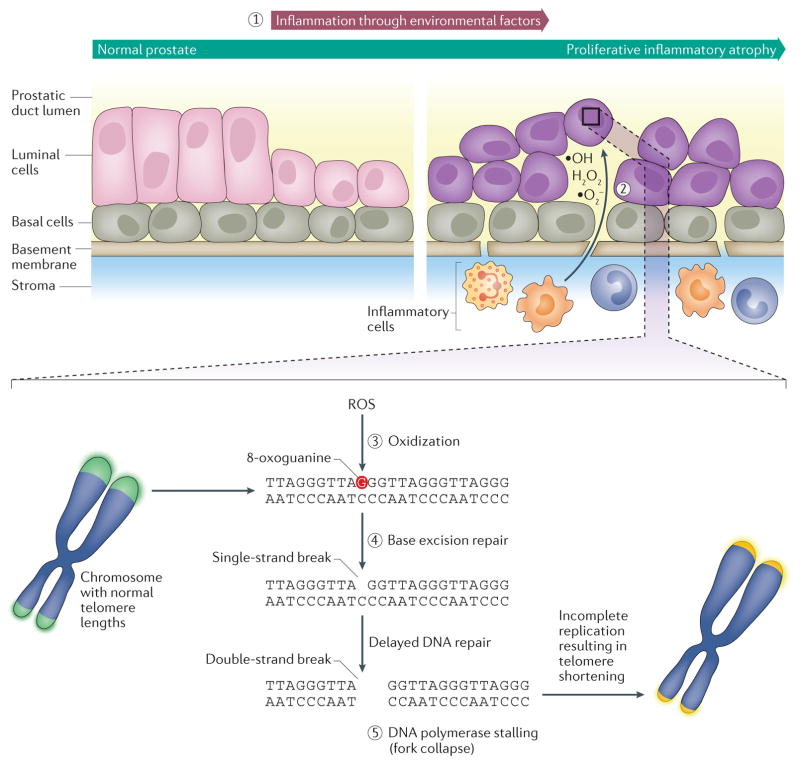
Figure 2. Reactive oxygen species as a cause of telomere shortening in prostate tumorigenesis.
Prostatic inflammation is common, can result in the formation of prostatic inflammatory atrophy (PIA), and has been associated with prostate cancer (1). PIA lesions are characterized by an increased proliferation of epithelial cells and contain activated inflammatory cells (predominately lymphocytes and macrophages), which produce reactive oxygen species (ROS), such as ·O2−, H2O2, and ·OH (2). ROS can cause oxidization of guanine to form 8-oxoguanine (3), and incomplete base excision repair of 8-oxoguanine (4) can trigger DNA polymerase replication stalling and replication fork collapse at telomeres (5). Because DNA damage response is repressed at chromosomal ends, resulting in incomplete telomere replication, telomeres are vulnerable to severe and unmitigated telomere erosion.
Telomere sequences are rich in guanine nucleotides and, consequently, telomeres are particularly susceptible to oxidative damage by ROS. Cells in culture exposed to increasing amounts of radiation have a dose-dependent increase in 8-oxoguanine abundance and telomere loss73. 8-Oxoguanine DNA lesions are recognized and excised by 8-oxoguanine glycosylase and the abasic site is repaired via the base excision repair (BER) pathway74. During BER, a single-strand DNA break is generated following the glycosylase step by AP endonuclease 1. BER operates during all phases of the cell cycle, including in dividing cells75, which is particularly relevant, as single-strand DNA breaks in telomeres seem to accelerate the rate of telomere shortening in proliferating cells76. Telomeres have a higher frequency of single-strand DNA breaks than any other part of the genome, single-strand breaks induced by oxidative damage in telomeres accumulate and repair is substantially delayed77. The reduced repair efficiency in telomeres78 might in part be explained by suppression of DNA repair by the shelterin complex to prevent telomere ends from being recognized as double-strand breaks79. In addition to oxidative damage, the abundance of guanine nucleotides in telomeres also makes them susceptible to the formation of G-quadruplexes, which interfere with replication80. The helicases BLM and RTEL1 resolve G-quadruplexes at telomeres and, in cooperation with the shelterin protein TRF1, help prevent replication fork stalling to ensure efficient replication at telomeres81. However, these processes are not perfect and telomeres are prone to develop DNA strand breaks; thus, they behave as fragile sites in the chromosome81. One hypothesis is that DNA damage, such as that caused by oxidative stress, exacerbates the fragile site phenotype of telomeres and that incomplete replication at telomeric ends caused by replication fork stalling at persistent sites of DNA lesions promotes critical telomere shortening82. In addition to metabolic sources of free radicals, inflammation can locally increase levels of ROS in tissues. ROS are generated by immune cells as part of the immune response against infection by pathogens, acting as signalling molecules, nonspecific antimicrobials, and mediators of inflammation83.
Inflammation is an important component in the initiation and progression of many cancers and accumulating evidence supports a similar role in prostate cancer84–86. The presence of inflammation, mostly chronic, in benign prostate biopsy tissues was found to be associated with prostate cancer, particularly high-grade disease87. Furthermore, use of anti-inflammatory medications, specifically acetylsalicylic acid and other nonsteroidal anti-inflammatory drugs, is associated with reduced risk of developing prostate cancer88. Prostatic inflammation is common86,89–93 and inflammatory triggers include infectious agents (for example, bacteria and viruses), dietary factors, oestrogen exposure, physical trauma from corpora amylacea, and the physical and chemical irritation from urine reflux85,94,95.
The histological sequelae, presumably caused by acute and/or chronic inflammation in the prostate, include prostatic inflammatory atrophy (PIA)85. These morphologically atrophic lesions are characterized by an increased proliferation of epithelial cells (to a mean proliferation rate of 10.7-fold above normal epithelial cells)96–98 and the presence of activated inflammatory cells (predominately lymphocytes and macrophages)96,99. These proliferation rates would lead epithelial cells in PIA lesions to have increased telomere shortening and, therefore, to reach replicative senescence considerably faster than normal prostate cells. Thus, PIA has been suggested to be a precursor to PIN and subsequent prostate cancer85. Intriguingly, PIA lesions tend to histologically merge into HGPIN in the prostate100, supporting the notion that PIA is a precursor to PIN. In addition, the relative frequency of prostatic inflammation is similar to the demographic frequency of prostate cancer in men. For example, American men of African origin are at increased risk of developing prostate cancer101 and have increased rates of prostatic inflammation compared with American men of European decent102. One interesting observation of prostate cancer epidemiological data is that American men with parents who have migrated from Asia have substantially increased rates of prostate cancer compared with Asian men who did not migrate, strongly implicating an environmental component103. Switching from an Asian, possibly anti-inflammatory diet including tea and soy products to a Western, pro-inflammatory diet has been suggested as a contributing factor to the increased prevalence of prostate cancer observed in men of Asian descent104.
Taken together, the link between telomere shortening and prostate cancer might, in part, be explained by connecting telomere shortening to oxidative stress: the generation of ROS owing to inflammation, the preponderance of inflammation in the prostate, and the association between prostatic inflammation and prostate cancer, potentially driven largely by environmental factors, such as microorganisms or diet. As a precursor to PIN and subsequently prostate cancer, an inflammatory environment potentially drives PIA towards malignant transformation.
Interestingly, in addition to PIN and prostate cancer cells, telomere shortening has also been observed in normal stromal cells and is associated with increased prostate cancer risk105. The specific stromal cell types that have short telomeres have yet to be identified, but the telomere shortening in these stromal cells might be a direct result of inflammation.
Inflammation also seems to have a role in the development of BPH106, but BPH does not display substantial telomere shortening in contrast to HGPIN and prostate cancer39,47. Presumably, the telomerase activity in the postulated epithelial progenitor cells and stem-like cells in BPH would not safeguard against genomic insults from ROS. However, glutathione S-transferase P (GST-P), which is expressed in normal prostate and BPH but not in HGPIN or prostate cancer107,108, does have activity against reactive oxidants and electrophiles that could damage DNA109,110. Perhaps, BPH is not susceptible to telomere shortening from hyper-proliferation and oxidative DNA damage because of the maintenance activity of telomerase in postulated epithelial progenitor cells and stem-like cells57 and the detoxification activity of GST-P50, respectively. By contrast, the short telomeres in HGPIN should result in considerable selective pressure for halting telomere loss. During tumorigenesis, critically short telomeres can precipitate many important cancer- promoting mutations, such as telomerase activation, by encouraging global genomic instability.
Genomic alterations due to short telomeres
Telomere shortening is one of the earliest molecular genomic events in prostate tumorigenesis and can generate genomic instability. Genomic translocation events are prevalent in prostate cancer and were traditionally thought to accumulate gradually during tumorigenesis. However, chromothripsis can occur, in which multiple translocation events occur in a single catastrophic event leading to imperfect rearrangement and repair of one or a few shattered chromosomes111–113. Mutations in the genome resulting from chromothripsis can be involved in tumour initiation or progression through the generation of fusion genes, inactivation of tumour suppressors, and amplification of oncogenes114–117. Cell culture experiments with artificially shortened telomeres have demonstrated that critically short telomeres can precipitate chromothripsis118. Approximately 30–45% of prostate cancers show DNA rearrangements resembling the translocation events typified by chromothripsis119.
Chromothripsis notwithstanding, early contributions of telomere shortening to genomic instability are evident in the form of telomere fusions, which have been reported to occur in >50% of assessed prostate cancer precursor lesions derived from radical prostatectomy specimens120. These chromosomal end-to-end fusions of dysfunctional short telomeres can elicit the canonical breakage-fusion-bridge (BFB) cycles, in which missegregation of fused chromosomes during mitosis perpetuates a cycle of fused chromosomes improperly breaking and fusing in an error-prone manner31. BFB cycles result in substantial chromosomal rearrangements, particularly duplications and deletions, and nonreciprocal translocations121. Studies in well-characterized prostate cancer cell lines have shown that short telomeres can drive the complex chromosomal rearrangements characteristic of prostate cancer through BFB cycles, despite telomerase activation122.
Telomerase activation in prostate cancer
Most cancer cells activate the enzyme telomerase for telomere maintenance to support unlimited replicative capacity and to prevent an intolerable level of genomic instability. In human cancers, the rate-limiting determinant for telomerase activity is thought to be expression of the catalytic protein subunit TERT. Telomerase-positive cultured human cells contain ~1,150 TERC molecules and ~500 TERT molecules per cell123. However, estimates of the number of functional, assembled telomerase complexes range from 20 to 240 complexes per cell123,124, suggesting an excess of unassembled components of telomerase123. The transcription of TERT is tightly regulated and experimental evidence indicates that telomerase activity directly corresponds with TERT expression125,126. Furthermore, in somatic cells, forced expression of TERT is sufficient to reactivate telomerase activity127–129.
The androgen receptor (AR) is essential for stimulating the expression of genes important for the male phenotype, including the development, maintenance, and function of the prostate130. The first in vivo study of telomerase activity in the prostate of rats showed that normal prostate glands lacked telomerase activity, but involuted prostate glands, following androgen deprivation via castration, had detectable levels of telomerase activity131. Reintroduction of androgen in castrated rats stimulated regrowth of the prostate and phenocopy of the precastration prostate in which no telomerase was detected, suggesting that androgen negatively regulates telomerase activity131. Similar observations were later made in rhesus monkeys132.
In normal human prostate epithelial cells, the AR binds to the TERT promoter and, in cooperation with the tumour suppressor p53, directly represses TERT expression133. By contrast, androgen activates TERT expression in the human prostate cancer cell line LNCaP, which has a point mutation in the AR gene that is also recurrently observed in prostate cancer133,134. Treatment of prostate cancer lines with methaneseleninic acid, which decreases AR protein levels135, resulted in a concomitant decrease in TERT expression in both AR-wild-type (LAPC-4) and AR-mutated (CWR22Rv1, LNCaP, and LNCaP sublines) cell lines, but not in an AR-negative (DU-145) cell line136. The decreased TERT expression in the AR-positive cell lines was reported to be a consequence of reduced AR occupancy at the TERT promoter.
Cancer cells can activate telomerase activity through upregulation of TERT expression in several ways (TABLE 1). Two well-documented mechanisms are hypermethylation of the TERT promoter, which has been reported in multiple cancer cell lines137, and activating point mutations in the TERT promoter, which have been observed in cancers of the central nervous system, bladder, thyroid, and skin138,139. The methylation status of the TERT promoter in prostate cancer has yet to be fully investigated; however, TERT promoter mutations have not been observed in prostate cancer138,140. Instead, the MYC oncogene has been implicated as a contributor to TERT overexpression in prostate cancer. Gene expression and immunohistochemical studies have shown overexpression of MYC RNA and protein in prostate cancer compared with BPH and normal prostate tissue141–146. Furthermore, TERT overexpression and MYC overexpression in prostate cancer correlate147, supporting previous observations that MYC stimulates expression of TERT148. The TERT promoter contains five GC-boxes, a consensus sequence recognized by the transcription factor SP1, and two E-boxes, a consensus sequence recognized by MYC. The transcription factors MYC and SP1 bind to the TERT promoter and cooperatively activate the expression of TERT149. Depending on the cell type, studies have shown that overexpression of either MYC or SP1 protein is sufficient to activate transcription of TERT149.
Table 1.
Cancer-associated activation of telomerase
| Molecular mechanism | Prostate cancer relevance | Refs |
|---|---|---|
| TERT amplification | Amplification of TERT has not been observed in prostate cancer, but has been reported in embryonal tumours of the central nervous system, cervical carcinoma, lung adenocarcinoma, and hepatocellular carcinoma. | 196–199 |
| TERT translocation | Genomic rearrangements of TERT have not been reported in prostate cancer, but have been tightly linked to high-risk neuroblastoma. | 200,201 |
| TERT promoter hypermethylation | The methylation status of the TERT promoter in prostate cancer has yet to be fully investigated, but promoter methylation has been reported to occur in multiple cancer cell lines and in some cancers. | 137,202 |
| TERT promoter activating mutations | TERT promoter mutations have not been observed in prostate cancer, but have been frequently observed in cancers of the central nervous system, bladder, thyroid, and skin. | 138,139 |
| MYC activation | MYC positively regulates TERT expression and MYC activation coincides with the earliest detectable appearance of abnormally short telomeres during prostate tumorigenesis. >80% of prostate cancers have nuclear overexpression of MYC. Thus, MYC activation is likely to be a mechanism of TERT activation in a high proportion of prostate cancers. | 141,203 |
Increased levels of MYC are one of the earliest somatic molecular alterations observed in prostate cancer precursor lesions. In histologically normal prostatic tissues, MYC expression is localized to the basal compartment. By contrast, in PIA and both low-grade and high-grade PIN, MYC expression abnormally localizes to the luminal compartment141. The precise molecular mechanisms underlying MYC activation in prostate cancer precursor lesions are not well understood. Gain of chromosome 8q, which includes MYC, is common in PIN, primary and metastatic prostate cancer, and specific amplification of the MYC locus is frequently observed in aggressive disease150,151. However, amplification of MYC as the cause for increased MYC protein levels in PIN is controversial152.
Clinical relevance
Telomere dysfunction might be a useful target for improved management of patients with prostate cancer (FIG. 3). Potential applications that are directed at telomeres and telomerase could facilitate disease diagnosis and patient prognosis. In addition, treatments that directly target telomerase or depend on its function, as well as agents that exploit shortened telomeres and disrupted DDR, could have clinical utility in the future.
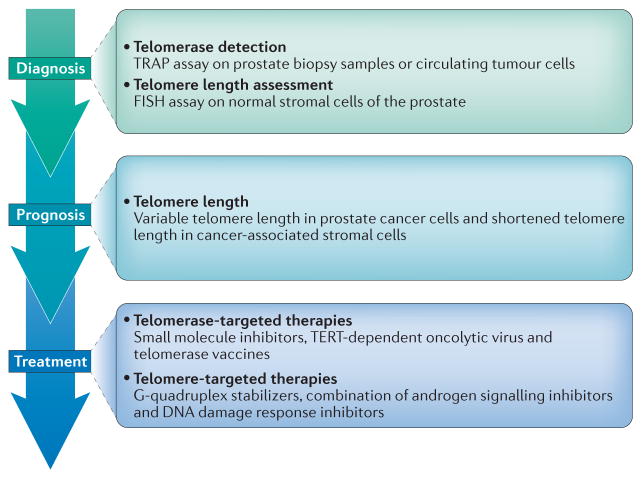
Figure 3. Potential applications directed at telomeres and telomerase in prostate cancer management.
At the diagnostic stage, detection of telomerase expression and measurement of telomere length might be useful, as telomerase activation and shortened telomeres are strongly associated with prostate cancer. At the prognostic level, determining telomere lengths might enable distinguishing men who will develop lethal metastatic disease from men whose disease is unlikely to advance beyond an organ-confined stage. At the therapeutic level, strategies targeting telomerase or telomeres might have clinical utility, particularly in combination with traditional prostate cancer therapies that target the androgen signalling pathway.
Diagnosis and prognosis of prostate cancer
Early detection is paramount to ensure the best outcomes for men diagnosed with prostate cancer, but currently available screening methods can result in overdiagnosis and overtreatment153,154. Detection of telomerase activation could potentially aid in prostate cancer screening, owing to its high cancer specificity. The primary obstacle to telomerase detection is the low abundance of the enzyme. Telomerase-positive human cancer cell lines contain 20–240 functional telomerase complexes per cell123,124. The earliest telomerase activity assays required the use of radiolabeled nucleotides to compensate for the relatively low abundance of the protein155. To improve assay sensitivity, a PCR-amplification-based approach termed the telomere repeat amplification protocol (TRAP) assay was developed and used successfully to detect telomerase in prostate biopsy samples156. In the TRAP assay, telomerase activity is detected by addition of a synthetic telomere end to a cell or tissue lysate. If telomerase is present, the enzyme will recognize and extend the synthetic substrate, and telomerase extension products can be detected by PCR amplification.
Currently, no telomerase assays for cancer detection are clinically validated. However, considerable effort has been made to incorporate technological advances in signal amplification, nanotechnology, and optical detection to improve the reliability and sensitivity of the TRAP assay157. The detection of circulating tumour cells (CTCs) in the blood is a noninvasive procedure that has potential utility in the clinic. Telomerase activity measurements could identify CTCs isolated from the blood of patients with prostate cancer. An ELISA-based TRAP assay was able to identify CTCs in 79% of patients with localized prostate cancer and in 0% of healthy men with no evidence of prostate cancer158. A subsequent study using a different version of the TRAP assay demonstrated sensitive and reliable detection of prostate cancer cells in blood collected on a microfilter platform159. Further studies evaluated the utility of telomerase detection in live CTCs as a prognostic tool for overall survival in patients with advanced castration-resistant prostate cancer. In a subset of patients with a baseline count of ≥5 CTCs per 7.5 ml of blood, increased telomerase activity was associated with worse outcomes160.
Alternatively, telomere length can potentially aid in prostate cancer diagnosis. A study using quantitative telomere-specific fluorescence in situ hybridization (FISH) in biopsy samples from men participating in the placebo arm of the Prostate Cancer Prevention Trial showed that telomere shortening in normal stromal cells was associated with an increased prostate cancer risk161. The findings of these studies need to be validated, but telomere length assessment in the stroma might be particularly useful in men with a negative biopsy but continued suspicion for prostate cancer (for example, owing to persistently elevated PSA levels)161.
Once prostate cancer has been definitively diagnosed, the currently available prognostic tools cannot reliably predict whether a man with organ-confined disease will or will not eventually develop lethal metastatic disease, particularly in patients with intermediate pathological grade. As a result, many men undergoing treatment are thought to have cancers that would not substantially progress within their lifetimes and could, therefore, forego treatment. The potential harm from the overtreatment of prostate cancer is considerable162 and new ways to identify the subset of men who will most probably benefit from aggressive treatment need to be identified. Telomere length measurements in biopsy samples could possibly augment current approaches to better inform clinicians and patients whether active surveillance or definitive treatment is more appropriate. In a prospective study using quantitative telomere-specific FISH to assess telomere length, the combination of more variable telomere length in prostate cancer cells and shorter telomere length in cancer-associated stromal cells correlated with progression to metastasis and disease-specific death, independently of existing clinicopathological indicators105. These findings indicate a translational potential of tissue-based telomere measurements for prognostication that might inform risk stratification for personalized therapeutic and surveillance strategies.
Telomerase-targeted therapies
Approximately 90% of cancers activate telomerase for telomere maintenance and to achieve unlimited replicative capacity35,163. Telomerase is an attractive target for anticancer therapy for two reasons. Firstly, unless cells acquire the ability to maintain telomeres in a telomerase-independent fashion, the lack of telomerase will impair the cancer cell’s ability for unlimited replicative capacity. Secondly, telomerase activation distinguishes normal somatic cells from cancer cells, and normal somatic cells, which lack telomerase, will not be affected by telomerase-targeted therapies.
However, targeting telomerase is not without caveats. Germ cells and some stem or progenitor cells in highly proliferative tissues rely on telomerase to maintain their telomeres; thus, their telomere lengths would be potentially affected by telomerase inhibition causing adverse effects164. However, such possible off-target effects were predicted to be minimal, partly owing to the large differential in telomere lengths in cancer versus normal cells. Preclinical studies showed favourable tolerance to telomerase-active agents. As a result, telomerase-directed agents, such as imetel-stat and GV1001, have advanced to clinical trials. Disappointingly, these treatments have shown no survival benefit, but investigation of their clinical potential has not been entirely abandoned165 (TABLE 2).
Table 2.
Therapies directed at telomerase or telomeres
| Mechanism of action | Example | Prostate cancer relevance | Refs |
|---|---|---|---|
| Telomerase inhibition with small molecules | Imetelstat is a lipid-conjugated 13-mer oligonucleotide that functions as a small molecular telomerase inhibitor by binding to the RNA template, TERC, and disrupts telomerase activity. | Clinical trials in breast and lung cancers have not been successful. No clinical trials of telomerase small molecule inhibitors in prostate cancer exist, but preclinical studies of prostate cancer cell lines show that imetelstat causes telomere shortening in tumour-initiating cells. | 167,169 |
| Telomerase vaccine | GV1001 is a 16-mer peptide vaccine containing a TERT amino acid sequence used to elicit immune responses against cells with telomerase activity. | No studies have investigated the use of GV1001 in prostate cancer to date. In pancreatic cancer, clinical trials of the vaccine have shown the agent to be immunogenic and well tolerated in patients; however, GV1001 was not efficacious as a single agent or in combination therapies. | 173,204 |
| TERT-regulated oncolytic virus | Telomelysin is a replication-selective adenovirus engineered to express the essential viral E1 replication genes under the control of the TERT promoter. | Telomelysin was effective in a LNCaP tumour model in nude mice. Completed phase I trials of telomelysin in various solid tumours (not including prostate cancer) have indicated no severe adverse effects following administration, but patient tumour response was limited. | 172,205 |
| Telomerase inhibition through AR down regulation | AR inhibitor bicalutamide and methaneseleninic acid downregulate AR protein level | AR inhibition using methaneseleninic acid in combination with bicalutamide decreased TERT expression and increased apoptosis in prostate cancer cells. | 136 |
| Telomere deprotection | G-Quadruplex stabilizers and telomestatin | No G-quadruplex stabilizers that specifically target telomeres have advanced to clinical trials. Quarfloxin, which has advanced to clinical trials, does not interact with telomeres, but with ribosomal DNA G-quadruplexes in the nucleolus. Telomestatin disrupts the interaction of shelterin protein TRF2 with telomeres in glioma stem cells, but the effects in prostate cancer have not been investigated. Prostate cancer mouse models responded to treatment with TMPyP4 and RHPS4. | 206–210 |
| Telomere dysfunction through AR inhibition | AR inhibitor (for example, bicalutamide or enzalutamide) and ATM inhibitor (KU-60019) | In prostate cancer cell culture studies, AR inhibition resulted in telomere dysfunction. ATM inhibition blocked cell cycle checkpoint arrest and prevented the repair of damaged telomeres caused by AR inhibition, promoting cell death. | 193 |
AR, androgen receptor; TERC, telomerase RNA component; TERT, telomerase reverse transcriptase.
Imetelstat is a lipid-conjugated 13-mer oligonucleotide that functions as a small molecular inhibitor by binding to the RNA template TERC to disrupt telomerase activity. Several, both completed and ongoing, clinical trials have evaluated imetelstat166. In patients with non-small-cell lung cancer, imetelstat did not improve progression-free survival; however, tumours with the shortest telomeres responded to treatment better than tumours with longer telomeres, providing support for target-specific efficacy167. One notable observation made in a failed phase II trial of imetelstat was a decrease in platelet levels in patients with breast and lung cancer following treatment with this agent165. In a small study, imetelstat was found to be particularly efficacious in patients with thrombocythaemia, a hyperproliferative blood disorder characterized by overproduction of platelets by megakaryocytes168. Notably, the mechanism of action of imetelstat in patients with thrombocythaemia is unclear, as telomere length was not associated with clinical response168.
The failure to establish telomerase-targeted treatments for cancer therapy might partly be explained by the fact that telomerase inhibition does not immediately kill cancer cells and is only efficacious when telomeres become critically short. Consequently, the benefits of telomerase inhibition depend on treatment duration, proliferation rate, and initial telomere lengths169. Thus, telomerase inhibition might be more effective in highly proliferative cancers or cancers with exceptionally short telomeres. Although prostate cancer is typically a relatively slow growing cancer, the telomeres are mostly substantially shortened. Thus, telomerase inhibition might have clinical utility. Studies in prostate cancer cell lines show that imetelstat causes considerable telomere shortening in the key subset of tumour-initiating cells170.
Oncolytic virus strategies targeting telomerase- expressing cells are also currently being investigated in clinical trials. Telomelysin is a replication-selective adenovirus in which expression of the viral E1 genes, which are essential for viral replication, are under the control of the TERT promoter. The oncolytic virus replicates selectively in cancer cells that express telomerase and not in normal cells lacking telomerase, resulting in selective killing of cancer cells171. Completed phase I trials of telomelysin have indicated no severe adverse effects following administration; however, tumour responses in patients have been limited to date172.
As telomerase-targeted therapies might not be effective as single agents, clinical trials are underway that investigate combination approaches. One study investigating the combination of the telomerase peptide vaccine GV1001 with gemcitabine and capecitabine did not show enhanced efficacy in patients with pancreatic cancer173. GV1001 is a 16-mer peptide containing a TERT amino acid sequence. In this study, the peptide was combined with granulocyte–macrophage colony- stimulating factor as an adjuvant173. Combination of GV1001 with gemcitabine and capecitabine treatment, which is the standard of care for patients with pancreatic cancer, was thought to potentially enhance the immune response generated by GV1001 vaccination.
The most synergistic combination therapy and optimal delivery method for telomerase inhibition might still have to be identified165. In prostate cancer, a potentially efficacious combination therapy inhibits both telomerase activity and AR activity. Wild-type AR in conjunction with p53 represses TERT expression in the prostate133. However, in prostate cancer, AR signalling acts in an opposite manner and upregulates the expression of TERT133,135. Conceivably, this reversal of AR-mediated TERT repression is a consequence of abrogated p53 and AR function. Metastatic castration-resistant prostate cancer is considerably enriched in TP53 and AR alterations compared with organ-confined tumours174, and increased AR function in the advanced disease setting potentially upregulates telomerase activity. Traditionally, telomerase-targeted treatments have largely focused on the telomere maintenance functions of telomerase; however, compelling evidence suggests additional contributions of TERT in cancer-relevant phenotypes independent of telomere maintenance175, including the promotion of cell proliferation176–179, drug resistance180, and epithelial–mesenchymal transition181. In the clinic, prostate cancer responds to antiandrogen treatments, albeit temporarily182. Targeting two essential pathways, AR signalling and telomerase, in prostate cancer simultaneously might be a promising strategy to kill tumour cells early and prevent the progression to metastatic disease. The potential synergy of combining AR-targeted and telomerase-targeted therapies warrants preclinical studies.
Therapies directed at shortened telomeres
Therapeutic approaches directed at telomeres have not advanced as far as those targeting telomerase, but warrant further investigation (TABLE 2). A limitation of telomerase-targeted therapies is the potential for cancers to develop resistance via telomerase-independent mechanisms, such as ALT, to maintain telomeres. Only ~10% of all cancers show de novo ALT mechanisms35,163 and no primary prostate cancers exhibit ALT. However, under selective pressure through, for example, telomerase inhibition, telomerase-positive cancers can adopt a telomerase-independent programme of telomere maintenance183.
When chromosomal ends are not properly protected, numerous proteins associated with the DDR are recruited to the site of telomere dysfunction20. In the DDR pathway, sites of DNA lesions, thought to be double-strand breaks, accumulate proteins and modifications to signal for repair. Histone H2AX is phosphorylated at Ser139 (γH2AX) by the kinases ATM, ATR or DNA-PK at the site of the DNA lesion. DNA damage signalling markers such as 53BP1, MDC1, and phosphorylated ATM aggregate at sites of γH2AX to form canonical DNA damage foci184. Cancers that employ ALT mechanisms to maintain their telomeres are characterized by a higher level of telomeric DDR compared with telomerase-positive cells185,186. One study showed that a small molecule inhibitor of ATR (VE-821) was effective against ALT-positive osteosarcoma and glioma cells187. These findings are encouraging for therapy of ALT-positive cancers, but focusing therapeutic efforts on telomeres might require a more comprehensive approach, as targeting telomeres might be efficacious in cancer regardless of telomerase dependency. To develop treatments with maximum therapeutic index, identifying exploitable differences between telomeres in tumours and those in normal cells will be a crucial objective.
Studies in a derivative of the human IMR-90 fibroblast cell line that expresses HPV16 E6 and E7 proteins, which inhibit tumour suppressors p53 and retinoblastoma protein, demonstrated that telomere fusion between chromosomes causes mitotic arrest188. Telomere deprotection that arose as a result of prolonged mitotic arrest acted as a molecular signal to trigger cell death. Thus, telomere deprotection might sensitize cancer cells to antimitotic drugs189. Intriguingly, a sub-population of prostate tumour cells with elevated telomerase activity and TERT expression were sensitive to strategies inducing apoptosis through telomere deprotection190. Expression of mutated TERC in these cells reprogrammed telomerase to extend telomeres with an incorrect sequence, effectively inducing telomere deprotection. Therapeutic strategies that promote telomere deprotection, such as G-quadruplex stabilizers191,192, might have clinical utility in prostate cancer, particularly in the setting of metastatic castration-resistant disease, in which p53 inactivation is common174.
Disruption of AR function in AR-positive prostate cancer cells results in telomere dysfunction, and activates the DDR proteins ATM and checkpoint kinase 2 (REF. 193). Mutational studies of the shelterin protein TIN2 showed that deletions in the C-terminal or N-terminal regions of TIN2 triggered cell death in the AR-negative and p53-deficient PPC-1 prostate cancer cell line but not in the AR-positive and p53-positive LNCaP prostate cancer cell line, suggesting that the telomere complex might be different between AR-negative and AR-positive prostate cancer194. Consistent with this notion, treating AR-positive LNCaP cells with the AR antagonists bicalutamide or enzalutamide resulted in γH2AX enrichment and recruitment of 53BP1 at telomeres. However, telomere dysfunction was not observed following bicalutamide treatment in the AR-negative PC-3 cell line188. Furthermore, AR inactivation by knockdown, androgen deprivation, or treatment with bicalutamide in LNCaP cells induced telomere breaks and sister chromatid telomere fusion195. Blocking the repair of these telomere DNA lesions with an ATM inhibitor enhanced cell killing by bicalutamide in both LNCaP (androgen-responsive) and CWR 22Rv1 cells (androgen-insensitive). In this setting, ATM inhibition blocked cell cycle checkpoint arrest, preventing the repair of damaged telomeres caused by AR inhibition, and as a result promoted cell death193. These studies suggest that combination treatments including an ATM inhibitor that potentiates the effects of existing androgen deprivation therapies are effective via telomere-directed mechanisms and might have clinical utility in both androgen-sensitive and androgen-insensitive prostate cancer.
Conclusions
Telomeres and telomerase seem to be integral to the initiation and progression of prostate cancer and, therefore, might also be relevant in the diagnosis, prognosis, and treatment of the disease (FIG. 3). At the earliest stages of prostate tumorigenesis, telomere shortening can be observed in the precursor lesion HGPIN. The exact causes of telomere shortening in the prostate are not yet well defined, but evidence suggests an inflammatory contribution that increases local ROS production, which accelerates telomere shortening in addition to the losses caused by cell proliferation.
Critically shortened telomeres compromise genomic integrity and can drive somatic copy number alterations, aneuploidy, and DNA rearrangements that are typically observed in the genomic landscape of prostate cancer. The ensuing genomic instability contributes to the somatic molecular alterations to sustain the unlimited replicative capacity in cancer. Essential to this process is the activation of telomerase or the adoption of telomerase-independent mechanisms to maintain telomeres. For the majority of prostate cancers, MYC is believed to be the main driver for telomerase activation.
The combination of short telomeres and telomerase activation in prostate cancer make telomerase an attractive therapeutic target. However, in clinical trials, telomerase-targeted therapies for other solid cancers have mostly proven to be ineffective as single agents. Clinical studies have not fully investigated the use of these treatments in prostate cancer, but the outcomes of single-agent treatment will probably be similarly disappointing. The utility of telomerase-targeted therapies in prostate cancer possibly exists in combination with other established treatment regimens, such as AR inhibitors or radiotherapy. In addition, a subset of lethal metastatic prostate cancer has been shown to employ the telomerase-independent telomere maintenance programme ALT for telomere maintenance. The potential for prostate cancer to employ ALT is particularly salient, as this mechanism is a way for advanced prostate cancer to circumvent telomerase-directed therapies and continue to maintain and extend telomeres. Thus, inhibiting telomerase activity might not be sufficient to treat advanced prostate cancer, as ALT mechanisms might become engaged to maintain telomeres and support continued cell division. New findings that connect telomere dysfunction and AR inhibition in prostate cancer cells are provocative, and re-emphasize the importance of the telomeres as a potential therapeutic target in prostate cancer. Telomere-directed therapies in combination with AR inhibition, perhaps also including targeting of the DDR, might have the potential to be effective in both telomerase-expressing and ALT-positive prostate cancers.
Key points.
Telomerase activation or the cancer-specific, telomerase-independent alternative lengthening of telomeres (ALT) mechanism are two telomere maintenance mechanisms in human cells. Most prostate cancers activate telomerase and a subset of lethal metastases use ALT
Substantial telomere shortening is common in prostate cancers and in the precursor lesion prostatic intraepithelial neoplasia (PIN). Moderate telomere shortening has also been observed in cancer-associated stroma
The mechanisms for telomere shortening in prostate cancer and PIN are not fully understood; in addition to replication-associated telomere loss, inflammation and reactive oxygen species might be contributors
Telomere length assessment might be useful in prostate cancer diagnosis and in current prognostic tools to more reliably predict whether organ-confined prostate cancer will progress to lethal metastatic disease
Telomerase-targeted single-agent treatments for solid cancers have, to date, been ineffective in clinical trials; these therapies have yet to be tested in prostate cancer and might potentially be useful in combination with established androgen receptor (AR)-targeted treatments
Disruption of AR function in AR-positive prostate cancer cells activates the DNA damage response (DDR) at telomeres; thus, DDR inhibitors might potentiate the effects of androgen deprivation therapy
Acknowledgments
The authors wish to thank Dr Christopher Michael Heaphy and Dr Karen Sandell Sfanos for critical reading of the manuscript. The authors’ research work was supported by NIH research grants R01CA172380 to A. M., NIH Training in Areas Fundamental to Cancer Research 5T32CA009110-38 to M. K. G., and the Prostate Cancer Foundation Young Investigators Award (granted to C. M. Heaphy and supported M. K. G.).
Glossary
- T-Loop
A structure stabilized by shelterin proteins at the end of telomeres, where the telomere double-stranded DNA loops onto itself to form a partial overlap between the 3′ G-rich telomere overhang and the complementary C-rich telomere strand upstream of the overhang.
- End replication problem
During DNA replication, synthesis on the lagging DNA strand of linear templates is incomplete, resulting in the loss of ~50 terminal nucleotides in each round of cellular division.
- Replicative senescence
In normal cells, cessation of cell division owing to substantial telomere shortening following ~50 cell divisions (Hayflick limit).
- BPH
Noncancerous enlargement of the prostate owing to hyperproliferation of epithelial and/or stromal cells in the prostate.
- Prostatic intraepithelial neoplasia (PIN)
A noncancerous lesion in the prostate with abnormal acinar architecture, observed as overcrowding of luminal cells with enlarged nuclei.
- High-grade PIN (HGPIN)
Considered a precursor lesion of prostate cancer, featuring cancer-like morphological abnormalities (for example, nuclear pleomorphism and prominent nucleoli), but no evidence of invasion.
- Reactive oxygen species (ROS)
Highly reactive, oxygen-containing free radicals that can damage cellular RNA, DNA, and proteins.
- 8-Oxoguanine
The best-characterized and highly abundant DNA lesion arising from the oxidation of guanine through reactive oxygen species.
- Base excision repair (BER)
The DNA repair pathway that employs specialized DNA glycosylases, N-glycosylase/DNA lyase and adenine DNA glycosylase, to repair 8-oxoguanine.
- G-Quadruplexes
Nucleic acid secondary structures arising from Hoogsteen base pairing (an alternative form of base pairing) interactions of guanine residues.
- Fragile sites
Unstable regions in the genome that are prone to break under replication stress.
- Prostatic inflammatory atrophy
Prostatic lesions characterized by increased proliferation and atrophic morphology of prostatic luminal epithelial cells, associated with local inflammatory cells.
- Chromothripsis
Multiple translocation events occurring in a single catastrophic event leading to imperfect rearrangement and repair of one or a few shattered chromosomes.
- Overdiagnosis and overtreatment
Diagnosing patients with a disease that will not give rise to symptoms or cause death, often leading to treatment that might have no benefit and might even be harmful to the patient.
- Fluorescence in situ hybridization (FISH)
A technique using fluorophore-conjugated oligonucleotide probes that bind to specific DNA sequences via complementary Watson–Crick base pairing, enabling detection of sequences of interest in intact cells or chromosomes by fluorescence microscopy.
- Prostate Cancer Prevention Trial
A study conducted from 1994–2003 to investigate if the 5α-reductase inhibitor finasteride reduces prostate cancer development in men ≥55 years of age.
- Peptide vaccine
A peptide conjugated with a vaccine adjuvant to stimulate an immune response against a target antigen that shares the same amino acid sequence of the peptide.
- Epithelial–mesenchymal transition
The biological process in which epithelial cells acquire characteristics more consistent with mesenchymal cells, including loss of cell polarity and adhesion, and enhanced migration and invasiveness.
- Telomere deprotection
Telomeres partially or completely unprotected by shelterin proteins, resulting in the activation of DDR.
Footnotes
Author contributions
Both authors researched data for the article and made substantial contributions to discussion of its content. Both authors wrote and reviewed and/or edited the manuscript before submission.
Competing interests statement
The authors declare no competing interests.
References
- 1.Murray AW, Szostak JW. Construction of artificial chromosomes in yeast. Nature. 1983;305:189–193. doi: 10.1038/305189a0. [DOI] [PubMed] [Google Scholar]
- 2.Hsiao CL, Carbon J. High-frequency transformation of yeast by plasmids containing the cloned yeast ARG4 gene. Proc Natl Acad Sci USA. 1979;76:3829–3833. doi: 10.1073/pnas.76.8.3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stinchcomb DT, Struhl K, Davis RW. Isolation and characterisation of a yeast chromosomal replicator. Nature. 1979;282:39–43. doi: 10.1038/282039a0. [DOI] [PubMed] [Google Scholar]
- 4.Clarke L, Carbon J. Isolation of a yeast centromere and construction of functional small circular chromosomes. Nature. 1980;287:504–509. doi: 10.1038/287504a0. [DOI] [PubMed] [Google Scholar]
- 5.Stinchcomb DT, Mann C, Davis RW. Centromeric DNA from Saccharomyces cerevisiae. J Mol Biol. 1982;158:157–190. doi: 10.1016/0022-2836(82)90427-2. [DOI] [PubMed] [Google Scholar]
- 6.Szostak JW, Blackburn EH. Cloning yeast telomeres on linear plasmid vectors. Cell. 1982;29:245–255. doi: 10.1016/0092-8674(82)90109-x. [DOI] [PubMed] [Google Scholar]
- 7.Blackburn EH, Challoner PB. Identification of a telomeric DNA sequence in Trypanosoma brucei. Cell. 1984;36:447–457. doi: 10.1016/0092-8674(84)90238-1. [DOI] [PubMed] [Google Scholar]
- 8.Moyzis RK, et al. A highly conserved repetitive DNA sequence, (TTAGGG)n, present at the telomeres of human chromosomes. Proc Natl Acad Sci USA. 1988;85:6622–6626. doi: 10.1073/pnas.85.18.6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Samassekou O, Gadji M, Drouin R, Yan J. Sizing the ends: normal length of human telomeres. Ann Anat. 2010;192:284–291. doi: 10.1016/j.aanat.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 10.de Lange T. Shelterin: the protein complex that shapes and safeguards human telomeres. Genes Dev. 2005;19:2100–2110. doi: 10.1101/gad.1346005. [DOI] [PubMed] [Google Scholar]
- 11.Zhong Z, Shiue L, Kaplan S, de Lange T. A mammalian factor that binds telomeric TTAGGG repeats in vitro. Mol Cell Biol. 1992;12:4834–4843. doi: 10.1128/mcb.12.11.4834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chong L, et al. A human telomeric protein. Science. 1995;270:1663–1667. doi: 10.1126/science.270.5242.1663. [DOI] [PubMed] [Google Scholar]
- 13.Bilaud T, et al. Telomeric localization of TRF2, a novel human telobox protein. Nat Genet. 1997;17:236–239. doi: 10.1038/ng1097-236. [DOI] [PubMed] [Google Scholar]
- 14.Broccoli D, Smogorzewska A, Chong L, de Lange T. Human telomeres contain two distinct Myb-related proteins, TRF1 and TRF2. Nat Genet. 1997;17:231–235. doi: 10.1038/ng1097-231. [DOI] [PubMed] [Google Scholar]
- 15.Kim SH, Kaminker P, Campisi J. TIN2, a new regulator of telomere length in human cells. Nat Genet. 1999;23:405–412. doi: 10.1038/70508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li B, Oestreich S, de Lange T. Identification of human Rap1: implications for telomere evolution. Cell. 2000;101:471–483. doi: 10.1016/s0092-8674(00)80858-2. [DOI] [PubMed] [Google Scholar]
- 17.Houghtaling BR, Cuttonaro L, Chang W, Smith S. A dynamic molecular link between the telomere length regulator TRF1 and the chromosome end protector TRF2. Curr Biol. 2004;14:1621–1631. doi: 10.1016/j.cub.2004.08.052. [DOI] [PubMed] [Google Scholar]
- 18.Liu D, et al. PTOP interacts with POT1 and regulates its localization to telomeres. Nat Cell Biol. 2004;6:673–680. doi: 10.1038/ncb1142. [DOI] [PubMed] [Google Scholar]
- 19.Ye JZ, et al. POT1-interacting protein PIP1: a telomere length regulator that recruits POT1 to the TIN2/TRF1 complex. Genes Dev. 2004;18:1649–1654. doi: 10.1101/gad.1215404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones M, et al. The shelterin complex and hematopoiesis. J Clin Invest. 2016;126:1621–1629. doi: 10.1172/JCI84547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Griffith JD, et al. Mammalian telomeres end in a large duplex loop. Cell. 1999;97:503–514. doi: 10.1016/s0092-8674(00)80760-6. [DOI] [PubMed] [Google Scholar]
- 22.Hayflick L. The limited in vitro lifetime of human diploid cell strains. Exp Cell Res. 1965;37:614–636. doi: 10.1016/0014-4827(65)90211-9. [DOI] [PubMed] [Google Scholar]
- 23.Hayflick L, Moorhead PS. The serial cultivation of human diploid cell strains. Exp Cell Res. 1961;25:585–621. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- 24.Watson JD. Origin of concatemeric T7 DNA. Nat New Biol. 1972;239:197–201. doi: 10.1038/newbio239197a0. [DOI] [PubMed] [Google Scholar]
- 25.Olovnikov AM. A theory of marginotomy. The incomplete copying of template margin in enzymic synthesis of polynucleotides and biological significance of the phenomenon. J Theor Biol. 1973;41:181–190. doi: 10.1016/0022-5193(73)90198-7. [DOI] [PubMed] [Google Scholar]
- 26.Levy MZ, Allsopp RC, Futcher AB, Greider CW, Harley CB. Telomere end-replication problem and cell aging. J Mol Biol. 1992;225:951–960. doi: 10.1016/0022-2836(92)90096-3. [DOI] [PubMed] [Google Scholar]
- 27.Fumagalli M, et al. Telomeric DNA damage is irreparable and causes persistent DNA-damage-response activation. Nat Cell Biol. 2012;14:355–365. doi: 10.1038/ncb2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.von Zglinicki T, Saretzki G, Ladhoff J, d’Adda di Fagagna F, Jackson SP. Human cell senescence as a DNA damage response. Mech Ageing Dev. 2005;126:111–117. doi: 10.1016/j.mad.2004.09.034. [DOI] [PubMed] [Google Scholar]
- 29.Zou Y, Sfeir A, Gryaznov SM, Shay JW, Wright WE. Does a sentinel or a subset of short telomeres determine replicative senescence? Mol Biol Cell. 2004;15:3709–3718. doi: 10.1091/mbc.E04-03-0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blasco MA, et al. Telomere shortening and tumor formation by mouse cells lacking telomerase RNA. Cell. 1997;91:25–34. doi: 10.1016/s0092-8674(01)80006-4. [DOI] [PubMed] [Google Scholar]
- 31.McClintock B. The stability of broken ends of chromosomes in Zea Mays. Genetics. 1941;26:234–282. doi: 10.1093/genetics/26.2.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Counter CM, et al. Telomere shortening associated with chromosome instability is arrested in immortal cells which express telomerase activity. EMBO J. 1992;11:1921–1929. doi: 10.1002/j.1460-2075.1992.tb05245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chin L, et al. p53 deficiency rescues the adverse effects of telomere loss and cooperates with telomere dysfunction to accelerate carcinogenesis. Cell. 1999;97:527–538. doi: 10.1016/s0092-8674(00)80762-x. [DOI] [PubMed] [Google Scholar]
- 34.Hackett JA, Feldser DM, Greider CW. Telomere dysfunction increases mutation rate and genomic instability. Cell. 2001;106:275–286. doi: 10.1016/s0092-8674(01)00457-3. [DOI] [PubMed] [Google Scholar]
- 35.Shay JW, Bacchetti S. A survey of telomerase activity in human cancer. Eur J Cancer. 1997;33:787–791. doi: 10.1016/S0959-8049(97)00062-2. [DOI] [PubMed] [Google Scholar]
- 36.Dunham MA, Neumann AA, Fasching CL, Reddel RR. Telomere maintenance by recombination in human cells. Nat Genet. 2000;26:447–450. doi: 10.1038/82586. [DOI] [PubMed] [Google Scholar]
- 37.Henson JD, Neumann AA, Yeager TR, Reddel RR. Alternative lengthening of telomeres in mammalian cells. Oncogene. 2002;21:598–610. doi: 10.1038/sj.onc.1205058. [DOI] [PubMed] [Google Scholar]
- 38.Haffner MC, et al. Tracking the clonal origin of lethal prostate cancer. J Clin Invest. 2013;123:4918–4922. doi: 10.1172/JCI70354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sommerfeld HJ, et al. Telomerase activity: a prevalent marker of malignant human prostate tissue. Cancer Res. 1996;56:218–222. [PubMed] [Google Scholar]
- 40.Greider CW, Blackburn EH. The telomere terminal transferase of Tetrahymena is a ribonucleoprotein enzyme with two kinds of primer specificity. Cell. 1987;51:887–898. doi: 10.1016/0092-8674(87)90576-9. [DOI] [PubMed] [Google Scholar]
- 41.Podlevsky JD, Bley CJ, Omana RV, Qi X, Chen JJ. The telomerase database. Nucleic Acids Res. 2008;36:D339–343. doi: 10.1093/nar/gkm700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Greider CW, Blackburn EH. A telomeric sequence in the RNA of Tetrahymena telomerase required for telomere repeat synthesis. Nature. 1989;337:331–337. doi: 10.1038/337331a0. [DOI] [PubMed] [Google Scholar]
- 43.Shippen-Lentz D, Blackburn EH. Functional evidence for an RNA template in telomerase. Science. 1990;247:546–552. doi: 10.1126/science.1689074. [DOI] [PubMed] [Google Scholar]
- 44.Batista LF. Telomere biology in stem cells and reprogramming. Prog Mol Biol Transl Sci. 2014;125:67–88. doi: 10.1016/B978-0-12-397898-1.00003-7. [DOI] [PubMed] [Google Scholar]
- 45.Allshire RC, Dempster M, Hastie ND. Human telomeres contain at least three types of G-rich repeat distributed non-randomly. Nucleic Acids Res. 1989;17:4611–4627. doi: 10.1093/nar/17.12.4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Berges RR, et al. Implication of cell kinetic changes during the progression of human prostatic cancer. Clin Cancer Res. 1995;1:473–480. [PMC free article] [PubMed] [Google Scholar]
- 47.Meeker AK, et al. Telomere shortening is an early somatic DNA alteration in human prostate tumorigenesis. Cancer Res. 2002;62:6405–6409. [PubMed] [Google Scholar]
- 48.Kyprianou N, Tu H, Jacobs SC. Apoptotic versus proliferative activities in human benign prostatic hyperplasia. Hum Pathol. 1996;27:668–675. doi: 10.1016/s0046-8177(96)90396-2. [DOI] [PubMed] [Google Scholar]
- 49.Helpap B. Cell kinetic studies on prostatic intraepithelial neoplasia (PIN) and atypical adenomatous hyperplasia (AAH) of the prostate. Pathol Res Pract. 1995;191:904–907. doi: 10.1016/S0344-0338(11)80975-1. [DOI] [PubMed] [Google Scholar]
- 50.De Marzo AM, Nelson WG, Meeker AK, Coffey DS. Stem cell features of benign and malignant prostate epithelial cells. J Urol. 1998;160:2381–2392. doi: 10.1097/00005392-199812020-00004. [DOI] [PubMed] [Google Scholar]
- 51.Heatfield BM, Sanefuji H, Trump BF. Studies on carcinogenesis of human prostate. III Long-term explant culture of normal prostate and benign prostatic hyperplasia: transmission and scanning electron microscopy. J Natl Cancer Inst. 1982;69:757–766. [PubMed] [Google Scholar]
- 52.Merchant DJ, Clarke SM, Ives K, Harris S. Primary explant culture: an in vitro model of the human prostate. Prostate. 1983;4:523–542. doi: 10.1002/pros.2990040511. [DOI] [PubMed] [Google Scholar]
- 53.Bonkhoff H, Stein U, Remberger K. The proliferative function of basal cells in the normal and hyperplastic human prostate. Prostate. 1994;24:114–118. doi: 10.1002/pros.2990240303. [DOI] [PubMed] [Google Scholar]
- 54.Bostwick DG, Brawer MK. Prostatic intra-epithelial neoplasia and early invasion in prostate cancer. Cancer. 1987;59:788–794. doi: 10.1002/1097-0142(19870215)59:4<788::aid-cncr2820590421>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 55.Rohr HP, Bartsch G. Human benign prostatic hyperplasia: a stromal disease? New perspectives by quantitative morphology. Urology. 1980;16:625–633. doi: 10.1016/0090-4295(80)90577-4. [DOI] [PubMed] [Google Scholar]
- 56.McNeal JE, Haillot O, Yemoto C. Cell proliferation in dysplasia of the prostate: analysis by PCNA immunostaining. Prostate. 1995;27:258–268. doi: 10.1002/pros.2990270505. [DOI] [PubMed] [Google Scholar]
- 57.Rane JK, et al. Telomerase activity and telomere length in human benign prostatic hyperplasia stem-like cells and their progeny implies the existence of distinct basal and luminal cell lineages. Eur Urol. 2016;69:551–554. doi: 10.1016/j.eururo.2015.09.039. [DOI] [PubMed] [Google Scholar]
- 58.Bostwick DG. Prostatic intraepithelial neoplasia (PIN): current concepts. J Cell Biochem Suppl. 1992;16H:10–19. doi: 10.1002/jcb.240501205. [DOI] [PubMed] [Google Scholar]
- 59.Mostofi FK, Sesterhenn IA, Davis CJ., Jr Prostatic intraepithelial neoplasia (PIN): morphological clinical significance. Prostate Suppl. 1992;4:71–77. doi: 10.1002/pros.2990210511. [DOI] [PubMed] [Google Scholar]
- 60.Koeneman KS, et al. Telomerase activity, telomere length, and DNA ploidy in prostatic intraepithelial neoplasia (PIN) J Urol. 1998;160:1533–1539. [PubMed] [Google Scholar]
- 61.Zhang W, Kapusta LR, Slingerland JM, Klotz LH. Telomerase activity in prostate cancer, prostatic intraepithelial neoplasia, and benign prostatic epithelium. Cancer Res. 1998;58:619–621. [PubMed] [Google Scholar]
- 62.National Cancer Institute (2016).
- 63.Thompson SJ, et al. P53 and Ki-67 immunoreactivity in human prostate cancer and benign hyperplasia. Br J Urol. 1992;69:609–613. doi: 10.1111/j.1464-410x.1992.tb15632.x. [DOI] [PubMed] [Google Scholar]
- 64.Shay JW, Wright WE. Hayflick, his limit, and cellular ageing. Nat Rev Mol Cell Biol. 2000;1:72–76. doi: 10.1038/35036093. [DOI] [PubMed] [Google Scholar]
- 65.Capper R, et al. The nature of telomere fusion and a definition of the critical telomere length in human cells. Genes Dev. 2007;21:2495–2508. doi: 10.1101/gad.439107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Muller F. The nature and mechanism of superoxide production by the electron transport chain: its relevance to aging. J Am Aging Assoc. 2000;23:227–253. doi: 10.1007/s11357-000-0022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kryston TB, Georgiev AB, Pissis P, Georgakilas AG. Role of oxidative stress and DNA damage in human carcinogenesis. Mutat Res. 2011;711:193–201. doi: 10.1016/j.mrfmmm.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 68.Halliwell B. Superoxide-dependent formation of hydroxyl radicals in the presence of iron salts. Its role in degradation of hyaluronic acid by a superoxide-generating system. FEBS Lett. 1978;96:238–242. doi: 10.1016/0014-5793(78)80409-8. [DOI] [PubMed] [Google Scholar]
- 69.Liochev SI, Fridovich I. The role of O2. - in the production of HO.: in vitro and in vivo. Free Radic Biol Med. 1994;16:29–33. doi: 10.1016/0891-5849(94)90239-9. [DOI] [PubMed] [Google Scholar]
- 70.McCord JM, Day ED., Jr Superoxide-dependent production of hydroxyl radical catalyzed by iron-EDTA complex. FEBS Lett. 1978;86:139–142. doi: 10.1016/0014-5793(78)80116-1. [DOI] [PubMed] [Google Scholar]
- 71.Burrows CJ, Muller JG. Oxidative nucleobase modifications leading to strand scission. Chem Rev. 1998;98:1109–1152. doi: 10.1021/cr960421s. [DOI] [PubMed] [Google Scholar]
- 72.Gajewski E, Rao G, Nackerdien Z, Dizdaroglu M. Modification of DNA bases in mammalian chromatin by radiation-generated free radicals. Biochemistry. 1990;29:7876–7882. doi: 10.1021/bi00486a014. [DOI] [PubMed] [Google Scholar]
- 73.Kawanishi S, Oikawa S. Mechanism of telomere shortening by oxidative stress. Ann NY Acad Sci. 2004;1019:278–284. doi: 10.1196/annals.1297.047. [DOI] [PubMed] [Google Scholar]
- 74.Fortini P, et al. 8-Oxoguanine DNA damage: at the crossroad of alternative repair pathways. Mutat Res. 2003;531:127–139. doi: 10.1016/j.mrfmmm.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 75.Wilson DM, 3rd, Bohr VA. The mechanics of base excision repair, and its relationship to aging and disease. DNA Repair (Amst) 2007;6:544–559. doi: 10.1016/j.dnarep.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 76.Sitte N, Saretzki G, von Zglinicki T. Accelerated telomere shortening in fibroblasts after extended periods of confluency. Free Radic Biol Med. 1998;24:885–893. doi: 10.1016/s0891-5849(97)00363-8. [DOI] [PubMed] [Google Scholar]
- 77.Petersen S, Saretzki G, von Zglinicki T. Preferential accumulation of single-stranded regions in telomeres of human fibroblasts. Exp Cell Res. 1998;239:152–160. doi: 10.1006/excr.1997.3893. [DOI] [PubMed] [Google Scholar]
- 78.Kruk PA, Rampino NJ, Bohr VA. DNA damage and repair in telomeres: relation to aging. Proc Natl Acad Sci USA. 1995;92:258–262. doi: 10.1073/pnas.92.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Webb CJ, Wu Y, Zakian VA. DNA repair at telomeres: keeping the ends intact. Cold Spring Harb Perspect Biol. 2013;5:a012666. doi: 10.1101/cshperspect.a012666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rhodes D, Lipps HJ. G-Quadruplexes and their regulatory roles in biology. Nucleic Acids Res. 2015;43:8627–8637. doi: 10.1093/nar/gkv862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sfeir A, et al. Mammalian telomeres resemble fragile sites and require TRF1 for efficient replication. Cell. 2009;138:90–103. doi: 10.1016/j.cell.2009.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.von Zglinicki T. Oxidative stress shortens telomeres. Trends Biochem Sci. 2002;27:339–344. doi: 10.1016/s0968-0004(02)02110-2. [DOI] [PubMed] [Google Scholar]
- 83.Mittal M, Siddiqui MR, Tran K, Reddy SP, Malik AB. Reactive oxygen species in inflammation and tissue injury. Antioxid Redox Signal. 2014;20:1126–1167. doi: 10.1089/ars.2012.5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 85.De Marzo AM, et al. Inflammation in prostate carcinogenesis. Nat Rev Cancer. 2007;7:256–269. doi: 10.1038/nrc2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sfanos KS, De Marzo AM. Prostate cancer and inflammation: the evidence. Histopathology. 2012;60:199–215. doi: 10.1111/j.1365-2559.2011.04033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gurel B, et al. Chronic inflammation in benign prostate tissue is associated with high-grade prostate cancer in the placebo arm of the prostate cancer prevention trial. Cancer Epidemiol Biomarkers Prev. 2014;23:847–856. doi: 10.1158/1055-9965.EPI-13-1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Vidal AC, et al. Aspirin, NSAIDs, and risk of prostate cancer: results from the REDUCE study. Clin Cancer Res. 2015;21:756–762. doi: 10.1158/1078-0432.CCR-14-2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Stimac G, et al. Aggressiveness of inflammation in histological prostatitis—correlation with total and free prostate specific antigen levels in men with biochemical criteria for prostate biopsy. Scott Med J. 2009;54:8–12. doi: 10.1258/RSMSMJ.54.3.8. [DOI] [PubMed] [Google Scholar]
- 90.Fujita K, et al. Prostatic inflammation detected in initial biopsy specimens and urinary pyuria are predictors of negative repeat prostate biopsy. J Urol. 2011;185:1722–1727. doi: 10.1016/j.juro.2010.12.058. [DOI] [PubMed] [Google Scholar]
- 91.Delongchamps NB, et al. Evaluation of prostatitis in autopsied prostates—is chronic inflammation more associated with benign prostatic hyperplasia or cancer? J Urol. 2008;179:1736–1740. doi: 10.1016/j.juro.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nickel JC, Downey J, Young I, Boag S. Asymptomatic inflammation and/or infection in benign prostatic hyperplasia. BJU Int. 1999;84:976–981. doi: 10.1046/j.1464-410x.1999.00352.x. [DOI] [PubMed] [Google Scholar]
- 93.Nickel JC, et al. The relationship between prostate inflammation and lower urinary tract symptoms: examination of baseline data from the REDUCE trial. Eur Urol. 2008;54:1379–1384. doi: 10.1016/j.eururo.2007.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sfanos KS, Isaacs WB, De Marzo AM. Infections and inflammation in prostate cancer. Am J Clin Exp Urol. 2013;1:3–11. [PMC free article] [PubMed] [Google Scholar]
- 95.Sfanos KS, Wilson BA, De Marzo AM, Isaacs WB. Acute inflammatory proteins constitute the organic matrix of prostatic corpora amylacea and calculi in men with prostate cancer. Proc Natl Acad Sci USA. 2009;106:3443–3448. doi: 10.1073/pnas.0810473106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.De Marzo AM, Marchi VL, Epstein JI, Nelson WG. Proliferative inflammatory atrophy of the prostate: implications for prostatic carcinogenesis. Am J Pathol. 1999;155:1985–1992. doi: 10.1016/S0002-9440(10)65517-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Feneley MR, Young MP, Chinyama C, Kirby RS, Parkinson MC. Ki-67 expression in early prostate cancer and associated pathological lesions. J Clin Pathol. 1996;49:741–748. doi: 10.1136/jcp.49.9.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ruska KM, Sauvageot J, Epstein JI. Histology and cellular kinetics of prostatic atrophy. Am J Surg Pathol. 1998;22:1073–1077. doi: 10.1097/00000478-199809000-00005. [DOI] [PubMed] [Google Scholar]
- 99.van Leenders GJ, et al. Intermediate cells in human prostate epithelium are enriched in proliferative inflammatory atrophy. Am J Pathol. 2003;162:1529–1537. doi: 10.1016/S0002-9440(10)64286-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Putzi MJ, De Marzo AM. Morphologic transitions between proliferative inflammatory atrophy and high-grade prostatic intraepithelial neoplasia. Urology. 2000;56:828–832. doi: 10.1016/s0090-4295(00)00776-7. [DOI] [PubMed] [Google Scholar]
- 101.Aizer AA, et al. Lack of reduction in racial disparities in cancer-specific mortality over a 20-year period. Cancer. 2014;120:1532–1539. doi: 10.1002/cncr.28617. [DOI] [PubMed] [Google Scholar]
- 102.Eastham JA, et al. Clinical characteristics and biopsy specimen features in African-American and white men without prostate cancer. J Natl Cancer Inst. 1998;90:756–760. doi: 10.1093/jnci/90.10.756. [DOI] [PubMed] [Google Scholar]
- 103.Peto J. Cancer epidemiology in the last century and the next decade. Nature. 2001;411:390–395. doi: 10.1038/35077256. [DOI] [PubMed] [Google Scholar]
- 104.Hsu A, Bray TM, Ho E. Anti-inflammatory activity of soy and tea in prostate cancer prevention. Exp Biol Med (Maywood) 2010;235:659–667. doi: 10.1258/ebm.2010.009335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Heaphy CM, et al. Prostate cancer cell telomere length variability and stromal cell telomere length as prognostic markers for metastasis and death. Cancer Discov. 2013;3:1130–1141. doi: 10.1158/2159-8290.CD-13-0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.De Nunzio C, Presicce F, Tubaro A. Inflammatory mediators in the development and progression of benign prostatic hyperplasia. Nat Rev Urol. 2016;13:613–626. doi: 10.1038/nrurol.2016.168. [DOI] [PubMed] [Google Scholar]
- 107.Cookson MS, Reuter VE, Linkov I, Fair WR. Glutathione S-transferase PI (GST-pi) class expression by immunohistochemistry in benign and malignant prostate tissue. J Urol. 1997;157:673–676. [PubMed] [Google Scholar]
- 108.Lee WH, et al. Cytidine methylation of regulatory sequences near the pi-class glutathione S-transferase gene accompanies human prostatic carcinogenesis. Proc Natl Acad Sci USA. 1994;91:11733–11737. doi: 10.1073/pnas.91.24.11733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mian OY, et al. GSTP1 Loss results in accumulation of oxidative DNA base damage and promotes prostate cancer cell survival following exposure to protracted oxidative stress. Prostate. 2016;76:199–206. doi: 10.1002/pros.23111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kanwal R, et al. Protection against oxidative DNA damage and stress in human prostate by glutathione S-transferase P1. Mol Carcinog. 2014;53:8–18. doi: 10.1002/mc.21939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Stephens PJ, et al. Massive genomic rearrangement acquired in a single catastrophic event during cancer development. Cell. 2011;144:27–40. doi: 10.1016/j.cell.2010.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Malhotra A, et al. Breakpoint profiling of 64 cancer genomes reveals numerous complex rearrangements spawned by homology-independent mechanisms. Genome Res. 2013;23:762–776. doi: 10.1101/gr.143677.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zack TI, et al. Pan-cancer patterns of somatic copy number alteration. Nat Genet. 2013;45:1134–1140. doi: 10.1038/ng.2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Storchova Z, Kloosterman WP. The genomic characteristics and cellular origin of chromothripsis. Curr Opin Cell Biol. 2016;40:106–113. doi: 10.1016/j.ceb.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 115.Rausch T, et al. Genome sequencing of pediatric medulloblastoma links catastrophic DNA rearrangements with TP53 mutations. Cell. 2012;148:59–71. doi: 10.1016/j.cell.2011.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Molenaar JJ, et al. Sequencing of neuroblastoma identifies chromothripsis and defects in neuritogenesis genes. Nature. 2012;483:589–593. doi: 10.1038/nature10910. [DOI] [PubMed] [Google Scholar]
- 117.Wu C, et al. Poly-gene fusion transcripts and chromothripsis in prostate cancer. Genes Chromosomes Cancer. 2012;51:1144–1153. doi: 10.1002/gcc.21999. [DOI] [PubMed] [Google Scholar]
- 118.Maciejowski J, Li Y, Bosco N, Campbell PJ, de Lange T. Chromothripsis and kataegis induced by telomere crisis. Cell. 2015;163:1641–1654. doi: 10.1016/j.cell.2015.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kovtun IV, Murphy SJ, Johnson SH, Cheville JC, Vasmatzis G. Chromosomal catastrophe is a frequent event in clinically insignificant prostate cancer. Oncotarget. 2015;6:29087–29096. doi: 10.18632/oncotarget.4900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tu L, et al. Widespread telomere instability in prostatic lesions. Mol Carcinog. 2016;55:842–852. doi: 10.1002/mc.22326. [DOI] [PubMed] [Google Scholar]
- 121.Feijoo P, Dominguez D, Tusell L, Genesca A. Telomere-dependent genomic integrity: evolution of the fusion-bridge-breakage cycle concept. Curr Pharm Des. 2014;20:6375–6385. doi: 10.2174/1381612820666140630085416. [DOI] [PubMed] [Google Scholar]
- 122.Vukovic B, et al. Correlating breakage-fusion-bridge events with the overall chromosomal instability and in vitro karyotype evolution in prostate cancer. Cytogenet Genome Res. 2007;116:1–11. doi: 10.1159/000097411. [DOI] [PubMed] [Google Scholar]
- 123.Xi L, Cech TR. Inventory of telomerase components in human cells reveals multiple subpopulations of hTR and hTERT. Nucleic Acids Res. 2014;42:8565–8577. doi: 10.1093/nar/gku560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Cohen SB, et al. Protein composition of catalytically active human telomerase from immortal cells. Science. 2007;315:1850–1853. doi: 10.1126/science.1138596. [DOI] [PubMed] [Google Scholar]
- 125.Kilian A, et al. Isolation of a candidate human telomerase catalytic subunit gene, which reveals complex splicing patterns in different cell types. Hum Mol Genet. 1997;6:2011–2019. doi: 10.1093/hmg/6.12.2011. [DOI] [PubMed] [Google Scholar]
- 126.Nakamura TM, et al. Telomerase catalytic subunit homologs from fission yeast and human. Science. 1997;277:955–959. doi: 10.1126/science.277.5328.955. [DOI] [PubMed] [Google Scholar]
- 127.Bodnar AG, et al. Extension of life-span by introduction of telomerase into normal human cells. Science. 1998;279:349–352. doi: 10.1126/science.279.5349.349. [DOI] [PubMed] [Google Scholar]
- 128.Counter CM, et al. Telomerase activity is restored in human cells by ectopic expression of hTERT (hEST2), the catalytic subunit of telomerase. Oncogene. 1998;16:1217–1222. doi: 10.1038/sj.onc.1201882. [DOI] [PubMed] [Google Scholar]
- 129.Vaziri H, Benchimol S. Reconstitution of telomerase activity in normal human cells leads to elongation of telomeres and extended replicative life span. Curr Biol. 1998;8:279–282. doi: 10.1016/s0960-9822(98)70109-5. [DOI] [PubMed] [Google Scholar]
- 130.Nieto CM, Rider LC, Cramer SD. Influence of stromal-epithelial interactions on androgen action. Endocr Relat Cancer. 2014;21:T147–160. doi: 10.1530/ERC-14-0138. [DOI] [PubMed] [Google Scholar]
- 131.Meeker AK, Sommerfeld HJ, Coffey DS. Telomerase is activated in the prostate and seminal vesicles of the castrated rat. Endocrinology. 1996;137:5743–5746. doi: 10.1210/endo.137.12.8940411. [DOI] [PubMed] [Google Scholar]
- 132.Ravindranath N, et al. Androgen depletion activates telomerase in the prostate of the nonhuman primate, Macaca mulatta. Prostate. 2001;49:79–89. doi: 10.1002/pros.1120. [DOI] [PubMed] [Google Scholar]
- 133.Moehren U, et al. Wild-type but not mutant androgen receptor inhibits expression of the hTERT telomerase subunit: a novel role of AR mutation for prostate cancer development. FASEB J. 2008;22:1258–1267. doi: 10.1096/fj.07-9360com. [DOI] [PubMed] [Google Scholar]
- 134.Guo C, Armbruster BN, Price DT, Counter CM. In vivo regulation of hTERT expression and telomerase activity by androgen. J Urol. 2003;170:615–618. doi: 10.1097/01.ju.0000074653.22766.c8. [DOI] [PubMed] [Google Scholar]
- 135.Cho SD, et al. Methyl selenium metabolites decrease prostate-specific antigen expression by inducing protein degradation and suppressing androgen-stimulated transcription. Mol Cancer Ther. 2004;3:605–611. [PubMed] [Google Scholar]
- 136.Liu S, et al. Telomerase as an important target of androgen signaling blockade for prostate cancer treatment. Mol Cancer Ther. 2010;9:2016–2025. doi: 10.1158/1535-7163.MCT-09-0924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Renaud S, et al. Dual role of DNA methylation inside and outside of CTCF-binding regions in the transcriptional regulation of the telomerase hTERT gene. Nucleic Acids Res. 2007;35:1245–1256. doi: 10.1093/nar/gkl1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Killela PJ, et al. TERT promoter mutations occur frequently in gliomas and a subset of tumors derived from cells with low rates of self-renewal. Proc Natl Acad Sci USA. 2013;110:6021–6026. doi: 10.1073/pnas.1303607110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Vinagre J, et al. Frequency of TERT promoter mutations in human cancers. Nat Commun. 2013;4:2185. doi: 10.1038/ncomms3185. [DOI] [PubMed] [Google Scholar]
- 140.Stoehr R, et al. Frequency of TERT Promoter Mutations in Prostate Cancer. Pathobiology. 2015;82:53–57. doi: 10.1159/000381903. [DOI] [PubMed] [Google Scholar]
- 141.Gurel B, et al. Nuclear MYC protein overexpression is an early alteration in human prostate carcinogenesis. Mod Pathol. 2008;21:1156–1167. doi: 10.1038/modpathol.2008.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lapointe J, et al. Gene expression profiling identifies clinically relevant subtypes of prostate cancer. Proc Natl Acad Sci USA. 2004;101:811–816. doi: 10.1073/pnas.0304146101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Dhanasekaran SM, et al. Molecular profiling of human prostate tissues: insights into gene expression patterns of prostate development during puberty. FASEB J. 2005;19:243–245. doi: 10.1096/fj.04-2415fje. [DOI] [PubMed] [Google Scholar]
- 144.Varambally S, et al. Integrative genomic and proteomic analysis of prostate cancer reveals signatures of metastatic progression. Cancer Cell. 2005;8:393–406. doi: 10.1016/j.ccr.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 145.Tomlins SA, et al. Integrative molecular concept modeling of prostate cancer progression. Nat Genet. 2007;39:41–51. doi: 10.1038/ng1935. [DOI] [PubMed] [Google Scholar]
- 146.Yu YP, et al. Gene expression alterations in prostate cancer predicting tumor aggression and preceding development of malignancy. J Clin Oncol. 2004;22:2790–2799. doi: 10.1200/JCO.2004.05.158. [DOI] [PubMed] [Google Scholar]
- 147.Latil A, et al. htert expression correlates with MYC over-expression in human prostate cancer. Int J Cancer. 2000;89:172–176. [PubMed] [Google Scholar]
- 148.Wu KJ, et al. Direct activation of TERT transcription by c-MYC. Nat Genet. 1999;21:220–224. doi: 10.1038/6010. [DOI] [PubMed] [Google Scholar]
- 149.Kyo S, et al. Sp1 cooperates with c-Myc to activate transcription of the human telomerase reverse transcriptase gene (hTERT) Nucleic Acids Res. 2000;28:669–677. doi: 10.1093/nar/28.3.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Jenkins RB, Qian J, Lieber MM, Bostwick DG. Detection of c-myc oncogene amplification and chromosomal anomalies in metastatic prostatic carcinoma by fluorescence in situ hybridization. Cancer Res. 1997;57:524–531. [PubMed] [Google Scholar]
- 151.Nupponen NN, Kakkola L, Koivisto P, Visakorpi T. Genetic alterations in hormone-refractory recurrent prostate carcinomas. Am J Pathol. 1998;153:141–148. doi: 10.1016/S0002-9440(10)65554-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Koh CM, et al. MYC and Prostate Cancer. Genes Cancer. 2010;1:617–628. doi: 10.1177/1947601910379132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Andriole GL, et al. Mortality results from a randomized prostate-cancer screening trial. N Engl J Med. 2009;360:1310–1319. doi: 10.1056/NEJMoa0810696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Schroder FH, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360:1320–1328. doi: 10.1056/NEJMoa0810084. [DOI] [PubMed] [Google Scholar]
- 155.Morin GB. The human telomere terminal transferase enzyme is a ribonucleoprotein that synthesizes TTAGGG repeats. Cell. 1989;59:521–529. doi: 10.1016/0092-8674(89)90035-4. [DOI] [PubMed] [Google Scholar]
- 156.Kim NW, et al. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994;266:2011–2015. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- 157.Zhou X, Xing D. Assays for human telomerase activity: progress and prospects. Chem Soc Rev. 2012;41:4643–4656. doi: 10.1039/c2cs35045a. [DOI] [PubMed] [Google Scholar]
- 158.Fizazi K, et al. High detection rate of circulating tumor cells in blood of patients with prostate cancer using telomerase activity. Ann Oncol. 2007;18:518–521. doi: 10.1093/annonc/mdl419. [DOI] [PubMed] [Google Scholar]
- 159.Xu T, Lu B, Tai YC, Goldkorn A. A cancer detection platform which measures telomerase activity from live circulating tumor cells captured on a microfilter. Cancer Res. 2010;70:6420–6426. doi: 10.1158/0008-5472.CAN-10-0686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Goldkorn A, et al. Circulating tumor cell telomerase activity as a prognostic marker for overall survival in SWOG 0421: a phase III metastatic castration resistant prostate cancer trial. Int J Cancer. 2015;136:1856–1862. doi: 10.1002/ijc.29212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Heaphy CM, et al. Prostate stromal cell telomere shortening is associated with risk of prostate cancer in the placebo arm of the Prostate Cancer Prevention Trial. Prostate. 2015;75:1160–1166. doi: 10.1002/pros.22997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Loeb S, et al. Overdiagnosis and overtreatment of prostate cancer. Eur Urol. 2014;65:1046–1055. doi: 10.1016/j.eururo.2013.12.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Shay JW, Reddel RR, Wright WE. Cancer. Cancer and telomeres—an ALTernative to telomerase. Science. 2012;336:1388–1390. doi: 10.1126/science.1222394. [DOI] [PubMed] [Google Scholar]
- 164.Shay JW, Zou Y, Hiyama E, Wright WE. Telomerase and cancer. Hum Mol Genet. 2001;10:677–685. doi: 10.1093/hmg/10.7.677. [DOI] [PubMed] [Google Scholar]
- 165.Williams SC. No end in sight for telomerase-targeted cancer drugs. Nat Med. 2013;19:6. doi: 10.1038/nm0113-6. [DOI] [PubMed] [Google Scholar]
- 166.Jafri MA, Ansari SA, Alqahtani MH, Shay JW. Roles of telomeres and telomerase in cancer, and advances in telomerase-targeted therapies. Genome Med. 2016;8:69. doi: 10.1186/s13073-016-0324-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Chiappori AA, et al. A randomized phase II study of the telomerase inhibitor imetelstat as maintenance therapy for advanced non-small-cell lung cancer. Ann Oncol. 2015;26:354–362. doi: 10.1093/annonc/mdu550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Baerlocher GM, et al. Telomerase inhibitor imetelstat in patients with essential thrombocythemia. N Engl J Med. 2015;373:920–928. doi: 10.1056/NEJMoa1503479. [DOI] [PubMed] [Google Scholar]
- 169.Rousseau P, Autexier C. Telomere biology: Rationale for diagnostics and therapeutics in cancer. RNA Biol. 2015;12:1078–1082. doi: 10.1080/15476286.2015.1081329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Marian CO, Wright WE, Shay JW. The effects of telomerase inhibition on prostate tumor-initiating cells. Int J Cancer. 2010;127:321–331. doi: 10.1002/ijc.25043. [DOI] [PubMed] [Google Scholar]
- 171.Kawashima T, et al. Telomerase-specific replication-selective virotherapy for human cancer. Clin Cancer Res. 2004;10:285–292. doi: 10.1158/1078-0432.ccr-1075-3. [DOI] [PubMed] [Google Scholar]
- 172.Nemunaitis J, et al. A phase I study of telomerase-specific replication competent oncolytic adenovirus (telomelysin) for various solid tumors. Mol Ther. 2010;18:429–434. doi: 10.1038/mt.2009.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Middleton G, et al. Gemcitabine and capecitabine with or without telomerase peptide vaccine GV1001 in patients with locally advanced or metastatic pancreatic cancer (TeloVac): an open-label, randomised, phase 3 trial. Lancet Oncol. 2014;15:829–840. doi: 10.1016/S1470-2045(14)70236-0. [DOI] [PubMed] [Google Scholar]
- 174.Robinson D, et al. Integrative clinical genomics of advanced prostate cancer. Cell. 2015;161:1215–1228. doi: 10.1016/j.cell.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Artandi SE, DePinho RA. Telomeres and telomerase in cancer. Carcinogenesis. 2010;31:9–18. doi: 10.1093/carcin/bgp268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Smith LL, Coller HA, Roberts JM. Telomerase modulates expression of growth-controlling genes and enhances cell proliferation. Nat Cell Biol. 2003;5:474–479. doi: 10.1038/ncb985. [DOI] [PubMed] [Google Scholar]
- 177.Park JI, et al. Telomerase modulates Wnt signalling by association with target gene chromatin. Nature. 2009;460:66–72. doi: 10.1038/nature08137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Choi J, et al. TERT promotes epithelial proliferation through transcriptional control of a Myc- and Wnt-related developmental program. PLoS Genet. 2008;4:e10. doi: 10.1371/journal.pgen.0040010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Jagadeesh S, Banerjee PP. Telomerase reverse transcriptase regulates the expression of a key cell cycle regulator, cyclin D1. Biochem Biophys Res Commun. 2006;347:774–780. doi: 10.1016/j.bbrc.2006.06.172. [DOI] [PubMed] [Google Scholar]
- 180.Beck S, et al. Telomerase activity-independent function of TERT allows glioma cells to attain cancer stem cell characteristics by inducing EGFR expression. Mol Cells. 2011;31:9–15. doi: 10.1007/s10059-011-0008-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Liu Z, et al. Telomerase reverse transcriptase promotes epithelial-mesenchymal transition and stem cell-like traits in cancer cells. Oncogene. 2013;32:4203–4213. doi: 10.1038/onc.2012.441. [DOI] [PubMed] [Google Scholar]
- 182.Imamura Y, Sadar MD. Androgen receptor targeted therapies in castration-resistant prostate cancer: bench to clinic. Int J Urol. 2016;23:654–65. doi: 10.1111/iju.13137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Hu J, et al. Antitelomerase therapy provokes ALT and mitochondrial adaptive mechanisms in cancer. Cell. 2012;148:651–663. doi: 10.1016/j.cell.2011.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Rothkamm K, et al. DNA damage foci: meaning and significance. Environ Mol Mutag. 2015;56:491–504. doi: 10.1002/em.21944. [DOI] [PubMed] [Google Scholar]
- 185.Cesare AJ, et al. Spontaneous occurrence of telomeric DNA damage response in the absence of chromosome fusions. Nat Struct Mol Biol. 2009;16:1244–1251. doi: 10.1038/nsmb.1725. [DOI] [PubMed] [Google Scholar]
- 186.Silvestre DC, et al. Alternative lengthening of telomeres in human glioma stem cells. Stem Cells. 2011;29:440–451. doi: 10.1002/stem.600. [DOI] [PubMed] [Google Scholar]
- 187.Flynn RL, et al. Alternative lengthening of telomeres renders cancer cells hypersensitive to ATR inhibitors. Science. 2015;347:273–277. doi: 10.1126/science.1257216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Kim SH, et al. Androgen receptor interacts with telomeric proteins in prostate cancer cells. J Biol Chem. 2010;285:10472–10476. doi: 10.1074/jbc.M109.098798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Hayashi MT, Cesare AJ, Rivera T, Karlseder J. Cell death during crisis is mediated by mitotic telomere deprotection. Nature. 2015;522:492–496. doi: 10.1038/nature14513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Xu T, He K, Wang L, Goldkorn A. Prostate tumor cells with cancer progenitor properties have high telomerase activity and are rapidly killed by telomerase interference. Prostate. 2011;71:1390–1400. doi: 10.1002/pros.21355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Muller S, Rodriguez R. G-Quadruplex interacting small molecules and drugs: from bench toward bedside. Expert Rev Clin Pharmacol. 2014;7:663–679. doi: 10.1586/17512433.2014.945909. [DOI] [PubMed] [Google Scholar]
- 192.Rizzo A, Salvati E, Biroccio A. Methods of studying telomere damage induced by quadruplex-ligand complexes. Methods. 2012;57:93–99. doi: 10.1016/j.ymeth.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 193.Reddy V, et al. ATM Inhibition Potentiates Death of Androgen Receptor-inactivated Prostate Cancer Cells with Telomere Dysfunction. J Biol Chem. 2015;290:25522–25533. doi: 10.1074/jbc.M115.671404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Kim SH, et al. Telomere dysfunction and cell survival: roles for distinct TIN2-containing complexes. J Cell Biol. 2008;181:447–460. doi: 10.1083/jcb.200710028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Zhou J, et al. Structural and functional association of androgen receptor with telomeres in prostate cancer cells. Aging (Albany NY) 2013;5:3–17. doi: 10.18632/aging.100524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Fan X, et al. hTERT gene amplification and increased mRNA expression in central nervous system embryonal tumors. Am J Pathol. 2003;162:1763–1769. doi: 10.1016/S0002-9440(10)64311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Zhang A, et al. Amplification of the telomerase reverse transcriptase (hTERT) gene in cervical carcinomas. Genes Chromosomes Cancer. 2002;34:269–275. doi: 10.1002/gcc.10071. [DOI] [PubMed] [Google Scholar]
- 198.Zhu CQ, et al. Amplification of telomerase (hTERT) gene is a poor prognostic marker in non-small-cell lung cancer. Br J Cancer. 2006;94:1452–1459. doi: 10.1038/sj.bjc.6603110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Totoki Y, et al. Trans-ancestry mutational landscape of hepatocellular carcinoma genomes. Nat Genet. 2014;46:1267–1273. doi: 10.1038/ng.3126. [DOI] [PubMed] [Google Scholar]
- 200.Peifer M, et al. Telomerase activation by genomic rearrangements in high-risk neuroblastoma. Nature. 2015;526:700–704. doi: 10.1038/nature14980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Valentijn LJ, et al. TERT rearrangements are frequent in neuroblastoma and identify aggressive tumors. Nat Genet. 2015;47:1411–1414. doi: 10.1038/ng.3438. [DOI] [PubMed] [Google Scholar]
- 202.Castelo-Branco P, et al. Methylation of the TERT promoter and risk stratification of childhood brain tumours: an integrative genomic and molecular study. Lancet Oncol. 2013;14:534–542. doi: 10.1016/S1470-2045(13)70110-4. [DOI] [PubMed] [Google Scholar]
- 203.Bethel CR, et al. Decreased NKX3.1 protein expression in focal prostatic atrophy, prostatic intraepithelial neoplasia, and adenocarcinoma: association with gleason score and chromosome 8p deletion. Cancer Res. 2006;66:10683–10690. doi: 10.1158/0008-5472.CAN-06-0963. [DOI] [PubMed] [Google Scholar]
- 204.Bernhardt SL, et al. Telomerase peptide vaccination of patients with non-resectable pancreatic cancer: a dose escalating phase I/II study. Br J Cancer. 2006;95:1474–1482. doi: 10.1038/sj.bjc.6603437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Huang P, et al. Direct and distant antitumor effects of a telomerase-selective oncolytic adenoviral agent, OBP-301, in a mouse prostate cancer model. Cancer Gene Ther. 2008;15:315–322. doi: 10.1038/cgt.2008.3. [DOI] [PubMed] [Google Scholar]
- 206.Drygin D, et al. Anticancer activity of CX-3543: a direct inhibitor of rRNA biogenesis. Cancer Res. 2009;69:7653–7661. doi: 10.1158/0008-5472.CAN-09-1304. [DOI] [PubMed] [Google Scholar]
- 207.Balasubramanian S, Hurley LH, Neidle S. Targeting G-quadruplexes in gene promoters: a novel anticancer strategy? Nat Rev Drug Discov. 2011;10:261–275. doi: 10.1038/nrd3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Hasegawa D, et al. G-Quadruplex ligand-induced DNA damage response coupled with telomere dysfunction and replication stress in glioma stem cells. Biochem Biophys Res Commun. 2016;471:75–81. doi: 10.1016/j.bbrc.2016.01.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Grand CL, et al. The cationic porphyrin TMPyP4 down-regulates c-MYC and human telomerase reverse transcriptase expression and inhibits tumor growth in vivo. Mol Cancer Ther. 2002;1:565–573. [PubMed] [Google Scholar]
- 210.Salvati E, et al. Telomere damage induced by the G-quadruplex ligand RHPS4 has an antitumor effect. J Clin Invest. 2007;117:3236–3247. doi: 10.1172/JCI32461. [DOI] [PMC free article] [PubMed] [Google Scholar]